- Department of Gastrointestinal Surgery, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Pregnancy-related gastric cancer is characterized by a refractory nature and poor prognosis; few gastric cancer cases during pregnancy achieved acceptable outcomes by using anti-PD-1 as a monotherapy. A 32-year-old pregnant female patient was admitted to the emergency department of the obstetrics and gynecology department and eventually diagnosed with gastric cancer. Radical surgery for gastric cancer was conducted after the termination of pregnancy. At 1-year postoperative follow-up, tumor recurrence was revealed. This patient has achieved a decrease in tumor burden after receiving anti-PD-1 as a monotherapy. This case documents tumor response to PD-1 monotherapy in pregnancy-related gastric cancer and highlights the potential for future use in specific clinical scenarios.
Introduction
The process of diagnosis and treatment of gastric cancer during pregnancy is quite challenging, which unavoidably presents patients with conflicting choices of individualized treatment and continued childbirth. This group of gastric cancer patients is characterized by a refractory nature and dismal prognosis (1–3).
As for the treatment options, immunotherapy, especially with immune checkpoint blockades, has changed the direction of cancer care. Programmed cell death protein-1 (PD-1) blockage is the most widely used method for immune checkpoint inhibition (4). Nevertheless, only some particular gastric cancer patients have achieved promising results after receiving anti-PD-1 therapy. There are very few gastric cancer patients during pregnancy who achieved satisfactory outcomes by using immunotherapy alone (5).
This was a case of a patient with gastric cancer during pregnancy who underwent radical gastric cancer surgery after elective induction of labor, yet postoperative tumor recurrence occurred. Nevertheless, the patient refused chemotherapy and was treated with anti-PD-1 therapy alone. Intriguingly, the recurrent lesion was found to have continued to shrink during the subsequent follow-up. The aim of this study is to provide experience and protocols for the comprehensive treatment of patients with pregnancy-related gastric cancer.
We present a case of a pregnancy-related gastric cancer patient who achieved promising results with immunotherapy alone. The study including the human participant was reviewed and approved by the Research Ethics Committee of Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University and was carried out in accordance with the ethical standards of the World Medical Association’s Declaration of Helsinki. The patient provided written informed consent.
Case presentation
A 32-year-old female patient was admitted to the department of Emergency Obstetrics and Gynecology, Ren Ji Hospital, Shanghai Jiao Tong University (Shanghai, China) with an emergency admission of 11 h for hematemesis with epigastric pain while at 21 + 4 weeks of pregnancy on August 10, 2018. The patient had regular menstruation, the age of menarche was 12 years old, and the menstrual cycle was 5–7/30 with moderate volume and no dysmenorrhea. Her fertility history was 0–0-0–0, LMP: 2018–03-18, and EDC: 2018–12-30. Early pregnancy ultrasound verified the gestational weeks, 4 months of menopause, and felt fetal movement. The patient experienced severe vomiting on May 10, 2018 with pink foam in the vomitus and no concomitant symptoms such as abdominal pain, diarrhea, or melena. The patient regarded it as morning sickness and did not pay enough attention to it. Despite that, the vomiting gradually worsened until the patient was unable to eat normally. On August 1, 2018, she was presented to one of the maternal and child health hospitals and was later recommended to be referred to Renji Hospital. Family and social history was non-contributory and unremarkable. Her past history included in vitro fertilization–embryo transfer.
Upon physical examination, the vital signs were stable, with a mild anemic appearance, a mid to lower abdominal bulge, and mild epigastric pain. The patient was admitted with black vomitus. The abdominal circumference was 94 cm and the fundal height was 30 cm. There were no contractions, and the fetal heart rate was 149 bpm.
At auxiliary examination, the emergency gynecological ultrasound revealed as follows: singleton cephalic position, fetal heart rate and fetal movement: visible, growth meridian: 51–188-158–33. The emergency ancillary tests revealed as follows: Hb: 95 g/L; gastric fluid occult blood: positive, fecal occult blood: negative; AFP: 90.20 ng/mL (normal range: 0–25 ng/mL); CA 724: 172.10 U/ml (normal range: 0–6 U/mL), CYFRA (21–1): 11.48 ng/ml (normal range: 0–3.3 ng/mL), and CA242: 23.5 U/ml (normal range: 0–20 U/mL). The upper abdomen enhanced MRI showed a space-occupying lesion of the gastric sinus, suspicious lymph node enlargement on the lateral side of the greater curvature of the gastric sinus, and a scan of the pregnant uterus (Figures 1A–C). Electron fiberoptic gastroscopy revealed a large, ulcerated lesion of the gastric sinus, involving the four walls and the gastric angle, and the lesser curvature of the gastric body (Supplementary Figure S1). The gastroscopic diagnosis was malignant ulcer of the gastric sinus with incomplete obstruction, and the pathological diagnosis was poorly differentiated adenocarcinoma. Moreover, the immunohistochemistry results indicated Her-2(-); the tumor was classified as cTNM:cT4N+M0. Based on the medical history and auxiliary examination results detailed above, the following diagnosis has been made: gastric adenocarcinoma, singleton pregnancy, and moderate anemia.
