
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Oncol. , 31 July 2024
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1303268
 Margaret M. Byrne1
Margaret M. Byrne1 Grerk Sutamtewagul1
Grerk Sutamtewagul1 William Zeitler1
William Zeitler1 Sarah L. Mott2
Sarah L. Mott2 Gideon K.D. Zamba2,3
Gideon K.D. Zamba2,3 Arsenije Kojadinovic1
Arsenije Kojadinovic1 Jun Zhang1†
Jun Zhang1† Taher Abu-Hejleh1†
Taher Abu-Hejleh1† Gerald Clamon1
Gerald Clamon1 Muhammad Furqan1*
Muhammad Furqan1*Background: Patients with small cell lung cancer (SCLC) often respond to first-line chemoimmunotherapy. However, relapse is inevitable and is associated with a poor prognosis. Treatments for relapsed SCLC, such as lurbinectedin and topotecan, are limited by modest efficacy and significant hematologic adverse events, leaving a need for newer therapeutic agents or regimens. The combination of gemcitabine and nab-paclitaxel is active and safe in other types of malignancies, such as pancreatic cancer.
Patients and methods: We conducted a phase II trial evaluating the efficacy and safety of gemcitabine and nab-paclitaxel in patients with relapsed/refractory SCLC. The primary endpoint was objective response rate (ORR), defined as the proportion of patients with confirmed complete or partial response. Secondary endpoints included time to progression (TTP), progression-free survival (PFS), overall survival (OS), and safety.
Results: Between October 2016 and May 2021, 32 patients were enrolled. Patients were followed for a median of 9.3 months (range 1.8–65.2). Median age was 65 years (range 48–81). Fifty percent of patients were female. Fifty-three percent of patients had platinum-resistant/refractory relapsed SCLC. The ORR was 28.1% (95% confidence interval [CI] 15.5–100%). Median PFS was 2.9 months (95% CI 2.4–3.6), and median OS was 9.3 months (95% CI 5.2–12.4). Seven patients (21.9%) developed grade 3 or 4 neutropenia.
Conclusion: Our study showed that the combination of gemcitabine and nab-paclitaxel led to encouraging outcomes in relapsed/refractory SCLC. Further studies are needed to compare this combination with other treatments used for relapsed SCLC, including lurbinectedin, temozolomide, and topotecan.
Clinical trial registration: https://clinicaltrials.gov/study/NCT02769832?cond=NCT02769832&rank=1, identifier NCT02769832.
Among the estimated 130,000 lung cancer deaths in 2023, approximately 15% will be due to small cell lung cancer (SCLC) (1). Nearly two-thirds of patients with SCLC will present with cancer that has metastasized beyond the thoracic cavity, known as extensive stage small cell lung cancer (ES-SCLC) (1, 2). For several decades, standard front-line therapy for patients with ES-SCLC has been platinum chemotherapy with etoposide (3). Recently, the addition of an immune checkpoint inhibitor (ICI), atezolizumab or durvalumab, to standard chemotherapy has shown a modest improvement in overall survival in patients with ES-SCLC (4–6). While ES-SCLC is initially sensitive to this combination of chemo-immunotherapy, responses are not durable, and almost all patients inevitably develop disease progression (1).
Effective therapies for patients with relapsed SCLC remain limited, particularly for those with resistant or refractory disease, defined as disease progression within 90 days of chemotherapy or while on chemotherapy (7–9). Since 1996, the primary treatment for patients with relapsed SCLC was topotecan, although it causes significant hematologic side effects (10). In 2020, lurbinectedin was the first treatment in many years to show promise in relapsed SCLC with an improvement in response rate to 35% and relatively fewer hematologic adverse events (11). However, a phase III study of lurbinectedin with doxorubicin failed to meet its primary endpoint of improved overall survival (OS) in patients with relapsed SCLC compared to the investigator’s choice of topotecan or the combination of cyclophosphamide, doxorubicin, or vincristine (CAV) (12). In addition, although lurbinectedin led to less hematologic toxicity than topotecan, grade 3 or 4 neutropenia developed in over 40% of patients (11). Studies evaluating single-agent chemotherapy for relapsed SCLC have demonstrated modest efficacy. Finally, with expanding indications for ICIs into front-line treatment, the benefit from ICIs in second or third-line is unclear, leaving a paucity of safe and effective treatment options for patients with relapsed SCLC.
