- Department of Rehabilitation Medicine, Changi General Hospital, Singapore, Singapore
Aim: This review aimed to evaluate the effectiveness and feasibility of cancer prehabilitation programs delivered through technological enablers compared to conventional face-to-face interventions.
Methods: A systematic review was conducted, searching PubMed, Embase, and CINAHL for studies published from inception to February 6, 2024. Studies were included if they involved adult cancer patients in primary research, utilized technology for prehabilitation, and assessed functional, psychological, and quality of life outcomes.
Results: Sixteen studies were included, encompassing wearables, apps, teleprehabilitation, and virtual reality. All studies reported feasibility, but challenges included technical issues, lack of supervision, and non-compliance. Effectiveness depended on intervention rigor and technology type. Wearables offered objective monitoring but faced compliance issues. Videoconferencing provided supervision and could mitigate compliance concerns. Multimodal programs and intervention-specific outcome measures were recommended.
Conclusion: Technology-based prehabilitation programs seem feasible, but effectiveness depends on intervention design and technology employed. Future research should focus on developing robust evidence to guide clinical practice and explore the potential of integrated technological solutions.
Systematic review registration: PROSPERO, identifier CRD42022376028.
Introduction
Cancer prehabilitation, defined as interventions delivered prior to the commencement of acute cancer treatments (surgery, chemotherapy, radiation therapy, or immunotherapy) (1), has seen an increase in the use of technology during the COVID-19 pandemic. This aligns with broader healthcare trends towards telemedicine and telehealth applications (2, 3). While some countries had established telehealth services pre-pandemic, facilitating easier scaling-up during the crisis (3), cancer prehabilitation also underwent adaptations (4, 5). This increased utilization of technology warrants a review of the different modes employed, their effectiveness, and feasibility.
Cancer prehabilitation programs can encompass single or multimodal interventions including exercise, nutritional guidance, and psychological support (6, 7). Specific diagnostic groups may benefit from additional targeted interventions such as respiratory muscle training and breathing exercises for pre-thoracic surgery or pelvic floor exercises and sexual well-being support in patients with prostate cancer (8, 9). Research also includes studies focusing solely on exercise-based (10) or nutritional interventions (11).
The pandemic necessitated adaptations to traditional in-person delivery models, shifting interactions to phone calls or web conferencing (12–14). Other approaches have implemented adaptive case management platforms integrated with electronic health records (15). Notably, a telehealth-delivered home-based prehabilitation program adapted from a face-to-face model has demonstrated feasibility and effectiveness (16). This raises questions about exploring other existing technologies, such as wearables and robotics, in cancer prehabilitation research.
Despite the increased use of technology in cancer prehabilitation, information on its efficacy, acceptability, and feasibility compared with conventional face-to-face interventions remains limited. While knowledge on telerehabilitation has existed for two decades (17), teleprehabilitation and other technology-enabled applications for remote prehabilitation are more recent developments (12). A systematic review of the cost-utility and cost-effectiveness of telemedicine, electronic, and mobile health systems yielded mixed results (18). Further understanding of diverse technology deployments and their impact on efficacy, acceptability, and feasibility is crucial before their widespread adoption.
Accessibility is another key consideration, particularly in areas with limited healthcare access due to geographical barriers or resource constraints. For patients already owning smartphones, technology-based interventions offer the potential to improve healthcare accessibility and reduce travel time, costs, and work disruptions (19, 20).
To date, no systematic review has comprehensively assessed the effectiveness and feasibility of cancer prehabilitation programs leveraging technological enablers. This review aims to address this gap.
The primary objective was to evaluate the effectiveness of cancer prehabilitation delivered through technological enablers compared with conventional face-to-face interventions or standard care. The secondary objective was to evaluate the feasibility of these technology-based programs.
Methods
Inclusion criteria
This systematic review included studies that met the following criteria.
1. Primary research (randomized and non-randomized experimental trials, cohort, or case-control studies).
2. Studied cancer prehabilitation as an intervention.
3. Applied cancer prehabilitation prior to surgical as well as non-surgical intervention (for example chemotherapy, radiation therapy, immunotherapy, hormonal therapy).
4. Utilised technology such as trackers, apps, telehealth, virtual or online platforms and robotic devices for cancer prehabilitation.
5. The participants were aged 18 years or older.
Both single and multimodal prehabilitation models were included due to the focus on technology use.
Exclusion criteria:
Studies were excluded if they were:
1. Presented as conference proceedings, poster abstracts, case reports or study protocols.
2. Animal studies.
3. Rehabilitation instead of prehabilitation studies.
Study registration
This protocol was developed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Protocols. This study was registered with the PROSPERO database, protocol number CRD42022376028.
Search methods
Population
Adult patients aged 18 years or older with cancer.
Interventions
The use of telehealth, wearables, smart phone applications, virtual or online platforms, robotics and virtual reality in the application of cancer prehabilitation, prior to any cancer surgery, chemotherapy, radiation therapy, immunotherapy, or hormonal therapy.
Comparison
Patients who received conventional face-to-face prehabilitation or standard care.
Outcomes
Functional, psychological, and quality of life domains.
Search Terms:
Searches were performed on PubMed, Embase, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) from inception until 6 Feb 2024.
The search strategy included “cancer” AND “prehabilitation” AND “technology”. We combined synonyms and MeSH terms with the “OR” operator (Table 1). This strategy was employed for PubMed and was adapted for use with other databases. In addition, we checked the reference lists of all the included trials and relevant systematic reviews to identify potentially eligible studies.
All titles and abstracts were reviewed for relevance. Full-text articles were read when they were eligible or when eligibility was unclear. Two independent reviewers (SST and FQZ) screened all articles to assess their eligibility. A third reviewer (EN) was available for discussion in case of disagreement.
Types of outcome measures
Primary outcomes
Physical function including the 6 min walk test (6MWT), time up and go test (TUG), and the 30 seconds sit-to-stand test (30 sec STS).
Psychological and quality of life outcomes were assessed using measures such as the Hospital Anxiety and Depression Scale (HADS) (21), EuroQol-5D (EQ5D) (22), and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C-30 (EORTC QLC-C30) (23).
Secondary outcomes
Outcome indicators such as length of stay, postsurgical complications, other patient-reported outcomes (PROM), readmission rates, safety, and feasibility will be reported if available.
Data extraction and outcomes
Data extraction of the included articles was conducted by two reviewers: SST and FQZ. FQZ collated the relevant features of each study using data extraction sheets and compiled them in a computerised database, which was counterchecked by SST for accuracy.
National Institutes of Health (NIH) quality assessment tools were deployed, and tailored quality assessment was applied separately for controlled interventions and for before-after (pre-post) studies with no control group (24). NIH tools facilitate a comprehensive assessment of a range of aspects of research quality, such as randomization, allocation, blinding, inter-group demographics, drop-outs, intervention adherence, missing data, and data analysis.
Each reviewer rated the studies as good, fair, or poor. No official published system is available for denoting the overall quality of NIH tools. As such, we deemed the individual quality indicators to have similar weights for internal validity and ascribed overall quality through cumulative scores. We followed this application in another review project (25). A cumulative score of <50% quality indicators present denoted a poor-quality study (significant risk of bias), while >80% present denoted a good-quality study (least risk of bias). A “fair” study (50-80% quality indicators present) would be susceptible to some bias deemed insufficient to invalidate its results. The range can be broad and each study would have its strength and weaknesses.
Two reviewers (SST and FQZ) were involved in the quality assessment and disagreements were escalated to a third reviewer (EN).
Statistical analysis
Data were qualitatively synthesized to evaluate changes in the three primary outcome domains (physical, psychological, and quality of life) between the pre- and post-intervention phases. Feasibility and safety of the interventions were also synthesized and reported. Attempts were made to contact the study authors if there were any cases of missing data. This study conformed to all Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and reported the required information accordingly.