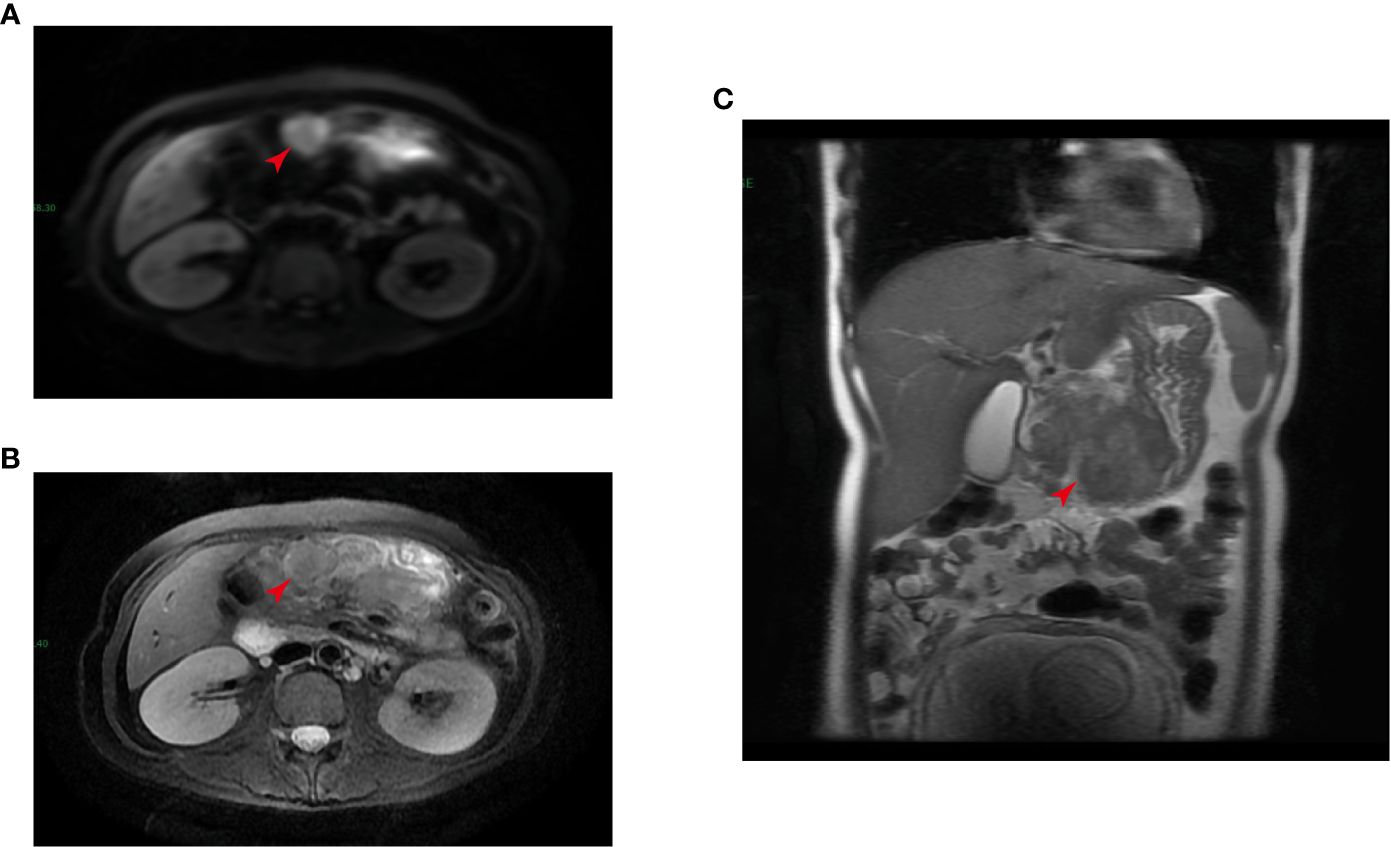
Figure 1 Magnetic resonance imaging (MRI) detected a tumor in the gastric sinus (as indicated by the red arrow, intersecting surface (A, B); coronal position (C)).
After conducting MDT discussions in Renji hospital, radical surgery for gastric cancer was recommended after termination of pregnancy. She was then induced in our Obstetrics and Gynecology Department (22 + 1 weeks gestation), and the placenta-fetal membranes were sent for pathology for the presence of tumor cells. The pathology suggested “placenta-fetal membranes” and placenta membrane tissue with degeneration; no tumor tissue was seen. On August 30, 2018, radical surgery for gastric cancer was performed (major distal gastrectomy with remnant gastrojejunostomy RY anastomosis, anterior colon, and gastric D2 lymph node dissection). Intraoperatively, the gross appearance of the resected specimen was a tumor located in the anterior wall of the gastric body and the gastric sinus, with a size of about 4 × 5 cm in diameter, stiff, infiltrative ulcerative type, with a central deep concave ulcer, with the tumor breaking through the plasma membrane layer and invading part of the transverse colonic mesentery and the pancreatic capsule, and perigastric nos. 6, 7, 8, 9, and 12a lymph nodes were accessible and enlarged. No obvious metastatic lesions were found in the abdominal and pelvic cavities.
The postoperative pathological examination showed a “poorly differentiated adenocarcinoma (diffuse infiltrative type, 6 × 5 × 1.2 cm) on the side of the lesser curvature of the gastric sinus, invading the plasma membrane, pancreatic adhesions, cancerous thrombus in the ducts, invasion of nerves, lymph nodes of the lesser curvature (5/7), lymph nodes of the greater curvature (1/5), lymph nodes of “no. 7” (1/4), lymph nodes of “no. 8” (1/5), and in the “transverse colonic mesentery”. The “upper and lower margins”, the omentum, the “hepatic ligament”, and the “nos. 4, 6, and 12a lymph nodes” were negative for fibrofatty tissue. The immunohistochemistry results were as follows: CEA (+), P16 (++), Ki67 (80%), P53 (-), ER (-), PR (-), HER2 (-), PD-L1 (-, interstitial 5%), MLH1 (-), PMS2 (-), MSH2 (+), and MSH6 (+) (Figures 2A–F). According to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (Springer International Publishing, 8th Edition 2018), the tumor was classified as pT4bN3M0, stage III C.
