- 1Center for Clinical Research, The Affiliated Hospital of Qingdao University, Qingdao, China
- 2Department of Hepatobiliary and Pancreatic Surgery, The Affiliated Hospital of Qingdao University, Qingdao, China
- 3Clinical Laboratory, The Affiliated Hospital of Qingdao University, Qingdao, China
Introduction: Lymph node metastasis (LNM) is a critical prognostic factor for colorectal cancer (CRC). Due to the potential influence of immune system on CRC progression, investigation into lymphocyte subsets as clinical markers has gained attention. The objective of this study was to assess the capability of lymphocyte subsets in evaluating the lymph node status and prognosis of CRC.
Methods: Lymphocyte subsets, including T cells (CD3+), natural killer cells (NK, CD3- CD56+), natural killer-like T cells (NK-like T, CD3+ CD56+), CD38+ NK cells (CD3- CD56+ CD38+) and CD38+ NK-like T cells (CD3+ CD56+ CD38+), were detected by flow cytometry. Univariate and multivariate analyses were used to assess the risk factors of LNM. The prognostic role of parameters was evaluated by survival analysis.
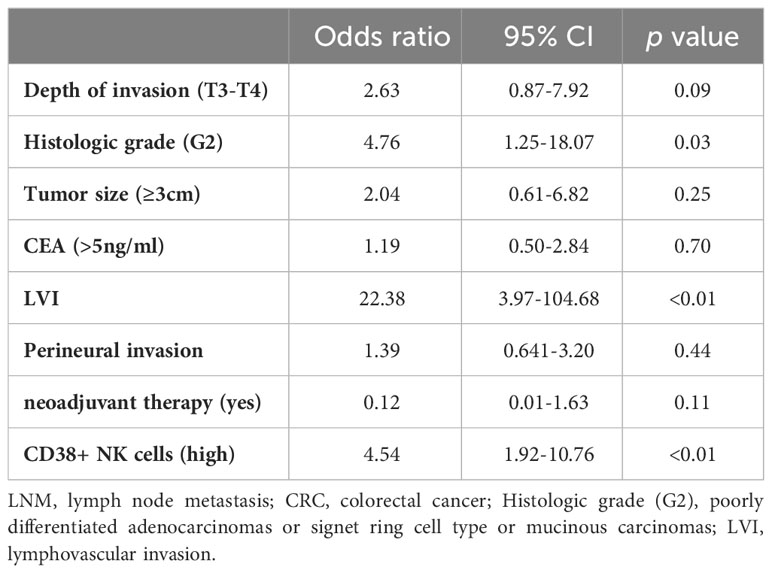
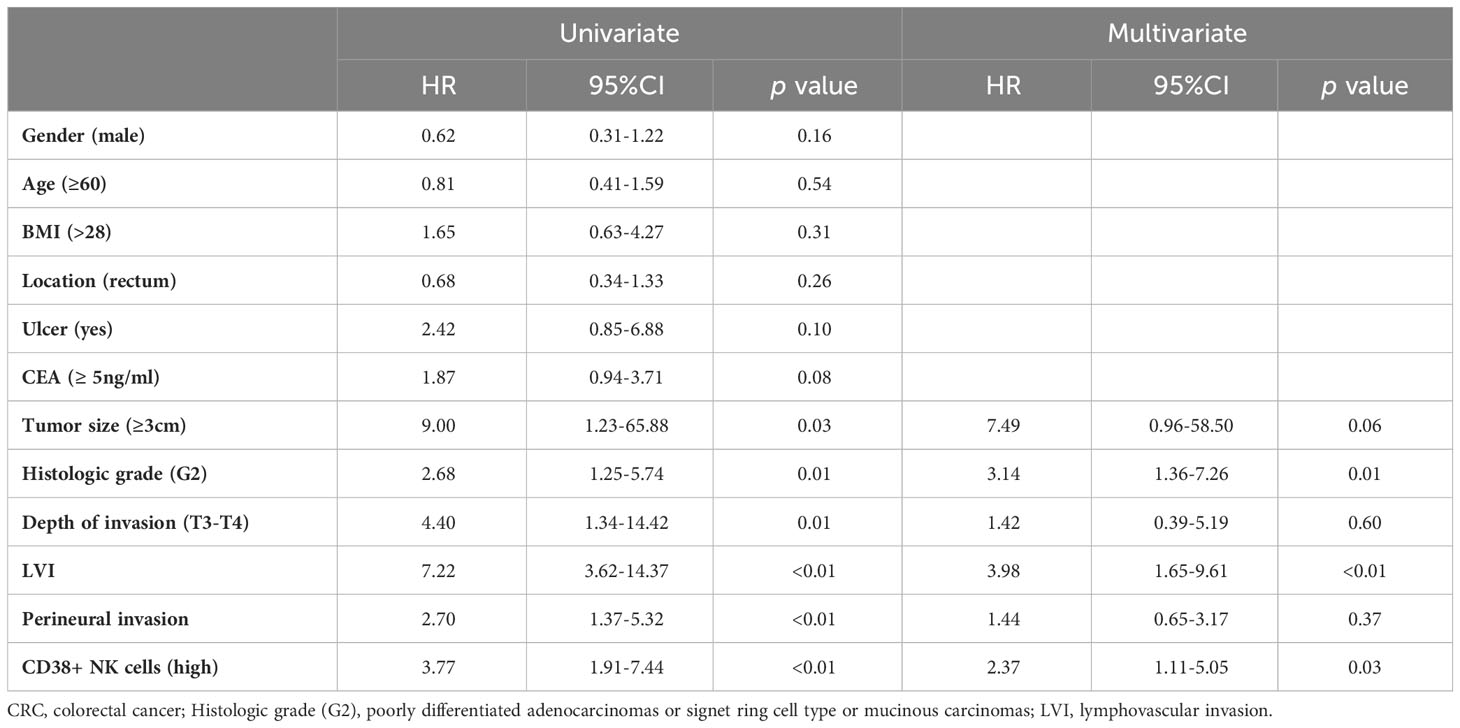
Results: The proportion of CD38+ NK cells within the NK cell population was significantly higher in LNM-positive patients (p <0.0001). However, no significant differences were observed in the proportions of other lymphocyte subsets. Poorer histologic grade (odds ratio [OR] =4.76, p =0.03), lymphovascular invasion (LVI) (OR =22.38, p <0.01), and CD38+ NK cells (high) (OR =4.54, p <0.01) were identified as independent risk factors for LNM. Furthermore, high proportion of CD38+ NK cells was associated with poor prognosis of CRC patients (HR=2.37, p =0.03).
Conclusions: It was demonstrated that the proportion of CD38+ NK cells was a marker overexpressed in LNM-positive patients compared with LNM-negative patients. Moreover, an elevated proportion of CD38+ NK cells is a risk factor for LNM and poor prognosis in CRC.