Both gemcitabine and paclitaxel are active in relapsed SCLC, although response rates to these agents individually are less than optimal (13–16). Nab-paclitaxel is an albumin-bound formulation of paclitaxel, created to decrease the rate of infusion reactions and potential side effects (17). Gemcitabine and nab-paclitaxel have distinct mechanisms of action and have been shown to have additive/synergistic activity and are relatively safe in the treatment of other types of malignancies, such as pancreatic cancer (18). In addition, in a phase I study, this combination showed potential activity in previously treated SCLC (19). We report a phase II clinical trial evaluating gemcitabine and nab-paclitaxel combination in patients with relapsed SCLC (NCT02303977).
We conducted an open-label, single-arm, phase II study to evaluate nab-paclitaxel and gemcitabine in patients with relapsed SCLC. Eligible participants were adults (≥18 years) with histologically or cytologically confirmed SCLC, Eastern Cooperative Oncology Group (ECOG) performance score 0–2, at least 1 measurable lesion as defined by Response Evaluation Criteria in Solid Tumors (RECIST v1.1), disease progression during or after first-line chemotherapy, including progression after chemoradiation for limited stage disease if progressed within 12 months of treatment, adequate hematologic function (ANC ≥1800/mm3, platelet count ≥100,000/mm3, and hemoglobin ≥9.0), hepatic function (bilirubin ≤1.5 x ULN, AST and ALT ≤2.5 x ULN or AST and ALT ≤5 x ULN if liver metastases were present), and renal function (serum creatinine ≤ 1.5 x ULN). Prior treatment with ICIs, either with first-line chemotherapy or as second-line therapy, was allowed after a protocol modification when these agents got the FDA approval.
Key exclusion criteria included previous receipt of a taxane, history of other invasive malignancy in the past 12 months, pre-existing peripheral neuropathy (grade ≥2 according to Common Terminology Criteria for Adverse Events version 4.03 (CTCAEv4.03), serious medical condition in the previous 6 months, or untreated brain metastases requiring radiation, surgery, or continued use of steroids. Treated brain metastases were required to be stable for at least 4 weeks and steroids were to be discontinued for at least 7 days before study therapy.
The study was performed at the University of Iowa Holden Cancer Center (HCCC). It was approved by the institutional review board (HawkIRB 201512799) and was performed per the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent. The HCCC Data Safety and Monitoring Committee provided study oversight.
Eligible patients received gemcitabine 1000 mg/m2 and nab-paclitaxel 100 mg/m2 on days 1 and 8 of a 21-day cycle. This was continued until disease progression or intolerable toxicity or withdrawal of consent. Dose modifications were allowed for low absolute neutrophil (ANC) and platelet counts. Dose delays due to toxicities, febrile neutropenia, and other illnesses were allowed for up to 3 weeks.
The primary endpoint of the study was to evaluate the objective response rate (ORR) according to RECIST version 1.1. Patients who achieved a partial or complete response underwent a confirmatory tumor assessment at least 4 weeks following the initial imaging demonstrating the response. Tumor assessments occurred at baseline and every 6 weeks while in the study. Secondary endpoints included time to progression (TTP), progression-free survival (PFS), OS, and safety. Adverse events (AEs) were graded using CTCAE v4.03.
The primary objective of this phase II trial was to evaluate the anti-tumor activity of nab-paclitaxel and gemcitabine by testing the null hypothesis that the best ORR is less than 15% versus the alternative that it is greater (20). Best response was defined as a confirmed complete or partial response. The trial was conducted as a single-stage design having 80% power to detect a response rate of 35% with one-sided statistical testing performed at the 5% level of significance and assuming 12.5% lost to follow-up.
Primary statistical analysis focused on the best objective response rate estimated as a binomial proportion along with a one-sided 95% confidence interval (CI). Secondary analyses focused on TTP, PFS, OS, and safety. TTP was defined as the time from treatment initiation to the date of first documentation of disease progression. PFS was defined as the time from treatment initiation to the date of first documentation of disease progression or death due to any cause. Patients were censored at the date of the last radiographic assessment for progression. OS was defined as the time from treatment initiation to death due to any cause. Patients still alive were censored at the last date known to be alive. Survival probabilities were estimated and plotted using the Kaplan-Meier method. Estimates along with 95% pointwise CIs are reported. Incidence of adverse events attributable to the study drugs was graded, with the most severe grade per patient being reported.
