- Cancer Institute, The Affiliated People’s Hospital of Jiangsu University, Zhenjiang, Jiangsu, China
Numerous studies have suggested a robust association between amylase and ovarian cancer. however, few amylase-producing ovarian cancers have been reported because amylase is a rare product of ovarian cancer. A case of an elderly female patient with an upper abdominal unfitness, intestinal wall along with uterine adnexal invasion, and high serum and urinary amylase is summarized in this article. The patient was initially suspected of having a gastrointestinal tumor. Initial laboratory findings showed markedly significantly raised serum and urinary amylase levels. Imaging showed invasion of the intestinal wall and uterine adnexa, and histology of the specimen taken through the abdominal wall lump and electron colonoscopy showed ovarian cancer. The patient’s blood amylase levels decreased to normal after 4 cycles of neoadjuvant chemotherapy with paclitaxel and carboplatin. Following this, she underwent interval debulking surgery, which included total hysterectomy, bilateral adnexectomy, great omentectomy, appendectomy, resection of pelvic and abdominal lesions, and partial rectal resection. Postoperative pathology and immunohistochemistry staining confirmed a diagnosis of high-grade serous ovarian cancer. This case suggests that in female patients, hyperamylasemia may indicate the presence of ovarian cancer. It is necessary to perform a multisite, multipoint histologic examination to identify the tumor’s origin in patients with multiple sites of invasion.
1 Introduction
Ovarian cancer is one of the three most common malignant tumors of the female genital system, which cannot be easily diagnosed at an early stage due to inconspicuous clinical symptoms (1). Serum amylase, one of the digestive enzymes, can be elevated in a variety of disease states, including pancreatic inflammation, pancreatic cancer, and other malignant tumors (2–4). In recent years, many studies have been dedicated to exploring the application of serum enzyme profiles in ovarian cancer (5, 6), with particular focus on the role of serum amylase in the diagnosis and prognostic evaluation of ovarian cancer (7).
We summarized a case of an ovarian cancer patient who had intestinal wall invasion along with high Serum and urinary amylase, and performed a literature review of amylase elevation arising from ovarian cancer. We encourage clinicians to consider ovarian cancer as one of the possible diagnoses of hyperamylasemia in female patients.
2 Case report
A 66-year-old woman was admitted to the oncology clinic with epigastric discomfort for more than a month. Her symptoms mainly consisted of epigastric fullness and discomfort in the abdomen, with occasional vague pain in the epigastric region, which could be relieved after feeding. She had no specific history or family history of tumors. The Patient’s initial gastroscopy at an outside hospital showed chronic superficial gastritis and duodenal bulbar inflammation, and she was poorly treated with acid-suppressing therapy. A computed tomography (CT) scan of the upper abdomen at the local hospital showed a slightly hypodense nodule in the right liver, thickened peritoneum, and multiple nodular and clumpy shadows. Subsequently, she presented to our outpatient clinic for consultation. Physical examination revealed abdominal distension with tenderness in the upper abdomen, but no palpable masses were detected. The outpatient doctor admitted her to the Gastrointestinal Oncology Unit of the Chemotherapy Department, suspecting a digestive tract tumor. After admission, the patient had no significant discomfort in the upper abdomen. However, the serum and urine amylase levels (Table 1), as well as serum carbohydrate antigen 125 (CA125) and carbohydrate antigen 72-4 (CA72-4) were distinctly elevated, while carcinoembryonic antigen (CEA) level was within the normal range. CT scans of the abdomen and pelvis (Figure 1) showed no pancreatic exudation, multiple hyperintense nodules in the abdominopelvic cavity and greater omental peritoneum, patchy dense shadows in the adnexal region of the pelvis, poorly displayed uterine region, thickened wall of the junction between the sigmoid colon and the rectum, and a nodule in the right lobe of the liver.