Introduction
Colorectal cancer (CRC) is one of the most common cancers worldwide, ranking third in terms of incidence and second in terms of mortality among all cancers (1). In CRC, the lymph node status is associated with prognosis and affects clinical treatment decisions. For example, patients with LNM identified during preoperative assessment are typically advocated for neoadjuvant chemotherapy (2). For patients with lesion invasion depth limited to the submucosa (cT1), endoscopic treatment is applicable only when the possibility of LNM is negligible (3). Although previous studies have indicated that pathological factors such as lymphovascular invasion (LVI) can effectively predict LNM in CRC patients (4), these indicators cannot predict lymph node status preoperatively.
Natural killer cells (NK cells) are integral component of the innate immune system and possess cellular cytotoxicity, making them effective immune cells against various threats (5). NK cells are TCR/CD3 complex-negative and are phenotypically defined by the expression of CD56 (6, 7). They exhibit a diverse range of functions that encompass immunoregulatory activities and immune responses against tumor cells and viral infections (8, 9). NK cells play a crucial role in the diagnosis and prognosis of diseases. Previous studies found the circulating CD3- CD56+ CD16+ NK cells were decreased in coronary artery disease (10). In the tumor microenvironment of papillary thyroid cancer, CD3- CD16- CD56bright NK cells were highly expressed (7). The proportion of circulating CD3- CD56+ CD16+ NK cells to lymphocytes was negatively correlated with the occurrence of CRC (11). Additionally, Cui et al. (12) discovered a negative correlation between circulating CD3- CD56+ CD16+ NK cells and the prognosis of CRC. CD38+ NK (CD3- CD56+ CD38+) cell was a subtype of NK cells. Morandi et al. (13) reported that NK cells generate adenosine through a CD38-mediated pathway, which serves as a regulatory mechanism to suppress the proliferation of CD4+ T cell. This suggested that the expression of CD38 on NK cells might had an immunosuppressive role. Indeed, the role of CD38+ NK cells in CRC remains unclear. In this study, flow cytometry was used to detected the proportions of lymphocyte subsets in the peripheral blood of CRC patients before initial treatment. The relationship between CD38+ NK cells and lymph node status was detected, and the risk factors for LNM in CRC patients was identified. In addition, utilizing the follow-up data, we evaluated the factors associated with adverse prognosis.
Materials and methods
Blood sample collection
Between March 2021 and August 2023, a total of 225 blood samples from CRC patients were collected. After excluding duplicates and patients with a history of tumor treatment and/or other neoplastic diseases, 165 patients were finally included in this study. All the enrolled patients were newly diagnosed and had not received any treatment, including surgery, radiation therapy, chemotherapy, and neoadjuvant chemoradiotherapy, at the time of blood sample collection. The blood samples were obtained by laboratory physicians at our hospital. The collection of samples followed standardized procedures and doctor’s prescriptions. Among all the participants, 72 patients were LNM-positive while 93 patients were LNM-negative, as confirmed by endoscopy and postoperative pathology (Figure 1). The design of this study adhered to the Declaration of Helsinki of the World Medical Association. The research protocol received approval from the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFYWZLL28002), and all patients provided written informed consent prior to participation.
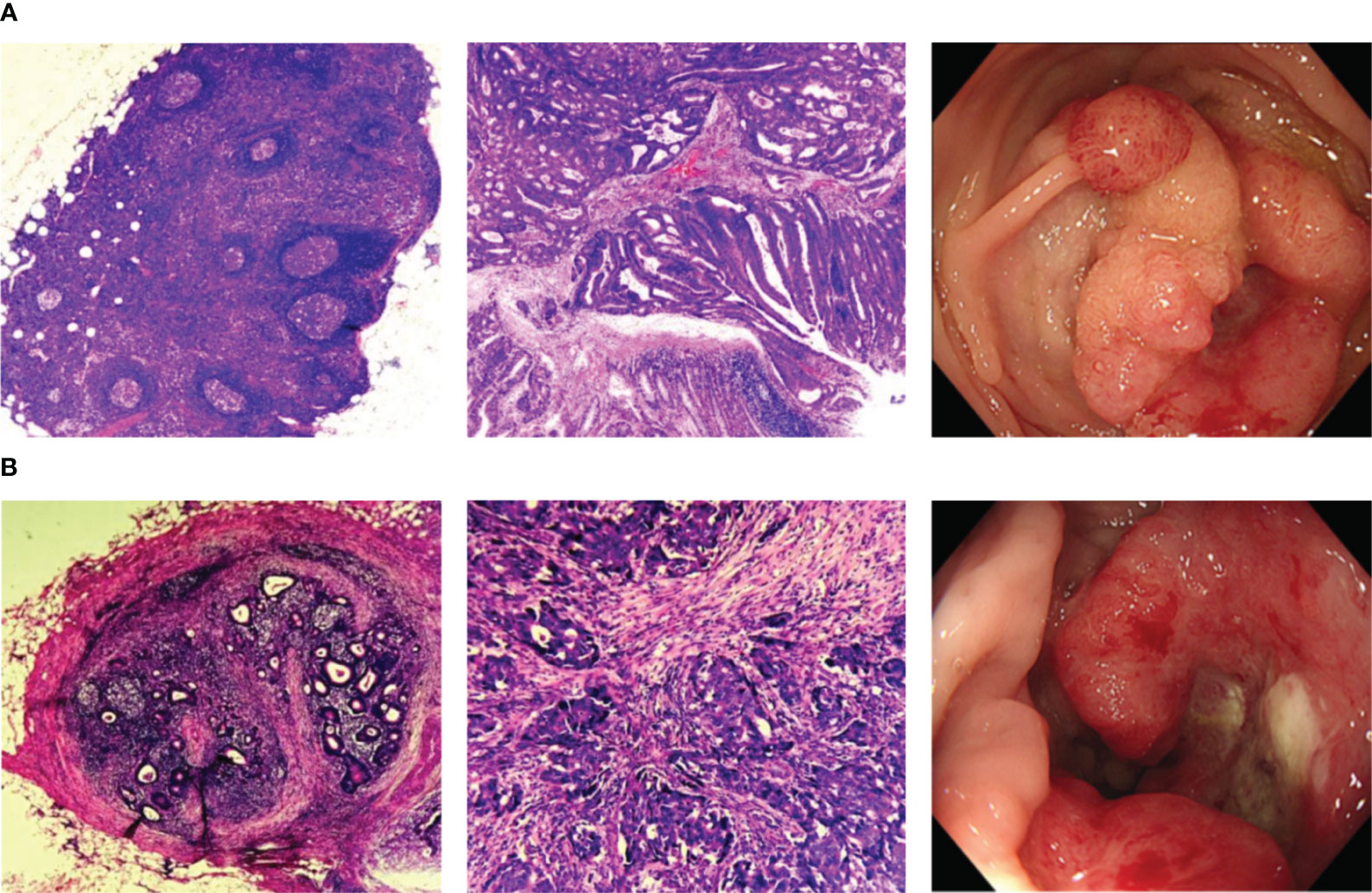
Figure 1 Representative histopathologic and endoscopic images. (A) Histopathologic and endoscopic images of CRC patients without LNM; (B) Histopathologic and endoscopic images of CRC patients with LNM. CRC, colorectal cancer; LNM, lymph node metastasis.
