
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 21 March 2024
Sec. Breast Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1296328
 Fuyu Guo1†
Fuyu Guo1† Shiwei Sun1,2†
Shiwei Sun1,2† Xiangnan Niu3,4†
Xiangnan Niu3,4† Yue Wang1
Yue Wang1 Wei Yao1
Wei Yao1 Peng Yue1
Peng Yue1 Xiaoqian Deng1
Xiaoqian Deng1 Jiwen Shang1,3,4
Jiwen Shang1,3,4 Yangang Zhang1,3,4*
Yangang Zhang1,3,4*Renal metastasis of breast angiosarcoma is rare. This article reports the medical records of a patient diagnosed with breast angiosarcoma who underwent radical mastectomy and was found to have multiple lung metastases 3 years after surgery and renal pelvic metastasis 4 years after surgery. The patient underwent robot-assisted laparoscopic radical nephroureterectomy and sleeve resection of the intramural segment of the ureter, and postoperative pathology and immunohistochemical staining confirmed the diagnosis of renal pelvic metastasis of breast angiosarcoma. The patient received anlotinib for lung metastases following surgery and was followed up for 4 months after surgery. Currently, the patient has symptoms of coughing and hemoptysis but no other discomfort. The diagnosis and treatment of this rare malignant tumor remain challenging.
Breast angiosarcoma (BA) is a highly invasive malignant tumor that originates from the endothelial cells of the breast blood vessels. It is most common in women aged 20-40 years and accounts for less than 1% of all breast cancers. The 5-year overall survival rate is only around 30% (1, 2). BA can metastasize hematogenously to the contralateral breast, lungs, bones, liver, ovaries, skin, and subcutaneous tissues, whereas metastasis to the orbital tissues and lymph nodes is rare (3, 4). Primary breast angiosarcoma (PBA) often presents as a rapidly enlarged round-like mass located in the depths of the breast in a short period of time, which can show traumatic purplish-red changes when the tumor is superficial or invades the skin (1, 5). However, there is currently no literature on PBA that has metastasized to the kidney or renal pelvis. Here, we describe the diagnosis and treatment of PBA in a 33-year-old female.
The patient is a 33-year-old female, with no family history of breast tumor. In October 2019, she underwent needle biopsy of a right breast mass and was diagnosed with PBA (Figure 1). Immunohistochemical staining showed AE1/AE3 (- [Indicates a negative result]), CK7 (-), GATA3 (-), Vimentin (+ [Indicates a positive result]), CD34 (+), CD31 (+), SMA (-), calponin (-), ER (-), PR (-), CerbB-2 (0), β-catenin (cytoplasmic +), CD10 (-), and Ki67 (approximately 70% +) (Figure 2). The patient received radical mastectomy and paclitaxel for 10 cycles. In January 2022, due to coughing and bloody sputum, she underwent computed tomography (CT) of the lungs and needle biopsy; she was subsequently diagnosed with multiple lung metastases. The patient received targeted therapy and showed a poor response to apatinib but achieved good control after switching to anlotinib.