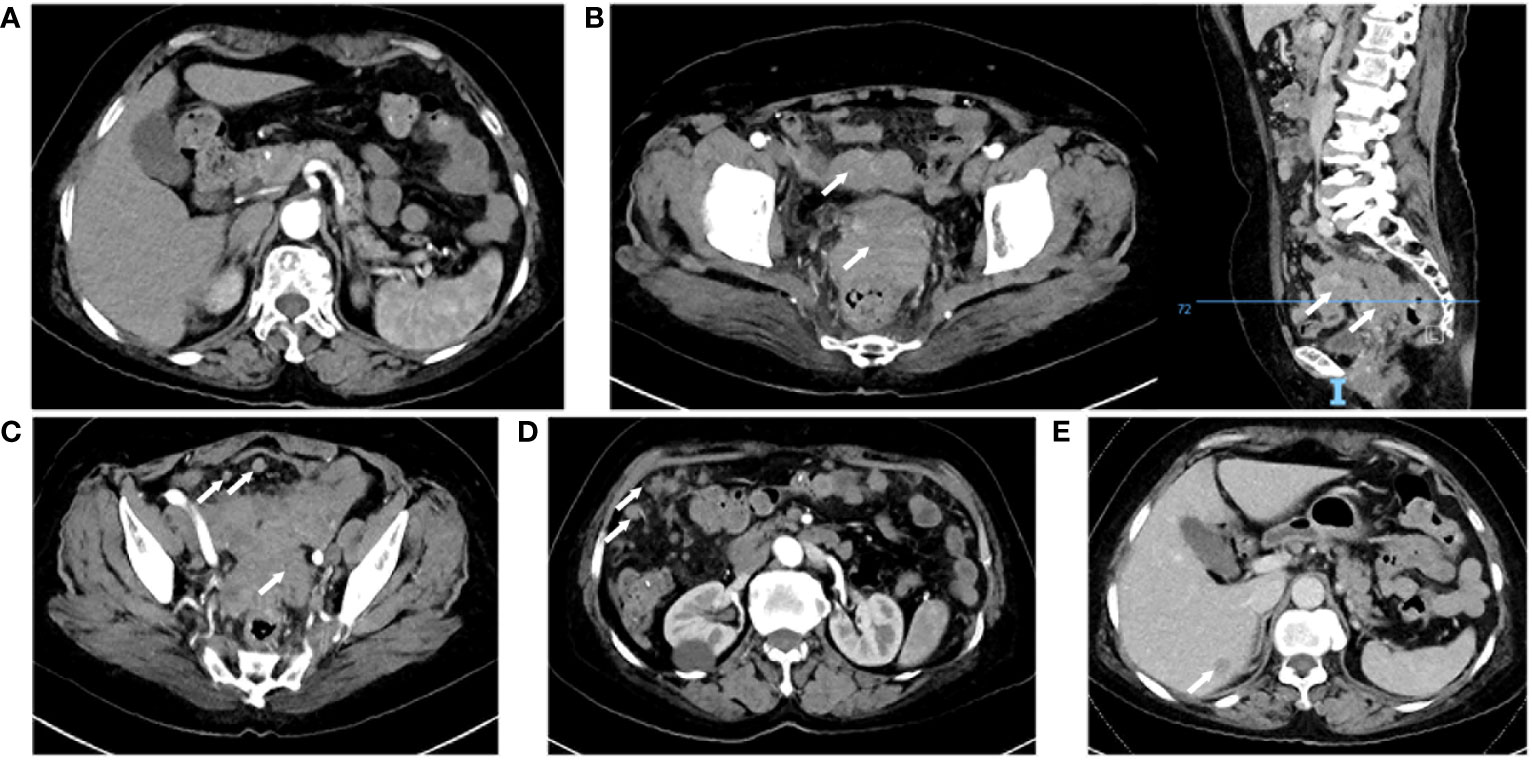
Figure 1 Computed tomography enhanced scan. (A) Normal pancreas. (B) Uterine adnexal mass and pelvic metastatic lesion, right: sagittal view. (C) Thickening of the junction area between the rectum and the sigmoid colon and multiple pelvic nodules. (D) Multiple nodules in the abdominal cavity. (E) Venous phase, a nodule in the right lobe of the liver.