Detection of lymphocyte subsets in peripheral blood
600μl red blood cell lysis buffer (Solarbio, China) was added to 200μl fresh peripheral blood and lysed for 2h at 4°C. After centrifugation, the pellets were resuspended by adding 100μl PBS. Antibodies including FITC anti-human CD3 (Biolegend), PE anti-human CD56 (Biolegend) and APC anti-human CD38 (BD Biosciences, USA) were added to the mixture and incubated for 30min at room temperature under light. After centrifugation, the pellets were resuspended with 300ul PBS. Then, T cells (CD3+), NK cells (CD3- CD56+), natural killer-like T (NK-like T) cells (CD3+ CD56+), CD38+ NK (CD3- CD56+ CD38+) and CD38+ NK-like T cells (CD3+ CD56+ CD38+) were detected by flow cytometry (Agilent, USA). CD38 fluorescence-minus-one (FMO) was used as control.
Data collection
Based on the treatment guidelines set by the Japanese Society for Cancer of the Colon and Rectum (JSCCR), the enrolled patients subsequently underwent colon/rectal resection with lymph node dissection. The histologic slides collected from the procedure were then independently evaluated by two proficient pathologists.
According to the TNM staging classification from the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC), the extent of tumor invasion can be categorized into four grades: T1 (no invasion beyond the submucosa), T2 (invasion into the muscularis propria), T3 (invasion reaching the subserosa), and T4 (invasion into the visceral peritoneum or adjacent organs or structures). In addition, tumors were categorized into well-differentiated, moderately-differentiated, and poorly-differentiated adenocarcinomas, as well as mucinous carcinoma or signet ring cell type, according to the most predominant histological feature (14). LVI was assessed using the D2-40 antibody (Dako, Denmark). Perineural invasion was determined by detecting the presence of the S100 protein.
Other clinical and histopathological informations of all patients were collected, including gender, age, presence of ulcers, body mass index (BMI), carcinoembryonic antigen (CEA), location, tumor size and neoadjuvant therapy.
Statistical analysis
The data analysis was conducted using GraphPad Prism 8.3.0 and SPSS statistical software (version 22.0). For quantitative data with a normal distribution, t-test was employed for analysis, otherwise, the Mann-Whitney U test was utilized. Receiver operating characteristic (ROC) curve analysis was performed to distinguish between LNM-positive and LNM-negative patients and establish the optimal cut-off value. The sensitivity and specificity for each point could be obtained in ROC curve analysis, and the cut-off value was determined based on the point that maximized the sum of sensitivity and specificity. Univariate analysis involved the application of the chi-square test or Fisher’s exact test. The variables that showed statistical significance in the univariate analysis were then included in a multivariate logistic regression analysis to determine the independent risk factors for LNM. For the purpose of survival analysis, the log-rank test was employed. To identify prognostic factors, both univariate and multivariate Cox proportional hazards models were utilized. All p-values were two-sided, and p< 0.05 was deemed statistically significant.
Results
The proportions of lymphocyte subsets according to LNM status
The proportions of lymphocyte subsets in the peripheral blood of CRC patients were detected by flow cytometry. The representative flow cytometry plot was shown in Figure 2A. There was no difference in lymphocyte counts between the LNM-positive and LNM-negative groups (Figure 2B). In CRC patients with LNM, the proportion of CD38+ NK cells to NK cells was significantly increased (p < 0.0001) (Figure 2C). However, in the proportion of CD38+ NK-like T cells to total NK-like T cells, no difference was observed between patients with LNM and those without (Figure 2D). In addition, the proportions of NK cells (CD3- CD56+), NK-like T cells (CD3+ CD56+) and CD3+ T cells to lymphocytes showed no significant difference between the two groups (Figures 2E–G).
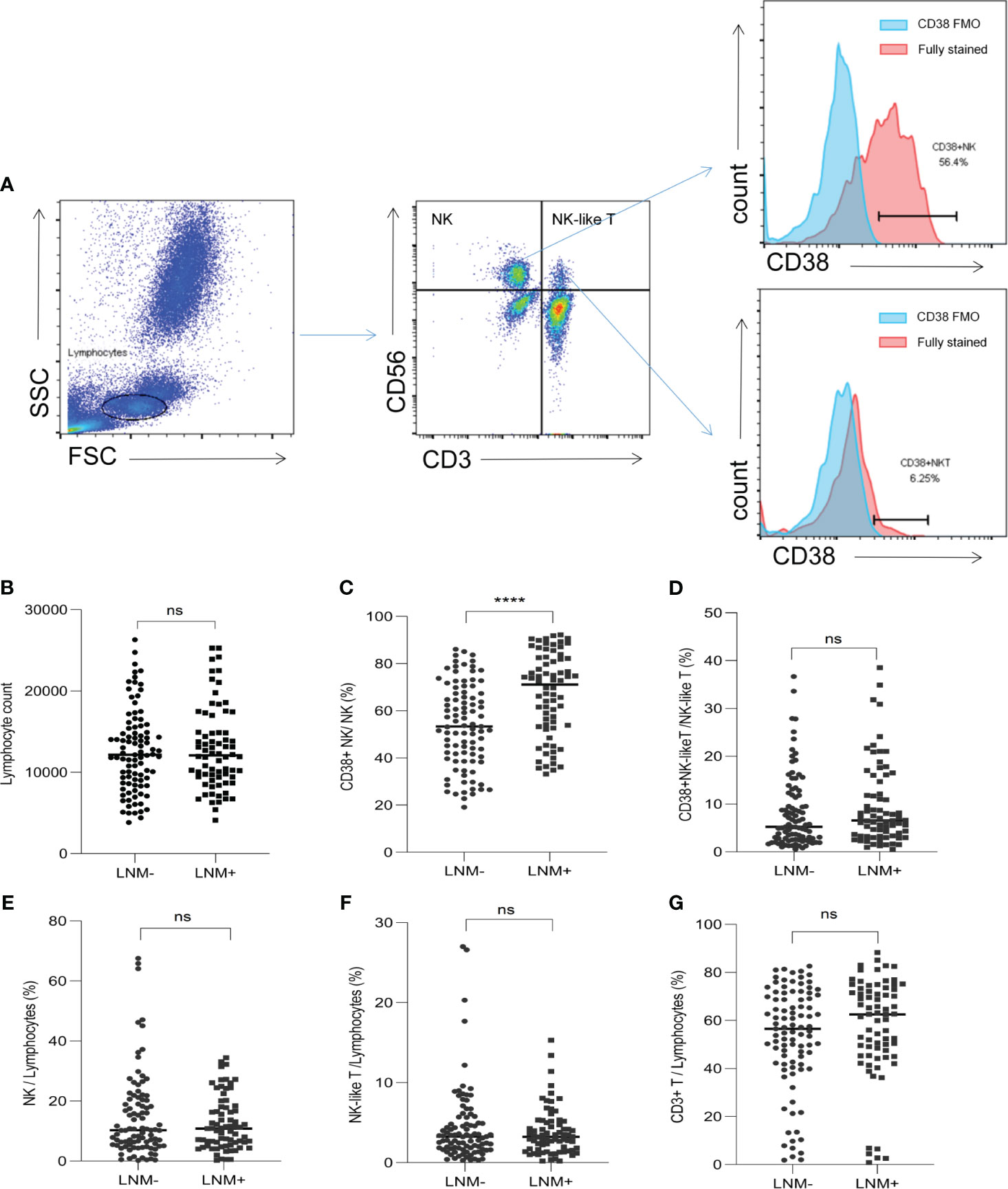
Figure 2 The proportions of lymphocyte subsets in CRC patients according to LNM status. (A) Flow cytometry plot showed the gating strategy for NK cells (CD3- CD56+), NK-like T cells (CD3+ CD56+), T cells (CD3+), CD38+ NK cells (CD3- CD56+ CD38+) and CD38+ NK-like T cells (CD3+ CD56+ CD38+). (B) The lymphocyte counts in LNM-positive and LNM-negative group. (C) The proportions of CD38+ NK cells to NK cells according to LNM; (D) The proportions of CD38+ NK-like T cells to NK-like T cells according to LNM; The proportion of NK cells (E), NK-like T cells (F) and T cells (G) to lymphocytes according to LNM status. CRC, colorectal cancer; LNM, lymph node metastasis; CD38 FMO: CD38 fluorescence-minus-one; LNM+, patients with lymph node metastasis; LNM-, patients without lymph node metastasis. ns, not significant, ****: p <0. 0001.
