- 1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Interventional Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Purpose: To investigate the prognostic value of platelet-to-lymphocyte ratio (PLR) in patients with unresectable hepatocellular carcinoma (uHCC) treated with transarterial chemoembolization (TACE) and tailored tyrosine kinase inhibitors (TKIs) plus immune checkpoints inhibitors (ICIs).
Materials and methods: Ninety-eight patients from May 2018 to January 2022 in our hospital were enrolled in this study. The receiver operating characteristic (ROC) curve analysis was performed and the corresponding Youden index was used to determine the optimal PLR cut-off. Overall survival (OS), progression-free survival (PFS), and adverse events (AEs) of patients were evaluated based on the PLR cut-off. The factors affecting survival were assessed using univariate and multivariate Cox proportional hazards regression analyses.
Results: The PLR cut-off was 98.89. There were 49 patients in the low pretreatment PLR group (PLR ≤ 98.89) and 49 patients in the high PLR group (PLR > 98.89). Patients with low pretreatment PLR had significantly longer median OS (25.7 months vs 16.1 months; P < 0.001) and PFS (14.9 months vs 10.2 months; P < 0.001) than those with high pretreatment PLR. The multivariate analysis revealed that ALT, tumor size, and PLR are risk factors affecting OS. The three independent factors affecting PFS are tumor size, AFP, and PLR. The AEs were tolerable and manageable.
Conclusion: The low pretreatment PLR (PLR ≤ 98.89) was an independent protective factor for the survival outcomes of patients in this study. PLR was helpful for clinicians to predict the prognosis and identify the patients with uHCC who were most likely to benefit from TACE + TKIs + ICIs.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death in the world (1). Patients who are diagnosed with early-stage HCC have the opportunity to undergo curative treatments (2, 3). Since the onset of HCC is insidious, a majority of patients with HCC are diagnosed at intermediate or advanced stage and are not suitable for curative resection (4).
According to the guidelines (5, 6), transarterial chemoembolization (TACE) has been recommended as a standard treatment for intermediate and advanced HCC. Since the efficacy of TACE is associated with tumor size, vascular invasion and distant metastasis (7), it is challenging to achieve complete tumor necrosis using TACE alone. In addition, TACE could increase the expression of programmed cell death ligand 1 (PD-L1) and vascular endothelial growth factors (VEGF) as a result of the hypoxic microenvironment after embolization, contributing to the tumor recurrence and metastasis (8, 9).
It is known that immune checkpoints, including programed cell death protein 1 (PD-1), programed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), can suppress the T-cell-mediated immune responses, which permits cancer cells to escape from the immune destruction (10). Immune checkpoint inhibitors (ICIs) such as camrelizumab and atezolizumab act to block the interaction of immune checkpoints and the corresponding ligands. As a result, tumor-reactive T cells are able to overcome the negatively regulatory mechanisms caused by immune checkpoints and facilitate an effective anti-tumor response (11).
Angiogenic factors such as VEGF can bind to VEGF receptors (VEGFRs) to suppress immune responses by inducing vascular abnormalities, inhibiting antigen presentation, or enhancing the activity of regulatory T cells to suppress the immune system (12, 13). Tyrosine kinase inhibitors (TKIs) can exactly block the intracellular domain of VEGFR to impede the immunosuppression effects of VEGF (14).
Thus, systemic therapy, including ICIs and TKIs, has been recommended as the first-line treatment for patients with advanced HCC (15). Based on the guidelines for primary liver cancer (16), it is recommended to combine TACE with systemic therapy to enhance the efficacy of TACE. And many studies have investigated the efficacy of TACE and TKIs plus ICIs, demonstrating significantly higher tumor response and survival benefits (17–19).
Some studies have shown that inflammatory and immune environments play an important role in the formation and progression of HCC (20, 21). And many studies have evaluated the effects of various inflammatory and immune biomarkers in predicting the outcomes of patients with malignant tumors (22–24). High platelet counts can stimulate angiogenesis and tumor proliferation by enhancing the secretion of growth factors, such as VEGF and platelet-derived growth factors (25). Decreased lymphocyte counts are related to an insufficient immunologic reaction to the tumor, which consequently enable tumor progression and metastasis (26). Increased platelet counts along with decreased lymphocyte counts lead to an elevated PLR, which is associated with unfavorable clinical outcome in HCC patients receiving TACE alone or TACE plus TKIs (27–29). However, the prognostic value of PLR for uHCC patients treated with TACE + TKIs + ICIs has not been evaluated.
This study aimed to investigate the effectiveness of pretreatment PLR in predicting the survival outcomes of uHCC patients treated with TACE + TKIs + ICIs.
Materials and methods
Patients
The Institutional Review Board in our hospital approved this retrospective study, and the informed consent was waived. This study was conducted in accordance with the Declaration of Helsinki.
Patients with uHCC received TACE + TKIs + ICIs between May 2018 and January 2022 in our hospital were enrolled in this study. HCC was diagnosed by pathological examination or noninvasive criteria based on the European Association for the Study of the Liver (EASL) guidelines (6). A multidisciplinary team determined the patients’ treatment decisions.
The inclusion criteria for this study were as follows: 1) age ≥ 18 years; 2) confirmed diagnosis with uHCC; 3) Eastern Cooperative Oncology Group (ECOG) scores ≤ 1; 4) Child-Pugh A or B; 5) adequate cardiac, renal and coagulation function; 6) treated with TACE + TKIs + ICIs.