During the patient’s hospitalization, there were no significant abdominal pain symptoms. Based on the imaging examinations, we ruled out the possibility of acute pancreatitis. However, follow-up blood and urine amylase tests the next day showed no decrease. To define the origin of the tumor, the patient underwent Positron Emission Tomography (PET) which revealed invasions of the abdominal wall and intestinal wall (Figure 2). The patient then underwent percutaneous biopsy of the abdominal wall mass and endoscopic biopsy to obtain tissue samples for immunohistochemical staining. The histological examination of the percutaneous biopsy of the abdominal wall mass showed poorly differentiated carcinoma, suggesting a possible adnexal origin (Figure 3A). Immunohistochemical staining results indicated positivity for Cytokeratin 18 (CK18), P53 (weak), Cytokeratin (CK), Estrogen receptor (ER), Wilms’ tumor (WT-1), Paired box 8 (PAX-8), and CA125 proteins, while Progesterone receptor (PR), Villin, and CDX-2 were negative. Further endoscopic biopsy was performed, and the histological examination revealed poorly differentiated carcinoma, suggesting a possible adnexal origin (Figure 3A). The immunohistochemical results showed positivity for PAX-8, WT-1, ER, and CK proteins, while Villin, CDX2, PR, and Cytokeratin 20 (CK20) were negative. Nonsense mutation was detected in P53. According to the WHO classification (8), the preoperative histopathological examination and immunohistochemical staining results suggest a potential diagnosis of high-grade serous ovarian cancer (HGSOC). Considering the absence of significant gastrointestinal symptoms during the patient’s hospitalization, elevated CA125 levels, increased amylase levels, and the location of the tumor as indicated by imaging, the patient was diagnosed with advanced-stage ovarian cancer with implantation metastasis (stage IV).
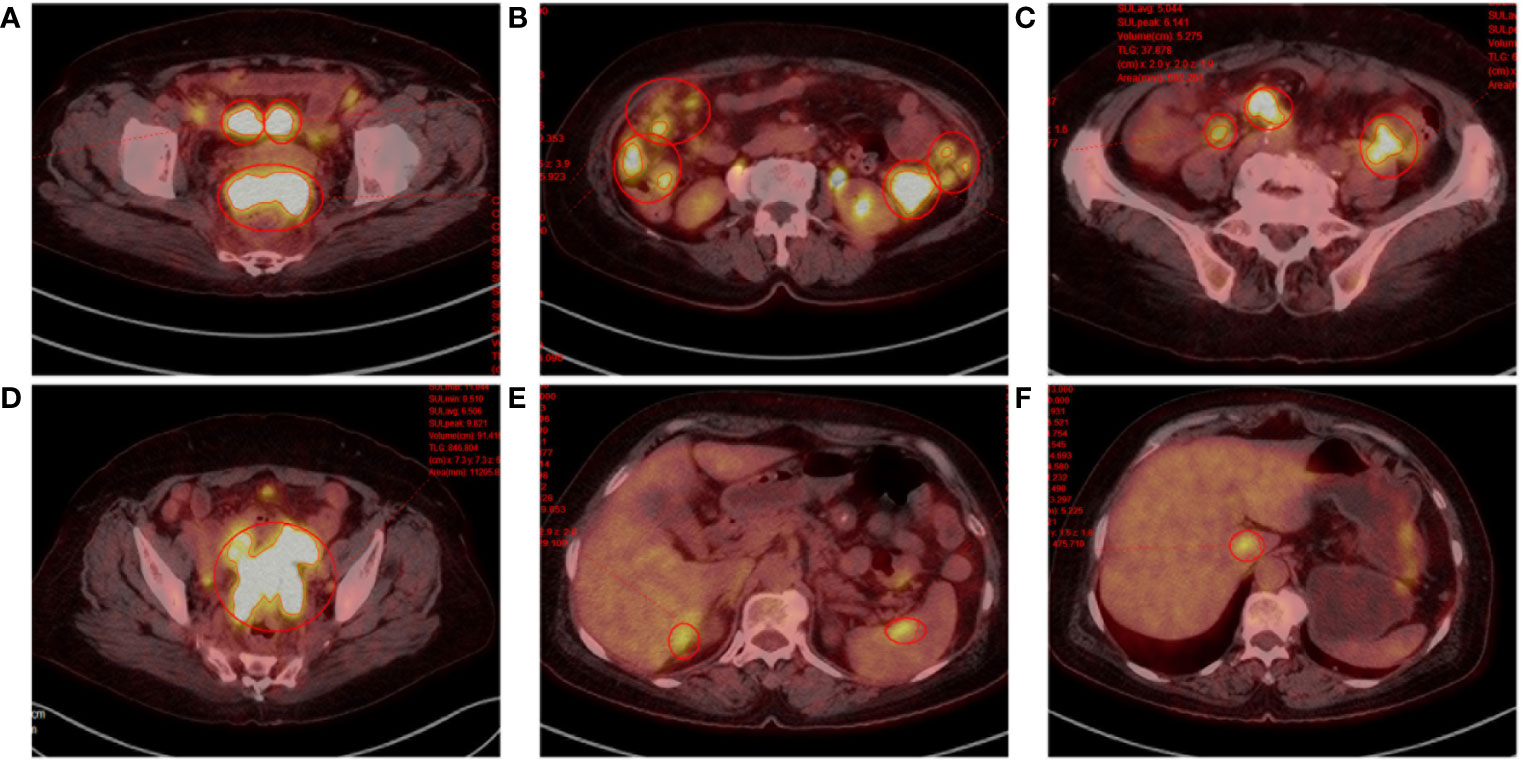
Figure 2 Positron emission tomography. (A) Rectouterine pouch and uterine adnexal mass. (B-D) Multiple nodular shadows in the abdominopelvic cavity and greater omentum, inhomogeneous thickening of the local peritoneum adjacent to the left colon. (E, F) Hypodense shadow in the right lobe of the liver with increased FDG metabolism; foci of increased FDG metabolism in the caudate lobe of the liver and spleen.