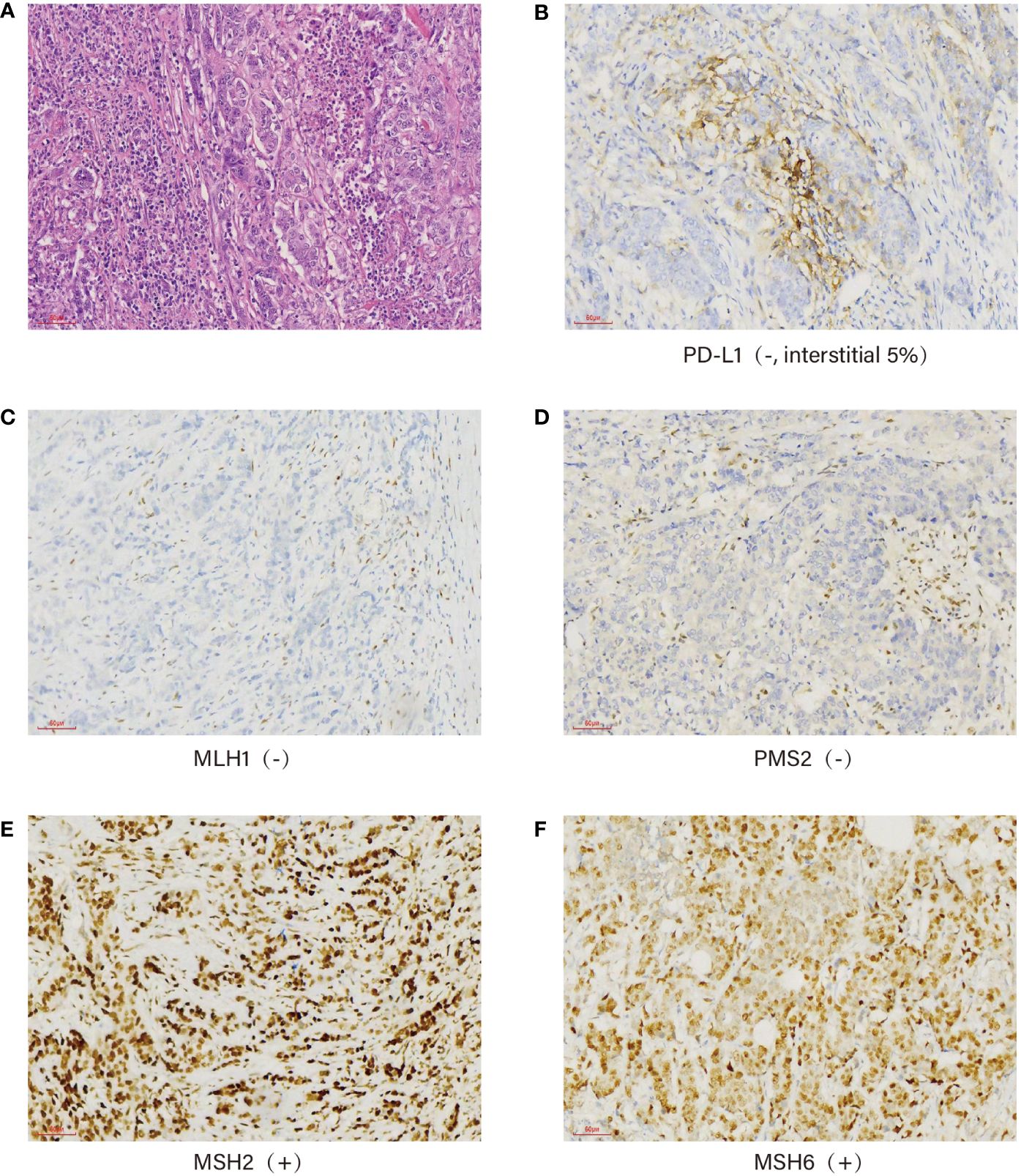
Figure 2 Immunohistochemistry: Pathological picture of the tumor confirmed under a ×40 microscope (A), PD-L1 (-, interstitial 5%) (B), mismatch repair protein expression: MLH1 (-) (C), PMS2 (-) (D), MSH2 (+) (E), and MSH6 (+) (F).
Unfortunately, postoperative follow-up at 1 year revealed tumor recurrence. CT enhancement of the abdomen showed a soft tissue mass measuring approximately 7.5 cm × 5.2 cm, considered as tumor recurrence/metastasis with a possible involvement of the duodenal stump, pancreatic head capsule, and adjacent transverse colon mesentery, with enlarged lymph nodes in the hilar region and retroperitoneum (Figure 3A). The PET-CT results similarly confirmed this result (Supplementary Figure S2). Due to the refusal of using any chemotherapy for personal reasons, the patient received a pembrolizumab injection of 100 mg (2 mg/kg Q3W) intravenous drip for the first time on September 3, 2019 (6–9). (In view of the scarcity of cases treated with PD-1 inhibitors alone, we referred to the relevant medication guidelines and empirically used the relevant doses).