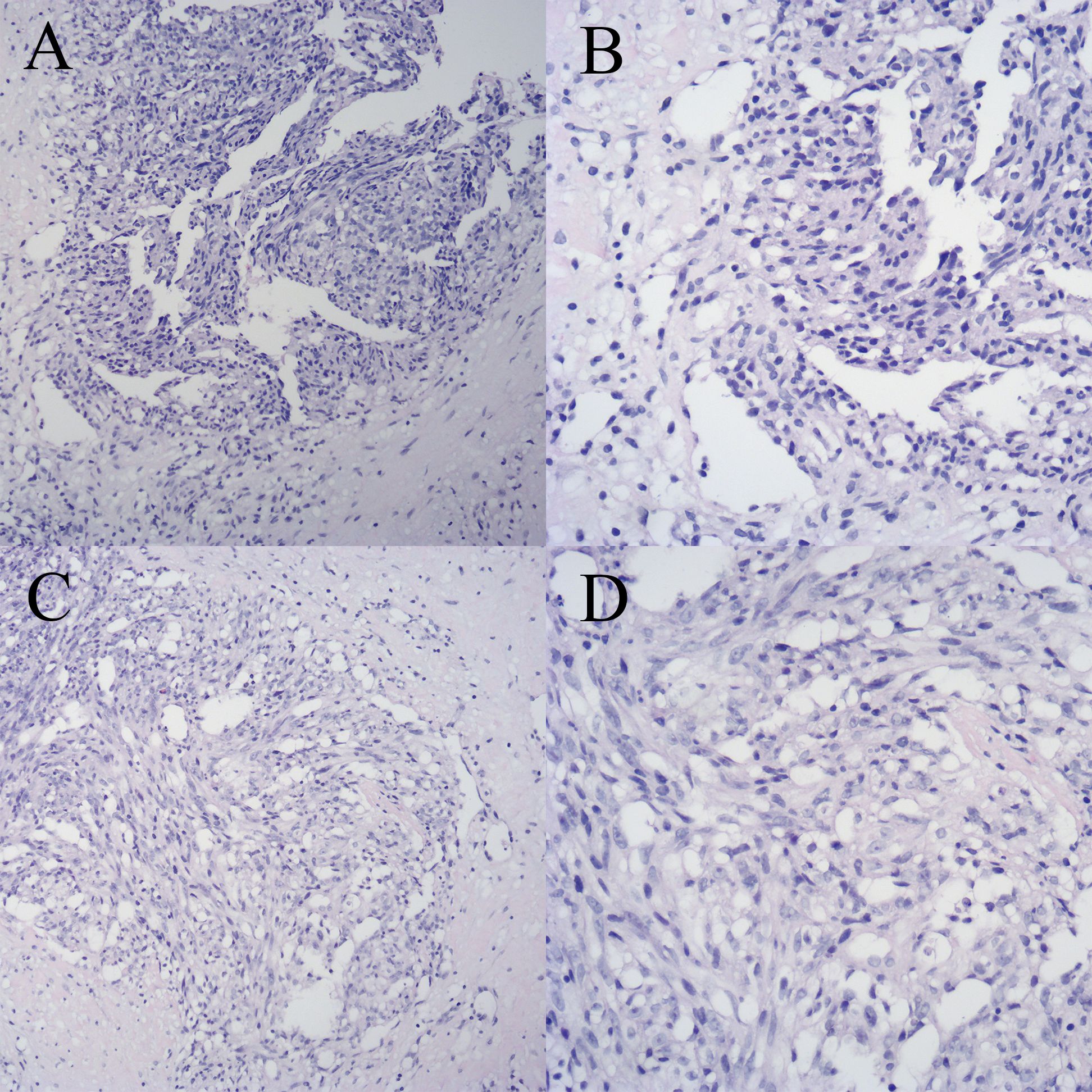
Figure 1 Breast biopsy specimen with hematoxylin and eosin (HE) staining. (A) Specimen 1 (×100). (B) Specimen 1 (×200). (C) Specimen 2 (×100). (D) Specimen 2 (×200).