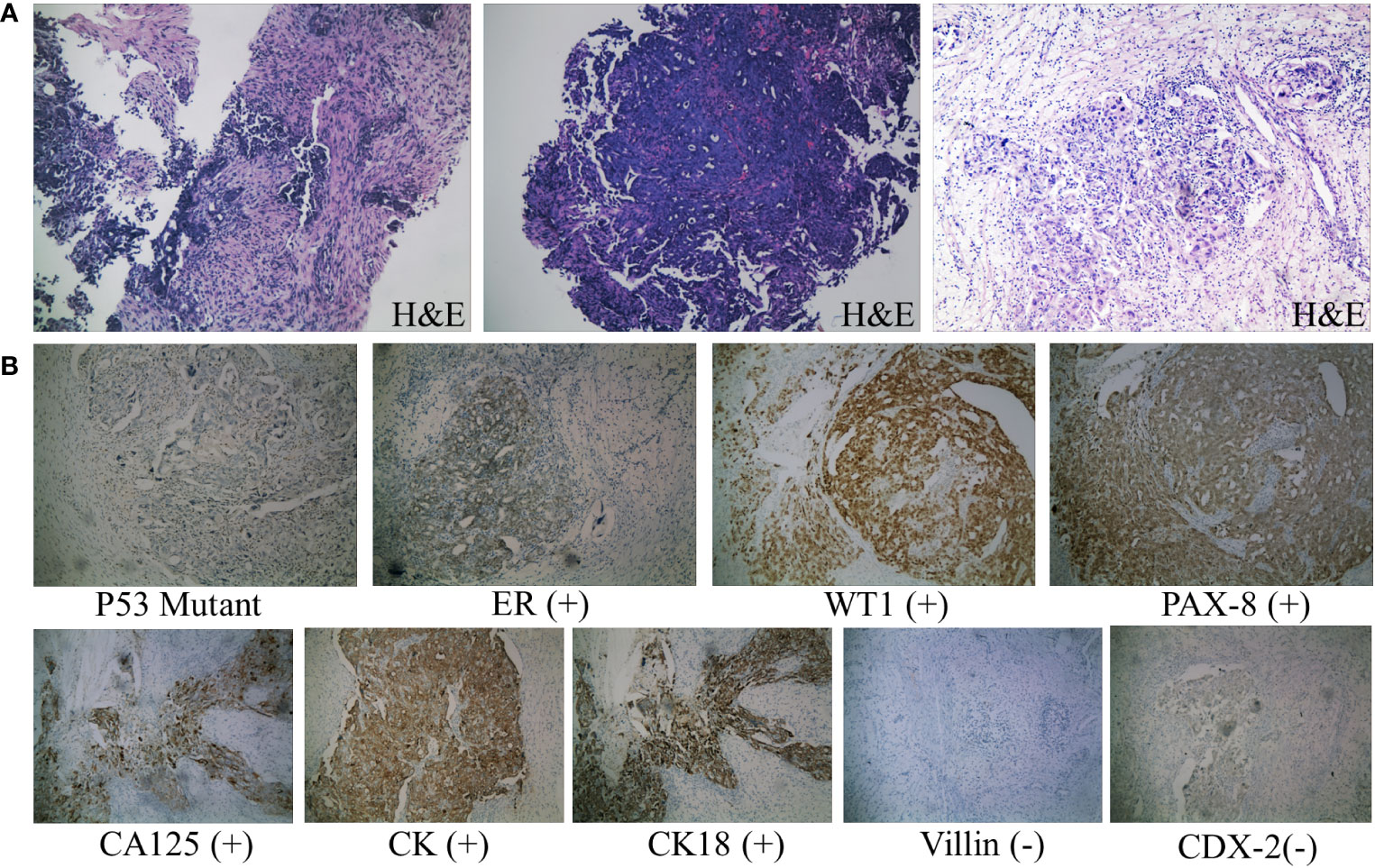
Figure 3 Pathological diagnosis. (A) From left to right: H&E staining results (×100) of needle biopsy of abdominal wall mass, endoscopic biopsy specimen, and surgical resection of ovarian tissue. The tumor cells are oval-shaped, varying in size, with large, deeply stained nuclei, prominent cell atypia, and easily observable mitotic figures. (B) Immunohistochemical staining results of ovarian tissue (×100). Consistent with the features of HGSOC according to the WHO classification: diffuse nuclear expression of WT1, mutant p53 expression, positive staining for CA125 and PAX8, and frequent ER expression. Negative staining for common immunohistochemical markers of colorectal cancer, such as Villin and CDX-2 proteins.