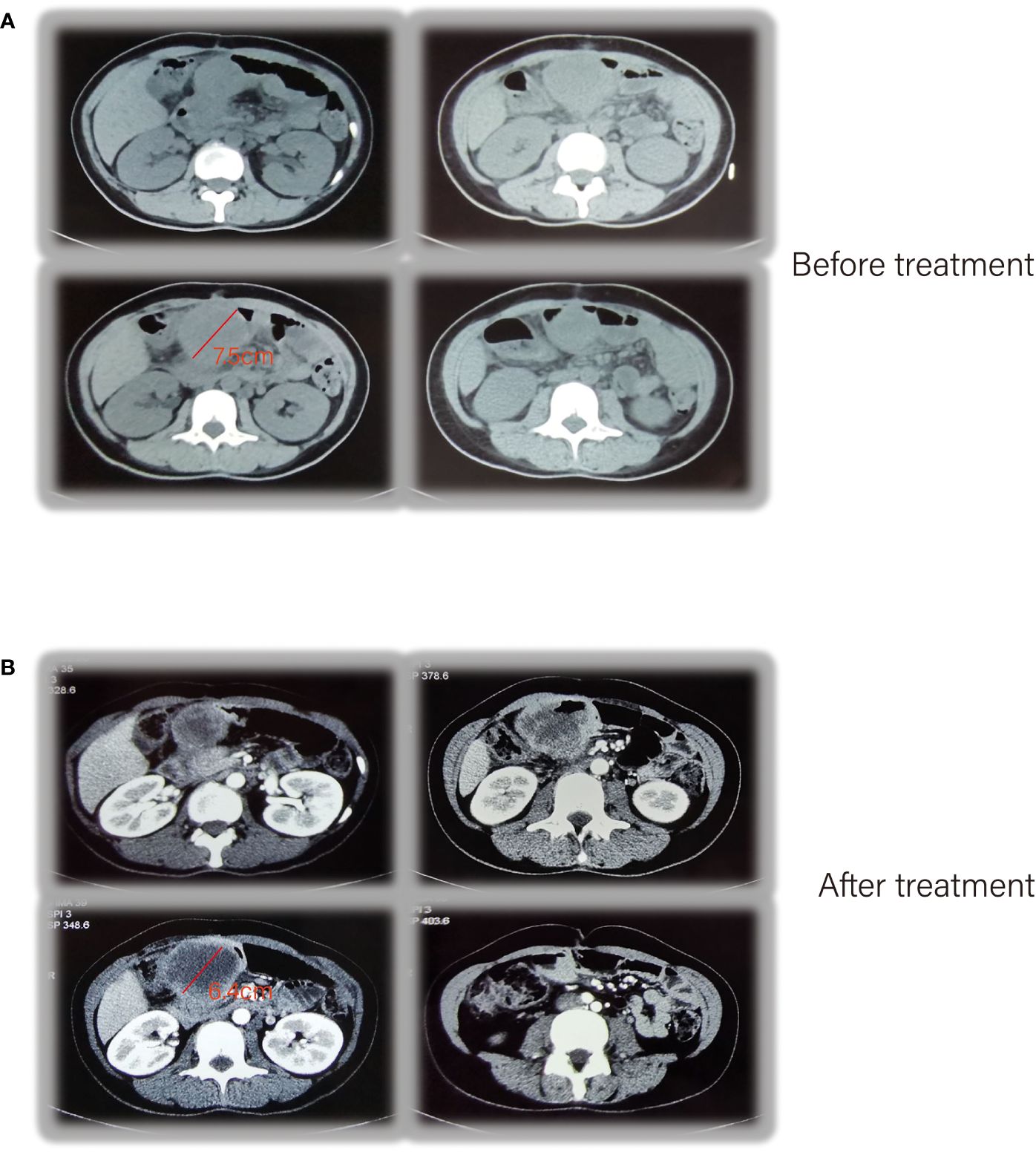
Figure 3 CT enhancement of the abdomen showed tumor recurrence (A), after receiving pembrolizumab therapy (B).
After the treatment, abdominal CTA was performed and, a mass measuring approximately 6.4 cm × 6 cm was seen on the right side of the mid-upper abdomen, which was significantly enhanced in a circular pattern with non-enhancing ground-density necrotic foci and gas shadows within, with scattered calcifications visible in the posterior superior wall and disappearance of the fatty gap with the surrounding intestinal canal (Figure 3B).
On the day of pembrolizumab administration, she suffered a high fever of 39°C –40°C with chills, cough, dyspnea, profuse sweating, malaise, hypotension (systolic blood pressure 70–60 mmHg), SpO2 80%–90%, WBC 19.87 × 109/L, and N% 80.3%, accompanied with normal hepatorenal function. Considering an infusion reaction or drug allergy to immunotherapy, aggressive symptomatic supportive therapy, physical hypothermia, oxygen inhalation, volume expansion, and rehydration were given. At 3 days later, the patient’s symptoms were significantly relieved, and the WBC (white blood cells) were also gradually decreasing in the routine blood tests. Then, on September 29, 2019, the patient received a pembrolizumab injection of 100 mg intravenous drip for the second time. On October 8, 2019, MRI enhancement of the abdomen suggested that the soft tissue mass in the right upper abdomen measured approximately 6.5 cm × 4.0 cm (Figure 4A). On October 19, 2019, the patient received a pembrolizumab injection of 100 mg intravenous drip for the third time. The CTA for the abdomen suggested a patchy shadow of the duodenum and peripancreatic head in the right upper abdomen, approximately 5.1 cm × 4.0 cm in diameter. The duodenum and peripancreatic head lesion was smaller than 19–10-8, multiple lymph nodes in the mesentery, hilar region, and retroperitoneum (Figure 4B). During the period November 11, 2019–February 24, 2020, the patient received a pembrolizumab injection of 100 mg intravenous drip regularly, and the lesion had shrunk to 1.7 cm in diameter (Figure 4C).