Further, the proportion of CD38+ CD56bright and CD38+ CD56dim NK cells between LNM-positive and LNM-negative patients were compared. The gating strategy for NK cell subtypes was shown in Supplementary Figure 1A. The proportions of CD38+ CD56bright (p<0.0001) and CD38+ CD56dim (p<0.0001) cells were increased in LNM-positive patients (Supplementary Figures 1B, C); And, the proportion of CD38 frequencies between CD56bright and CD56dim fractions showed no significant difference (p=0.11) (Supplementary Figure 1D).
ROC curve analysis was employed to differentiate LNM-positive patients and LNM-negative patients. The area under the curve (AUC) was determined to be 0.70, with a cut-off value of 72.85% (Figure 3A). The proportion of CD38+ NK cells had a superior ability in evaluating lymphnode status compared to CEA (AUC = 0.59) (Figure 3B).
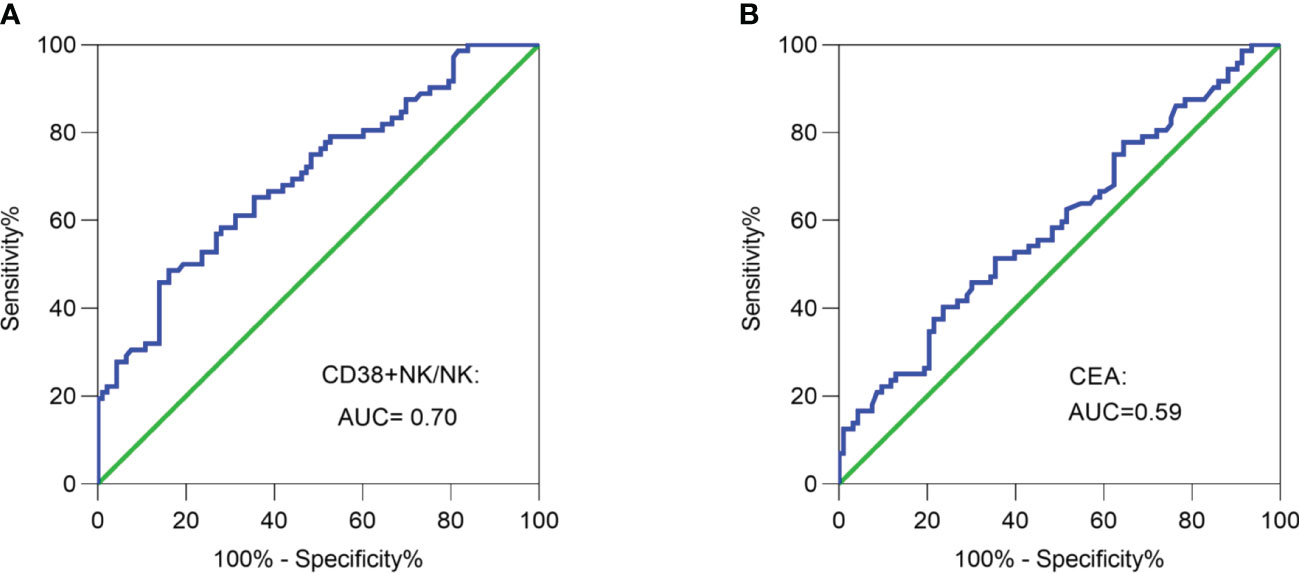
Figure 3 Receiver operating characteristic (ROC) curve analysis. (A) ROC curve analysis of the proportion of CD38+ NK cells was performed to differentiate LNM-positive patients and LNM-negative patients. (B) ROC curve analysis of CEA was performed to differentiate LNM-positive patients and LNM-negative patients. CRC, colorectal cancer; LNM, lymph node metastasis; CD38+ NK/NK, the proportions of CD38+ NK cells to NK cells; CEA, carcinoembryonic antigen.
Clinical and pathological features of the CRC patients included in this study
The incidence of LNM in patients with tumor size ≥ 3 cm was 49.25% (66/134), which was significantly higher than in patients with tumor size < 3 cm (19.35%, 6/31) (p < 0.01). In terms of histologic grade, there was a higher incidence of LNM in patients with G2 (p < 0.01) compared to those with G1. Regarding depth of invasion, LNM was found in 19.44% (7/36) of the patients with T1/T2 stage, whereas 50.391% (65/129) of the patients with T3/T4 stage were LNM-positive (p <0.01). The incidence of LNM in patients with LVI was higher than those without (p < 0.01). The patients with perineural invasion demonstrated an increased likelihood of LNM (p <0.01). And, patients with CEA>5ng/ml had a higher incidence of LNM (p = 0.04). Considering that neoadjuvant therapy might affect the LNM status of patients, we included it in this analysis. The incidence of LNM in patients who did not receive neoadjuvant therapy was 45.81% (71/155), which was significantly higher than those recdeived (10.00%, 1/10) (p =0.03). Based on the results depicted in Figure 2, we included the proportion of CD38+ NK cells in the analysis of risk factors for LNM. And high proportion of CD38+ NK cells were associated with the incidence of LNM (p <0.01). No significant differences was observed in terms of age, sex, BMI, presence of ulcer and tumor location between LNM-positive patients and LNM-negative patients. The detailed results were shown in Table 1. Additionally, we conducted separate analyses on the relationship between LNM and clinicopathological factors in colon cancer and rectal cancer. The results showed the LNM in both colon cancer and rectal cancer was associated with tumor size, histologic grade, depth of invasion, LVI, perineural invasion, CEA, and CD38+ NK cells; Besides, LNM in rectal cancer was also associated with neoadjuvant therapy (Supplementary Table 1).