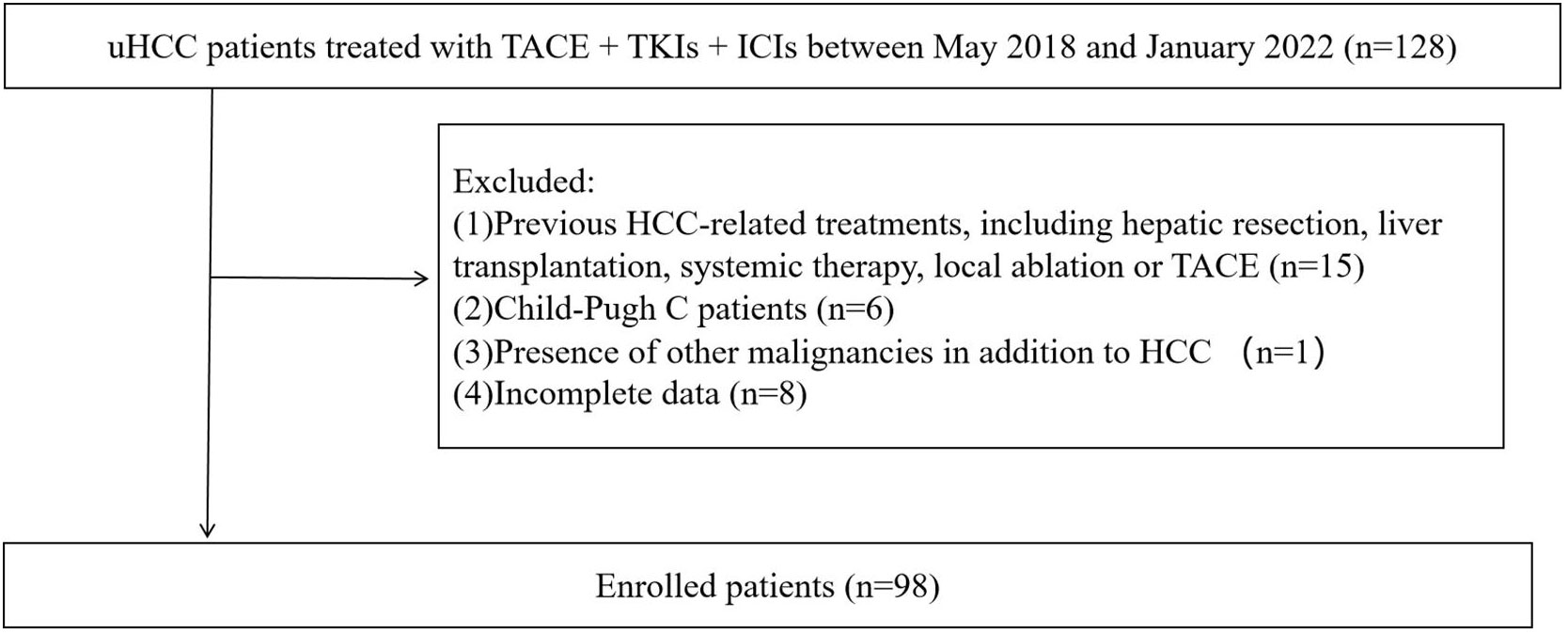
The exclusion criteria were as follows: 1) previous HCC-related treatments, including hepatic resection, liver transplantation, systemic therapy, local ablation or TACE; 2) Child-Pugh C; 3) presence of other malignancies in addition to HCC; 4) incomplete data.
Treatment protocol
TACE was performed under local anesthesia via right femoral artery. The Seldinger technique and angiography were performed to identify the tumor-feeding arteries and assess the tumor burden. According to the tumor burden, 5-15mL of emulsion containing 10-20 mg of doxorubicin hydrochloride (Hisun Pharmaceutical Co.LTD, Zhejiang, China) was mixed with 5-10 mL of lipiodol (Lipiodol Ultrafluido, Guerbet, France) and injected into the tumor-feeding arteries through a 3-F microcatheter. Finally, an appropriate amount of gelatin sponge particles (350-560 µm; Cook) was injected into the tumor-feeding arteries to induce embolization.
TKIs including sorafenib (800 mg), lenvatinib (8 or 12 mg), and apatinib (500 mg) were administered orally daily. ICI immunotherapy with intravenous fixed-dose camrelizumab (200 mg) was performed every 3 weeks until disease progression or unexpected toxicity was observed. The dose and interval of TKIs were adjusted according to the toxicity and disease conditions. The administration of TKIs and ICIs should be stopped when unacceptable toxicity occurred or no clinical benefits were observed. What’s more, TKIs and ICIs were discontinued for 3 days before and after TACE.
Outcomes and follow-up
All laboratory indicators and radiological data were collected within 7 days of initial treatment. PLR was calculated as absolute platelet count divided by absolute lymphocyte count prior to the initial treatment. All patients were followed up every 4-8 weeks. The laboratory and imaging information of patients were recorded at each appointment. Two radiologists with more than 10 years experience in abdominal radiology evaluated the imaging examinations. Both of them were blinded to the patients’ clinical information. TACE was recommended if the patient had a residual tumor or disease progression during the follow-up. Adverse events (AEs) in this study were monitored and recorded by experienced nurses according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (30).
Overall Survival (OS) and Progression-Free Survival (PFS) were outcomes of this study. OS was defined as the interval from the initial treatment to death or the last follow-up. PFS was defined as the time between the initial treatment and disease progression according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (31), death or the last follow-up.
Statistical analysis
SPSS version 24.0 (IBM, Chicago, Illinois, USA) was used for the statistical analyses. Continuous variables and categorical variables were presented as median (interquartile range) and frequencies (percentages), respectively. The time-dependent ROC curve analysis was performed and the corresponding Youden index was used to determine the optimal PLR cut-off for patients with uHCC. Continuous variables at baseline for the high and low PLR groups were compared using Student’s t-test or Mann-Whitney U test. Chi-square test or Fisher’s exact test was used to compare categorical variables. OS and PFS curves were drawn by the Kaplan-Meier method and were compared using log-rank tests. Risk factors related to OS and PFS were identified by univariate and multivariate Cox proportional hazards regression analysis. Factors with P < 0.05 at univariate analysis were included in the multivariate analysis. P < 0.05 was considered statistically significant.