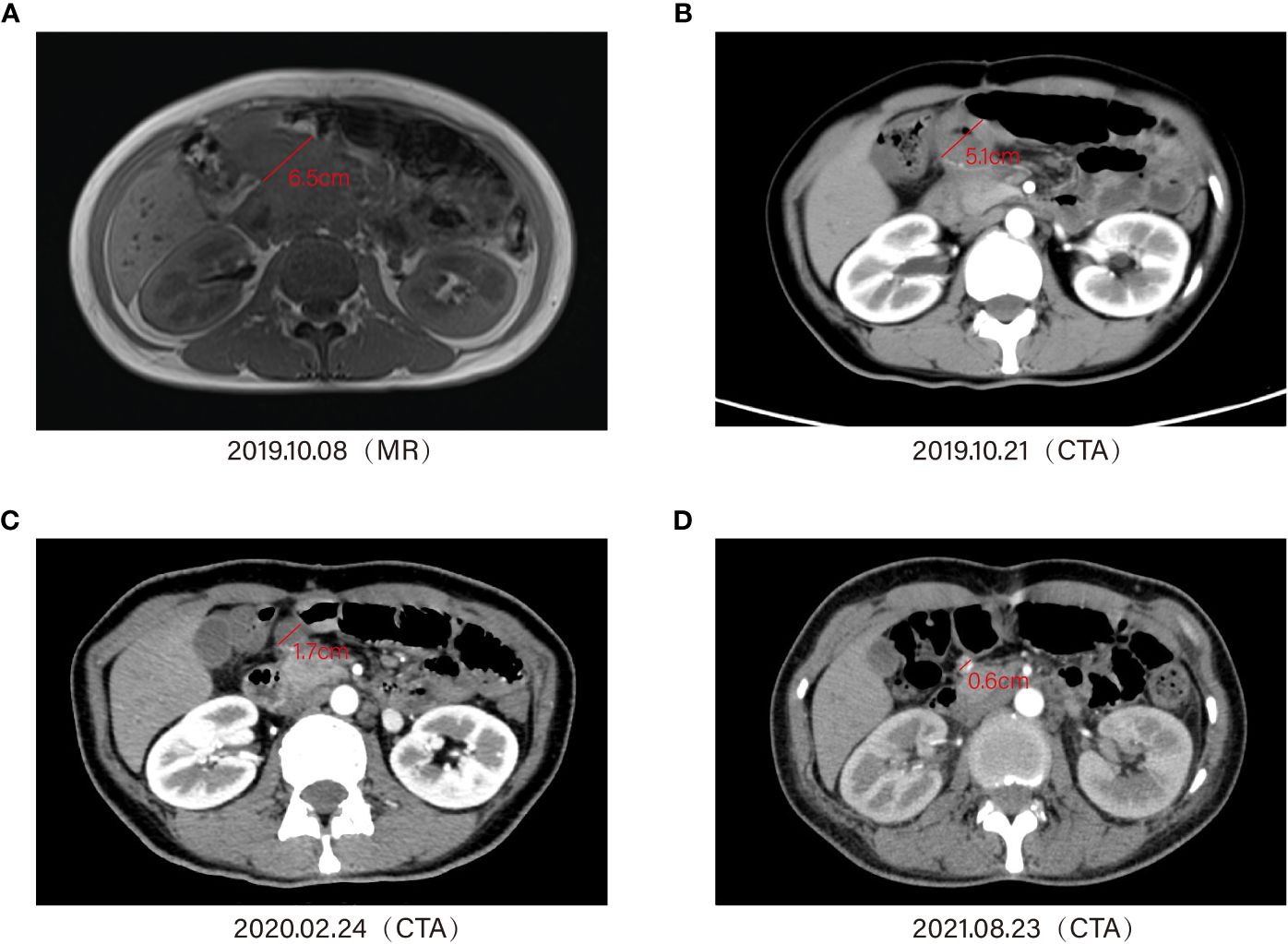
Figure 4 Timeline of treatment and diagnostic assessment about the patient who received a pembrolizumab injection of 100 mg, intravenous drip regularly. Magnetic resonance imaging (MRI) and enhanced computed tomography (CT) showed the lesion shrinking gradually (A–D).
The patient developed complications such as rash and vitiligo during this period. In addition, during the last application of immunotherapy, the patient experienced sudden hypotension (85/42 mmHg) with oxygen saturation of 88.4% and was transferred to the surgical ICU ward of our hospital. In addition, the patient’s troponin level was 1.30 ng/ml, the myoglobin level was 11.60 ng/ml, and the creatine kinase isoenzymes were 15.8 ng/ml, indicating drug-responsive hypotension and myocarditis. After receiving norepinephrine, dobutamine, and glucocorticoids to boost the heart pressure and anti-inflammatory effects, the symptoms were improved and the patient was discharged. At subsequent follow-up, the whole abdomen CTA (August 23, 2021) suggested that the duodenal and peripancreatic head lesion reduced to 0.6 cm, with a bilateral ovarian pattern in full view and multiple lymph nodes in the mesentery, hilar region, and retroperitoneum (Figure 4D).