Results
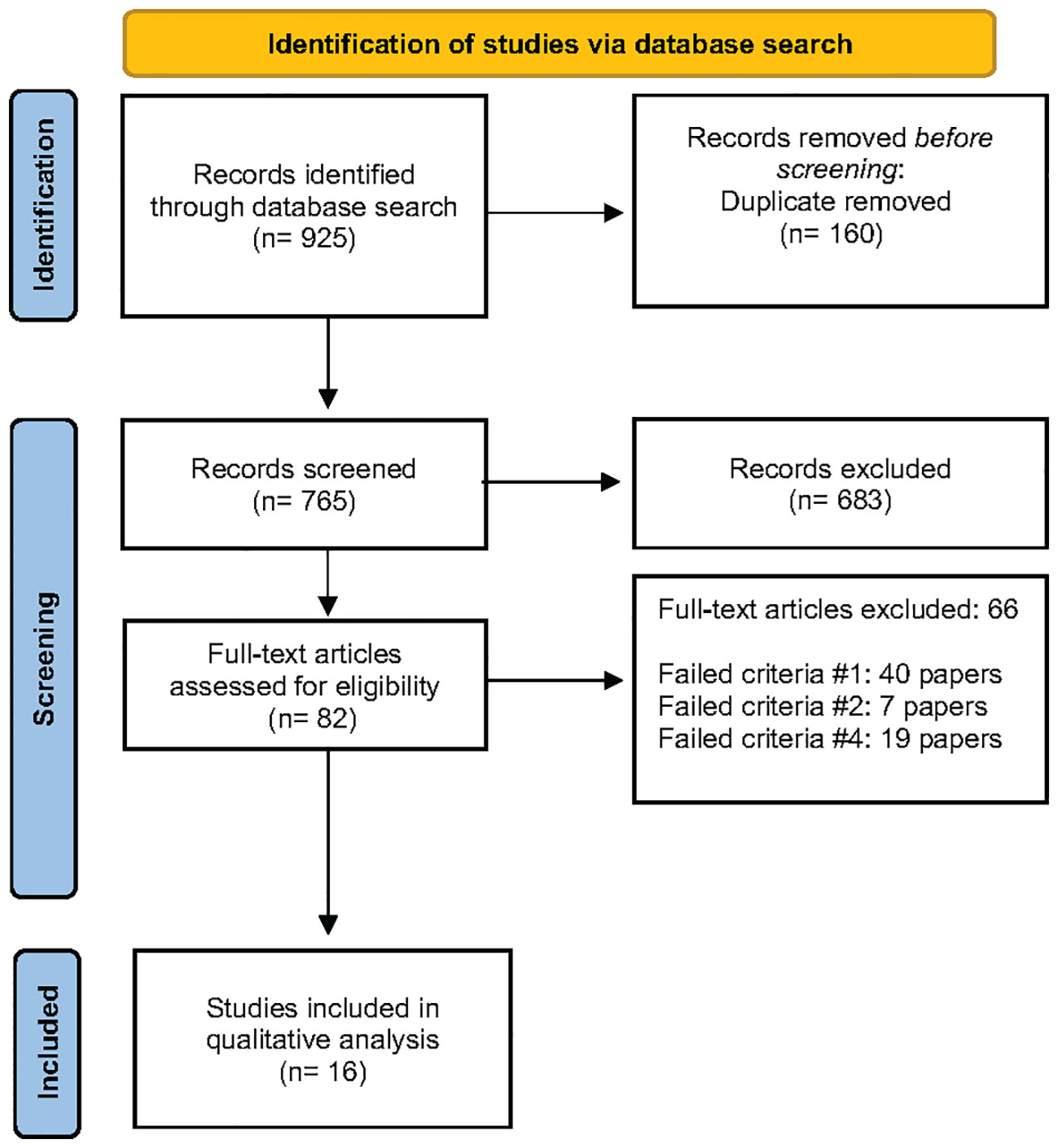
A total of 765 records were screened after the computer-assisted removal of duplicates. After screening the titles, 115 were chosen to be screened for abstracts, of which 82 were chosen to have the full-text articles assessed for eligibility. After a full-text review, 66 studies were excluded, and 16 were included in the qualitative synthesis. Forty papers failed criteria #1, (14, 19, 26–63), seven papers failed criteria #2, (64–70) and 19 papers failed criteria #4, (12, 17, 71–87) and all were excluded from the systematic review (Supplementary Table 1). The PRISMA flow diagram is found in Figure 1.
Methodological quality
Two studies initially categorized as cohort studies were reclassified as pre-post due to clear interventions with pre- and post-intervention data (88, 89). Most studies had small participant numbers (< 50), except for one (16) with 100 participants.
Among the 16 included studies, four were controlled intervention studies [one rated good (90), two fair (91, 92), and one poor (93)]. Twelve were pre-post studies [ten were fair with some bias risk (16, 88, 94–101) and two with significant risk (89, 102)]. Due to their heterogeneity and limited large-scale studies, three poor-quality studies were included in the qualitative analysis (Table 2).
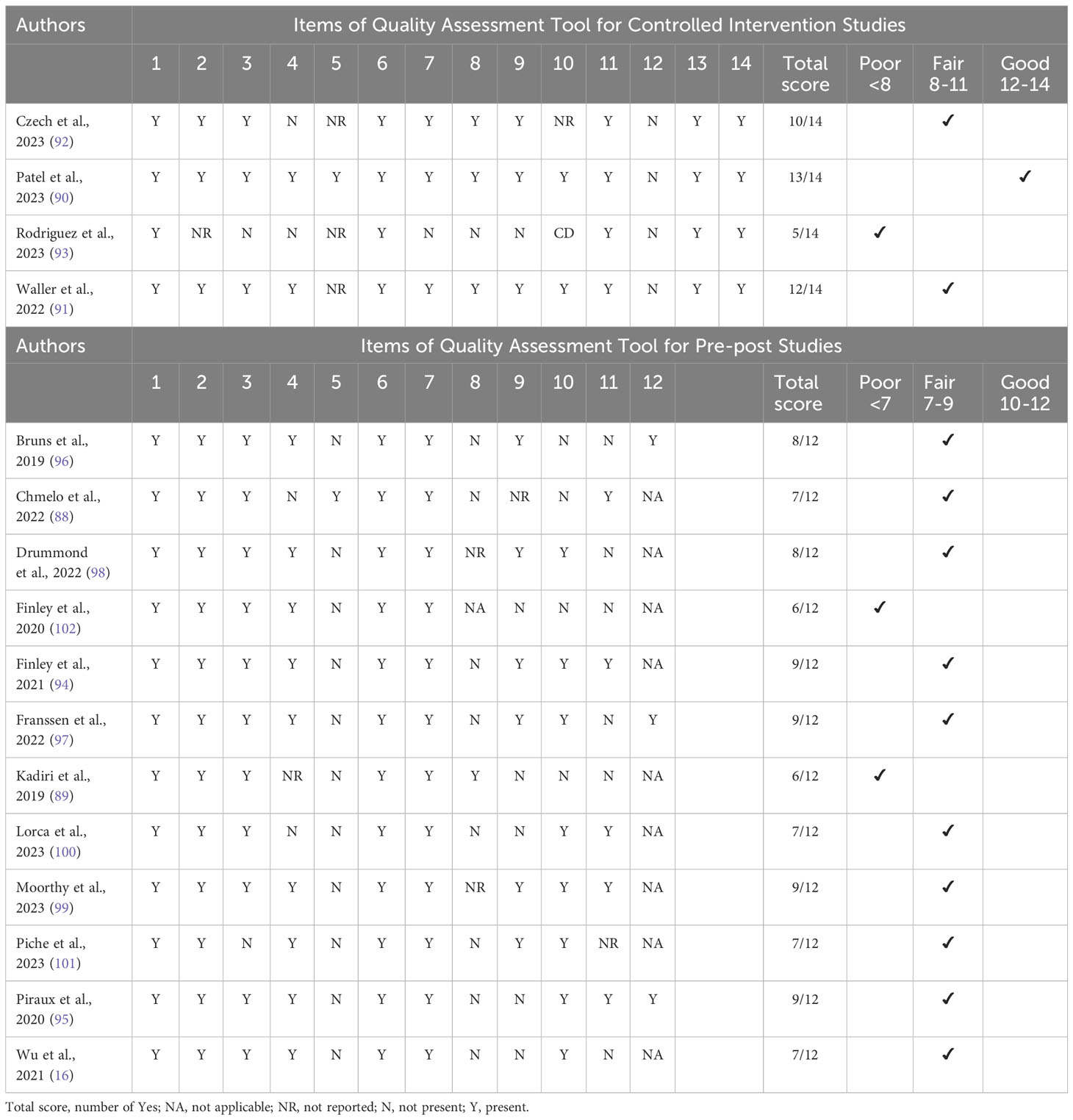
Table 2 Study quality assessed by quality assessment tools for controlled intervention and pre-post studies.