Risk factors associated with the LNM in CRC patients
The univariate analysis revealed that several factors were associated with LNM, including histologic grade (G2), tumor size (≥3cm), depth of invasion (T3-T4), perineural invasion, LVI, CEA and high proportion of CD38+ NK cells. Subsequent stepwise logistic analysis revealed independent risk factors for LNM in CRC. These factors included histologic grade (G2) (OR = 4.76, p = 0.03), LVI (OR = 22.38, p < 0.01), and high proportion of CD38+ NK cells (OR = 4.54, p < 0.01). Multivariate analysis demonstrated neoadjuvant therapy tended to be a protective factor against LNM (OR=0.12), but this finding was not statistically significant (p = 0.11). The detailed results of the multivariate analysis were provided in Table 2.
The relationship between CD38+ NK cells and prognosis in CRC patients
The survival status of the CRC patients in this study was obtained from hospitalization records and telephone follow-up. The survival analysis demonstrated a significant association between the proportion of CD38+ NK cells to NK cells and poor prognosis in CRC patients (p < 0.001), as depicted in Figure 4A. The capability of CD38+ NK cells to evaluate prognosis was superior to CEA (p = 0.06) (Figure 4B). Furthermore, we investigated the relationship between clinical pathological factors and prognosis in CRC patients. Following both univariate and multivariate Cox regression analyses, the histologic grade (G2) (HR = 3.14; p = 0.01), LVI (HR = 3.98; p < 0.01), and higher proportion of CD38+ NK cells (HR = 2.37; p = 0.03) were identified as independent risk factors for poor prognosis of CRC. The details were shown in Table 3.

Figure 4 Log-rank analysis about the proportion of CD38+ NK cells and CEA. (A) The proportion of CD38+ NK cells was associated with poor prognosis of CRC patients. (B) No significant correlation was observed between serum CEA levels and prognosis of CRC. CRC, colorectal cancer; CD38+ NK/NK, the proportions of CD38+ NK cells to NK cells; CEA, carcinoembryonic antigen.
CD38+ NK cells were overexpressed in early-stage CRC with LNM compared to those without
In order to investigate the ability of CD38+ NK cells and CEA to evaluate lymph node status in early-stage CRC, we compared the proportions of CD38+ NK cells between T1/T2-stage patients with LNM and those without. The proportion of CD38+ NK cells to NK cells was significantly increased in LNM-positive group (p = 0.03 (Figure 5A). The levels of CEA was also elevated in CRC patients with LNM (p = 0.005) (Figure 5B). The ROC curve analysis showed that the AUC for the proportion of CD38+ NK cells was 0.69 (Figure 5C), while the AUC for CEA was 0.54 (Figure 5D).
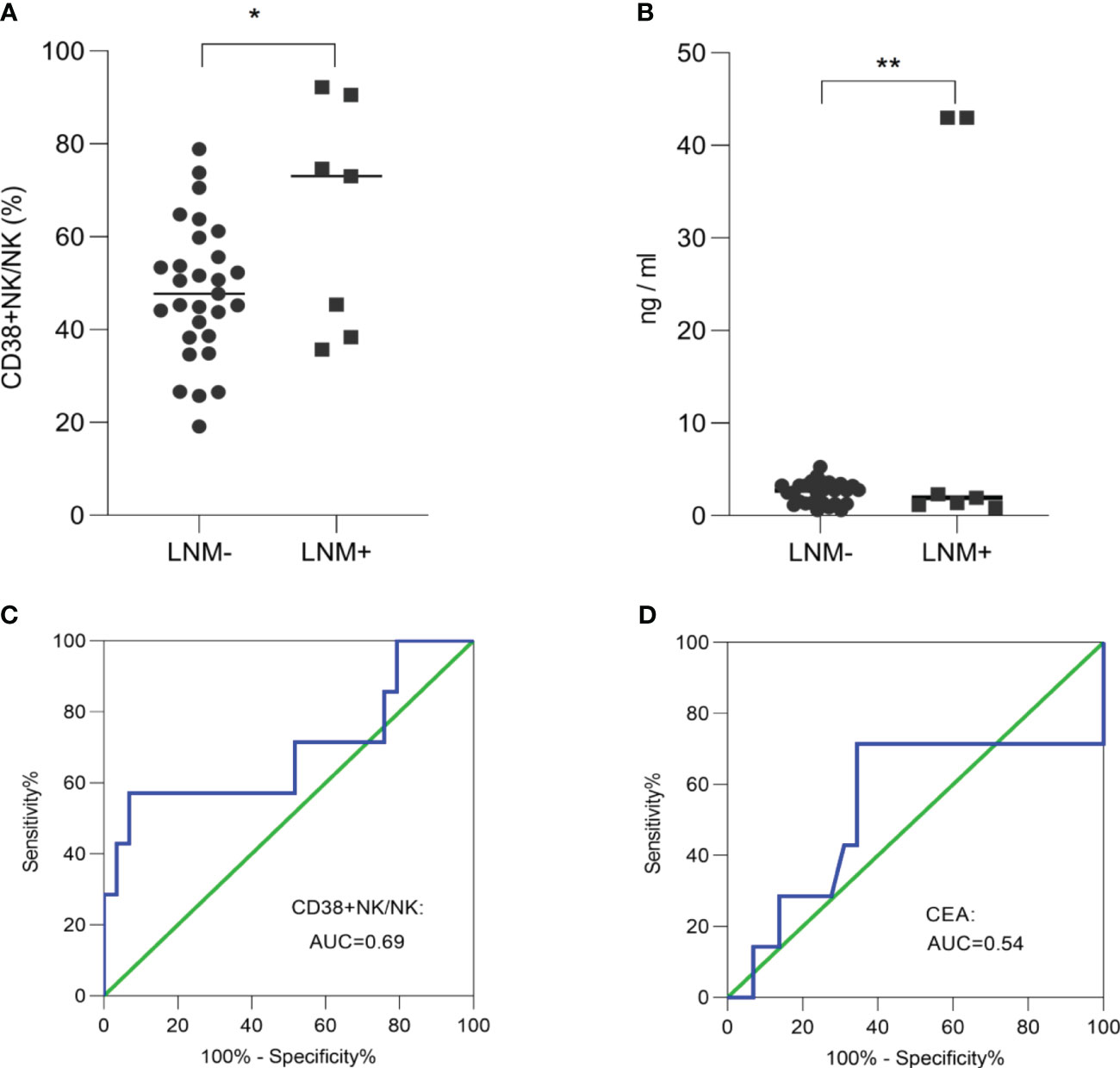
Figure 5 The ability of CD38+ NK cells and CEA to evaluate lymph node status in early-stage CRC. The proportion of CD38+ NK cells to NK cells (A) and the levels of CEA (B) was elevated in early-stage CRC patients with LNM. (C) ROC curve analysis of the proportion of CD38+ NK cells was performed to distinguish between early-stage CRC patients with LNM and those without LNM. (D) ROC curve analysis of CEA was performed to distinguish between early-stage CRC patients with LNM and those without LNM. CRC, colorectal cancer; LNM, lymph node metastasis; CD38+ NK/NK, the proportions of CD38+ NK cells to NK cells; CEA, carcinoembryonic antigen. *: p <0.05; **: p <0.01.
