- 1Department of Nuclear Medicine and PET, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Pathology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Perivascular epithelioid cell tumor (PEComa), an uncommon mesenchymal neoplasm, arises from specialized perivascular epithelioid cells exhibiting distinct features of smooth muscle and melanocytic differentiation with unpredictable behavior. PEComa tends to occur more commonly in the uterus and kidneys; its occurrence in the liver is exceedingly rare. We presented a case of a 29-year-old woman with hepatic PEComa and evaluated the tumor with MRI, integrated 18F-fluorodeoxyglucose (FDG), and 68Ga-fibroblast activation protein inhibitor (FAPI) PET/CT scans at presentation. The patient had a history of intermittent utilization of oral contraceptive drugs for several years. An abdominal ultrasound in a physical examination from an outside institution revealed a mass in the liver. A contrast-enhanced abdominal MRI revealed restricted diffusion on diffusion-weighted imaging (DWI) and rapid contrast enhancement and washout patterns in the hepatic lesion, suggesting hepatic adenoma (HA) or hepatocellular carcinoma (HCC). Further assessment was carried out using 18F-FDG and 68Ga-FAPI PET/CT scans. The hepatic lesion was non-FDG avid, whereas increased tracer uptake was observed on the 68Ga-FAPI PET/CT. Subsequently, laparoscopic partial resection of liver segment V was performed. Immunohistochemical analyses demonstrated positive staining for HMB45, Melan-A, and SMA while showing negative results for AFP, glypican-3, hepatocyte, and arginase-1. The results were indicative of a hepatic PEComa diagnosis based on these findings. We also review the current literature on the clinical characteristics, pathological features, and challenges in the diagnosis of hepatic PEComa.
1 Introduction
Perivascular epithelioid cell tumor (PEComa) is an infrequent but distinct type of mesenchymal neoplasm. The PEComa family mainly includes the renal and extrarenal types of angiomyolipoma (AML), the pulmonary and extrapulmonary types of clear-cell “sugar” tumors (CCST) or lymphangioleiomyomatosis (LAMs), and the PEComa not otherwise specified (PEComa NOS) (1). In 1992, Bonnetti initiated the concept of perivascular epithelioid cells (PEC) (2). Zamboni subsequently coined the term PEComa in 1996 to designate the group of tumors distinguished by these specific cell types and further elucidated their pathological features (3). Typically, spindle cells may be found adjacent to epithelioid cells, and the PEComa may accumulate a great amount of lipids (4). Previous research suggested a potential association between PEComa and dysfunction of TSC complex 1 and complex 2, particularly TSC2, which negatively regulated mTORC1 and contributed to tumor development (5, 6). Immunohistochemical analysis reveals positive staining for melanocytic-related biomarkers HMB45 and Melan-A and smooth muscle marker SMA (7, 8). PEComas are tumors with unpredictable behavior. Primary hepatic PEComa is extremely rare. Data regarding hepatic PEComa evaluation with PET/CT are limited. This case highlights the diagnostic challenges regarding identifying and thoroughly evaluating hepatic PEComa preoperatively. As indicated by our case, 18F-FDG and 68Ga-FAPI dual-tracer PET/CT imaging may play a significant role in the detection, differentiation, and whole-body evaluation of hepatic PEComa.
2 Case presentation
During a routine medical examination, an abdominal ultrasound was performed on a 29-year-old female patient with no tumor history. Incidentally, a focal solid hypoechoic lesion measuring 3.5 cm in size was discovered in the right lobe of the liver. A review of the medication use history revealed that she had been taking oral contraceptives for several years. Her laboratory data indicated normal values of coagulation and liver function. The tumor marker tests, including α-fetoprotein, carcinoembryonic antigen, Des-γ-carboxyprothrombin, and carbohydrate antigen 19-9, showed values within the normal range. MRI demonstrated a 3.5-cm-sized circular lesion displaying slightly prolonged T1 and T2 signals in the liver with high signal intensity on diffusion-weighted imaging (DWI). Moreover, arterial enhancement was observed with rapid clearance during the delayed phase (Figure 1). With these imaging characteristics and the medication use history of oral contraceptives, the lesion was highly suspicious for liver tumors of hepatic adenoma (HA) or hepatocellular carcinoma (HCC).