According to the National Comprehensive Cancer Network (NCCN) guidelines (9), the patient received neoadjuvant chemotherapy with the specific regimen of paclitaxel 120mg and cisplatin 200mg administered by intravenous infusion on days 1 and 8, respectively. After three cycles of chemotherapy, her blood CA125 and amylase levels gradually decreased (Table 1), and a follow-up CT scan showed an improvement in the pelvic and abdominal lesions compared to before. Due to the complexity of the surgery, the patient underwent an additional cycle of the original chemotherapy regimen. Subsequently, she underwent interval debulking surgery, which involved the removal of the uterus, bilateral adnexa (fallopian tubes and ovaries), great omentum, appendix, pelvic and abdominal lesions, and partial rectum. The postoperative pathology report revealed the presence of cancerous tissue in the bilateral adnexa, pelvic floor, omentum majus, left colon, appendiceal fat tissue, and rectum. Immunohistochemistry staining was performed, and the results showed positive expression for CK18, CK, ER, P53 mutation type, WT1, PAX-8, and CA125, while PR, Villin, and CDX2 were negative. These findings further confirmed the diagnosis of HGSOC (Figure 3B). Following surgery, the patient had the original chemotherapy therapy schedule for two cycles. She is still under close follow-up with no signs of tumor progression. The patient has given informed consent to publish the case.
3 Discussion
Ovarian cancer is one of the most common malignant tumors in the female reproductive system (1). However, due to atypical early symptoms, such as abdominal distension and dyspepsia, it is frequently disregarded or confused with other diseases, resulting in missing the best time for treatment. There are two isoenzymes of amylase: the S-type (also known as salivary gland type amylase) and the P-type (also known as pancreatic amylase) (3). Previous studies have suggested an association between amylase and ovarian cancer (10), however, there have been few reports on ovarian cancer that produce amylase since amylase is a rare product of ovarian cancer. The first case of hyperamylasemia related to ovarian cancer was reported in 1975 (11), followed by several cases of amylase elevation arising from ovarian cancer (12–14).
Pathologically, ovarian cancers associated with elevated amylase levels are mostly serous carcinomas, and the increased amylase is of salivary type. However, the specific mechanism remains unclear. The fallopian tubes themselves can secrete amylase, and the epithelium of serous ovarian carcinoma shares similar tissue structure with the epithelium of the fallopian tube, which may be one of the reasons (15, 16). Some scholars have presented amylase isoenzymes as possible biomarkers for assessing the prognosis and efficacy of ovarian cancer. For example, Zakrzewska (17, 18) found that total amylase activity and its salivary isoforms in serum were decreased in patients with ovarian cancer after radiotherapy and surgery. Vuković (7) found remarkable elevation of amylase levels correlated with poorer survival in ovarian malignancies.