Subsequently, due to the patient’s resistance to treatment, she did not receive further immunotherapy. Until at follow-up in August 2023, except for being diagnosed with undifferentiated connective tissue disease (UCTD) in February 2023, there were no signs of tumor recurrence. During the follow-up, the corresponding tumor markers showed a gradual downward trend (Supplementary Figure S3).
Discussion
The group of pregnancy-related gastric cancer patients has received considerable attention in recent years. In general, gastric cancer, perceived to have the fifth highest incidence of malignancies worldwide, is one of the four leading causes of cancer-related deaths (10). Actually, gastric cancer is the most commonplace cancer in the middle-aged and elderly population (average age of prevalence is 60 years old) and is less common in people under 40 years of age. Less than 15% of all gastric adenocarcinomas occur in adults younger than 41 years of age, and some scholars believed that the rare gastric cancer during pregnancy has the same features as in other patients under 40 years (11, 12).
Due to a great quantity of factors, the difficulty of diagnosing gastric cancer during pregnancy is significantly increased, leading to pregnant patients with gastric cancer being diagnosed at a progressive or even advanced stage, which gives birth to an extremely poor prognosis for survival (13–15). This has alerted the requirements to attach importance to gastrointestinal symptoms during pregnancy, which includes conducting timely and comprehensive physical examinations, checking for tumor markers, and performing fiber optic gastroscopy to exclude gastric lesions (16–18). For pregnant patients with gastric cancer, the timing of systemic chemotherapy intervention, surgical intervention to reduce the impact on the fetus during delivery, and treatment for the fetus during different trimesters are key areas for further research (19–21). According to previous experience, most of the patients reported in China with gastric cancer in pregnancy are treated with SOX regimen chemotherapy after surgery. The first-line chemotherapy regimens for patients with advanced disease are dominated by a combination of platinum- and fluorouracil-based regimens, and the second-line chemotherapy regimens contain irinotecan or raltitrexed (22).
Additionally, the results of several studies demonstrated that HER-2-positive patients with advanced gastric cancer will benefit from trastuzumab treatment. There are no significant differences in HER-2 expression and amplification between gastric cancer patients in pregnancy and the non-pregnant ones. Therefore, HER-2 testing should be routinely recommended for patients with gastric cancer during pregnancy (23). With the advent of the era of immunotherapy, immunotherapy combined with chemotherapy has gradually become the first-line treatment choice for advanced gastric cancer. The efficacy of immunotherapy in MSI-H patients is satisfactory (24–26).
Immune checkpoint blockade is now regarded as a strategy for chemorefractory gastric cancer. The phase 2 non-randomized KEYNOTE-059 trial resulted in improved overall survival for an international group of chemorefractory gastric cancer patients treated with pembrolizumab (27). Currently, more antibodies targeting PD-L1 have also become commercially available (28, 29). Novel pharmaceutics development like computational analysis of PD-L1 may be allowed for the accurate determination of antigen–antibody interactions, which could improve the efficiency of immunotherapy (30, 31). However, the clinical practice of anti-PD-1 monotherapy only involves some special cancer species, and the efficacy is still controversial (32, 33). There have been only very few reports suggesting the use of immune checkpoint inhibitors in pregnant patients with melanoma (34).
To date, there are a few reports of satisfactory outcomes with PD-1 inhibitors alone after surgery in pregnancy-related gastric cancer patients, and there are no precise clinical guidelines recommending the use of PD-1 inhibitors alone. Furthermore, relevant studies have shown that the use of immune checkpoint inhibitors leads to an increased incidence of spontaneous abortion, stillbirth, and preterm birth from the onset of fetal organ development to delivery (35, 36). To the best of our knowledge, we provide the first case report of a satisfactory outcome with immunotherapy alone in a pregnancy-related gastric cancer patient. This patient opted for the termination of pregnancy and radical surgery, given a variety of subjective or objective factors. This case also refused conventional chemotherapy and received only immunotherapy alone. As a matter of fact, the discovery of tumor recurrence after surgical treatment in this case poses a greater challenge to the clinical surgeon. How should the treatment after recurrence be chosen? How about the choice of chemotherapy regimen? Is there an opportunity to consider re-surgical intervention? Does the patient still have a requirement for fertility and will she consider in vitro fertilization–embryo transfer again? It is pleasing to note that immunotherapy alone has shown promising results in controlling the recurrent lesions during the subsequent follow-up. The favorable outcome of the pregnancy-related gastric cancer patient treated with PD-1 inhibitors alone may be related to the alteration of the immune microenvironment by pregnancy-related hormones. Moreover, some related reports have yet to mention a novel mechanism in trophoblasts to create a tolerant fetal–maternal interface by upregulating PD-L1. Tumors may also use PD-L1 expression to evade the host’s immune response, thereby promoting their survival. Moreover, it has been hypothesized that age-related changes to the immune system may impact the efficacy and toxicity of immunotherapy. Nevertheless, there have been few studies in younger adults (e.g., under 40 years). Thus, it remains unclear whether immunotherapy has a different efficacy in this subgroup (37).Therefore, the application of PD-1 inhibitors may partially serve to disarm immune escape and strengthen anti-tumor immunity in gravida, but the exact mechanism needs to be further investigated (38).
In conclusion, the diagnosis and treatment of a pregnancy-related gastric cancer patient need to be balanced with multiple factors. This case report has provided some treatment options for related cases.