Results
Baseline statistics
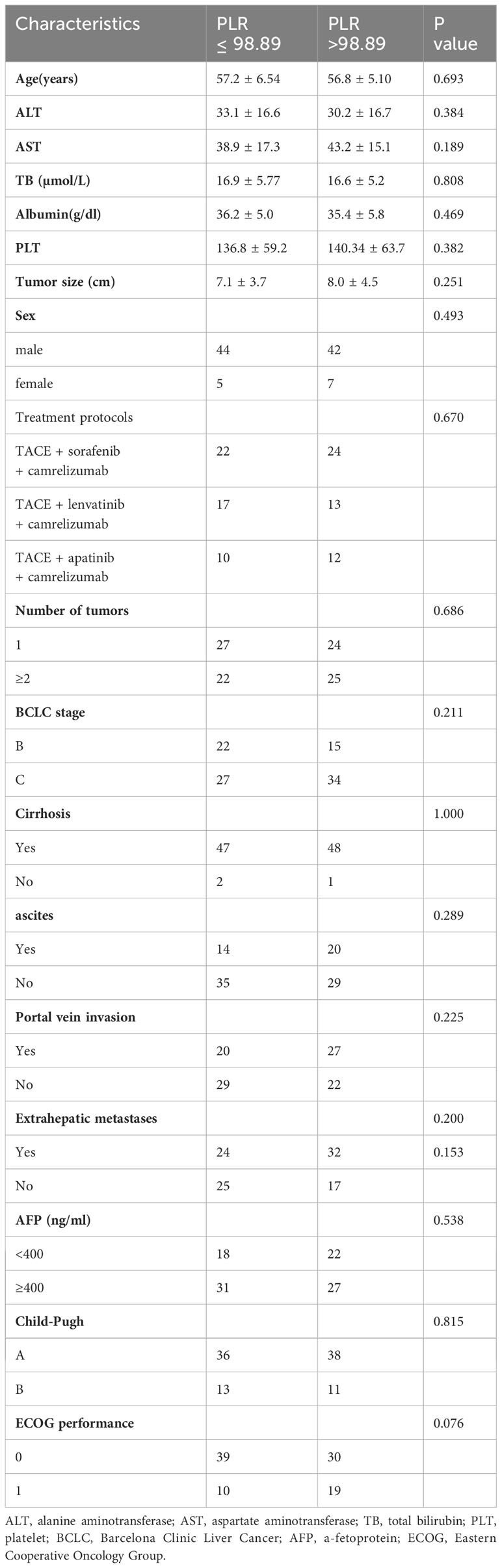
During the follow-up, a total of 128 uHCC patients treated with TACE + TKIs + ICIs were enrolled in this study. However, thirty patients were excluded according to the exclusion criteria (Figure 1). The ROC curve analysis was performed and the Youden index suggested that the optimal PLR cut-off was 98.89. The area under the ROC (AUC) curve was 0.77 (Figure 2). According to the cut-off, forty-nine (50.0%) patients with PLR > 98.89 were divided into the high PLR group and the rest 49 (50.0%) patients with PLR ≤ 98.89 were divided into the low PLR group. The baseline characteristics of these patients were presented in Table 1, with no statistical difference between the two groups.
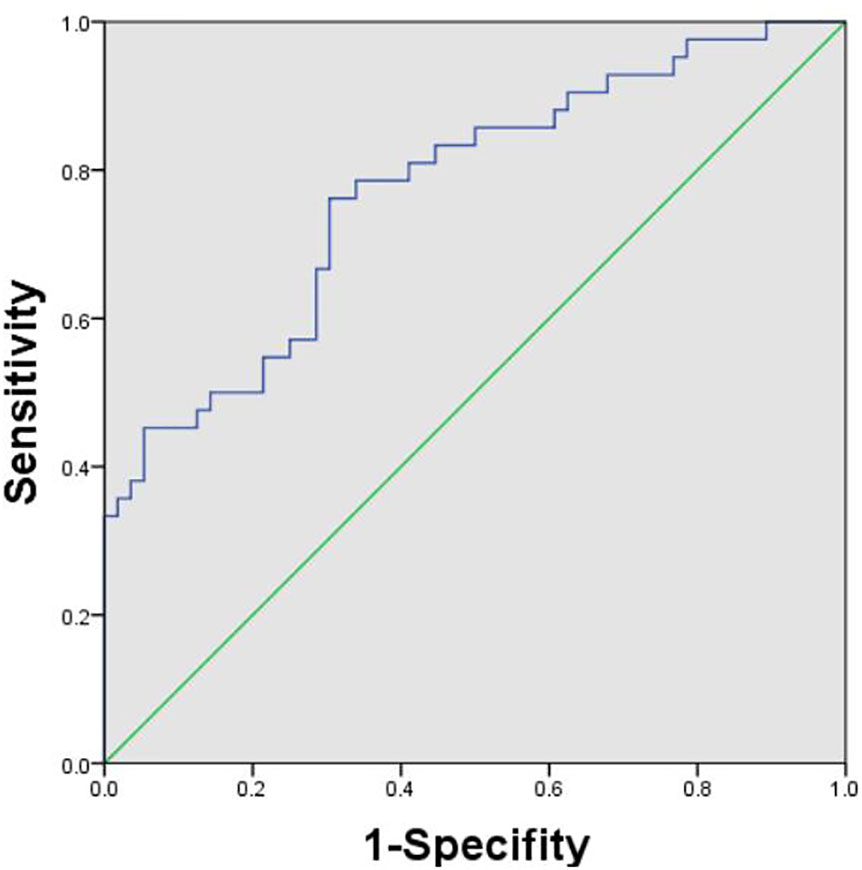
Figure 2 Receiver operating characteristic curve analysis was performed to determine the optimal cut-off for PLR. The cut-off was 98.89. PLR, platelet-to-lymphocyte ratio.
OS and PFS
The Kaplan–Meier analysis showed that the median OS (mOS) of patients in the low PLR group was higher than that of those in the high PLR group (25.7 months vs 16.1 months; P < 0.001) (Figure 3A). Similarly, the median PFS of patients in the low PLR group was also higher than the high PLR group (14.9 months vs 10.2 months; P < 0.001) (Figure 3B).