Between October 2016 and May 2021, 32 patients were enrolled (Figure 1). Median follow-up was 9.3 months (range 1.8–65.2). Fifty percent of the patients were male, and all patients were White (Table 1). The median age was 65 years (range 48–81). There were 12.5% of patients with an Eastern Cooperative Oncology Group (ECOG) performance score of 2. At diagnosis, 87.5% of patients had extensive disease. At the time of enrollment, patients had a median of 3 sites of disease involvement (range 1–5), 50% had bone metastases, and about 40% had treated brain metastases. Half of the patients had previously received an ICI, and over 60% had previously received radiation therapy to the thoracic cavity. Fifty-three percent of patients had platinum-resistant/refractory disease.
Patients received a median of 4.0 cycles (range 2.0–13.0) of chemotherapy. In total, 46.9% of patients required a level 1 dose reduction (defined as gemcitabine 800 mg/m2 or nab-paclitaxel 80 mg/m2), and 40.6% required a level 2 dose reduction (defined as gemcitabine 600 mg/m2 or nab-paclitaxel 60 mg/m2), most commonly due to bone marrow toxicity. Twenty patients (62.5%) received growth factor support. Median dose received on treatment days for gemcitabine was 835.7 mg/m2 and 84.8 mg/m2 for abraxane.
All patients were included in the efficacy analysis. ORR was 28.1%, demonstrating a statistically significant increase compared to a historical control of 15.0% (p=0.04; Figure 2). ORR was 33.3% in patients with platinum-sensitive disease and 23.5% in patients with platinum-resistant/refractory disease. Fifty percent of patients (n=16) had stable disease, of which 25.0% had an unconfirmed PR (n=4). The disease control rate (DCR) was 78.1%. Thirty patients had disease progression during the follow-up period, while 2 patients died before progression. Median TTP was 2.9 months (95% CI 2.4–3.8), and median PFS was 2.9 months (95% CI 2.4–3.6; Figure 3). Median PFS was 2.8 months (95% CI 1.5–5.6) in patients with platinum-sensitive disease, and 2.9 months (95% CI 1.7–3.6) in patients with platinum-resistant/refractory disease. Median OS was 9.3 months (95% CI 5.2–12.4; Figure 3). In patients with platinum-sensitive disease, median overall survival was 10.8 months (95% CI 3.3–12.6), and median OS was 6.7 months (95% CI 3.5–12.4) in patients with platinum-resistant/refractory disease. Overall survival at 9, 12, and 18 months was 53%, 34%, and 6%, respectively. At 18 months, 2 patients (6.2%) were still alive; one patient (3.1%) was still alive at the time of data cut-off for the trial. Outcomes were not different with regards to presence or absence of baseline brain metastases
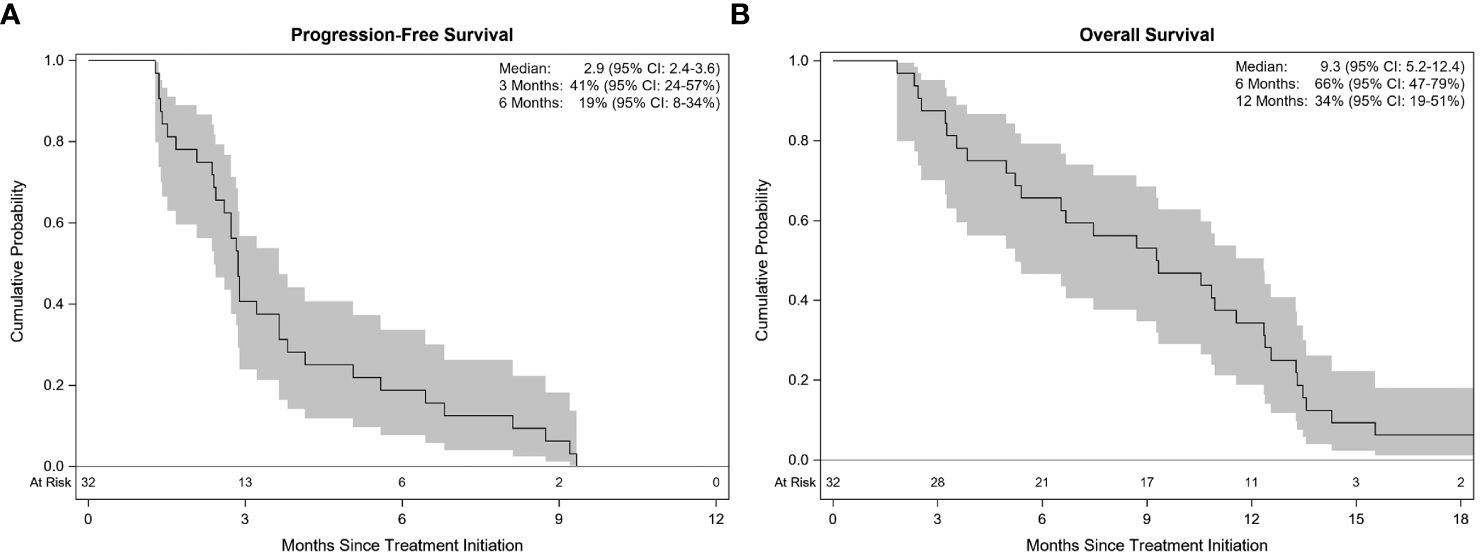
Figure 3 Kaplan-Meier estimates with 95% confidence. Progression-free survival (A) and Overall survival (B).