Conclusions
The treatment of pregnancy-related gastric cancer is quite tricky in clinical practice.
In this case report, we focus on a 32-year-old pregnant patient diagnosed with gastric cancer. Radical surgery was conducted after the termination of pregnancy. At 1-year postoperative follow-up, tumor recurrence was shown. Fortunately, this patient has achieved a quite favorable outcome after having received anti-PD-1 alone. For similar cases, questions such as when to intervene with systemic chemotherapy in pregnant patients with gastric cancer, when to perform surgical intervention with minimal impact on fetal delivery, how to weigh the timing of fetal treatments in different trimesters, and whether single-agent immunotherapy results in favorable outcomes deserved to be studied in depth. This case report provides some treatment options for related cases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XuL: Writing – original draft, Writing – review & editing. XiL: Writing – review & editing. CZ: Writing – review & editing. LJ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (grant number: 82203503).
Acknowledgments
The authors thank the patient who was involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1321149/full#supplementary-material
Supplementary Figure 1 | Electron fiber optic gastroscopy: large, ulcerated lesion of the gastric sinus, involving the four walls and the gastric angle, and the lesser curvature of the gastric body.
Supplementary Figure 2 | PET-CT detected a tumor recurrence in the right upper abdomen.
Supplementary Figure 3 | CA724, CA199, and CA125 decreased gradually during follow-up.
References
1. Constantin A, Constantin R, Achim F, Socea B, Predescu D. Pregnancy and gastric cancer: A narrative review. Diagnostics (Basel). (2023) 13:1909. doi: 10.3390/diagnostics13111909
2. Sakamoto K, Kanda T, Ohashi M, Kurabayashi T, Serikawa T, Matsunaga M, et al. Management of patients with pregnancy-associated gastric cancer in Japan: a mini-review. Int J Clin Oncol. (2009) 14:392–96. doi: 10.1007/s10147–009-0903–6
3. Lee H-J, Lee InK, Kim JW, Lee KUk, Choe KJ, Yang H-K. Clinical characteristics of gastric cancer associated with pregnancy. Dig Surg. (2009) 26:31–6. doi: 10.1159/000193330
4. Alsina M, Arrazubi V, Diez M, Tabernero J. Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat Rev Gastroenterol Hepatol. (2023) 20:155–70. doi: 10.1038/s41575-022-00703-w
5. Tanda ET, Croce E, Spagnolo F, Zullo L, Spinaci S, Genova C, et al. Immunotherapy in adolescents and young adults: what remains in cancer survivors? Front Oncol. (2021) 11:736123. doi: 10.3389/fonc.2021.736123
6. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039
7. Lala M, Li TR, de Alwis DP, Sinha V, Mayawala K, Yamamoto N, et al. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer. (2020) 131:68–75. doi: 10.1016/j.ejca.2020.02.016
8. Brahmer JR, Long GV, Hamid O, Garon EB, Herbst RS, Andre T, et al. Safety profile of pembrolizumab monotherapy based on an aggregate safety evaluation of 8937 patients. Eur J Cancer. (2024) 199:113530. doi: 10.1016/j.ejca.2024.113530
9. Schutte T, Derks S, van Laarhoven HWM. Pembrolizumab plus chemotherapy for advanced gastric cancer. Lancet Oncol. (2024) 25:e51. doi: 10.1016/S1470–2045(23)00621–6
10. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
11. Buffart TE, Carvalho B, Hopmans E, Brehm V, Klein Kranenbarg E, Schaaij-Visser TBM, et al. Gastric cancers in young and elderly patients show different genomic profiles. J Pathol. (2007) 11:45–51. doi: 10.1002/path.2085
12. Jaspers VK, Gillessen A, Quakernack K. Gastric cancer in pregnancy: do pregnancy, age or female sex alter the prognosis? Case reports and review. Eur J Obstet Gynecol Reprod Biol. (1999) 87:13–22. doi: 10.1016/S0301-2115(99)00072-X
13. Cappell MS. The fetal safety and clinical efficacy of gastrointestinal endoscopy during pregnancy. Gastroenterol Clin North Am. (2003) 32:123–79. doi: 10.1016/s0889–8553(02)00137–1
14. Miura T, Yamada S, Maruyama G, Nakamura J, Miura T, Yanagi M, et al. A pregnant woman with inoperable advanced gastric cancer who received systemic anti-cancer chemotherapy after the non-full term fetus delivery by cesarean section. Nihon Shokakibyo Gakkai Zasshi. (2009) 106:1500–7
15. Yildiz M, Akgun Y, Ozer H, Mihmanli V. A rare case presentation: pregnancy and gastric carcinoma. BMC Gastroenterol. (2020) 20:33. doi: 10.1186/s12876–020-1184–9
16. O’mahony S. Endoscopy in pregnancy. Best Pract Res Clin Gastroenterol. (2007) 21:893–99. doi: 10.1016/j.bpg.2007.05.007
17. Lee LC, Lee HY, Lee YH, Young YC, Huang SC. Pregnancy associated with gastric carcinoma. J Formos Med Assoc. (1998) 97:866–68.