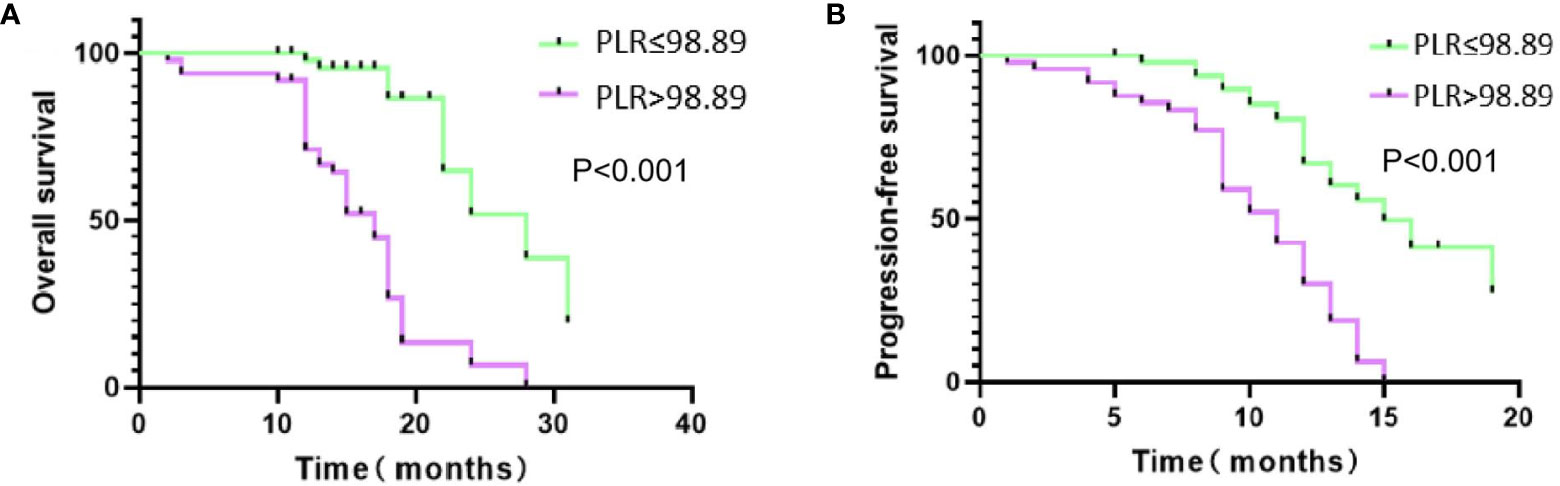
Figure 3 Kaplan-Meire (K-M) curves of two groups. (A) K-M curve for overall survival (OS); (B) K-M curve for progression-free survival (PFS).
Risk factors affecting OS and PFS
Univariate Cox proportional hazards regression analysis revealed that alanine transaminase (ALT) (hazard ratio [HR]: 1.035; 95% confidence interval [CI]: 1.002-1.069; P = 0.039), tumor size (HR: 1.186; 95% CI: 1.059-1.328; P = 0.003), and PLR (HR: 8.547; 95% CI: 2.902-25.170; P = 0.000) were risk factors affecting OS (Table 2). The factors related to PFS (Table 3) included tumor size (HR: 1.135; 95% CI: 1.031-1.249; P = 0.010), alpha-feto protein (AFP) (HR: 2.516; 95% CI: 1.281-4.940; P = 0.007), and PLR (HR: 4.882; 95% CI: 2.336-10.205; P = 0.000). Multivariate Cox proportional hazards regression analysis identified three risk factors affecting OS: ALT (HR: 1.022; 95% CI: 1.006-1.038; P = 0.006), tumor size (HR: 1.121; 95% CI: 1.045-1.202; P = 0.001), and PLR (HR: 6.680; 95% CI: 3.055-14.606; P = 0.000). Three independent factors affected PFS: tumor size (HR: 1.110; 95% CI: 1.037-1.188; P = 0.003), AFP (HR: 1.940; 95% CI: 1.095-3.437; P = 0.023) and PLR (HR: 3.540; 95% CI: 2.004-6.254; P = 0.000).