All patients developed a treatment-related adverse event (Table 2). The most common adverse events included fatigue, hematologic events, gastrointestinal complaints, neuropathy, pneumonitis, loss of appetite/weight loss, changes in electrolytes and/or liver function tests, and fever. The most common grade 3 or higher adverse events were hematologic. Grade 3 or 4 neutropenia occurred in 21.9% of patients (n=7). Pneumonitis occurred in 18.8% of patients (n=6); one was grade 1 (3.1%), four were grade 2 (12.5%), and one was grade 5 (3.1%; Table 3). Of the 5 patients who developed grade 1 or 2 pneumonitis, 4 patients were treated with steroids, all of whom had resolution of pneumonitis. The patient with grade 5 pneumonitis had received radiation to the thoracic cavity and an ICI before enrollment on the trial. This patient was admitted to another facility with shortness of breath and was found to have concurrent disease progression. Hence, subject decided to pursue hospice, and pneumonitis remained untreated. Treatment discontinuation occurred in 6.3% of patients (n=2) due to grade 2 pneumonitis and patient preference.
Out of 32, twenty-nine subjects developed RECIST progression. Twenty-one (72.4%) patients progressed systemically, six (1.75%) progressed in the brain while two progressed both systemically and in the CNS. Those who progressed in the CNS only, three develop new brain metastases while remaining three develop disease progression in the previously treated lesions. Nineteen patients received further systemic therapy with a median of 2 lines of treatment (range 1–4). Nine patients enrolled in another clinical trial. Four patients received radiation following progression, 3 extracranial and 1 intracranial. One patient underwent surgical resection of a brain metastasis.
This study met its primary endpoint of an improvement in ORR to 28.1%, showcasing the activity of the combination of gemcitabine and nab-paclitaxel in patients with relapsed SCLC. In addition, we report an overall survival of 9.3 months, which is promising in a patient population with several poor prognostic factors, including 12% with an ECOG performance score of 2, over half of the population with a platinum-resistant/refractory disease, and 40% with treated brain metastases at the time of enrollment.
While there have been slight improvements in outcomes for patients with relapsed SCLC in the past few years, the ORR in our patient population is comparable to ORR for treatments for relapsed SCLC, including treatments that are incorporated in the National Cancer Consortium Network guidelines (Table 4). In addition to an improvement in ORR, our study showed a median OS of 9.3 months, which is comparable to commonly used treatments for relapsed SCLC, including lurbinectedin and topotecan (Table 4) (10, 11, 34, 35). While we recognize that these studies cannot be directly compared, we believe that these results are encouraging. Our findings highlight the need for future studies to compare gemcitabine and nab-paclitaxel with current available treatments for relapsed SCLC.