It is easy to mistake ovarian cancer with hyperamylasemia for acute pancreatitis. Acute pancreatitis is a common cause of increased blood amylase, and the elevated amylase it causes is predominantly pancreatic type. An episode of acute pancreatitis is characterized by acute, persistent upper and middle abdominal pain and imaging showing morphological changes in the pancreas. In this case, neither pancreatic exudation on CT nor the usual indications of abdominal pain were present in the patient. So excluding the diagnosis of acute pancreatitis, hyperamylasemia was considered to be caused by ovarian cancer. Given the lack of definitive basis, doctors were still monitoring the patient’s blood and urine amylase levels and paying attention to her abdominal signs during the course of treatment. The decrease in serum amylase to the normal range after chemotherapy further confirmed that the hyperamylasemia resulted from ovarian cancer and was consistent with previous studies of serum amylase as a marker for assessing the prognosis and efficacy of ovarian cancer (17, 18). Although elevated serum amylase levels cannot specifically indicate ovarian cancer, female patients with hyperamylasemia should be vigilant about the presence of ovarian cancer after common causes of elevated amylase have been excluded.
Although this is not the first reported case of ovarian cancer causing elevated blood amylase levels, our case is more unique. The patient did not exhibit the typical clinical signs of ovarian cancer, such as abdominal mass and ascites, and the imaging showed simultaneous invasion of the intestinal wall and uterine adnexa, which was readily mistaken as plantation metastasis of gastrointestinal tumor. Ovarian cancer commonly metastasizes to the pelvic and abdominal cavities, with the most common and serious site of metastasis being the intestinal tract. Additionally, about 10-25 percent of ovarian tumors are metastatic, mainly from the gastrointestinal tract (19). Identifying the primary tumor source is essential since it affects the best approach to treatment and prognosis for cancers. For cases presenting with simultaneous involvement of the intestinal wall and adnexa during initial diagnosis, it is difficult to clinically differentiate between ovarian and gastrointestinal sources. The comprehensive application of methods such as serum amylase, tumor markers, colonoscopy, histopathology, and immunohistochemistry can assist in differential diagnosis. Studies have demonstrated the significant value of the CA125/CEA ratio in differentiating between ovarian and non-ovarian tumors (20, 21). When the CA125/CEA ratio is greater than 25, it is important to be cautious about the presence of primary ovarian cancer. The combined use of immunohistochemical markers can provide better guidance in distinguishing primary ovarian cancer from secondary ovarian tumors of gastrointestinal origin (22, 23), such as CK20, Villin, CDX-2, CEA, CA125, etc. (24, 25). Typical secondary ovarian tumors of gastrointestinal origin present with positive staining for Villin, CDX-2, CK20, and CEA, while they are negative for CA125. In contrast, primary ovarian cancer often exhibits the opposite pattern of staining. After acute pancreatitis was ruled out in this individual, abnormally elevated blood amylase and CA125 indicated the likelihood of ovarian cancer. Therefore, we have implemented additional measures including PET, percutaneous biopsy of abdominal wall masses, specimen retrieval through electronic colonoscopy, and immunohistochemical staining. According to the WHO classification (8), based on the histopathology and immunohistochemical staining of preoperative biopsy samples, it is considered as HGSOC. According to the patient’s lack of obvious gastrointestinal symptoms during hospitalization, elevated levels of CA125 and amylase, tumor distribution shown by imaging, as well as the histopathological and immunohistochemical staining results of preoperative biopsy, the patient was diagnosed with primary ovarian cancer. For patients with malignant tumors involving multiple sites, it is necessary to conduct comprehensive PET-CT scans and histological examinations at multiple sites in order to accurately determine the primary tumor origin, especially to exclude multiple primary malignant tumors (MPMT). MPMT refer to the simultaneous or sequential detection of two or more primary malignant tumors in single or multiple organs of the same patient (26). MPMT are typically linked with worse malignant behavior and prognosis than a single primary tumor. The treatment of MPMT is also relatively complex and individualized as it addresses the decision of which tumor to treat initially. For multisite malignancies, clinicians should consider the possibility of multiple primary cancers existing. In this particular case, clinically, we cannot exclude the possibility of MPMT. Therefore, we conducted further comprehensive PET-CT scans and histological examinations at multiple sites. The results indicated that the malignancy originated from the ovaries. There are some limitations to this study. The specific type of elevated amylase in the serum was not identified through amylase isoenzyme electrophoresis during the diagnostic and treatment process. Additionally, the expression of salivary-type amylase in the tumor was not confirmed through immunohistochemical staining.
4 Conclusion
Hyperamylasemia is a rare manifestation of ovarian cancer, and we encourage clinicians to consider ovarian cancer as one of the possible diagnoses for female patients with elevated serum amylase after common causes of hyperamylasemia have been ruled out. Histomorphological examinations of multiple sites and points are required to eliminate the possibility of MPMT in patients with tumors that show multiple sites of invasion on imaging.