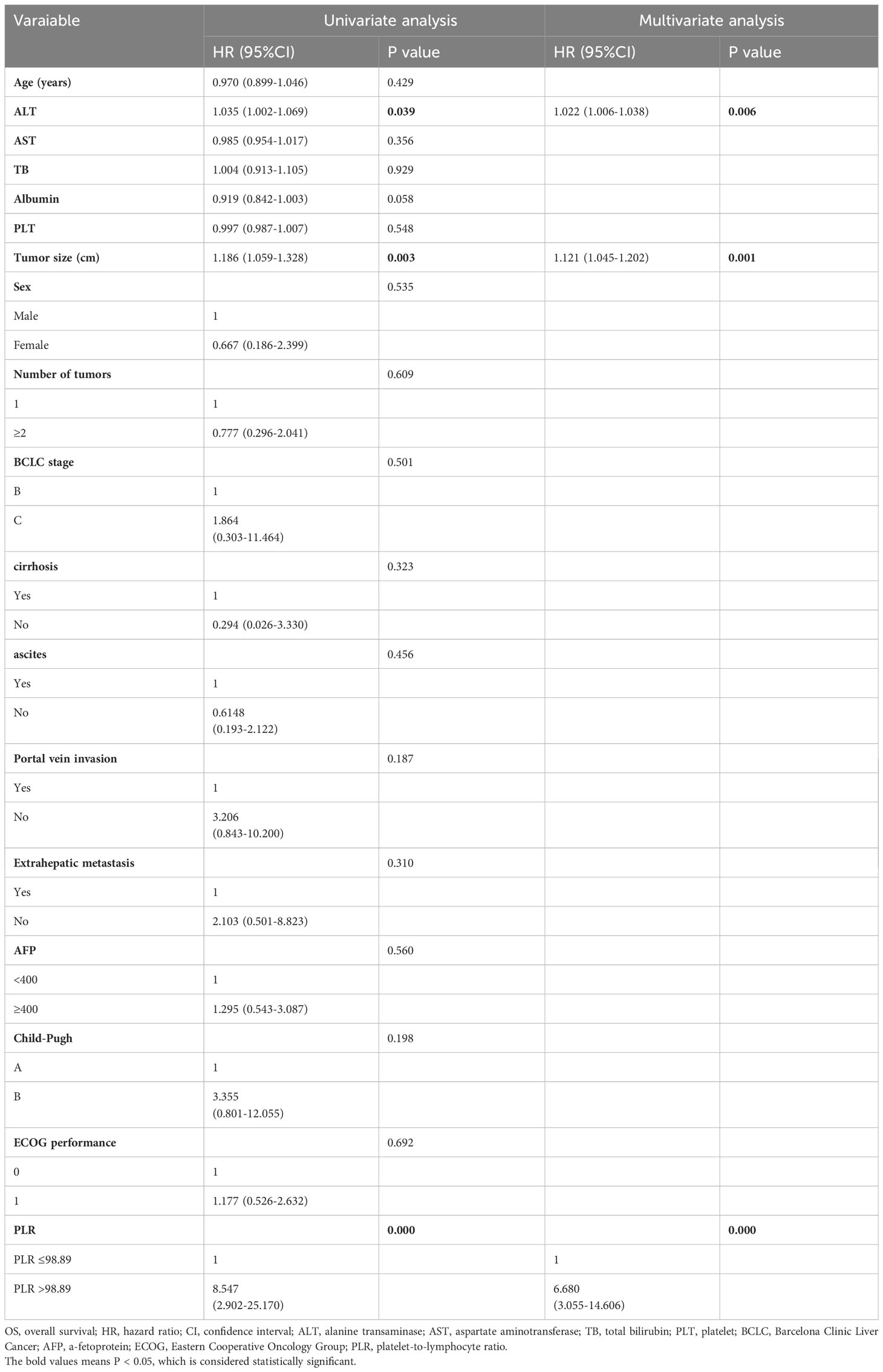
Table 2 Univariate and multivariate Cox proportional hazards regression analysis of risk factors for OS.
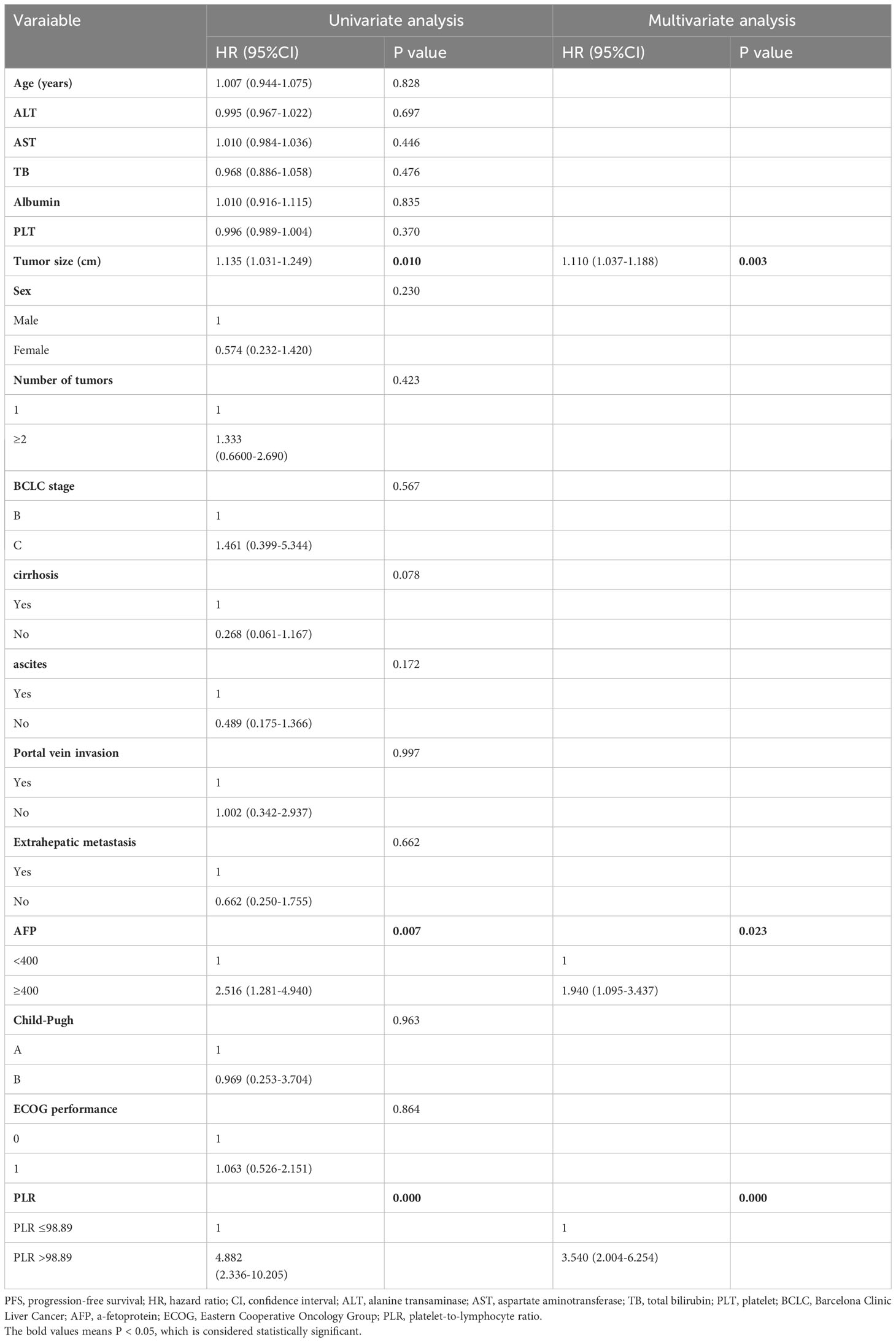
Table 3 Univariate and multivariate Cox proportional hazards regression analysis of risk factors for PFS.