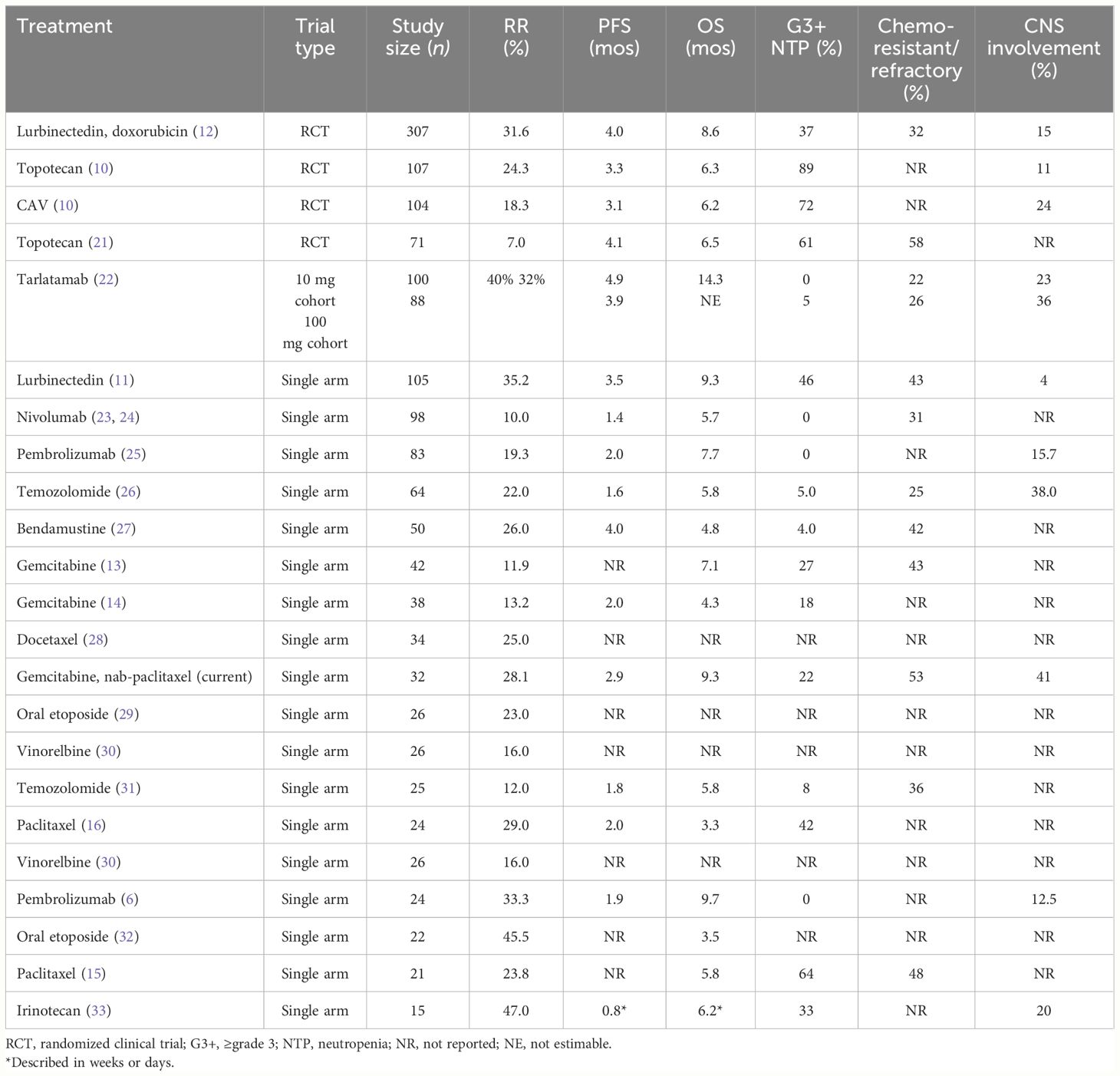
Table 4 Comparison of treatment options mentioned in NCCN guidelines for ES-SCLC in second-line or beyond except for tarlatamab.
Treatments for relapsed SCLC are commonly limited by hematologic toxicities. For example, topotecan has been reported to cause grade 4 neutropenia in 70% of patients. While lurbinectedin causes less neutropenia, grade 3 or 4 neutropenia has been reported to occur in 46% of patients. We found that gemcitabine and nab-paclitaxel led to less grade 3 or 4 neutropenia (22%; Table 4). In addition, 6.3% of patients developed febrile neutropenia in our study, which is comparable to febrile neutropenia in 5% of patients treated with lurbinectedin and lower than the reported 3–28% of patients on topotecan. Patients enrolled in lurbinectedin monotherapy and combinational therapies received prophylactic growth factor support while 62.5% of patients in this trial required growth factor support (11). Finally, only 6.3% of patients in this trial required treatment discontinuation due to adverse events, indicating that the combination is relatively tolerable.
In this study, nearly 19% of patients developed pneumonitis, of which 1 required treatment discontinuation and 1 was grade 5. Pneumonitis has been reported with this combination in pancreatic cancer but occurred less frequently (4%) in patients receiving this combination (18). The patient population included in this study may have increased risk factors for pneumonitis, such as a history of smoking (current or former = 100%), underlying lung disease (COPD 100%), previous receipt of radiation to the chest (62%), and previous exposure to ICI (56%). We could not find any other known predisposing factor for pneumonitis in these cases. A similar observation was made in a study that recruited non-small cell lung cancer patients who received the combination of gemcitabine and nab-paclitaxel, which led to grade 2–3 pneumonitis in 11% of patients (36).