Discussion
In humans, NK cells are a widely distributed type of innate lymphoid cells, which specifically expresses CD56 but not CD3 (6). These cells play a significant role in the immune response against viral infections and tumors by releasing cytokines, chemokines, and displaying cytotoxic abilities (6). Previous studies had reported that NK cells play a significant role in the diagnosis and prognosis of coronary artery disease (10), papillary thyroid cancer (7), and CRC (11, 12). CD38 was expressed on the surface of NK cells (15); And CD38+ NK cell was a subtype of NK cells. Morandi et al. (13) demonstrated that CD38+ NK cells could exert immunosuppressive effects by inhibiting the proliferation of CD4+ T cells. Our research group has found that CD38+ NK cells in the peripheral blood of rheumatoid arthritis (RA) patients play an important role in regulating immune balance (16). In this study, we found that the proportion of CD38+ NK cells was associated with LNM and it was an independent risk factor for LNM and poor prognosis in CRC patients.
We investigated the proportion of lymphocyte subsets in the peripheral blood of CRC patients. The results showed there was no significant difference in the proportion of NK cells between LNM-positive and LNM-negative patients. Krijgsman, et al. (17) reported that the proportion of NK cells in the blood of CRC patients did not differ significantly from that of healthy donors. However, in CRC tumor microenvironment, NK cells were generally scarce (18–20), which might be related to the impaired migration of NK cells into CRC tumor tissue (18). Further research is warranted to investigate the disparities in expression and functionality of NK cells between peripheral blood and the tumor microenvironment.
Among the lymphocyte subsets measured in this study, the proportion of CD38+ NK cells to NK cells was increased in CRC patients with LNM. And, the proportion of CD38 frequencies between CD56bright and CD56dim fractions showed no significant difference. Additionally, the elevated proportion of CD38+ NK cells was associated with adverse outcomes of CRC. Moreover, it served as an independent risk factor for both LNM and poor prognosis. In tumor cells, CD38 had been shown to facilitate tumor proliferation and migration in lung and cervical cancer (21, 22). Additionally, Deckert et al. (23) discovered that inhibiting CD38 in multiple myeloma cells promoted cellular apoptosis. As widely recognized, CD38 is an enzyme responsible for the hydrolysis of nicotinamide adenine dinucleotide (NAD+), leading to the generation of adenosine diphosphate ribose (ADPR) or cyclic ADPR (cADPR) (24–26). This enzymatic activity has been shown to impact calcium signaling and release, resulting in the decrease of extracellular NAD+ levels, alteration of calcium cascade reactions, and facilitation of adenosine-mediated immune suppression (24–26). In immune cells, inhibiting CD38 expression on T cells enhanced their anti-tumor effect through the CD38-NAD+ axis. In addition, CD38KO NK cells produced higher amounts of IFN-γ production and exhibited enhanced anti-tumor activity (27). In addition, Wang et al. (16) found that CD38 on NK cells could modulate T cell immune balance by regulating cytokine secretion.
Further, the previous studies had proposed that the impact of CD38 on immune cells is primarily mediated through the regulation of FasL expression (28); Alterations in CD38/FasL-mediated NK cell apoptosis have been reported in gastric cancer (29). These evidences emphasized the role of CD38 in malignant tumors and underscored its significance as a target in cancer immunotherapy (30).
CEA was commonly used as the primary marker for diagnosis and monitor of CRC. The levels of CEA in serum could also be used as an indicator to assess LNM in CRC (31), but its effectiveness was not optima (32). Our results demonstrated that the proportion of CD38+ NK cells had a superior ability in evaluating lymph node status and prognosis of CRC compared to CEA. Cells that co-express CD3 and CD56 are referred to as NK-like T cells (33, 34). NK-like T cells possess cytotoxicity but are primarily recognized for their significant regulatory functions, amplifying or suppressing immune responses through the secretion of abundant pro-inflammatory or anti-inflammatory cytokines upon activation (35). Zhou et al. (36) found that an elevated level of CD3+ CD56+ CD16+ NK-like T cells was associated with increased pregnancy rates and live birth rates in in vitro fertilization treatment. Krijgsman et al. (17) pointed out that CD3+ CD56+ NK-like T cells in CRC patients were not associated with disease-free survival, which was consistent with our research findings. Furthermore, this study demonstrated that there was no significant difference in the proportion of CD38+ NK-like T cells between CRC patients with and without LNM.
Regarding the assessment of clinicopathologic factors, the findings of this study indicated that the poorer histologic grade and LVI were independent risk factors for LNM in CRC patients; Additionally, it was found the poorer histologic grade and presence of LVI were also associated with adverse prognosis. These findings were in concordance with previous research (37, 38). Tumor size and depth of invasion were sometimes considered risk factors for LNM, but in this study, independent risk factors for LNM did not include either. Indeed, there were studies reported that tumor size was not correlated with LNM (39, 40). Additionally, previous studies found the depth of invasion was not associated with LNM (4, 40, 41). These were consistent with our findings. The differing critical thresholds for tumor size, inconsistent diagnostic criteria for invasion depth, and individual variations among clinical doctors might contribute to the disparate outcomes. Additionally, in univariate analysis, neoadjuvant therapy was associated with LNM in CRC patients. However, multivariate analysis suggested that neoadjuvant therapy was not a protective factor against LNM in CRC patients. The proportion of rectal cancer patients receiving neoadjuvant therapy in this study was relatively low. This might be attributed to a relatively higher proportion of early-stage rectal cancer in the collected samples, discrepancies between clinical staging and pathological staging, local symptoms such as bleeding and obstruction, and patient or family refusal of neoadjuvant therapy. Further studies can be conducted by expanding the collection of patients who have received neoadjuvant therapy to investigate the effects of neoadjuvant therapy on LNM in CRC, especially in rectal cancer patients.