Included studies
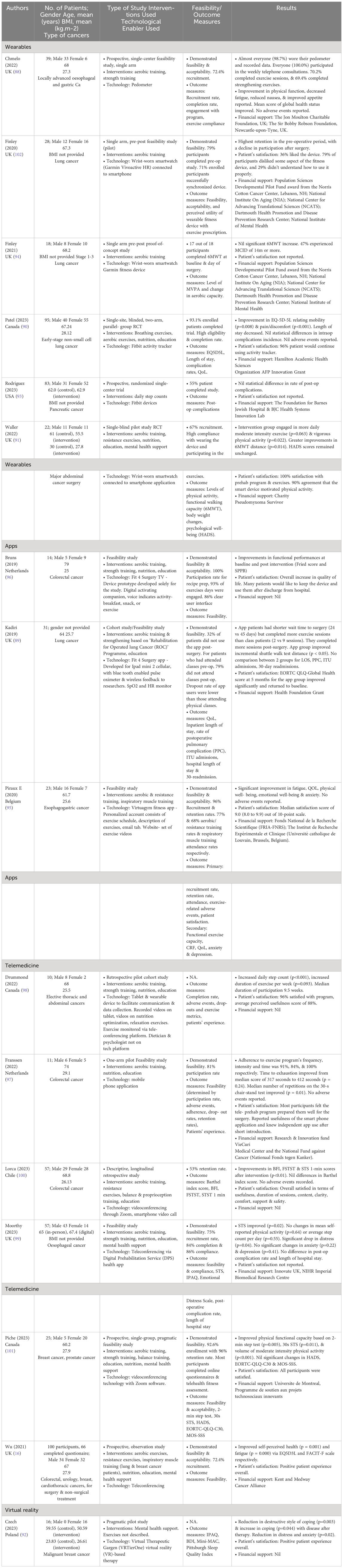
Of the 16 included studies, they were divided into 4 groups of technological enablers, namely wearables, apps, teleprehabilitation and virtual reality (Table 3).
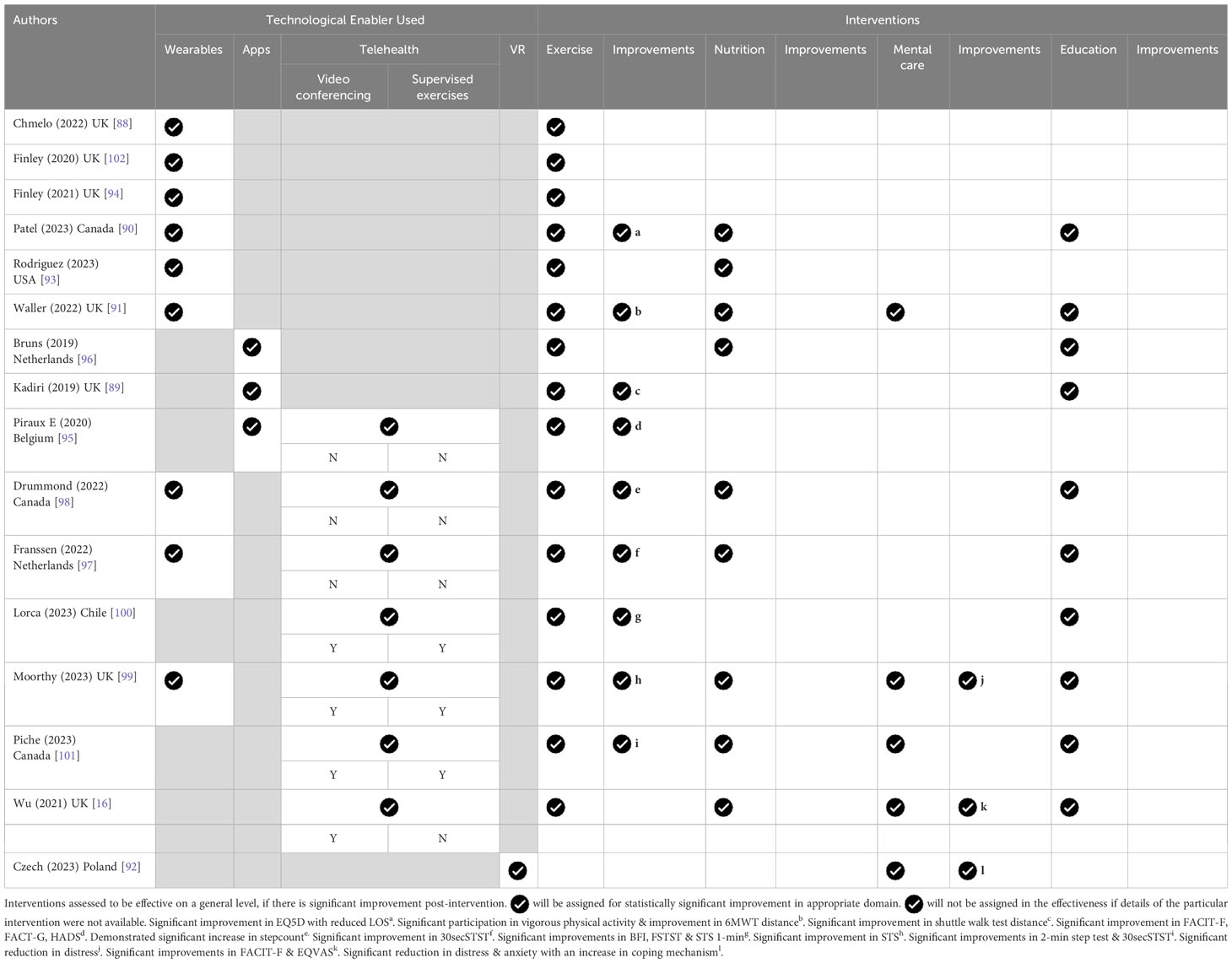
Six studies involved wearables such as a wrist-worn smartwatch (90, 91, 93, 94, 102) and pedometers (88). Three studies involved smart applications, such as Fit 4 Surgery TV (96), which was a prototype designed solely for the study; an exercise app (89); or a virtual gym app (95) that consisted of an exercise schedule, description of exercises, and an email tab. The exercise videos were hosted on a website.
Teleprehabilitation was conducted in 7 studies. One involved a tablet (98) and wearable device to facilitate communication and data collection. Recorded videos of nutrition and relaxation techniques were available on the tablet, whereas exercise was monitored on a teleconferencing platform. Another study conducted home physical exercise training using a mobile phone application with a heart rate monitor (97). The third study involved telehealth that delivered a multimodal home-based prehabilitation program involving exercise, nutrition, medical optimization and psychological support (16). Another study utilized video-conferencing for teleprehabilitation via the zoom platform or a videocall on the smart phone (100). The fifth involved tele-conferencing either through an app or on the web coupled with a wearable tracker for remote monitoring (99). Finally, group exercises and educational programs were carried out via videoconferencing (101).
Teleprehabilitation is broadly encompassing. In most of these studies, it involves the provision of digital information or material, and there is a feedback loop, be it through emails or phone calls. In most cases, there may be more than 1 type of technology provided, eg app and wearable (98). It is noteworthy that teleprehabilitation that provided supervision through videoconferencing trended towards improvements in physical outcome measures (99–101) (Table 4).
There was only 1 study on the the use of virtual reality therapy in helping mood and distress (92). The Virtual Reality Garden was applied through VR goggles, providing intense visual, auditory and kinestheic stimuli targeted at producing a calming effect with mood elevation. A variety of cancer patients were involved in all these studies involving technology-enabled prehabilitation.
Outcomes
Primary Outcomes
Functional
Physical functional outcomes reported were heterogenous, with 6MWT (91, 94), the incremental shuttle walk (89), sit-to-stand test (STST) (99–101), five-times STST (5STST) (100), 2 min step test (101), heart rate recovery (HRR) (99), step count (93, 99), all reported.
There is a trend towards a lower proportion of improvement in the physical domain when wearables are deployed as the sole technological enabler. Physical intervention may translate into improvements in the psychological and quality of life domains without mental health interventions (95, 100) (Table 4).
Psychological
Changes in HADS (91, 95), Emotional Distress Scale (99), and Beck Depression Inventory (92) have been reported.
Virtual reality therapy resulted in a significant reduction in the amount of distress post-intervention but did not show a significant difference between the intervention and control groups (92).
A study that involved exercise as the sole mode of intervention resulted in improvements in HADS (95). A study that provided psychological support through a coach did so through a video-conferencing platform and that resulted in a significant drop in distress (99).
Quality of life and others
QOL-EORTC (89, 96, 101), FACIT-F (16, 95), FACT-G (95), EQ-5D (16, 90) and BFI (100) were reported.
In the prehabilitation studies that targeted mental health, there were improvements in the FACIT-F and EQVAS (16). Prehabilitation that mainly targeted exercise also resulted in improvements in BFI, FACIT-F and FACT-G (95).
Secondary Outcomes
Length of stay (LOS) (90, 99) and postoperative complications (90, 93, 99) were studied. There was a reduction in the length of stay in a controlled study that intervened in exercise, diet, smoking cessation, and breathing exercises for lung cancer. In the 3 studies that reported postoperative complication rates, no differences were found.
Data pertaining to funding for the studies were extracted (Table 3). The funding sources were mostly academic or government institutions, or philanthropy. None of these were industrially sponsored.
Quantitative synthesis was not performed as none of the studies were sufficiently powered to inform the efficacy/effectiveness conclusions. Consequently, we were unable to perform a meta-analysis.
Adverse events
These studies reported no significant adverse events (AEs).