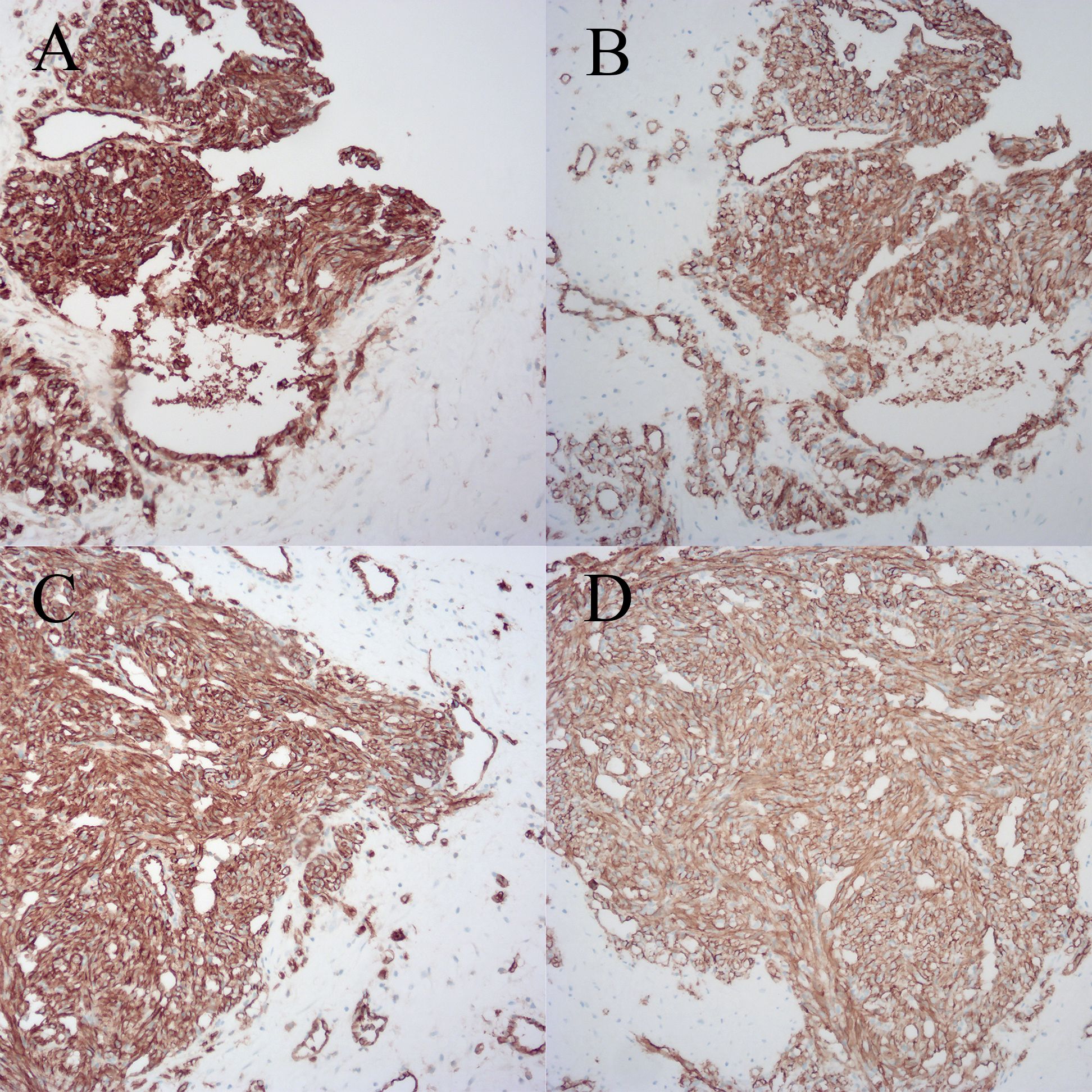
Figure 2 Breast biopsy specimen with immunohistochemical staining. (A) Specimen 1, CD31. (B) Specimen 1, CD34. (C) Specimen 2, CD31. (D) Specimen 2, CD34.