Patient perspective
The patient expressed satisfaction with the outcome of the treatment and was motivated to undergo postoperative chemotherapy as scheduled.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Affiliated People’s Hospital of Jiangsu University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YJ: Data curation, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing. JL: Conceptualization, Data curation, Investigation, Resources, Supervision, Validation, Writing – review & editing. C-FM: Data curation, Investigation, Resources, Supervision, Validation, Writing – review & editing. YF: Conceptualization, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Jiangsu Maternal and Child Health Research Project (F202030).
Acknowledgments
The authors would like to express their sincere gratitude to Dr. Lin Chen from the Department of Gynecology at the First People’s Hospital of Zhenjiang for her assistance during the diagnostic and treatment process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CT, Computed Tomography; PET, Positron Emission Tomography; CA125, carbohydrate antigen 125; CA72-4, carbohydrate antigen 72-4; CEA, carcinoembryonic antigen; CK18, Cytokeratin 18; CK20, Cytokeratin 20; CK, Cytokeratin; ER, Estrogen receptor; PR, Progesterone receptor; WT, Wilms’ tumor; PAX-8, Paired box 8; HGSOC, high-grade serous ovarian cancer; MPMT, multiple primary malignant tumors.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
2. Hansen SEJ, Langsted A, Varbo A, Madsen CM, Tybjærg-Hansen A, Nordestgaard BG. Low and high pancreatic amylase is associated with pancreatic cancer and chronic pancreatitis. Eur J Epidemiol (2021) 36(9):975–84. doi: 10.1007/s10654-021-00801-0
3. Swensson EE, King ME, Malekpour A, Maull KI. Serum amylase isoenzyme alterations in acute abdominal conditions. Ann Emergency Med (1985) 14(5):421–3. doi: 10.1016/s0196-0644(85)80285-7
4. Stein L, Bank S, Rai K. Hyperamylasemia and hematologic Malignancies. Ann Internal Med (1992) 116(3):266–7. doi: 10.7326/0003-4819-116-3-266_2
5. Murthi P, Barker G, Nowell CJ, Rice GE, Baker MS, Kalionis B, et al. Plasminogen fragmentation and increased production of extracellular matrix-degrading proteinases are associated with serous epithelial ovarian cancer progression. Gynecologic Oncol (2004) 92(1):80–8. doi: 10.1016/j.ygyno.2003.09.016
6. Hoffmann I, Dragomir MP, Monjé N, Keunecke C, Kunze CA, Schallenberg S, et al. Increased expression of ido1 is associated with improved survival and increased number of tils in patients with high-grade serous ovarian cancer. Neoplasia (New York NY) (2023) 44:100934. doi: 10.1016/j.neo.2023.100934
7. Vuković A, Kuna K, Lončar Brzak B, Vučičević Boras V, Šeparović R, Šekerija M, et al. The role of salivary and serum ca125 and routine blood tests in patients with ovarian Malignancies. Acta clinica Croatica (2021) 60(1):55–62. doi: 10.20471/acc.2021.60.01.08
8. WHO Classification of Tumours Editorial Board. WHO Classification of Tumors: Female Genital Tumors, 5th edition . Lyon: International Agency for Research on Cancer (2020).
9. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, nccn clinical practice guidelines in oncology. J Natl Compr Cancer Network: JNCCN (2021) 19(2):191–226. doi: 10.6004/jnccn.2021.0007
10. Moriyama T. Sialyl salivary-type amylase associated with ovarian cancer. Clinica chimica acta; Int J Clin Chem (2008) 391(1-2):106–11. doi: 10.1016/j.cca.2008.01.025
11. Tsunashima T, Arima T, Ohashi Y, Kita S, Shiba K. [Case of ovarian cancer associated with hyperamylasemia]. Nihon Shokakibyo Gakkai zasshi = Japanese J gastro-enterology (1976) 73(9):1090–96.