Feasibility and acceptability
Twelve of the 16 studies discussed the feasibility of conducting these studies (Table 3). All of these have been reported to be feasible. Generally, recruitment or attendance was above or close to 70%. Notably, the study population size was generally small, with the majority consisting of 30 patients or fewer (range, 11–100). One study reported more exercise sessions in the application group than in the conventional group despite a shorter wait time to surgery (89). One unintentional convenience sample reported 54% completion and 53% retention, deemed feasible but raising potential selection bias concerns (100). Another also raised concerns due to high drop-out rate of 48% with reasons of inadequate step data obtained, withdrawal and protocol deviation (93). There was acceptance by the participants in the studies, as indicated by patient satisfaction. One study reported the acceptance of technology by the data received (102). However, the patients provided feedback on the fitness device itself, some of which were negative.
Challenges in implementation
Challenges identified from various studies fell into three categories: technical barriers, lack of direct supervision, and non-compliance.
Technical barriers
Some participants in one study cited a self-perceived lack of digital ability and literacy (16). However, this was not a significant barrier to participation in the study. The absence of group sessions was cited as a reason for the lack of opportunities for peer support. Another study reported challenges such as patients requiring assistance for the use of technology, the multidisciplinary team needing to adjust to new technologies, loss to follow-up, and extra costs involved in the use of technology (98). One study reported that some patients were excluded from the study for reasons such as inability to operate a smart phone (97).
Lack of direct supervision
A possible lower quality in the execution of exercises owing to their unsupervised nature was a concern (96).
Non-compliance
One study faced the challenge of missing data, which could be device malfunction versus a choice not to wear, charge, or synchronize the device or an inability to operate the device (102). Similarly, in another study, patients failed to put on the wearable device daily, thus underestimating the activity level of the participants (94).
Discussion
This systematic review evaluates the feasibility and effectiveness of technology-based cancer prehabilitation programs, offering recommendations for future research and practice.
Feasibility
Twelve of sixteen studies evaluated feasibility and all 12 of them reported feasibility, although one raised concerns due to low completion and retention rates (100). Challenges included adapting face-to-face programs to remote delivery during the pandemic without proper piloting (99). Technical issues included wearable-app synchronization failures (102), device malfunctions (102), and user-friendliness concerns (98). Early prototype testing with team members or healthy volunteers is recommended to identify and address technical issues before implementation with cancer patients. Remote prehabilitation’s lack of supervision and compliance might be addressed through teleconferencing for individual or group support (101).
Determinants of effectiveness
Two key factors contribute to program effectiveness:
1. Intervention Rigor: Effective prehabilitation interventions form the foundation for success. For example, an individualized, structured program adhering to FITT principles with additional physical activity encouragement resulted in significant 6MWT improvement compared to controls (91). Conversely, programs lacking specific exercise prescription and delayed telephonic supervision (93) showed no difference in hospital complication rates. The intervention’s rigor and fidelity significantly impact its success (100).
2. Technology’s Nature and Effectiveness: Wearable technology offers advantages like remote monitoring of step count and heart rate zones, objectively quantifying exercise intensity and frequency without direct supervision. However, disadvantages include potential compliance issues due to lack of supervision and data capture failures. Wearables with real-time feedback to healthcare teams for daily audits and patient feedback during technical disruptions or non-compliance can address these concerns. Videoconferencing can further mitigate compliance issues by providing supervised individual or group exercise sessions (101). Real-time videoconferencing has been accepted as an effective and non-inferior alternative to face-to-face delivery of postsurgical rehabilitation (103) and has been effective in delivering nutritional and psychological counselling (104, 105). The results are seen in one of the studies in our review (99) and its utility should be increased.
Assessments and outcome measures
Remote prehabilitation’s lack of physical contact limits physical outcome measure collection. One study conducted the first outcome measure in-person and subsequent ones virtually (100). Others opted for virtual assessments only, measuring quality of life outcomes instead (90). One study solely employed virtual physical assessments (99). It is recommended that outcome measures align with the intervention mode and that further validation of virtual physical assessments be conducted for program pragmatism and timely implementation.
Multimodal prehabilitation
Limited information exists regarding nutritional, mental health, and educational interventions in most studies, except for a few (16, 96). This is concordant with conventional multimodal prehabilitation studies. Additionally, outcome measures related to nutrition, like serum albumin, muscle mass, and handgrip strength, were not commonly reported, except for frailty scores (96). These omissions might be due to the lack of face-to-face consultations. However, ensuring interventions follow established conventional protocols might address this issue, avoiding the need for entirely new intervention designs. Designs can then be tweaked to address the lack of physical contact thereafter.
An all-in-one system
Based on identified challenges like supervision, compliance, fidelity, and device usability, and insights from large-scale virtual cancer rehabilitation programs (106), remote prehabilitation might benefit from integrating multiple technologies instead of relying on single devices, especially for multimodal programs. Therefore, an “all-in-one system” concept was developed based on the reviewed studies. This system aims to combine features from various studies, potentially offering recorded exercise videos, educational and nutritional guides, teleconferencing capabilities, exercise output measurements, and two-way communication channels. Ideally, this would be a user-friendly, intuitive, and device-agnostic application (Figure 2).
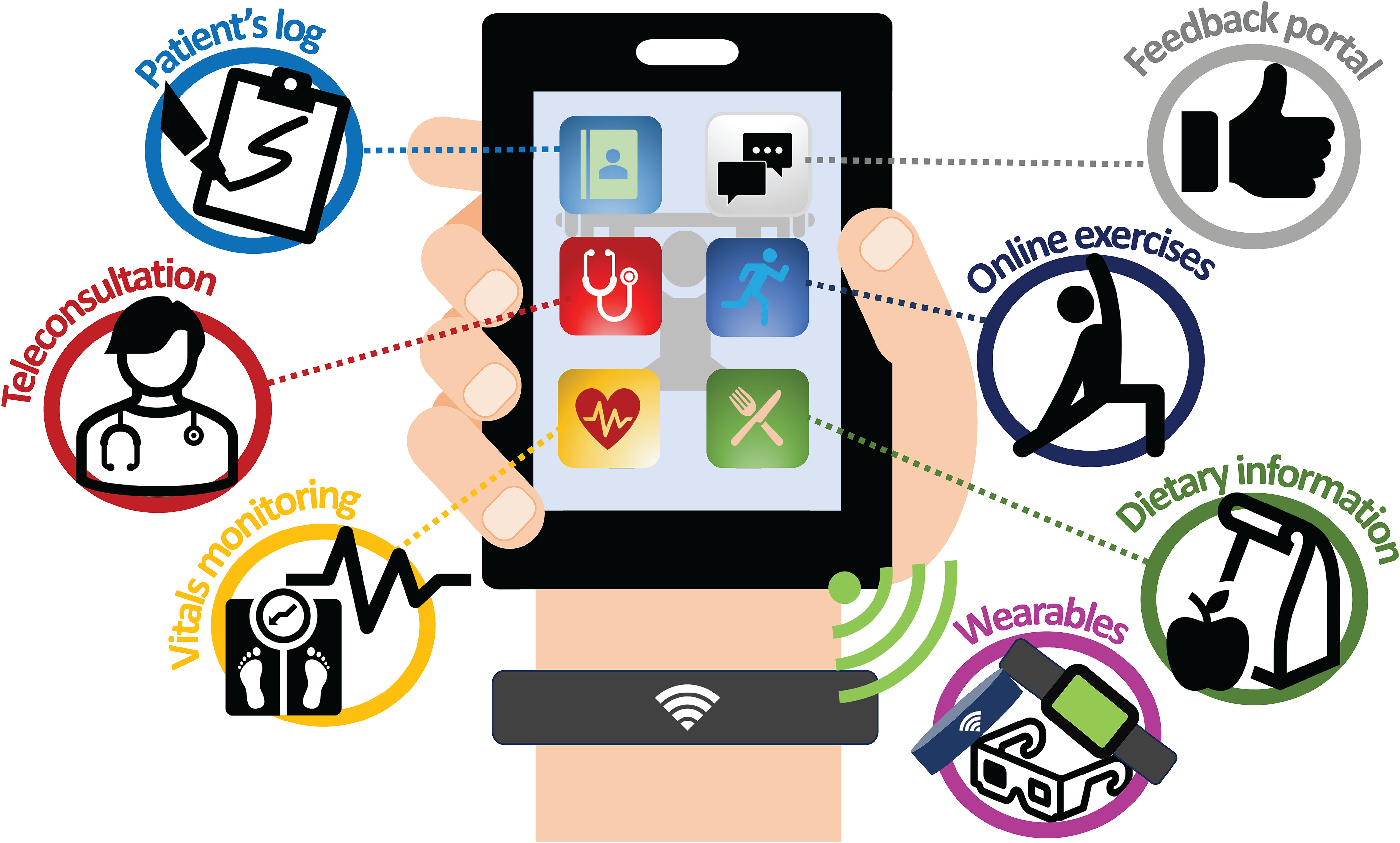
Figure 2 All-in-one system. This figure represents our authors’ impression of an all-in-one system, where patients can easily gain access to a plethora of services at the touch of a screen from a common handheld device. The authors envisioned a virtual platform with the ability to allow teleconsultation/feedback with physicians or allied health workers, either synchronous or asynchronous (provider and user do not have to be online simultaneously to interact), or allowing online exercises to be conducted in a supervised manner in either a 1-on-1 or group setting. In addition, information on diet, psychological well-being, exercise videos and other materials will also be made readily accessible for patients on this platform. This device will also be platform-agnostic as well as Bluetooth and Wi-Fi compatible, allowing seamless syncing and loading of software across many different devices and wearables. Other possibilities for this device include the ability to monitor vital signs, activity/exercise and these information gathered can be successfully shared selectively between user and healthcare providers to allow for server-side analytics.