On November 15, 2022, the patient was admitted to the hospital due to gross hematuria for 10 h, accompanied by dizziness and fatigue. The blood cell analysis revealed hemoglobin (Hb) at 76 g/L and a red blood cell (RBC) count of 2.45 × 10¹²/L. Urinalysis showed an entire field of RBCs. The chest CT scan showed multiple solid nodules in both lungs, with the larger one located in the apicoposterior segment of the upper lobe of the left lung, measuring approximately 1.79 × 1.39 cm. The edge of the nodule was lobulated and uniformly enhanced on a contrast-enhanced scan. Abdominal CT suggested destruction of the structure of the right renal pelvis and calyces, with a lump-like and slightly high-density shadow in the renal sinus area, with the largest cross-sectional size approximately 4.46 × 4.81 cm. Contrast-enhanced scanning revealed multiple irregularly enhanced areas inside the mass in the arterial phase with multiple small blood vessels visible. The adjacent renal parenchyma exhibited decreased enhancement. CT revealed a malignant tumor in the right renal pelvis that had metastasized from other origins, or a bleeding pseudoaneurysm (Supplementary Figures 1, 2). Cystoscopy revealed no ureteral bleeding, and a biopsy of the bladder tissue revealed chronic inflammation of the mucosa. Urine cytology results were grade II and urine fluorescence in situ hybridization (FISH) was negative. Owing to the patient’s wishes, no further treatment was administered.
After 3 months, CT showed an increase in the number and size of the lung lesions, and the tumor in the right renal pelvis was slightly larger, with the largest cross-sectional size of approximately 6.60 × 8.10 cm (Figure 3). Renal angiographic ultrasound showed that the tumor began to enhance at 17 s and then rapidly increased as a whole, with no enhancement visible inside the tumor throughout, reaching a peak at 20 s with high enhancement, and subsiding slightly earlier than the surrounding renal parenchyma (Supplementary Figure 3). The patient underwent robot-assisted laparoscopic right nephroureterectomy and transurethral cystoscopy for bladder clot removal (Supplementary Figure 4). Bleeding was observed at the right ureteral orifice during surgery. Postoperative pathology showed a tumor measuring 7.00 × 5.50 × 3.70 cm in the right renal pelvis, with cancerous tissue penetrating the perirenal fat and visible cancerous tissue in the blood vessels. Immunohistochemical staining showed CK20 (-), 34βE12 (-), β-catenin (membrane and cytoplasmic +), p63 (-), GATA-3 (focally weak +), S100P (-), Uroplakin II (-), CK7 (-), Ki67 (approximately 70% +), AE1/AE3 (-), EMA (-), Vimentin (+), CD31 (partial +), CD34 (+), SMA (-), Desmin (-), PAX-8 (-), INI-1 (expression), OCT3/4 (-), ALK D5F3 (-), CyclinD1 (partial +), HMB45 (-), Melan-A (partial +), S100 (-), Myogenin (-), Fli-1 (+), TLEl (+), and CD99 (partial cytoplasmic +) (Supplementary Figures 5, 6). The pathological diagnosis was consistent with angiosarcoma, partially presenting with an epithelial-like morphology. Follow-up at 4 months postoperatively showed symptoms of coughing and hemoptysis, with no other discomfort. CT revealed no abnormalities in the surgical area of the right kidney, but showed aggravation of lung metastasis (Supplementary Figure 7).
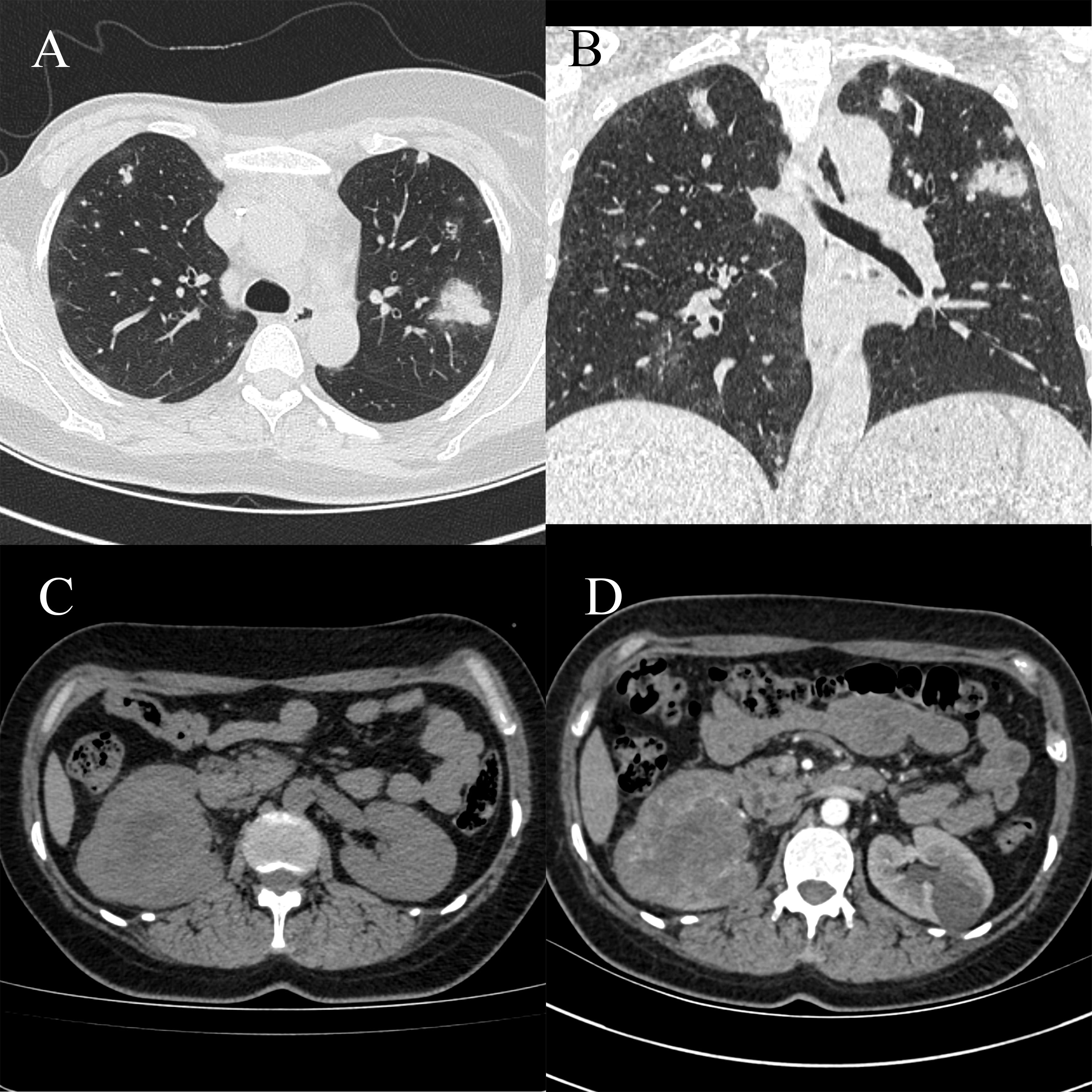
Figure 3 Computed tomography (CT) scan showing an increase in the number and size of lung lesions compared to previous results, with a slight increase in the right renal sinus mass. (A) Chest CT scan, horizontal position. (B) Chest CT scan, coronal position. (C) Abdominal CT scan, horizontal position. (D) Abdominal CT scan, arterial phase, horizontal position.
BA is a rare malignant tumor. There are only a few retrospective studies and case reports but no large randomized controlled trials. Therefore, there are still no clear guidelines and evidence to guide the treatment of this disease. Surgery is still the main treatment at present (1). Due to the aggressive and multifocal nature of angiosarcoma, the prognosis is poor when the margins are positive (6). Currently, breast angiosarcoma is mainly treated with mastectomy to achieve as negative a margin as possible, and the recurrence rate after breast-conserving surgery is high (7, 8). There is still no definite evidence that adjuvant chemotherapy and radiotherapy can improve patient survival.
Angiosarcoma may present local recurrence and distant metastasis in the early stage, and the prognosis is significantly worse than that of other breast tumors. Studies have shown that DFS and OS of low - and medium-grade breast tumors are significantly longer than those of high-grade tumors (7, 9). PBA is prone to metastasize to the contralateral breast, lungs, bones, liver, ovaries, skin, and subcutaneous tissues hematogenously. However, there is currently no record of metastasis to the kidneys or renal pelvis. This disease can be differentiated from the following conditions.