Safety
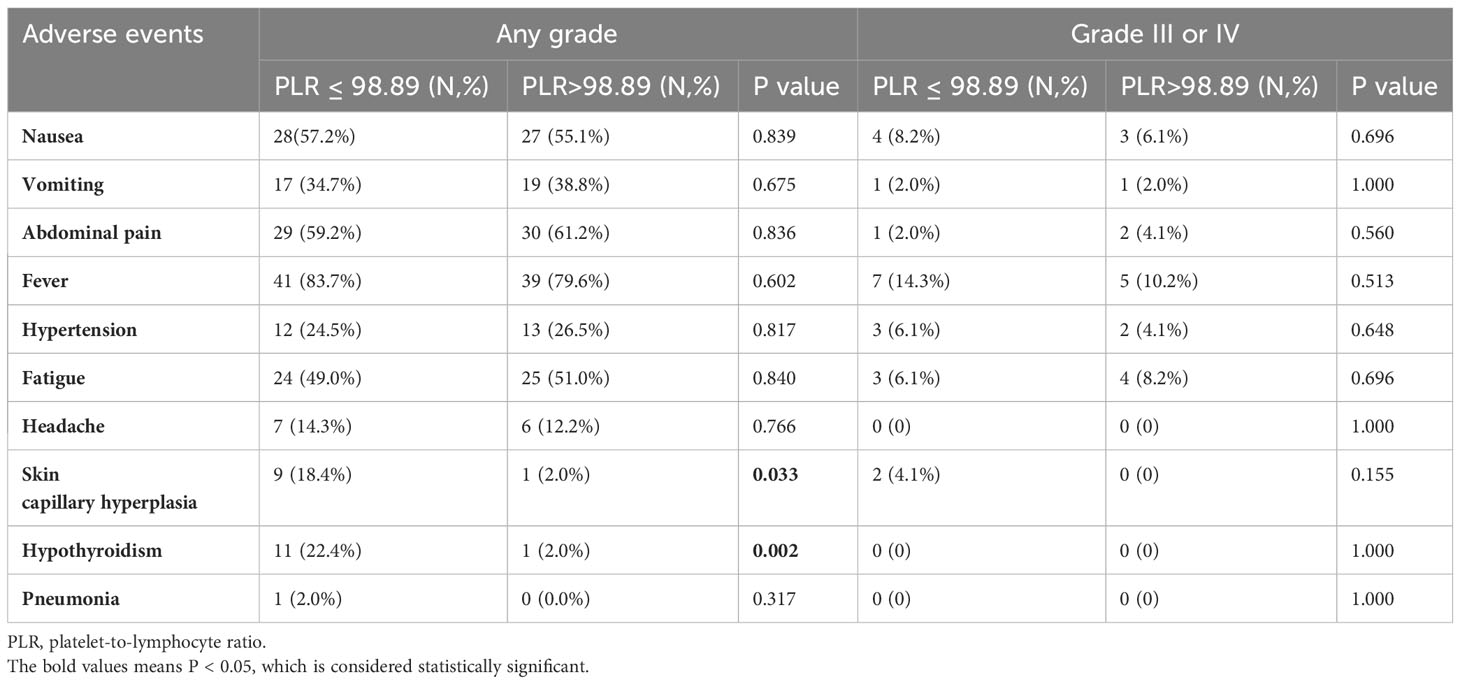
All AEs were presented in Table 4. There was no treatment-related death observed in this study. The most common TACE-related AEs were postembolization syndrome that included nausea (57.2%), vomiting (34.7%), abdominal pain (59.2%), and fever (83.7%). And the most common drug-related AEs were hypertension (24.5%), fatigue (49.0%), headache (14.3%), skin capillary hyperplasia (18.4%), hypothyroidism (22.4%), and pneumonia (2.0%). For grade 3 or 4 AEs, only nausea, fever, hypertension and fatigue had incidences of >5%. (Table 4).
There was no statistical difference in the incidence of most AEs between the two groups. However, the incidence of some immunotherapy-related adverse events (irAEs) of any grade in the low PLR group was significantly higher than that in the high PLR group, with no statistical difference in grade 3 or 4 AEs (Table 4).
Discussion
It is known that the prognosis of patients with uHCC is poor due to drug resistance, frequent recurrence, and metastasis [32]. With the advent of immunomodulatory antibodies and molecular-targeted drugs, a new combination strategy combining TACE + TKIs + ICIs has shown favorable results for uHCC patients (17, 19). However, given that the biological heterogeneity of uHCC and the tumor microenvironment might impair treatment effectiveness, not all patients can benefit from this treatment and the high medical cost is also a worrisome issue. Therefore, it is warranted to identify the patients who are most likely to benefit from this triple therapy.
Accumulating evidence indicates that the inflammatory tumor microenvironment contributes to tumor occurrence and progression and may affect the prognosis of patients with malignancies (33, 34). PLR, as a biomarker that correlates systemic inflammation and immune function, has been shown to be a prognostic factor in various tumors (Chen et al., 2020; 35; 36). In the present study, we evaluated the prognostic value of pretreatment PLR for uHCC patients treated with TACE + TKIs + ICIs.
Our results suggested that patients with low pretreatment PLR had better prognosis than those with high pretreatment PLR. The mOS increased from 16.1 to 25.7 months (P < 0.001), and the corresponding median PFS increased from 10.2 to 14.9 months (P < 0.001). This indicated that pretreatment PLR grading could predict the survival outcomes of uHCC patients treated with TACE + TKIs + ICIs.