12. Srivastava R, Fraser C, Gentleman D, Jamieson LA, Murphy MJ. Hyperamylasaemia: not the usual suspects. BMJ (Clinical Res ed) (2005) 331(7521):890–1. doi: 10.1136/bmj.331.7521.890
13. Kawakita T, Sasaki H, Hoshiba T, Asamoto A, Williamson E. Amylase-producing ovarian carcinoma: A case report and a retrospective study. Gynecologic Oncol Case Rep (2012) 2(3):112–4. doi: 10.1016/j.gynor.2012.06.002
14. Guo S, Lv H, Yan L, Rong F. Hyperamylasemia may indicate the presence of ovarian carcinoma: A case report. Medicine (2018) 97(49):e13520. doi: 10.1097/md.0000000000013520
15. Bruns DE, Mills SE, Savory J. Amylase in fallopian tube and serous ovarian neoplasms: immunohistochemical localization. Arch Pathol Lab Med (1982) 106(1):17–20.
16. Mc GR, Hargan LA, Potter BA, Daus AT Jr. Amylase in fallopian tubes. Proc Soc Exp Biol Med Soc Exp Biol Med (New York NY) (1958) 99(1):130–1. doi: 10.3181/00379727-99-24270
17. Zakrzewska I, Pietryńczak M. [Changes in activity of alpha amylase and its salivary isoenzyme in serum and urine after surgical treatment of ovarian neoplasms]. Ginekologia polska (1996) 67(10):504–9.
18. Zakrzewska I, Pietryńczak M. The alterations in the activity of amylase and its salivary isoenzyme in the serum of patients with ovarian carcinoma, submitted to radiotherapy. Roczniki Akademii Medycznej w Bialymstoku (1995) 1997) 42(1):229–35.
19. de Waal YR, Thomas CM, Oei AL, Sweep FC, Massuger LF. Secondary ovarian Malignancies: frequency, origin, and characteristics. Int J gynecological Cancer (2009) 19(7):1160–5. doi: 10.1111/IGC.0b013e3181b33cce
20. Nunes Pereira P, Françoise Derchain S, Yoshida A, Hoelz de Oliveira Barros R, Menezes Jales R, Sarian LO. Diffusion-weighted magnetic resonance sequence and ca125/cea ratio can be used as add-on tools to ultrasound for the differentiation of ovarian from non-ovarian pelvic masses. PloS One (2023) 18(3):e0283212. doi: 10.1371/journal.pone.0283212
21. Stiekema A, Boldingh QJ, Korse CM, van der Noort V, Boot H, van Driel WJ, et al. Serum human epididymal protein 4 (He4) as biomarker for the differentiation between epithelial ovarian cancer and ovarian metastases of gastrointestinal origin. Gynecologic Oncol (2015) 136(3):562–6. doi: 10.1016/j.ygyno.2014.12.037
22. Kir G, Gurbuz A, Karateke A, Kir M. Clinicopathologic and immunohistochemical profile of ovarian metastases from colorectal carcinoma. World J gastrointestinal Surg (2010) 2(4):109–16. doi: 10.4240/wjgs.v2.i4.109
23. Heatley MK. Immunohistochemical biomarkers of value in distinguishing primary ovarian carcinoma from gastric carcinoma: A systematic review with statistical meta-analysis. Histopathology (2008) 52(3):267–76. doi: 10.1111/j.1365-2559.2007.02824.x
24. Kim MJ. The usefulness of cdx-2 for differentiating primary and metastatic ovarian carcinoma: an immunohistochemical study using a tissue microarray. J Korean Med Sci (2005) 20(4):643–8. doi: 10.3346/jkms.2005.20.4.643
25. Nishizuka S, Chen ST, Gwadry FG, Alexander J, Major SM, Scherf U, et al. Diagnostic markers that distinguish colon and ovarian adenocarcinomas: identification by genomic, proteomic, and tissue array profiling. Cancer Res (2003) 63(17):5243–50.
Keywords: ovarian cancer, hyperamylasemia, neoplasm metastasis, diagnosis, case reports
Citation: Jie Y, Li J, Man C-f and Fan Y (2024) Ovarian cancer with intestinal wall invasion and hyperamylasemia: a case report. Front. Oncol. 14:1299226. doi: 10.3389/fonc.2024.1299226
Received: 22 September 2023; Accepted: 25 January 2024;
Published: 09 February 2024.
Edited by:
Andrea Tinelli, Veris delli Ponti Hospital, ItalyReviewed by:
Raghunadharao Digumarti, GSL Medical College, IndiaMingyuan Wang, Central South University, China
Copyright © 2024 Jie, Li, Man and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Fan, eXVmMTIzNDVAdWpzLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Yu Jie†
Yu Jie† Chang-feng Man
Chang-feng Man Yu Fan
Yu Fan