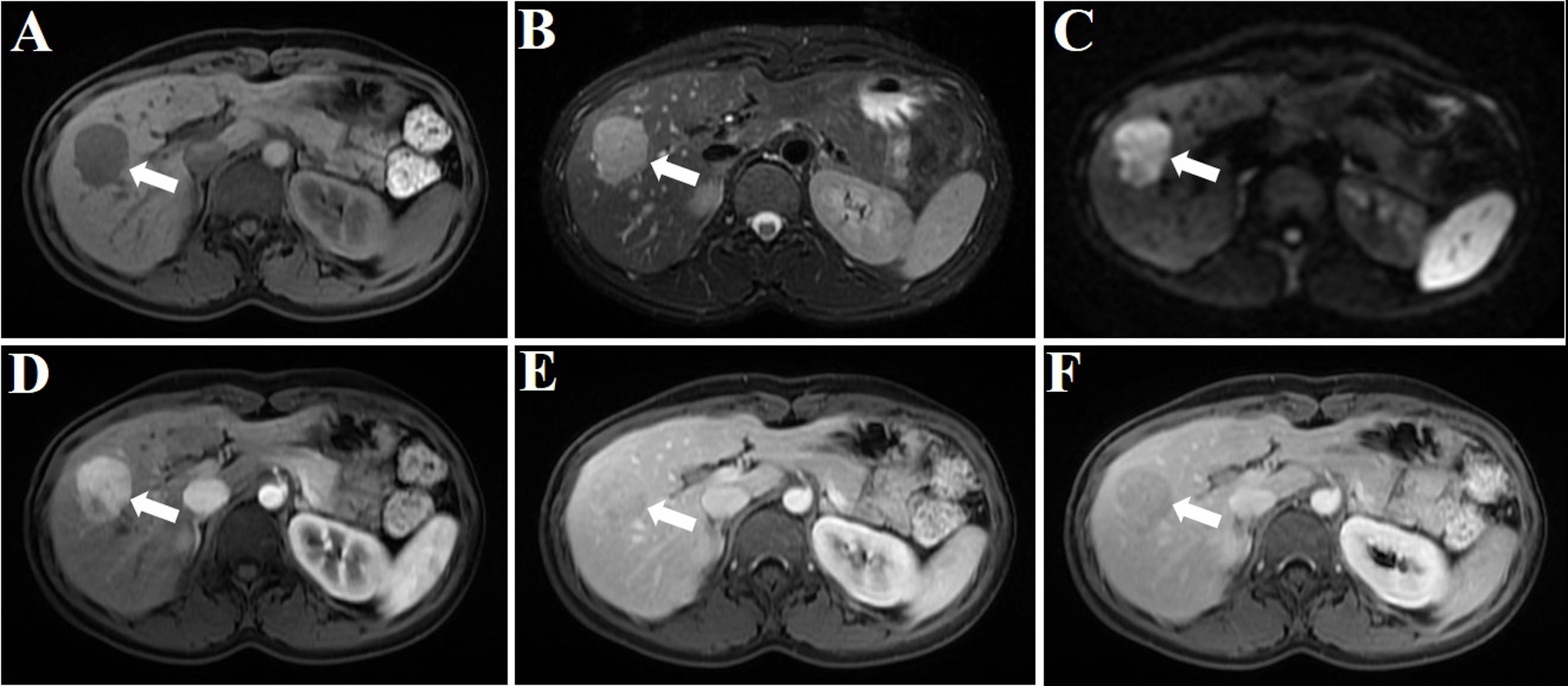
Figure 1 MRI plain scan, DWI, and enhanced image of the hepatic PEComa are presented. The hepatic PEComa exhibited a slightly prolonged T1 signal (A) and a slightly prolonged T2 signal (B). The DWI showed restricted diffusion (C). The enhancement pattern was characterized by fast-in and fast-out. During the arterial phase, a significant enhancement was observed, with a considerably higher degree of enhancement compared to the adjacent normal liver parenchyma (D). Subsequently, in the portal vein phase (E) and delayed phase (F), the enhancement gradually subsided and ultimately became less pronounced than the surrounding liver parenchyma (white arrows indicate the tumor).
An 18F-FDG PET/CT scan was performed for further whole-body evaluation. There was no focal activity typical of hypermetabolic malignancy on the MIP image (Figure 2A). The transaxial CT and FDG PET/CT images revealed a well-defined, round, slightly hypodense lesion in the lower portion of the anterior right liver lobe. The lesion was non-FDG avid with an SUVmax of 1.31 (in comparison to the normal hepatic SUVmax of 1.58), and the maximum tumor-to-background ratio (TBRmax) was 0.83 (Figures 2B, C). Next, the patient was enrolled in our hospital’s institutional review board-approved clinical trial utilizing 68Ga-fibroblast activation protein inhibitor (FAPI) PET/CT to rule out the non-FDG avidity of low-grade liver tumors. The patient provided written consent for participation. The 68Ga-FAPI PET/CT scan was conducted 6 days following the 18F-FDG PET/CT scan. Interestingly, 68Ga-FAPI PET/CT MIP, transaxial CT, and transaxial fused images (Figures 2D–F) demonstrated intense FAPI uptake (SUVmax, 5.28; TBRmax, 10.56) in the hepatic lesion. No other masses were observed, and there was no abnormally increased uptake of FDG and FAPI in the remaining organs.