Primary carcinoma of the renal pelvis: This is a subtype of urothelial carcinoma. Ureteroscopy and histopathological examinations are the gold standard for this form of carcinoma. Urine cytology and urine FISH examination are economical and convenient non-invasive methods with good sensitivity and specificity (10). Immunohistochemical (IHC) Staining is an application of immunostaining. It is based on antigen-antibody reaction, where it uses antibodies to determine the tissue distribution of an antigen of interest, such as tumor antigens. IHC is an important tool in diagnostic and research. The most important markers for malignant urothelial carcinoma are GATA3, CK7, CK20, and p63 (11), whereas for angiosarcoma, CD31 is the most specific marker for endothelial cell differentiation, and CD34 is more sensitive. Ki-67 reflects the mitotic rate of cells and is higher in sarcomas with invasive characteristics (12). The transcription factors ETS-related gene (ERG) and friend leukemia integration 1 (FLI-1) are new antibodies with high sensitivity and specificity compared with CD34 and CD31 (13).
Renal pelvic hematoma, otherwise known as renal pseudoaneurysm, is a renal pseudotumor caused by the rupture and bleeding of small renal parenchymal arteries. CT scans showed a slightly high-density mass with a clear boundary from the renal parenchyma, which could cause compression of the renal pelvis and calyces without signs of destruction. In the acute or subacute phase, the density of the mass may increase slightly owing to the entry of contrast agents into the lesion through the ruptured blood vessels.
Renal artery aneurysm: On CT, it appears as a uniform, slightly high-density mass with clear edges and calcifications on the wall. The tumor body was clearly enhanced. The difference is that the degree of aneurysm enhancement is the same as that of the abdominal aorta and renal artery at any time, and renal artery angiography remains the gold standard for diagnosis and treatment (14).
Nephroureterectomy is the gold standard treatment for upper urinary tract tumors, including total resection of the affected kidney and ureter to the ureteral orifice. Due to the long learning time for laparoscopic techniques, high surgical difficulty, and high tension in bladder incision suture reconstruction, its further promotion and application are limited. Robot-assisted laparoscopy has many advantages over traditional laparoscopy, including a three-dimensional high-definition surgical view, simulated arms with seven degrees of freedom, and the ability to automatically filter hand tremors (15). Currently, there are few reports of single-position robot-assisted laparoscopic nephroureterectomy for renal pelvic carcinoma in China. In this case, the patient had a minor intraoperative injury, good postoperative recovery, and no adverse complications.
This study is the first reported case of renal metastasis from a PBA to date. The diagnosis and treatment of this rare malignant tumor remain challenging.
The patient is satisfied with the treatment effect. The personal information related to the patient has been hidden from this manuscript. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
FG: Conceptualization, Data curation, Project administration, Writing – original draft. SS: Conceptualization, Data curation, Writing – original draft. XN: Conceptualization, Data curation, Writing – original draft. YW: Data curation, Writing – original draft. WY: Data curation, Writing – original draft. PY: Data curation, Writing – original draft. XD: Data curation, Writing – original draft. JS: Supervision, Writing – review & editing. YZ: Conceptualization, Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1296328/full#supplementary-material