The potential of telerehabilitation in specific conditions suggests its applicability to cancer prehabilitation. Notably, studies have successfully implemented telerehabilitation for various conditions, providing valuable insights for its potential use in cancer care. Barriers to accessing traditional prehabilitation programs, such as social isolation, transportation difficulties, limited support networks, inadequate infrastructure, and capacity constraints, can be alleviated with technology-based solutions. Moreover, the looming challenges of healthcare workforce shortages and budgetary limitations, exacerbated by potential future pandemics, highlight the vulnerabilities of traditional models unable to adapt. Challenges can exist for the all-in-one system as well, including technology disruption, necessitating a support team to be accessible. Other challenges include funding and cost of implementation, as well as problems with technology adoption. However, embracing technological innovation and leveraging its capabilities may be a viable path forward for developing accessible and sustainable cancer prehabilitation programs in the future.
Limitations of the review
This review acknowledges several methodological limitations:
Inclusion of diverse study types: Unlike typical systematic reviews often focused on controlled trials, this review included a broader range of studies due to the preliminary finding of limited high-quality evidence. While this approach ensures comprehensiveness, it may compromise the strength of conclusions drawn.
Choice of quality assessment tools: Employing the NIH tools instead of more widespread options like PEDro or Cochrane RoB 2 facilitated evaluation of diverse study types but may limit comparability with other reviews.
Arbitrary quality score cut-offs: The arbitrary thresholds of 50% and 80% for “good” and “poor” ratings, while informed by previous research, lack formal justification and may require refinement.
We anticipate that future iterations of this review, as the field generates more robust evidence, will be able to address these limitations and provide more definitive conclusions.
Authors’ conclusions
This systematic review investigated the feasibility and effectiveness of technology-based cancer prehabilitation programs. While the studies included were diverse in quality and design, they collectively offer valuable insights for future research and practice. All studies reported feasibility, although challenges related to technical issues, lack of supervision, and non-compliance were identified. Due to the diversity of the studies, no firm conclusion on effectiveness can be made, although trends are observed. Effectiveness depends on intervention rigor and the nature of the technology employed. Multimodal programs and outcome measures aligned with the intervention mode are crucial. An “all-in-one system” integrating various technologies may be promising for addressing identified challenges. Integrating teleprehabilitation strategies holds potential for wider applicability.
As the field matures, future reviews are expected to provide more definitive conclusions. Embracing technological innovation can pave the way for developing accessible and sustainable cancer prehabilitation programs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
ST: Writing – original draft, Writing – review & editing, Conceptualization, Methodology, Resources. FZ: Writing – original draft, Writing – review & editing. EN: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1321493/full#supplementary-material
Abbreviations
6MWT, The 6min walk test; 30 Sec STS, 30 seconds sit-to-stand test; BDI, Beck Depression Inventory; BFI, Brief Fatigue Inventory; EORTC QLC-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C-30; EQ5D, EuroQol-5D; FACIT-F, The Functional Assessment of Chronic Illness Therapy-Fatigue Scale; FACT-G, The Functional Assessment of Cancer Therapy-General; FSTST, Five Times Sit-To-Stand Test; HADS-A, The Hospital Anxiety and Depression Scale-anxiety; IPAQ, International Physical Activity Questionaire; Mini-MAC, Mini-Mental Adjustment to Cancer; MOS-SSS, Medical Outcome Study- Social Support Survey; TUG, Timed Up and Go Test.
References
1. Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. (2013) 92:715–27. doi: 10.1097/PHM.0b013e31829b4afe
2. Kichloo A, Albosta M, Dettloff K, Wani F, El-Amir Z, Singh J, et al. Telemedicine, the current COVID-19 pandemic and the future: a narrative review and perspectives moving forward in the USA. Fam Med Commun Health. (2020) 8:e000530. doi: 10.1136/fmch-2020-000530
3. Taylor A, Caffery LJ, Gesesew HA, King A, Bassal AR, Ford K, et al. How Australian health care services adapted to telehealth during the COVID-19 pandemic: A survey of telehealth professionals. Front Public Health. (2021) 9:648009. doi: 10.3389/fpubh.2021.648009
4. López-Rodríguez-Arias F, Sánchez-Guillén L, Aranaz-Ostáriz V, Triguero-Cánovas D, Lario-Pérez S, Barber-Valles X, et al. Effect of home-based prehabilitation in an enhanced recovery after surgery program for patients undergoing colorectal cancer surgery during the COVID-19 pandemic. Support Care Cancer. (2021) 29:7785–91. doi: 10.1007/s00520-021-06343-1
5. European Institute of Innovation and Technology. Patient Empowerment for Major Surgery Preparation at Home (2022). Available online at: https://eithealth.eu/product-service/paprika/ (Accessed 9 August 2022).
6. van Rooijen S, Carli F, Dalton S, Thomas G, Bojesen R, Le Guen M, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. (2019) 19:98. doi: 10.1186/s12885-018-5232-6
7. Chabot K, Gillis C, Minnella EM, Ferreira V, Awasthi R, Baldini G, et al. Functional capacity of prediabetic patients: effect of multimodal prehabilitation in patients undergoing colorectal cancer resection. Acta Oncol. (2021) 60:1025–31. doi: 10.1080/0284186X.2021.1937307
8. Goldsmith I, Chesterfield-Thomas G, Toghill H. Pre-treatment optimization with pulmonary rehabilitation in lung cancer: Making the inoperable patients operable. EclinicalMedicine. (2020) 31:100663. doi: 10.1016/j.eclinm.2020.100663
9. Paterson C, Roberts C, Toohey K, McKie A. Prostate cancer prehabilitation and the importance of multimodal interventions for person-centered care and recovery. Semin Oncol Nurs. (2020) 36:151048. doi: 10.1016/j.soncn.2020.151048
10. Sheill G, Guinan E, O’Neill L, Normand C, Doyle SL, Moore S, et al. Preoperative exercise to improve fitness in patients undergoing complex surgery for cancer of the lung or oesophagus (PRE-HIIT): protocol for a randomized controlled trial. BMC Cancer. (2020) 20:321. doi: 10.1186/s12885-020-06795-4
11. Cantwell LA, Fahy E, Walters ER, Patterson JM. Nutritional prehabilitation in head and neck cancer: a systematic review. Support Care Cancer. (2022) 30:8831–43. doi: 10.1007/s00520-022-07239-4
12. Tay SS. Perspectives on the direction of cancer prehabilitation in the pandemic and beyond. Arch Rehabil Res Clin Transl. (2022) 4:100236. doi: 10.1016/j.arrct.2022.100236
13. Santa Mina D, Sellers D, Au D, Alibhai SMH, Clarke H, Cuthbertson BH, et al. A pragmatic non-randomized trial of prehabilitation prior to cancer surgery: study protocol and COVID-19-related adaptations. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.629207
14. Gonella F, Massucco P, Perotti S, Monasterolo S, Vassallo D, Laezza A, et al. Telemedicine prehabilitation as a result of COVID-19: disruptive technological solutions. Br J Surg. (2021) 108:e215–6. doi: 10.1093/bjs/znab066
15. ClinicalTrials.gov. PAPRIKA—Patients Empowerment for Major Surgery Preparation, in: Home (PAPRIKA) (2022). Available online at: https://www.clinicaltrials.gov/ct2/show/NCT04295668 (Accessed April 25, 2022).