Univariate and multivariate Cox proportional hazards regression analysis showed that ALT, tumor size, and PLR were independent risk factors for OS and that tumor size, AFP (≥400 ug/L) and PLR were predictors for PFS. The results suggested that patients with larger tumors had a higher risk of all-cause mortality and tumor progression than those with smaller ones. It may be accounted for that the larger HCC generally has significant necrosis and inflammation pathophysiologically, which contribute to carcinogenesis and tumor progression (33, 37). What’s more, the larger HCCs have poorer response to TACE than smaller ones (38).
Immunotherapy related hepatotoxicity often presents as an increase in ALT or AST (39). Our results suggested that elevated ALT levels before TACE + TKIs + ICIs could predict the OS of uHCC patients, which was consistent with previous research results (40). Therefore, it is challenging for clinicians to manage the patients’ liver function. AFP is one of the biomarkers of HCC, and we found that elevated AFP levels were correlated with tumor progression. It indicated that AFP could be used as a potential biomarker to predict the tumor progression in uHCC patients treated with TACE + TKIs + ICIs. In addition, our results showed that patients with low pretreatment PLR had lower risks for tumor progression and all-cause mortality than those with high PLR, indicating that PLR is a promising biomarker to predict the survival outcomes of uHCC patients treated with TACE + TKIs + ICIs.
In reference to AEs, this study suggested that TACE + TKIs + ICIs was well-tolerated and its side effects were manageable. The incidences of some irAEs including skin capillary hyperplasia and hypothyroidism were significantly higher in the low PLR group than those in the high PLR group (P = 0.033, P = 0.002, respectively). It might be accounted for the stronger antitumor immune response in the low PLR group. And this result indicated that a low pretreatment PLR might be a predictor of the occurrence of irAEs. As the triple therapy may elicit strong immune responses, these irAEs should be carefully supervised in clinical practice.
While our study showed that the combination therapy of TACE + TKIs + ICIs was a promising approach to treat uHCC, it was also significant to delve into the potential role of second-line immunotherapy in uHCC if patients’ responses to the combination therapy were inadequate or the disease progressed. Some studies (41, 42) had investigated the efficacy of second-line immunotherapy, such as pembrolizumab and nivolumab, in advanced HCC and showed favorable results. However, given the variability in treatment response observed in immunotherapy, it is crucial to understand the responses to second-line treatments to optimize treatment selection and sequencing (43). In addition, it is also warranted to identify predictive biomarkers to aid in stratifying patients who are most likely to benefit from second-line immunotherapy.
Our study had some limitations. First, it was a retrospective and single-center study, which might cause selection bias. Second, the number of patients enrolled in this study was limited. Third, the cut-off value of PLR in our study was determined by the ROC curve analysis, which might not be representative. Therefore, further randomized case-controlled trials with a larger sample size are demanded to validate our findings.
Conclusion
Our study suggested that the low pretreatment PLR (PLR ≤ 98.89) was an independent protective factor for the prognosis with uHCC patients treated with TACE + TKIs + ICIs. What’s more, the lower pretreatment PLR might also be an indicator of the occurrence of irAEs. Considering that PLR is an easily accessible indicator in clinical practice, it was helpful for clinicians to predict the prognosis and identify the patients with uHCC who were most likely to benefit from TACE + TKIs + ICIs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Institutional Review Board of Wuhan Union Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study was a retrospective study.
Author contributions
YG: Investigation, Writing – original draft. WW: Investigation, Writing – review & editing. BS: Investigation, Writing – review & editing. TG: Funding acquisition, Writing – review & editing. KS: Visualization, Writing – review & editing. CZ: Conceptualization, Supervision, Writing – review & editing. XL: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No.82001788).
Acknowledgments
We would like to thank all medical workers in our department for their assistance with this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet (2022) 400(10360):1345–62. doi: 10.1016/S0140-6736(22)01200-4
2. Karaman B, Battal B, Sari S, Verim S. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol (2014) 20(47):18059–60. doi: 10.3748/wjg.v20.i47.18059
3. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology (2018) 68(2):723–50. doi: 10.1002/hep.29913
4. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
5. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int (2015) 35(9):2155–66. doi: 10.1111/liv.12818
6. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
7. Qin J, Huang Y, Zhou H, Yi S. Efficacy of sorafenib combined with immunotherapy following transarterial chemoembolization for advanced hepatocellular carcinoma: A propensity score analysis. Front Oncol (2022) 12:807102. doi: 10.3389/fonc.2022.807102
8. Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol (2008) 103(4):914–21. doi: 10.1111/j.1572-0241.2007.01712.x
9. Pinato DJ, Murray SM, Forner A, Kaneko T, Fessas P, Toniutto P, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer (2021) 9(9):e003311. doi: 10.1136/jitc-2021-003311
10. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discovery (2018) 8(9):1069–86. doi: 10.1158/2159-8290.CD-18-0367
11. Xin Yu J, Hodge JP, Oliva C, Neftelinov ST, Hubbard-Lucey VM, Tang J. Trends in clinical development for PD-1/PD-L1 inhibitors. Nat Rev Drug Discovery (2020) 19(3):163–4. doi: 10.1038/d41573-019-00182-w
12. Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol (2016) 17(10):611–25. doi: 10.1038/nrm.2016.87
13. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell (2019) 176(6):1248–64. doi: 10.1016/j.cell.2019.01.021