1. Abdou Y, Elkhanany A, Attwood K, Ji W, Takabe K, Opyrchal M. Primary and secondary breast angiosarcoma: single center report and a meta-analysis. Breast Cancer Res Treat. (2019) 178:523–33. doi: 10.1007/s10549-019-05432-4
2. Hodgson NC, Bowen-Wells C, Moffat F, Franceschi D, Avisar E. Angiosarcomas of the breast. Am J Clin Oncol. (2007) 30:570–3. doi: 10.1097/COC.0b013e3181131d62
3. Ooe Y, Terakawa H, Kawashima H, Ikeda H, Inaki N. Bilateral primary angiosarcoma of the breast: A case report. J Med Case Rep. (2023) 17:1–6. doi: 10.1186/s13256-023-03791-7
4. Champeaux-Orange E, Bonneau C, Raharimanana B, Favre A, Ibrahim M, Breteau N. Angiosarcome mammaire primitif: à Propos de deux cas. Cancer/Radiothérapie. (2009) 13:209–12. doi: 10.1016/j.canrad.2009.02.004
5. Fraga-Guedes C, André S, Mastropasqua MG, Botteri E, Toesca A, Rocha RM, et al. Angiosarcoma and atypical vascular lesions of the breast: diagnostic and prognostic role of myc gene amplification and protein expression. Breast Cancer Res Treat. (2015) 151:131–40. doi: 10.1007/s10549-015-3379-2
6. Gervais MK, Burtenshaw SM, Maxwell J, Dickson Bc, Catton CN, Blackstein M, et al. Clinical outcomes in breast angiosarcoma patients: A rare tumor with unique challenges. J Surg Oncol. (2017) 116:1056–61. doi: 10.1002/jso.24780
7. Taffurelli M, Pellegrini A, Meattini I, Orzalesi L, Tinterri C, Roncella M, et al. Secondary breast angiosarcoma: A multicentre retrospective survey by the national Italian association of breast surgeons (Anisc). Breast. (2019) 45:56–60. doi: 10.1016/j.breast.2019.02.011
8. Li GZ, Fairweather M, Wang J, Orgill DP, Bertagnolli MM, Raut CP. Cutaneous radiation-associated breast angiosarcoma. Ann Surg. (2017) 265:814–20. doi: 10.1097/sla.0000000000001753
9. McClelland S, Hatfield J, Degnin C, Chen Y, Mitin T. Extent of resection and role of adjuvant treatment in resected localized breast angiosarcoma. Breast Cancer Res Treat. (2019) 175:409–18. doi: 10.1007/s10549-019-05172-5
10. Liu J, Peng X, Ning X, Li T, Peng S, Wang J, et al. Clinical value of fluorescence in situ hybridization positive of exfoliated urothelial cells in urothelial carcinoma. J PEKING Univ (HEALTH SCIENCES). (2017) 49:585–9. doi: 10.3969/j.issn.1671-167X.2017.04.006
11. Vlajnic T, Bubendorf L. Diagnostische und prädiktive marker in der harntraktzytologie. Der Pathologe. (2022) 43:99–104. doi: 10.1007/s00292-022-01053-9
12. Nahed AS, Shaimaa MY. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol Med. (2016) 13:496–504. doi: 10.20892/j.issn.2095-3941.2016.0066
13. Varghese B, Deshpande P, Dixit S, Koppiker C, Jalnapurkar N. Primary angiosarcoma of the breast: A case report. J Radiol Case Rep. (2019) 13:15–25. doi: 10.3941/jrcr.v13i2.3449
14. LI S, Xiao J. Renal pseudotumors: ct diagnosis and differential diagnosis. J Med Postgrad. (2006) 19:788–92. doi: 10.16571/j.cnki.1008-8199.2006.09.008
Keywords: breast angiosarcoma, renal pelvis cancer, tumor metastasis, diagnosis, treatment, robot-assisted laparoscopic surgery
Citation: Guo F, Sun S, Niu X, Wang Y, Yao W, Yue P, Deng X, Shang J and Zhang Y (2024) Renal pelvis metastasis following surgery for breast angiosarcoma: a case report and literature review. Front. Oncol. 14:1296328. doi: 10.3389/fonc.2024.1296328
Received: 18 September 2023; Accepted: 12 March 2024;
Published: 21 March 2024.
Edited by:
Nosheen Masood, Fatima Jinnah Women University, PakistanReviewed by:
Anam Nayab, University of Science and Technology of China, ChinaCopyright © 2024 Guo, Sun, Niu, Wang, Yao, Yue, Deng, Shang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yangang Zhang, dXJvenlnQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.