16. Wu F, Rotimi O, Laza-Cagigas R, Rampal T. The feasibility and effects of a telehealth-delivered home-based prehabilitation program for cancer patients during the pandemic. Curr Ocnol. (2021) 28:2248–59. doi: 10.3390/curroncol28030207
17. Rogante M, Grigioni M, Cordella D, Giacomozzi C. Ten years of telerehabilitation: A literature overview of technologies and clinical applications. NeuroRehabilitation. (2010) 27:287–304. doi: 10.3233/NRE-2010-0612
18. de la Torre-Díez I, López-Coronado M, Vaca C, Aguado JS, de Castro C. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: A systematic review. Telemed J E Health. (2015) 21:81–5. doi: 10.1089/tmj.2014.0053
19. Waterland JL, Chahal R, Ismail H, Sinton C, Riedel B, Francis JJ, et al. Implementing a telehealth prehabilitation education session for patients preparing for major cancer surgery. BMC Health Serv Res. (2021) 21:443. doi: 10.1186/s12913-021-06437-w
20. Pew Research Center. Mobile fact sheet (2021). Available online at: https://www.pewresearch.org/internet/fact-sheet/mobile (Accessed August 11, 2023).
21. Antonietta M, Barbara A, Bidoli ME, Flaiban C, Bomben F, Piccinin M, et al. Hospital Anxiety and Depression Scale (HADS) accuracy in cancer patients. Support Care Cancer. (2020) 28:3921–6. doi: 10.1007/s00520-019-05244-8
22. Rabin R, De Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. (2001) 33:337–43. doi: 10.3109/07853890109002087
23. Wintner LM, Sztankay M, Aaronson N, Bottomley A, Giesinger JM, Groenvold M, et al. The use of EORTC measures in daily clinical practiced -A synopsis of a newly developed manual. Eur J Cancer. (2016) 68:73–81. doi: 10.1016/j.ejca.2016.08.024
24. National Heart Lung and Blood Institute (NIH). Study Quality Assessment Tools. Available online at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (Accessed 7 Sept 2023).
25. Tan YL, Neo EJR, Wee TC. Ultrasound-guided genicular nerve blockade with pharmacological agents for chronic knee osteoarthritis: A systematic review. Pain Physician. (2022) 25:E489–502.
26. Rammant E, Deforche B, Va Hecke A, Verhaeghe S, Van Ruymbeke B, Bultijnck R, et al. Development of a pre-and postoperative physical activity promotion integrated in the electronic health system of patients with bladder cancer (The POPEYE study): An intervention mapping approach. Eur J Cancer Care (Engl). (2021) 30:e13363. doi: 10.1111/ecc.13363
27. Sell NM, Silver JK, Rando S, Draviam AC, Mina DS, Qadan M. Prehabilitation telemedicine in neoadjuvant surgical oncology patients during the novel COVID-19 coronavirus pandemic. Ann Surg. (2020) 272:e81–3. doi: 10.1097/SLA.0000000000004002
28. Wu F, Laza-Cagigas R, Rampal T. Understanding patient’s experiences and perspectives of tele-prehabilitation: A qualitative study to inform study design and delivery. Clin Pract. (2022) 12:640–52. doi: 10.3390/clinpract12040067
29. Lambert G, Drummond K, Ferreira V, Carli F. Teleprehabilitation during COVID-19 pandemic: the essentails of “what” and “how”. Support Care Cancer. (2021) 29:551–4. doi: 10.1007/s00520-020-05768-4
30. Durrand JW, Moore J, Danjoux G. Prehabilitation and preparation for surgery: has the digital revolution arrived? Anaesthesia. (2022) 77:635–9. doi: 10.1111/anae.15622
31. Steffans D, Delbaere K, Young J, Solomon M, Denehy L. Evidence on technology-driven preoperative exercise interventions: are we there yet? Br J Anaesth. (2020) 125:646–9. doi: 10.1016/j.bja.2020.06.050
32. Asberg K. Bendtsen Perioperative digital behaviour change interventions for reducing alcohol consumption, improving dietary intake, increasing physical activity and smoking cessation: a scoping review. Perioper Med (Lond). (2021) 10:18. doi: 10.1186/s13741-021-00189-1
33. Barberan-Garcia A, Cano I, Bongers BC, Seyfried S, Ganslandt T, Herrle F, et al. Digital support to multimodal community- based prehabilitation: looking for optimization of health value generation. Front Oncol. (2021) 11:662013. doi: 10.3389/fonc.2021.662013
34. Kelly AE. Telehealth: only a click away. J Psychosoc Oncol. (2021) 39:3:(337–339). doi: 10.1080/07347332.2021.1892895
35. Townsend WB, Worrilow WM, Riggs SB. The benefit of prehabilitation and enhanced recovery in robot-assisted radical prostatectomy and the promising future of these protocols in the field of urologic oncology. Cancer. (2020) 126:4107–9. doi: 10.1002/cncr.33059
36. Huston A. Development of virtual integrative oncology centre. Oncol Issues. (2022) 37:2(38–45). doi: 10.1080/10463356.2022.2029112
37. Robinson A, Oksuz U, Slight R, Slight S, Husband A. Digital and mobile technologies to promote physical health behaviour change and provide psychological support for patients undergoing elective surgery: meta-ethnography and systematic review. JMIR Mhealth Uhealth. (2020) 8:e19237. doi: 10.2196/19237
38. Karlsson E, Dahl O, Rydwik E, Nygren-Bonnier M, Bergenmar M. Older patient’s attitudes towards, and perceptions of, preoperative physical activity and exercise prior to colorectal cancer surgery- a gap between awareness and action. Supportive Care Cancer. (2020) 28:3945–53. doi: 10.1007/s00520-019-05237-7
39. Seery T, Wu F, Hendricks E, McDonald J, Laza-Cagigas R, Elliott S, et al. Patients, experiences of virtual prehabilitation during the COVID-19 pandemic. Patient Educ Counsel. (2022) 105:2:488–489. doi: 10.1016/j.pec.2021.07.052
40. Li T-C, Yang M-C, Tseng AH, Lee HHC. Prehabilitation and rehabilitation for surgically treated lung cancer patients. J Cancer Res Pract. (2017) 4;3:89–94. doi: 10.1016/j.jcrpr.2017.06.001
41. Zhou YB. Prehabilitation for gastrointestinal cancer patients. Zhonghua Wei Chang Wai Ke Za Zhi. (2021) 24:122–7. doi: 10.3760/cma.j.cn.441530-20200318-00152
42. MaChado PFA, Oliveiros B, Martins RA, Cruz J. ASO author reflections: impact of a preoperative home-based exercise program on quality of life after lung cancer resection. Ann Surg Oncol. (2024) 31:897–8. doi: 10.1245/s10434-023-14620-y
43. Neuendorf T, Haase R, Schroeder S, Schumann M, Nitzsche N. Effects of high-intensity interval training on functional performance and maximal oxygen uptake in comparison with moderate intensity continuous training in cancer patients: a systematic review and meta-analysis. Support Care Cancer. (2023) 31:643. doi: 10.1007/s00520-023-08103-9
44. Guo Y, Ding L, Miao X, Jiang X, Xu T, Xu X, et al. Effects of prehabilitation on postoperative outcomes in frail cancer patients undergoing elective surgery: a systematic review and meta-analysis. Support Care Cancer. (2022) 31:57. doi: 10.1007/s00520-022-07541-1
45. Edbrooke L, Bowman A, Granger CL, Burgess N, Abo S, Connolly B, et al. Exercise across the lung cancer care continuum: an overview of systematic reviews. J Clin Med. (2023) 12:1871. doi: 10.3390/jcm12051871
46. Christopher CN, Kang DW, Wilson RL, Gonzalo-Encabo P, Ficarra S, Heislein D, et al. Exercise and nutrition interventions for prehabilitation in hepato-pancreato-biliary cancers: A narrative review. Nutrients. (2023) 15:5044. doi: 10.3390/nu15245044
47. Gillman A, Hayes M, Sheaf G, Walshe M, Reynolds JV, Regan J. Exercise-based dysphagia rehabilitation for adults with oesophageal cancer: a systematic review. BMC Cancer. (2022) 22:53. doi: 10.1186/s12885-021-09155-y
48. Whish-Wilson GA, Edbrooke L, Cavalheri V, Denehy L, Seller D, Granger CL, et al. Physiotherapy and exercise management of people undergoing surgery for lung cancer: A survey of current practice across Australia and New Zealand. J Clin Med. (2023) 12:2146. doi: 10.3390/jcm12062146
49. Raff C, Dörr-Harim C, Otto S, Thiele J, Mihaljevic A, Kramer K. Prehabilitation in an integrative medicine day clinic for patients undergoing neoadjuvant treatment: single-center feasibility pilot study. JMIR Res Protoc. (2023) 12:e46765. doi: 10.2196/46765