14. Qin S, Li A, Yi M, Yu S, Zhang M, Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol (2019) 12(1):27. doi: 10.1186/s13045-019-0718-5
15. Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology (2011) 53(3):1020–2. doi: 10.1002/hep.24199
16. Chen LT, Martinelli E, Cheng AL, Pentheroudakis G, Qin S, Bhattacharyya GS, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol (2020) 31(3):334–51. doi: 10.1016/j.annonc.2019.12.001
17. Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A multicenter retrospective study. J Hepatocell Carcinoma (2021) 8:1233–40. doi: 10.2147/JHC.S332420
18. Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: A retrospective cohort study. Front Immunol (2022) 13:848387. doi: 10.3389/fimmu.2022.848387
19. Sun B, Zhang L, Sun T, Ren Y, Cao Y, Zhang W, et al. Safety and efficacy of lenvatinib combined with camrelizumab plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A two-center retrospective study. Front Oncol (2022) 12:982948. doi: 10.3389/fonc.2022.982948
20. Sitia G, Aiolfi R, Di Lucia P, Mainetti M, Fiocchi A, Mingozzi F, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U.S.A. (2012) 109(32):E2165–72. doi: 10.1073/pnas.1209182109
21. Endig J, Buitrago-Molina LE, Marhenke S, Reisinger F, Saborowski A, Schutt J, et al. Dual role of the adaptive immune system in liver injury and hepatocellular carcinoma development. Cancer Cell (2016) 30(2):308–23. doi: 10.1016/j.ccell.2016.06.009
22. Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol (2013) 58(1):58–64. doi: 10.1016/j.jhep.2012.08.017
23. Kao WY, Su CW, Chiou YY, Chiu NC, Liu CA, Fang KC, et al. Hepatocellular carcinoma: nomograms based on the albumin-bilirubin grade to assess the outcomes of radiofrequency ablation. Radiology (2017) 285(2):670–80. doi: 10.1148/radiol.2017162382
24. Feng X, Li L, Wu J, Zhang L, Sun Z, Li X, et al. Complete blood count score model integrating reduced lymphocyte-monocyte ratio, elevated neutrophil-lymphocyte ratio, and elevated platelet-lymphocyte ratio predicts inferior clinical outcomes in adult T-lymphoblastic lymphoma. Oncologist (2019) 24(11):e1123–31. doi: 10.1634/theoncologist.2018-0789
25. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost (2011) 9(2):237–49. doi: 10.1111/j.1538-7836.2010.04131.x
26. Stotz M, Pichler M, Absenger G, Szkandera J, Arminger F, Schaberl-Moser R, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer (2014) 110(2):435–40. doi: 10.1038/bjc.2013.785
27. Chen L, Ke Z, Xiong F, Kan X, Ren Y, Cao Y, et al. Platelet-to-lymphocyte ratio predicts therapy outcomes of transarterial chemoembolization plus apatinib in the treatment of advanced hepatocellular carcinoma. Anticancer Drugs (2020) 31(9):966–72. doi: 10.1097/CAD.0000000000000913
28. Schobert IT, Savic LJ, Chapiro J, Bousabarah K, Chen E, Laage-Gaupp, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of tumor response in hepatocellular carcinoma after DEB-TACE. Eur Radiol (2020) 30(10):5663–73. doi: 10.1007/s00330-020-06931-5
29. Zhang L, Yan ZP, Hou ZH, Huang P, Yang MJ, Zhang S, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of outcomes in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization plus sorafenib. Front Mol Biosci (2021) 8:624366. doi: 10.3389/fmolb.2021.624366
30. Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, et al. Validity and reliability of the US national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol (2015) 1(8):1051–9. doi: 10.1001/jamaoncol.2015.2639
31. Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol (2020) 72(2):288–306. doi: 10.1016/j.jhep.2019.09.026
32. Oura K, Morishita A, Tani J, Masaki T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: A review. Int J Mol Sci (2021) 22(11):5801. doi: 10.3390/ijms22115801
33. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer (2013) 13(11):759–71. doi: 10.1038/nrc3611
34. Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest (2015) 125(9):3347–55. doi: 10.1172/JCI80007
35. Sidaway P. Prostate cancer: Platelet-to-lymphocyte ratio predicts prostate cancer prognosis. Nat Rev Urol (2015) 12(5):238. doi: 10.1038/nrurol.2015.69
36. Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol (2012) 23(4):265–73. doi: 10.3802/jgo.2012.23.4.265
37. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
38. Kim DJ, Clark PJ, Heimbach J, Rosen C, Sanchez W, Watt K, et al. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transplant (2014) 14(6):1383–90. doi: 10.1111/ajt.12684
39. De Martin E, Michot JM, Rosmorduc O, Guettier C, Samuel D. Liver toxicity as a limiting factor to the increasing use of immune checkpoint inhibitors. JHEP Rep (2020) 2(6):100170. doi: 10.1016/j.jhepr.2020.100170
40. Li X, Sun W, Ding X, Li W, Chen J. Prognostic model of immune checkpoint inhibitors combined with anti-angiogenic agents in unresectable hepatocellular carcinoma. Front Immunol (2022) 13:1060051. doi: 10.3389/fimmu.2022.1060051
41. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2
42. Kudo M, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer DH, et al. Updated efficacy and safety of KEYNOTE-224: a phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur J Cancer (2022) 167:1–12. doi: 10.1016/j.ejca.2022.02.009
Keywords: platelet-to-lymphocyte ratio, hepatocellular carcinoma, transarterial chemoembolization, tailored tyrosine kinase inhibitors, immune checkpoint inhibitors
Citation: Guo Y, Wu W, Sun B, Guo T, Si K, Zheng C and Li X (2024) Prognostic value of platelet-to-lymphocyte ratio in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization and tyrosine kinase inhibitors plus immune checkpoints inhibitors. Front. Oncol. 14:1293680. doi: 10.3389/fonc.2024.1293680
Received: 14 September 2023; Accepted: 04 January 2024;
Published: 23 January 2024.
Edited by:
Gianluca Rompianesi, University of Naples Federico II, ItalyReviewed by:
Valerio Rosato, Ospedale Evangelico Betania, ItalyAntonio Giovanni Solimando, University of Bari Aldo Moro, Italy
Copyright © 2024 Guo, Wu, Sun, Guo, Si, Zheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuansheng Zheng, hqzcsxh@sina.com; Xin Li, lxwsry2014@163.com
†These authors have contributed equally to this work and share first authorship
‡ORCID: Chuansheng Zheng, orcid.org/0000-0002-2435-1417
Xin Li, orcid.org/0000-0002-7381-1352
 Yiwan Guo
Yiwan Guo