According to the latest guidelines for CRC treatment (3), the JSCCR stated that endoscopic resection is recommended for cases of intramucosal carcinoma or slight submucosal carcinoma with negligible risk of LNM. Therefore, assessing LNM in early-stage CRC becomes crucial in guiding clinical decision-making. This study revealed that early-stage CRC patients with LNM had a higher proportion of CD38+ NK cells, suggesting that the CD38+ NK cells was expected to be a potential marker for LNM in early-stage CRC. ROC curve analysis showed the ability of the proportion of CD38+ NK cells to evaluate lymph node status in early-stage CRC outperformed CEA. However, the analysis conducted in this study lacked in-depth detail due to the small sample size of early-stage patients. It will be crucial for future studies to employ larger sample sizes and continue with long-term follow-ups to obtain more detailed results. Indeed, the samples enrolled in this study were screened by rigorous inclusion and exclusion criteria, indicating the results might be reproducible and reliable. Due to the potential impact of chemotherapy on the immune system, which might affect the levels of immune cells (12), we had collected pre-treatment samples in this study. In the subsequent phase, we will further enrich this data set and analyze the effects of chemotherapy on the proportion of lymphocyte subsets. In addition, the functional mechanism of CD38+ NK cells was not extensively investigated in this study. Our research group had previously reported CD38+ NK cells in blood and synovial fluids mediated T-cell immune imbalance in RA, whereas CD38- NK cells had no such role (16). Further exploration can be conducted on the role of CD38+ NK cells in modulating T-cell immune balance in blood and tumor microenvironment (TME) of CRC patients. Moreover, CD38 mediated the production of adenosine under the synergistic action of CD203a and CDD73, thereby exerting immunosuppressive effects (42–44). Analyzing the expression of these molecules in NK cells might serve as a promising strategy to unravel the underlying mechanisms of CD38+ NK cell functionality.
Conclusions
The proportion of CD38+ NK cells to total NK cells was increased in CRC patients with LNM compared to those without. A high proportion of CD38+ NK cells is an independent risk factor for LNM, and it is associated with poor prognosis in CRC patients. The proportion of CD38+ NK cells is expected to be a promising marker for LNM and prognosis in patients with CRC before initial treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Affiliated Hospital of Qingdao University (QYFYWZLL28002). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XW: Conceptualization, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. HL: Data curation, Validation, Writing – review & editing. HC: Formal analysis, Writing – review & editing. KF: Resources, Supervision, Writing – review & editing. XC: Conceptualization, Funding acquisition, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Shandong Provincial Key R & D programs (2017GSF18174).
Acknowledgments
We thank all the staff and patients in our hospital who made the current study possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1309785/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Kiehl L, Kuntz S, Höhn J, Jutzi T, Krieghoff-Henning E, Kather JN, et al. Deep learning can predict lymph node status directly from histology in colorectal cancer. Eur J Cancer (Oxford Engl 1990). (2021) 157:464–73. doi: 10.1016/j.ejca.2021.08.039
3. Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. (2020) 25:1–42. doi: 10.1007/s10147-019-01485-z
4. Zwager LW, Bastiaansen BAJ, Montazeri NSM, Hompes R, Barresi V, Ichimasa K, et al. Deep submucosal invasion is not an independent risk factor for lymph node metastasis in T1 colorectal cancer: A meta-analysis. Gastroenterology. (2022) 163:174–89. doi: 10.1053/j.gastro.2022.04.010
5. Kaszubowska L, Foerster J, Kaczor JJ, Schetz D, Ślebioda TJ, Kmieć Z. NK cells of the oldest seniors represent constant and resistant to stimulation high expression of cellular protective proteins SIRT1 and HSP70. Immun Ageing I A. (2018) 15:12. doi: 10.1186/s12979-018-0115-x
6. Crinier A, Narni-Mancinelli E, Ugolini S, Vivier E. SnapShot: natural killer cells. Cell. (2020) 180:1280–.e1. doi: 10.1016/j.cell.2020.02.029
7. Gogali F, Paterakis G, Rassidakis GZ, Liakou CI, Liapi C. CD3(-)CD16(-)CD56(bright) immunoregulatory NK cells are increased in the tumor microenvironment and inversely correlate with advanced stages in patients with papillary thyroid cancer. Thyroid. (2013) 23:1561–8. doi: 10.1089/thy.2012.0560
8. Artis D, Spits H. The biology of innate lymphoid cells. Nature. (2015) 517:293–301. doi: 10.1038/nature14189
9. Cording S, Medvedovic J, Aychek T, Eberl G. Innate lymphoid cells in defense, immunopathology and immunotherapy. Nat Immunol. (2016) 17:755–7. doi: 10.1038/ni.3448
10. Jabir NR, Firoz CK, Ahmed F, Kamal MA, Hindawi S, Damanhouri GA, et al. Reduction in CD16/CD56 and CD16/CD3/CD56 natural killer cells in coronary artery disease. Immunol investigations. (2017) 46:526–35. doi: 10.1080/08820139.2017.1306866
11. Cui F, Qu D, Sun R, Tao H, Si J, Xu Y. The role of circulating CD16+CD56+ Natural killer cells in the screening, diagnosis, and staging of colorectal cancer before initial treatment. Dis Markers. (2019) 2019:7152183. doi: 10.1155/2019/7152183
12. Cui F, Qu D, Sun R, Nan K. Circulating CD16+CD56+ nature killer cells indicate the prognosis of colorectal cancer after initial chemotherapy. Med Oncol (Northwood London England). (2019) 36:84. doi: 10.1007/s12032-019-1307-8
13. Morandi F, Horenstein AL, Chillemi A, Quarona V, Chiesa S, Imperatori A, et al. CD56brightCD16- NK cells produce adenosine through a CD38-mediated pathway and act as regulatory cells inhibiting autologous CD4+ T cell proliferation. J Immunol (Baltimore Md 1950). (2015) 195:965–72. doi: 10.4049/jimmunol.1500591
14. Kang J, Choi YJ, Kim IK, Lee HS, Kim H, Baik SH, et al. LASSO-based machine learning algorithm for prediction of lymph node metastasis in T1 colorectal cancer. Cancer Res Treat. (2021) 53:773–83. doi: 10.4143/crt.2020.974
15. de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol (Baltimore Md 1950). (2011) 186:1840–8. doi: 10.4049/jimmunol.1003032
16. Wang H, Fang K, Yan W, Chang X. T-cell immune imbalance in rheumatoid arthritis is associated with alterations in NK cells and NK-like T cells expressing CD38. J innate Immun. (2022) 14:148–66. doi: 10.1159/000516642