50. Flores LE, Westmark D, Katz NB, Hunter TL, Silver EM, Bryan KM, et al. Prehabilitation in radiation therapy: a scoping review. Support Care Cancer. (2024) 32:83. doi: 10.1007/s00520-023-08262-9
51. Meneses-Echavez JF, Loaiza-Betancur AF, Díaz-López V, Echavarría-Rodríguez AM, Triana-Reina HR. Prehabilitation programs for individuals with cancer: a systematic review of randomized-controlled trials. Syst Rev. (2023) 12:219. doi: 10.1186/s13643-023-02373-4
52. Rozenberg D. Rehabilitation pre- and post thoracic surgery: Progress and future opportunities. Chron Respir Dis. (2023) 20:14799731231165305. doi: 10.1177/14799731231165305
53. Lippi L, Turco A, Moalli S, Gallo M, Curci C, Maconi A, et al. Role of prehabilitation and rehabilitation on functional recovery and quality of life in thyroid cancer patients: A comprehensive review. Cancers (Basel). (2023) 15:4502. doi: 10.3390/cancers15184502
54. Jurys T, Kupilas A, Rajwa P, Bryniarski P, Burzyński B. Role of preoperative patient education among prostate cancer patients treated by radical prostatectomy. Cent Eur J Urol. (2022) 75:272–6. doi: 10.5173/ceju.2022.0037
55. Mareschal J, Hemmer A, Douissard J, Dupertuis YM, Collet TH, Koessler T, et al. Surgical prehabilitation in patients with gastrointestinal cancers: impact of unimodal and multimodal programs on postoperative outcomes and prospects for new therapeutic strategies-A systematic review. Cancers (Basel). (2023) 15:1881. doi: 10.3390/cancers15061881
56. Raz DJ, Kim JY, Erhunwmunesee L, Hite S, Varatkar G, Sun V. The value of perioperative physical activity in older patients undergoing surgery for lung cancer. Expert Rev Respir Med. (2023) 17:691–700. doi: 10.1080/17476348.2023.2255133
57. McCann L, Hewitt C, McMillan KA. Developing an e-prehabilitation system of care for young adults diagnosed with cancer: user-centered design study. JMIR Cancer. (2023) 9:e41441. doi: 10.2196/41441
58. Cooper M, Chmelo J, Sinclair RCF, Charman S, Hallsworth K, Welford J, et al. Exploring factors influencing uptake and adherence to a home-based prehabilitation physical activity and exercise intervention for patients undergoing chemotherapy before major surgery (ChemoFit): a qualitative study. BMJ Open. (2022) 12:e062526. doi: 10.1136/bmjopen-2022-062526
59. Hunter H, Bennington-McKay N, Sher J, Psutka SP, Lin C. Emerging role of mobile applications and wearable devices for prehabilitation in urologic oncology. Eur Urol Focus. (2023) S2405-4569:00227–4. doi: 10.1016/j.euf.2023.10.010
60. Steffens D, Denehy L, Solomon M, Koh C, Ansari N, McBride K, et al. Consumer perspectives on the adoption of a prehabilitation multimodal online program for patients undergoing cancer surgery. Cancers (Basel). (2023) 15:5039. doi: 10.3390/cancers15205039
61. van Deursen L, van der Vaart R, Alblas EE, Struijs JN, Chavannes NH, Aardoom JJ. Improving the colorectal cancer care pathway via e-health: a qualitative study among Dutch healthcare providers and managers. Support Care Cancer. (2023) 31:203. doi: 10.1007/s00520-023-07653-2
62. Ip N, Zhang K, Karimuddin AA, Brown CJ, Campbell KL, Puyat JH, et al. Preparing for colorectal surgery: a feasibility study of a novel web-based multimodal prehabilitation programme in Western Canada. Colorectal Dis. (2024). doi: 10.1111/codi.16851
63. Parraguez LAL, Ribeiro IL, Hinojosa MP, Troncoso JP. Implementation of a teleprehabilitation program for oncosurgical patients during the COVID-19 pandemic: perspectives and user satisfaction. Support Care Cancer. (2023) 31:346. doi: 10.1007/s00520-023-07799-z
64. Granger CL, Irving L, Antippa P, Edbrooke L, Parry SM, Krishnasamy M, et al. CAPACITY: A physical activity self-management program for patients undergoing surgery for lung cancer, a phase 1 feasibility study. Lung Cancer. (2018) 124:102–9. doi: 10.1016/j.lungcan.2018.07.034
65. Cuadros L, Ismail H, Ho K. Evaluation of Reliability of MYZONE MZ-3 Heart Rate Monitor: A Study for the future of Telephysiotherapy for preoperative Prehabilitation in Cancer Patients. Tele JE Health. (2017) 23:334–8. doi: 10.1089/tmj.2016.0138
66. Cos H, Zárate Rodríguez JG, Srivastava R, Bewley A, Raper L, Li D, et al. 4,300 steps per day prior to surgery are associated with improved outcomes after pancreatectomy. HPB (Oxford). (2023) 25:91–9. doi: 10.1016/j.hpb.2022.09.011
67. Amari T, Matta D, Makita Y, Fukuda K, Miyasaka H, Kimura M, et al. Early ambulation shortened the length of hospital stay in ICU patients after abdominal surgery. Clin Pract. (2023) 13:1612–23. doi: 10.3390/clinpract13060141
68. Chen CCG, Malpani A, Waldram MM, Romanczyk C, Tanner EJ, Fader AN, et al. Effect of pre-operative warm-up on trainee intraoperative performance during robot-assisted hysterectomy: a randomized controlled trial. Int Urogynecol J. (2023) 34:2751–8. doi: 10.1007/s00192-023-05595-1
69. Wu Y, Wang X, Gao F, Liao J, Zeng J, Fan L. Mobile nutrition and health management platform for perioperative recovery: an interdisciplinary research achievement using WeChat Applet. Front Med (Lausanne). (2023) 10:1201866. doi: 10.3389/fmed.2023.1201866
70. Alverdy JC. Rationale for colonic pre-habilitation prior to restoration of gastrointestinal continuity. Surg Infect (Larchmt). (2023) 24:265–70. doi: 10.1089/sur.2023.001
71. van Gestel T, Groen LCB, Puik JR, van Rooijen SJ, van der Zaag-Loonen HJ, Schoonmade LJ, et al. Fit4Surgery for cancer patients during covid-19 lockdown - A systematic review and meta-analysis. Eur J Surg Oncol. (2022) S0748-7983:0085–3. doi: 10.1016/j.ejso.2022.02.010
72. Steffans D, Solomon M, Denehy L. Is preoperative exercise training the new holy grail for patients undergoing major surgery? Ann Am Thorac Soc. (2021) 18:4(587–589). doi: 10.1513/AnnalsATS.202011-1388ED
73. Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiol Clinics. (2015) 33:1(17–33). doi: 10.1016/j.anclin.2014.11.002
74. Bingham SL, Small S, Semple CJ. A qualitative evaluation of a multi-modal cancer prehabilitation programme for colorectal, head and neck and lung cancers patients. PloS One. (2023) 18:e0277589. doi: 10.1371/journal.pone.0277589
75. MaChado P, Pimenta S, Garcia AL, Nogueira T, Silva S, Dos Santos CL, et al. Effect of preoperative home-based exercise training on quality of life after lung cancer surgery: A multicenter randomized controlled trial. Ann Surg Oncol. (2024) 31:847–59. doi: 10.1245/s10434-023-14503-2
76. Molenaar CJL, Minnella EM, Coca-Martinez M, Ten Cate DWG, Regis M, Awasthi R, et al. Effect of multimodal prehabilitation on reducing postoperative complications and enhancing functional capacity following colorectal cancer surgery: the PREHAB randomized clinical trial. JAMA Surg. (2023) 158:572–81. doi: 10.1001/jamasurg.2023.0198
77. Akdemir E, Sweegers MG, Vrieling A, Rundqvist H, Meijer RP, Leliveld-Kors AM, et al. EffectiveNess of a multimodal preHAbilitation program in patieNts with bladder canCEr undergoing radical cystectomy: protocol of the ENHANCE multicentre randomised controlled trial. BMJ Open. (2023) 13:e071304. doi: 10.1136/bmjopen-2022-071304
78. McCourt O, Fisher A, Ramdharry G, Land J, Roberts AL, Rabin N, et al. Exercise prehabilitation for people with myeloma undergoing autologous stem cell transplantation: results from PERCEPT pilot randomised controlled trial. Acta Oncol. (2023) 62:696–705. doi: 10.1080/0284186X.2023.2178326
79. Bradley P, Merchant Z, Rowlinson-Groves K, Taylor M, Moore J, Evison M. Feasibility and outcomes of a real-world regional lung cancer prehabilitation programme in the UK. Br J Anaesth. (2023) 130:e47–55. doi: 10.1016/j.bja.2022.05.034