Recent developments and an improved understanding of SCLC disease mechanisms are leading biomarker-driven drug development in SCLC. In particular, clinical trials are underway evaluating therapies targeted at delta-like ligand-3 through a bispecific antibody (tarlatamab) and targeting seizure-related homolog 6 (SEZ6) protein through an antibody-drug conjugate (22, 37). In addition, there has been interest in poly (ADP ribose) polymerase inhibitors in patients with Shlafen 11 expression (38). As more innovative therapies emerge, further developments of the combination of gemcitabine and nab-paclitaxel can be considered accordingly.
Limitations of this study include a single-institution study including only a small number of White patients, which may make our findings less generalizable. In addition, outcomes in patients with relapsed SCLC have improved modestly since the design and conduct of this study, which may make comparisons to our trial more difficult. As biomarker-based therapies emerge, sequencing of treatments for relapsed/refractory ES-SCLC is yet to be defined.
In patients with SCLC, relapse is inevitable and is associated with a poor prognosis. Treatment options for patients with relapsed SCLC remain limited. The combination of gemcitabine and nab-paclitaxel may be an effective and safe treatment option albeit with higher incidence of hematologic toxicities if utilized after topotecan or lurbinectedin. Further studies are needed to validate the therapeutic value of this regimen in larger patient populations and directly compare this combination with other approved options.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by Hawk IRB, University of Iowa, Iowa City, IA 52246. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MB: Visualization, Validation, Data curation, Writing – review & editing, Writing – original draft, Investigation. GS: Methodology, Funding acquisition, Conceptualization, Writing – review & editing, Writing – original draft. WZ: Resources, Investigation, Writing – review & editing. SM: Writing – original draft, Visualization, Formal analysis, Data curation, Writing – review & editing. GZ: Validation, Methodology, Writing – review & editing, Formal analysis. AK: Writing – original draft, Data curation, Writing – review & editing. JZ: Resources, Methodology, Investigation, Writing – review & editing, Writing – original draft. TA-H: Conceptualization, Writing – review & editing, Writing – original draft, Resources, Methodology, Investigation. GC: Funding acquisition, Writing – review & editing, Resources, Methodology, Conceptualization. MF: Visualization, Validation, Supervision, Project administration, Investigation, Formal analysis, Data curation, Writing – review & editing, Writing – original draft, Resources, Methodology, Funding acquisition, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. TThe author declared that institutional funding was received from Celegene (now Bristol Myers Squibb) to support the study conduct. However, funder is not involved in study design, data collection, analysis, interpretation of data, writing of the manuscript or its submission for publication.
We wish to thank all the patients, family members, and research staff that participated in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. (2006) 24:4539–44. doi: 10.1200/JCO.2005.04.4859
3. Farago AF, Keane FK. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. (2018) 7:69–79. doi: 10.21037/tlcr.2018.01.16
4. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
5. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. (2019) 394:1929–39. doi: 10.1016/S0140-6736(19)32222-6
6. Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. (2017) 35:3823–9. doi: 10.1200/JCO.2017.72.5069
7. Asai N, Ohkuni Y, Kaneko N, Yamaguchi E, Kubo A. Relapsed small cell lung cancer: treatment options and latest developments. Ther Adv Med Oncol. (2014) 6:69–82. doi: 10.1177/1758834013517413
8. Lara PN Jr., Moon J, Redman MW, Semrad TJ, Kelly K, Allen JW, et al. Relevance of platinum-sensitivity status in relapsed/refractory extensive-stage small-cell lung cancer in the modern era: a patient-level analysis of southwest oncology group trials. J Thorac Oncol. (2015) 10:110–5. doi: 10.1097/JTO.0000000000000385
9. Chouaid C, Baize N, Monnet I. Second-line therapy for disseminated small-cell lung cancer: optimal management remains to be defined. Transl Lung Cancer Res. (2020) 9:1732–5. doi: 10.21037/tlcr-20-362
10. von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. (1999) 17:658–67. doi: 10.1200/JCO.1999.17.2.658
11. Trigo J, Subbiah V, Besse B, Moreno V, Lopez R, Sala MA, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. (2020) 21:645–54. doi: 10.1016/S1470-2045(20)30068-1
12. Aix SP, Ciuleanu TE, Navarro A, Cousin S, Bonanno L, Smit EF, et al. Combination lurbinectedin and doxorubicin versus physician's choice of chemotherapy in patients with relapsed small-cell lung cancer (ATLANTIS): a multicentre, randomised, open-label, phase 3 trial. Lancet Respir Med. (2023) 11:74–86. doi: 10.1016/S2213-2600(22)00309-5
13. Masters GA, Declerck L, Blanke C, Sandler A, DeVore R, Miller K, et al. Phase II trial of gemcitabine in refractory or relapsed small-cell lung cancer: Eastern Cooperative Oncology Group Trial 1597. J Clin Oncol. (2003) 21:1550–5. doi: 10.1200/JCO.2003.09.130
14. van der Lee I, Smit EF, van Putten JW, Groen HJ, Schlosser NJ, Postmus PE, et al. Single-agent gemcitabine in patients with resistant small-cell lung cancer. Ann Oncol. (2001) 12:557–61. doi: 10.1023/A:1011104509759
15. Yamamoto N, Tsurutani J, Yoshimura N, Asai G, Moriyama A, Nakagawa K, et al. Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res. (2006) 26:777–81.