18. Pectasides M, Sekhar A, Dighe MK, Schwartz G, Shah SN, Mulcahy MF, et al. Gastrointestinal Malignancies in pregnancy. Abdom Radiol (NY). (2023) 48:1709–23. doi: 10.1007/s00261–022-03788–8
19. Auger N, Ayoub A, Piché N. First trimester general anaesthesia and risk of central nervous system defects in offspring. Br J Anaesth. (2020) 124:e92–4. doi: 10.1016/j.bja.2020.01.002
20. Nishie H, Mizushima T, Suzuki Y, Fukusada S, Inoue T, Kachi K, et al. Chemotherapy treatment of a pregnant woman with progressive gastric cancer. Intern Med. (2015) 54:1207–12. doi: 10.2169/internalmedicine.54.3973
21. Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. (2004) 5:283–91. doi: 10.1016/S1470–2045(04)01466–4
22. Jiang ZC, Chi Y. Data review and analysis of 22 cases of gastric cancer associated with pregnancy. Zhonghua Zhong Liu Za Zhi. (2018) 40:631–35. doi: 10.3760/cma.j.issn.0253–3766.2018.08.013
23. Song MJ, Park YS, Song HoJ, Park SeJ, Ahn JiY, Choi KD, et al. Prognosis of pregnancy-associated gastric cancer: an age-, sex-, and stage-matched case-control study. Gut Liver. (2016) 10:731–38. doi: 10.5009/gnl15323
24. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
25. Wang F-H, Zhang X-T, Li Y-F, Tang L, Qu X-J, Ying J-E, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). (2021) 41:747–95. doi: 10.1002/cac2.12193
26. Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Cutsem EV, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol. (2021) 7:895–902. doi: 10.1001/jamaoncol.2021.0275
27. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, MaChado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. (2018) 4:e180013. doi: 10.1001/jamaoncol.2018.0013
28. Wang Y, Zhou Y, Yang L, Lei L, He B, Cao J, et al. Challenges coexist with opportunities: spatial heterogeneity expression of PD-L1 in cancer therapy. Adv Sci (Weinh). (2024) 11:e2303175. doi: 10.1002/advs.202303175
29. Hu H, Wang Ke, Jia R, Zeng Z-X, Zhu M, Deng Y-L, et al. Current status in rechallenge of immunotherapy. Int J Biol Sci. (2023) 19:2428–42. doi: 10.7150/ijbs.82776
30. Kalim M, Ali H, Rehman AUr, Lu Y, Zhan J. Bioengineering and computational analysis of programmed cell death ligand-1 monoclonal antibody. Front Immunol. (2022) 13:1012499. doi: 10.3389/fimmu.2022.1012499
31. Xu X, Luo S, Zhao X, Tang B, Zhang E, Liu J. Computational analysis of PD-L1 dimerization mechanism induced by small molecules and potential dynamical properties. Int J Biol Macromol. (2024) 265:130921. doi: 10.1016/j.ijbiomac.2024.130921
32. Pai SI, Faivre S, Licitra L, Machiels JP, Vermorken JB, Bruzzi P, et al. Comparative analysis of the phase III clinical trials of anti-PD1 monotherapy in head and neck squamous cell carcinoma patients (CheckMate 141 and KEYNOTE 040). J Immunother Cancer. (2019) 7:96. doi: 10.1186/s40425-019-0578-0
33. Pires da Silva I, Zakria D, Ahmed T, Trojanello C, Dimitriou F, Allayous C, et al. Efficacy and safety of anti-PD1 monotherapy or in combination with ipilimumab after BRAF/MEK inhibitors in patients with BRAF mutant metastatic melanoma. J Immunother Cancer. (2022) 10:e004610. doi: 10.1136/jitc-2022-004610
34. Bucheit AD, Hardy JT, Szender JB, Glitza Oliva IC. Conception and viable twin pregnancy in a patient with metastatic melanoma while treated with CTLA-4 and PD-1 checkpoint inhibition. Melanoma Res. (2020) 30:423–25. doi: 10.1097/CMR.0000000000000657
35. Chen Z, Huang J, Kwak-Kim J, Wang W. Immune checkpoint inhibitors and reproductive failures. J Reprod Immunol. (2023) 156:103799. doi: 10.1016/j.jri.2023.103799
36. Garutti M, Lambertini M, Puglisi F. Checkpoint inhibitors, fertility, pregnancy, and sexual life: a systematic review. ESMO Open. (2021) 6:100276. doi: 10.1016/j.esmoop.2021.100276
37. Wong SK, Nebhan CA, Johnson DB. Impact of patient age on clinical efficacy and toxicity of checkpoint inhibitor therapy. Front Immunol. (2021) 12:786046. doi: 10.3389/fimmu.2021.786046
Keywords: pregnancy-related gastric cancer, tumor recurrence, immunotherapy, anti-PD-1, monotherapy
Citation: Liu X, Li X, Zhu C and Ji L (2024) Effective control of postoperative recurrence of pregnancy-related gastric cancer using anti-PD-1 as a monotherapy: a case report. Front. Oncol. 14:1321149. doi: 10.3389/fonc.2024.1321149
Received: 13 October 2023; Accepted: 23 April 2024;
Published: 10 May 2024.
Edited by:
Utpreksha Vaish, University of Alabama at Birmingham, United StatesReviewed by:
Sagnik Giri, University of Arizona, United StatesHem Chandra Jha, Indian Institute of Technology Indore, India
Dhanisha Sulekha Suresh, University of Alabama at Birmingham, United States
Copyright © 2024 Liu, Li, Zhu and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linhua Ji, MTM1NjQzODM3OTNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xu Liu
Xu Liu Xiaoqi Li
Xiaoqi Li Chunchao Zhu
Chunchao Zhu Linhua Ji*
Linhua Ji*