80. Halliday LJ, Doganay E, Wynter-Blyth VA, Hanna GB, Moorthy K. The impact of prehabilitation on post-operative outcomes in oesophageal cancer surgery: a propensity score matched comparison. J Gastrointest Surg. (2021) 25:2733–41. doi: 10.1007/s11605-020-04881-3
81. Smits A, Agius CM, Blake D, Ang C, Kucukmetin A, Ham MV, et al. Is cardiopulmonary exercise testing predictive of surgical complications in patients undergoing surgery for ovarian cancer? Cancers (Basel). (2023) 15:5185. doi: 10.3390/cancers15215185
82. Strijker D, Meijerink WJHJ, van Heusden-Schotalbers LAG, van den Berg MGA, van Asseldonk MJMD, Drager LD, et al. Multimodal prehabilitation in patients undergoing complex colorectal surgery, liver resection, and hyperthermic intraperitoneal chemotherapy (HIPEC): A pilot study on feasibility and potential efficacy. Cancers (Basel). (2023) 15:1870. doi: 10.3390/cancers15061870
83. Wu J, Chi H, Kok S, Chua JMW, Huang XX, Zhang S, et al. Multimodal prerehabilitation for elderly patients with sarcopenia in colorectal surgery. Ann Coloproctol. (2023) 40(1):3–12. doi: 10.3393/ac.2022.01207.0172
84. Christodoulidis G, Halliday LJ, Samara A, Bhuva N, Park WE, Moorthy K. Personalized prehabilitation improves tolerance to chemotherapy in patients with oesophageal cancer. Curr Oncol. (2023) 30:1538–45. doi: 10.3390/curroncol30020118
85. Sole-Sedeno JM, Miralpeix E, Muns MD, Rodriguez-Cosmen C, Fabrego B, Kanjou N, et al. Protein supplementation in a prehabilitation program in patients undergoing surgery for endometrial cancer. Int J Environ Res Public Health. (2023) 20:5502. doi: 10.3390/ijerph20085502
86. Kilinc F, Setzer M, Prinz V, Jussen D, Marquardt G, Gessler F, et al. The beneficial effect of preoperative exercise on postoperative clinical outcome, quality of life and return to work after microsurgical resection of spinal meningiomas. J Clin Med. (2023) 12:2804. doi: 10.3390/jcm12082804
87. Mawson S, Keen C, Skilbeck J, Ross H, Smith L, Dixey J, et al. Feasibility and benefits of a structured prehabilitation programme prior to autologous stem cell transplantation (ACST) in patients with myeloma; a prospective feasibility study. Physiotherapy. (2021) 113:88–99. doi: 10.1016/j.physio.2021.08.001
88. Chmelo J, Philips AW, Greystoke A, Charman SJ, Avery L, Hallsworth K, et al. A feasibility trial of prehabilitation before oesophaogastric cancer surgery using a multi-component home-based exercise programme: the ChemoFit study. Pilot Feasibility Stud. (2022) 8:173. doi: 10.1186/s40814-022-01137-6
89. Kadiri SB, Kerr AP, Oswald NK, Budacan AM, Flanagan S, Golby C, et al. Fit 4 Surgery, a bespoke app with biofeedback delivers prehabilitation at home before and after elective lung resection. J Cardiothorac Surg. (2019) 14:132. doi: 10.1186/s13019-019—0951-6
90. Patel YS, Sullivan KA, Churchll IF, Beauchamp MK, Wald J, Mbuagbaw L, et al. Preconditioning program reduces the incidence of prolonged hospital stay after lung cancer surgery: Results from the Move for Surgery randomized clinical trial. BJS. (2023) 110:1467–72. doi: 10.1093/bjs/znad252
91. Waller E, Sutton P, Rahman S, Allen J, Saxton J, Aziz O. Prehabilitation with wearables versus standard of care before major abdominal cancer suegery: a randomised controlled pilot study. Surg Endosc. (2022) 36:1008–17. doi: 10.1007/s00464-021-08365-6
92. Czech O, Siewierska K, Krzywinska A, Skórniak J, Maciejczyk A, Matkowski R, et al. Virtual therapy complementary prehabilitation of women diagnosed with breast cancer-A pilot study. Int J.Environ Res Public Health. (2023) 20:722. doi: 10.3390/ijerph20010722
93. Rodriguez J, Cos H, Srivastava R, Bewley A, Raper L, Li D, et al. Preoperative levels of physical activity can be increased in pancreatectomy patients via a remotely monitored, telephone-based intervention: A randomized trial. Surg Pract Sci. (2023) 15:100212. doi: 10.1016/j.sipas.2023.100212
94. Finley DJ, Stevens CJ, Emond JA, Batsis JA, Fay KA, Darabos C, et al. Potential effectiveness of a surgeron-delivered exercise prescription and an activity tracker on pre-operative exercise adherence and aerobic capacity of lung cancer patients. Surg Oncol. (2021) 37:101525. doi: 10.1016/j.suronc.2021.101525
95. Piraux E, Caty G, Reychler G, Forget P, Deswysen Y. Feasibility and preliminary effectiveness of a tele-prehabilitation program in esophagogastric cancer patients. J Clin Med. (2020) 9:2176. doi: 10.3390/jcm9072176
96. Bruns ERJ, Argillander TE, Schuijt HJ, van Duijvendijk P, van der Zaag ES, Wassenaar EB, et al. Fit4SurgeryTV at-home prehabilitation for frail older patients planned for colorectal cancer surgery: A pilot study. Am J Phys Med Rehabil. (2019) 98:399–406. doi: 10.1097/PHM.0000000000001108
97. Franssen RFW, Bongers BC, Vogelaar FJ, Janssen-Heijnen MLG. Feasibility of a tele-prehabilitation program in high-risk patients with colon or rectal cancer undergoing elective surgery: a feasibility study. Perioper Med (Lond). (2022) 11:28. doi: 10.1186/s13741-022-00260-5
98. Drummond K, Lambert G, Tahasildar B, Carli F. Success and challenges of implementing teleprehabilitation for onco-surgical candidates and patients’ experience: a retrospective pilot-cohort study. Sci Rep. (2022) 12:6775. doi: 10.1038/s41598-022-10810-y
99. Moorthy K, Halliday L, Noor N, Peters CJ, Wynter-Blyth V, Urch CE. Feasibility of implementation and the impact of a digitalPrehabilitation service in patients undergoing treatment for oesophago-gastric cancer. Curr Oncol. (2023) 30:1673–82. doi: 10.3390/curroncol30020128
100. Lorca L, Riberiro I, Pizarro M, Martínez M, Vivallos J. Functional results and feasibility of a teleprehabilitation program. Asia-Pac J Clin Oncol. (2023), 1–8. doi: 10.1111/ajco.13939
101. Piche A, Santa Mina D, Lambert S, Doré I. Assessing real-world implementability of a multimodal group-based tele-prehabilitation program in cancer care: a pragmatic feasibility study. Front Oncol. (2023) 13:1271812. doi: 10.2289/fonc.2023.1271812
102. Finley DJ, Fay KA, Batsis JA, Stevens CJ, Sacks OA, Darabos C, et al. A feasibility study on an unsupervised, pre-operative exercise program for adults with lung cancer. Eur J Cancer Care (Engl). (2020) 29:e13254. doi: 10.1111/ecc.13254
103. van Edmond MA, van der Schaaf M, Vredeveld T, Vollenbroek-Hutten MMR, van Berge Henegouwen MI, Klinkenbijl JHG, et al. Effectiveness of physiotherapy with telerehabilitation in surgical patients: a systematic review and meta-analysis. Physiotherapy. (2018) 104:277–98. doi: 10.1016/j.physio.2018.04.004
104. Lungu A, Boone MS, Chen SY, Chen CE, Walser RD. Effectiveness of a cognitive behavioral coaching program delivered via video in real world settings. Telemed e-Health. (2020) 27(1):47–54. doi: 10.1089/tmj.2019.0313
105. Haas K, Hayoz S, Maureer-Wiesner S. Effectiveness and feasibility of a remote lifestyle intervenmtion by dieticians for overweight and obese adults: pilot study. J Med Internet Res. (2019) 1:21(4). doi: 10.2196/12289
Keywords: cancer prehabilitation, technology, feasibility, effectiveness, telemedicine, wearables
Citation: Tay SS, Zhang F and Neo EJR (2024) The use of technology in cancer prehabilitation: a systematic review. Front. Oncol. 14:1321493. doi: 10.3389/fonc.2024.1321493
Received: 23 October 2023; Accepted: 22 March 2024;
Published: 19 April 2024.
Edited by:
Konstantinos Kaliarntas, Edinburgh Napier University, United KingdomReviewed by:
Arianna Folli, Università degli Studi del Piemonte Orientale, ItalyJuliette Hussey, Trinity College Dublin, Ireland
Copyright © 2024 Tay, Zhang and Neo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: San San Tay, dGF5LnNhbi5zYW5Ac2luZ2hlYWx0aC5jb20uc2c=
 San San Tay
San San Tay Fuquan Zhang
Fuquan Zhang Edmund Jin Rui Neo
Edmund Jin Rui Neo