16. Smit EF, Fokkema E, Biesma B, Groen HJ, Snoek W, Postmus PE. A phase II study of paclitaxel in heavily pretreated patients with small-cell lung cancer. Br J Cancer. (1998) 77:347–51. doi: 10.1038/bjc.1998.54
17. Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release. (2013) 170:365–72. doi: 10.1016/j.jconrel.2013.05.041
18. Von Hoff DD, Goldstein D, Renschler MF. Albumin-bound paclitaxel plus gemcitabine in pancreatic cancer. N Engl J Med. (2014) 370:479–80. doi: 10.1056/NEJMc1314761
19. Stinchcombe TE, Socinski MA, Lee CB, Hayes DN, Moore DT, Goldberg RM, et al. Phase I trial of nanoparticle albumin-bound paclitaxel in combination with gemcitabine in patients with thoracic Malignancies. J Thorac Oncol. (2008) 3:521–6. doi: 10.1097/JTO.0b013e31816de2a7
20. Horita N, Yamamoto M, Sato T, Tsukahara T, Nagakura H, Tashiro K, et al. Topotecan for relapsed small-cell lung cancer: systematic review and meta-analysis of 1347 patients. Sci Rep. (2015) 5:15437. doi: 10.1038/srep15437
21. O'Brien ME, Ciuleanu TE, Tsekov H, Shparyk Y, Cucevia B, Juhasz G, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. (2006) 24:5441–7. doi: 10.1200/JCO.2006.06.5821
22. Ahn MJ, Cho BC, Felip E, Korantzis I, Ohashi K, Majem M, et al. Tarlatamab for patients with previously treated small -Cell Lung Cancer. N Engl J Med. (2023) 389:2063–75. doi: 10.1056/NEJMoa2307980
23. Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. (2016) 17:883–95. doi: 10.1016/S1470-2045(16)30098-5
24. Ready NE, Ott PA, Hellmann MD, Zugazagoitia J, Hann CH, de Braud F, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the checkMate 032 randomized cohort. J Thorac Oncol. (2020) 15:426–35. doi: 10.1016/j.jtho.2019.10.004
25. Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens JA, Kao S, Miller WH, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol. (2020) 15:618–27. doi: 10.1016/j.jtho.2019.12.109
26. Pietanza MC, Kadota K, Huberman K, Sima CS, Fiore JJ, Sumner DK, et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res. (2012) 18:1138–45. doi: 10.1158/1078-0432.CCR-11-2059
27. Lammers PE, Shyr Y, Li CI, Hutchison AS, Sandler A, Carbone DP, et al. Phase II study of bendamustine in relapsed chemotherapy sensitive or resistant small-cell lung cancer. J Thorac Oncol. (2014) 9:559–62. doi: 10.1097/JTO.0000000000000079
28. Smyth JF, Smith IE, Sessa C, Schoffski P, Wanders J, Franklin H, et al. Activity of docetaxel (Taxotere) in small cell lung cancer. The Early Clinical Trials Group of the EORTC. Eur J Cancer. (1994) 30A:1058–60. doi: 10.1016/0959-8049(94)90455-3
29. Einhorn LH, Pennington K, McClean J. Phase II trial of daily oral VP-16 in refractory small cell lung cancer: a Hoosier Oncology Group study. Semin Oncol. (1990) 17:32–5.