17. Krijgsman D, de Vries NL, Skovbo A, Andersen MN, Swets M, Bastiaannet E, et al. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile. Cancer immunology immunotherapy CII. (2019) 68:1011–24. doi: 10.1007/s00262-019-02343-7
18. Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. (2011) 17:678–89. doi: 10.1158/1078-0432.ccr-10-2173
19. André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. (2018) 175:1731–43.e13. doi: 10.1016/j.cell.2018.10.014
20. Eugène J, Jouand N, Ducoin K, Dansette D, Oger R, Deleine C, et al. The inhibitory receptor CD94/NKG2A on CD8(+) tumor-infiltrating lymphocytes in colorectal cancer: a promising new druggable immune checkpoint in the context of HLAE/β2m overexpression. Modern Pathol. (2020) 33:468–82. doi: 10.1038/s41379-019-0322-9
21. Bu X, Kato J, Hong JA, Merino MJ, Schrump DS, Lund FE, et al. CD38 knockout suppresses tumorigenesis in mice and clonogenic growth of human lung cancer cells. Carcinogenesis. (2018) 39:242–51. doi: 10.1093/carcin/bgx137
22. Liao S, Xiao S, Zhu G, Zheng D, He J, Pei Z, et al. CD38 is highly expressed and affects the PI3K/Akt signaling pathway in cervical cancer. Oncol Rep. (2014) 32:2703–9. doi: 10.3892/or.2014.3537
23. Deckert J, Wetzel MC, Bartle LM, Skaletskaya A, Goldmacher VS, Vallée F, et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic Malignancies. Clin Cancer Res. (2014) 20:4574–83. doi: 10.1158/1078-0432.ccr-14-0695
24. Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. (2008) 88:841–86. doi: 10.1152/physrev.00035.2007
25. Malavasi F, Funaro A, Roggero S, Horenstein A, Calosso L, Mehta K. Human CD38: a glycoprotein in search of a function. Immunol Today. (1994) 15:95–7. doi: 10.1016/0167-5699(94)90148-1
26. Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, et al. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry Part B Clin cytometry. (2013) 84:207–17. doi: 10.1002/cyto.b.21092
27. Chatterjee S, Daenthanasanmak A, Chakraborty P, Wyatt MW, Dhar P, Selvam SP, et al. CD38-NAD(+)Axis regulates immunotherapeutic anti-tumor T cell response. Cell Metab. (2018) 27:85–100.e8. doi: 10.1016/j.cmet.2017.10.006
28. van Rensburg IC, Loxton AG. Killer (FASL regulatory) B cells are present during latent TB and are induced by BCG stimulation in participants with and without latent tuberculosis. Tuberculosis (Edinburgh Scotland). (2018) 108:114–7. doi: 10.1016/j.tube.2017.11.010
29. Saito H, Takaya S, Osaki T, Ikeguchi M. Increased apoptosis and elevated Fas expression in circulating natural killer cells in gastric cancer patients. Gastric Cancer. (2013) 16:473–9. doi: 10.1007/s10120-012-0210-1
30. Konen JM, Fradette JJ, Gibbons DL. The good, the bad and the unknown of CD38 in the metabolic microenvironment and immune cell functionality of solid tumors. Cells. (2019) 9(1):52. doi: 10.3390/cells9010052
31. Luo H, Shen K, Li B, Li R, Wang Z, Xie Z. Clinical significance and diagnostic value of serum NSE, CEA, CA19-9, CA125 and CA242 levels in colorectal cancer. Oncol Lett. (2020) 20:742–50. doi: 10.3892/ol.2020.11633
32. Yu Y, Xue W, Liu Z, Chen S, Wang J, Peng Q, et al. A novel DNA methylation marker to identify lymph node metastasis of colorectal cancer. Front Oncol. (2022) 12:1000823. doi: 10.3389/fonc.2022.1000823
33. Peng LS, Mao FY, Zhao YL, Wang TT, Chen N, Zhang JY, et al. Altered phenotypic and functional characteristics of CD3+CD56+ NKT-like cells in human gastric cancer. Oncotarget. (2016) 7:55222–30. doi: 10.18632/oncotarget.10484
34. Li XF, Dai D, Song XY, Liu JJ, Zhu L, Zhu X, et al. A different representation of natural T cells and natural killer cells between tumor-infiltrating and periphery lymphocytes in human hepatocellular carcinoma. Oncol Lett. (2017) 13:3291–8. doi: 10.3892/ol.2017.5808
35. Krijgsman D, Hokland M, Kuppen PJK. The role of natural killer T cells in cancer-A phenotypical and functional approach. Front Immunol. (2018) 9:367. doi: 10.3389/fimmu.2018.00367
36. Zhou J, Zhao X, Wang Z, Wang J, Sun H, Hu Y. High circulating CD3+CD56+CD16+ natural killer-like T cell levels predict a better IVF treatment outcome. J Reprod Immunol. (2013) 97:197–203. doi: 10.1016/j.jri.2012.12.006
37. Bernescu I, Reichstein AC, Luchtefeld M, Ogilvie JW. Does CD10 expression predict lymph node metastasis in colorectal cancer? Dis colon rectum. (2016) 59:22–7. doi: 10.1097/dcr.0000000000000498
38. Gao Y, Wang J, Zhou Y, Sheng S, Qian SY, Huo X. Evaluation of serum CEA, CA19-9, CA72-4, CA125 and ferritin as diagnostic markers and factors of clinical parameters for colorectal cancer. Sci Rep. (2018) 8:2732. doi: 10.1038/s41598-018-21048-y
39. Choi PW, Yu CS, Jang SJ, Jung SH, Kim HC, Kim JC. Risk factors for lymph node metastasis in submucosal invasive colorectal cancer. World J Surg. (2008) 32:2089–94. doi: 10.1007/s00268-008-9628-3
40. Sugai T, Yamada N, Osakabe M, Hashimoto M, Uesugi N, Eizuka M, et al. Microenvironmental markers are correlated with lymph node metastasis in invasive submucosal colorectal cancer. Histopathology. (2021) 79:584–98. doi: 10.1111/his.14388
41. Cao Y, Zhang J, Bao H, Zhang G, Yan X, Wang Z, et al. Development of a nomogram combining clinical risk factors and dual-energy spectral CT parameters for the preoperative prediction of lymph node metastasis in patients with colorectal cancer. Front Oncol. (2021) 11:689176. doi: 10.3389/fonc.2021.689176
42. Ferretti E, Horenstein AL, Canzonetta C, Costa F, Morandi F. Canonical and non-canonical adenosinergic pathways. Immunol Lett. (2019) 205:25–30. doi: 10.1016/j.imlet.2018.03.007
43. Morandi F, Horenstein AL, Rizzo R, Malavasi F. The role of extracellular adenosine generation in the development of autoimmune diseases. Mediators Inflammation. (2018) 2018:7019398. doi: 10.1155/2018/7019398
Keywords: CD38+ NK cells, colorectal cancer, lymph node metastasis, prognosis, marker
Citation: Wang X, Li H, Chen H, Fang K and Chang X (2024) Overexpression of circulating CD38+ NK cells in colorectal cancer was associated with lymph node metastasis and poor prognosis. Front. Oncol. 14:1309785. doi: 10.3389/fonc.2024.1309785
Received: 08 October 2023; Accepted: 31 January 2024;
Published: 23 February 2024.
Edited by:
Anne Jarry, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Fabio Malavasi, University of Turin, ItalyNadine Gervois, INSERM U1232 Centre de Recherche en Cancérologie et Immunologie Nantes Angers (CRCINA), France
Copyright © 2024 Wang, Li, Chen, Fang and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kehua Fang, a2VodWEuZmFuZ0BxZHUuZWR1LmNu; Xiaotian Chang, Y2hhbmd4dEBxZHUuZWR1LmNu
 Xueling Wang
Xueling Wang Haoran Li
Haoran Li Huixian Chen1
Huixian Chen1