30. Jassem J, Karnicka-Mlodkowska H, van Pottelsberghe C, van Glabbeke M, Noseda MA, Ardizzoni A, et al. Phase II study of vinorelbine (Navelbine) in previously treated small cell lung cancer patients. EORTC Lung Cancer Cooperative Group. Eur J Cancer. (1993) 29A:1720–2. doi: 10.1016/0959-8049(93)90112-S
31. Zauderer MG, Drilon A, Kadota K, Huberman K, Sima CS, Bergagnini I, et al. Trial of a 5-day dosing regimen of temozolomide in patients with relapsed small cell lung cancers with assessment of methylguanine-DNA methyltransferase. Lung Cancer. (2014) 86:237–40. doi: 10.1016/j.lungcan.2014.08.007
32. Johnson DH, Greco FA, Strupp J, Hande KR, Hainsworth JD. Prolonged administration of oral etoposide in patients with relapsed or refractory small-cell lung cancer: a phase II trial. J Clin Oncol. (1990) 8:1613–7. doi: 10.1200/JCO.1990.8.10.1613
33. Masuda N, Fukuoka M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, et al. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol. (1992) 10:1225–9. doi: 10.1200/JCO.1992.10.8.1225
34. Farago AF, Drapkin BJ, Lopez-Vilarino de Ramos JA, Galmarini CM, Nunez R, Kahatt C, et al. ATLANTIS: a Phase III study of lurbinectedin/doxorubicin versus topotecan or cyclophosphamide/doxorubicin/vincristine in patients with small-cell lung cancer who have failed one prior platinum-containing line. Future Oncol. (2019) 15:231–9. doi: 10.2217/fon-2018-0597
35. Baize N, Monnet I, Greillier L, Geier M, Lena H, Janicot H, et al. Carboplatin plus etoposide versus topotecan as second-line treatment for patients with sensitive relapsed small-cell lung cancer: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. (2020) 21:1224–33. doi: 10.1016/S1470-2045(20)30461-7
36. Ciunci CA, Reibel JB, Evans TL, Mick R, Bauml JM, Aggarwal C, et al. Phase II trial of combination nab-paclitaxel and gemcitabine in non-squamous non-small cell lung cancer after progression on platinum and pemetrexed. Clin Lung Cancer. (2022) 23:e310–6. doi: 10.1016/j.cllc.2022.02.004
37. Morgensztern D, Ready NE, Johnson ML, Dowlati A, Choudhury N, Carbone DP, et al. First-in-human study of ABBV-011, a seizure-related homolog protein 6 (SEZ6)–targeting antibody-drug conjugate, in patients with small cell lung cancer. J Clin Oncol. (2023) 41:3002–2. doi: 10.1200/JCO.2023.41.16_suppl.3002
38. Karim NFA, Miao J, Reckamp KL, Gay CM, Byers LA, Zhao Y, et al. SWOG S1929: Phase II randomized study of maintenance atezolizumab (A) versus atezolizumab + talazoparib (AT) in patients with SLFN11 positive extensive stage small cell lung cancer (ES-SCLC). J Clin Oncol. (2023) 41:8504. doi: 10.1200/JCO.2023.41.16_suppl.8504
Keywords: small cell lung cancer, gemcitabine, nab-paclitaxel, clinical trial, single-arm
Citation: Byrne MM, Sutamtewagul G, Zeitler W, Mott SL, Zamba GKD, Kojadinovic A, Zhang J, Abu-Hejleh T, Clamon G and Furqan M (2024) Phase II study of nab-paclitaxel with gemcitabine for relapsed/refractory small cell lung cancer. Front. Oncol. 14:1303268. doi: 10.3389/fonc.2024.1303268
Received: 27 September 2023; Accepted: 08 April 2024;
Published: 31 July 2024.
Edited by:
Luigi Cavanna, Ospedaliera di Piacenza, ItalyReviewed by:
Shetal Arvind Patel, University of North Carolina at Chapel Hill, United StatesCopyright © 2024 Byrne, Sutamtewagul, Zeitler, Mott, Zamba, Kojadinovic, Zhang, Abu-Hejleh, Clamon and Furqan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Furqan, bXVoYW1tYWQtZnVycWFuQHVpb3dhLmVkdQ==
†Present address: Jun Zhang, Division of Medical Oncology, Department of Internal Medicine
Department of Cancer Biology, University of Kansas Medical Center, Kansas City, KS, United States
Taher Abu-Hejleh, Department of Internal Medicine, King Hussein Cancer Center, Amman, Jordan
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.