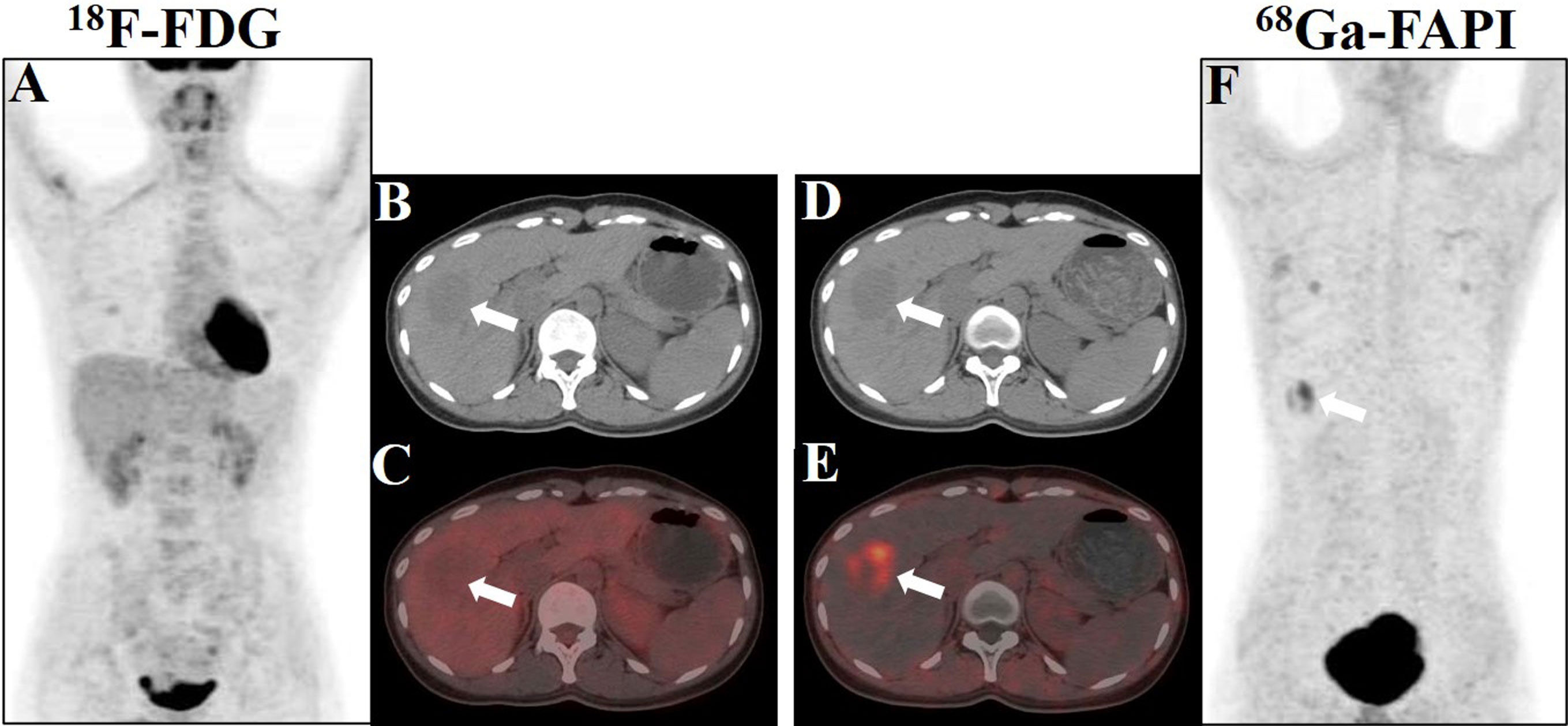
Figure 2 Showcase hepatic PEComa images obtained from 18F-FDG and 68Ga-FAPI PET/CT scans. (A) The maximum intensity projection (MIP) image generated from the FDG scan revealed a lack of tumor uptake in the liver. (B, C) Axial images acquired from CT and fused FDG PET/CT scans exhibited a mildly hypodense lesion in liver segment V, accompanied by minimal tumor radioactivity uptake, characterized by SUVmax = 1.31 and TBRmax = 0.83. (D, E) Axial images derived from CT and fused FAPI PET/CT scans demonstrated robust tumor radioactivity uptake at the same location, with SUVmax = 5.28 and TBRmax = 10.56. (F) The MIP image generated from the FAPI scan displayed avid tumor uptake in the liver (white arrows indicate the tumor).
Subsequently, the patient underwent laparoscopic partial hepatectomy of segment V, and hematoxylin and eosin (HE) staining indicated predominantly epithelioid tumor cells without significant atypical features, tumor necrosis, or pathological mitosis (Figure 3A). Immunohistochemistry analyses yielded positive results for HMB45, Melan-A, SMA, and FAP (Figures 3B–E), but negative for glypican-3, hepatocyte, arginase-1, AFP, CK19, CK7, desmin, SOX-10, S-100, and PCK (Figures 3F–O). The hepatic PEComa also presented a low Ki-67 labeling index (LI) of 3% (Figure 3P). Thus, the final diagnosis was primary hepatic PEComa with no indications of malignancy. At 12 months of follow-up, the patient remained asymptomatic and showed no signs of disease recurrence on MRI monitoring.
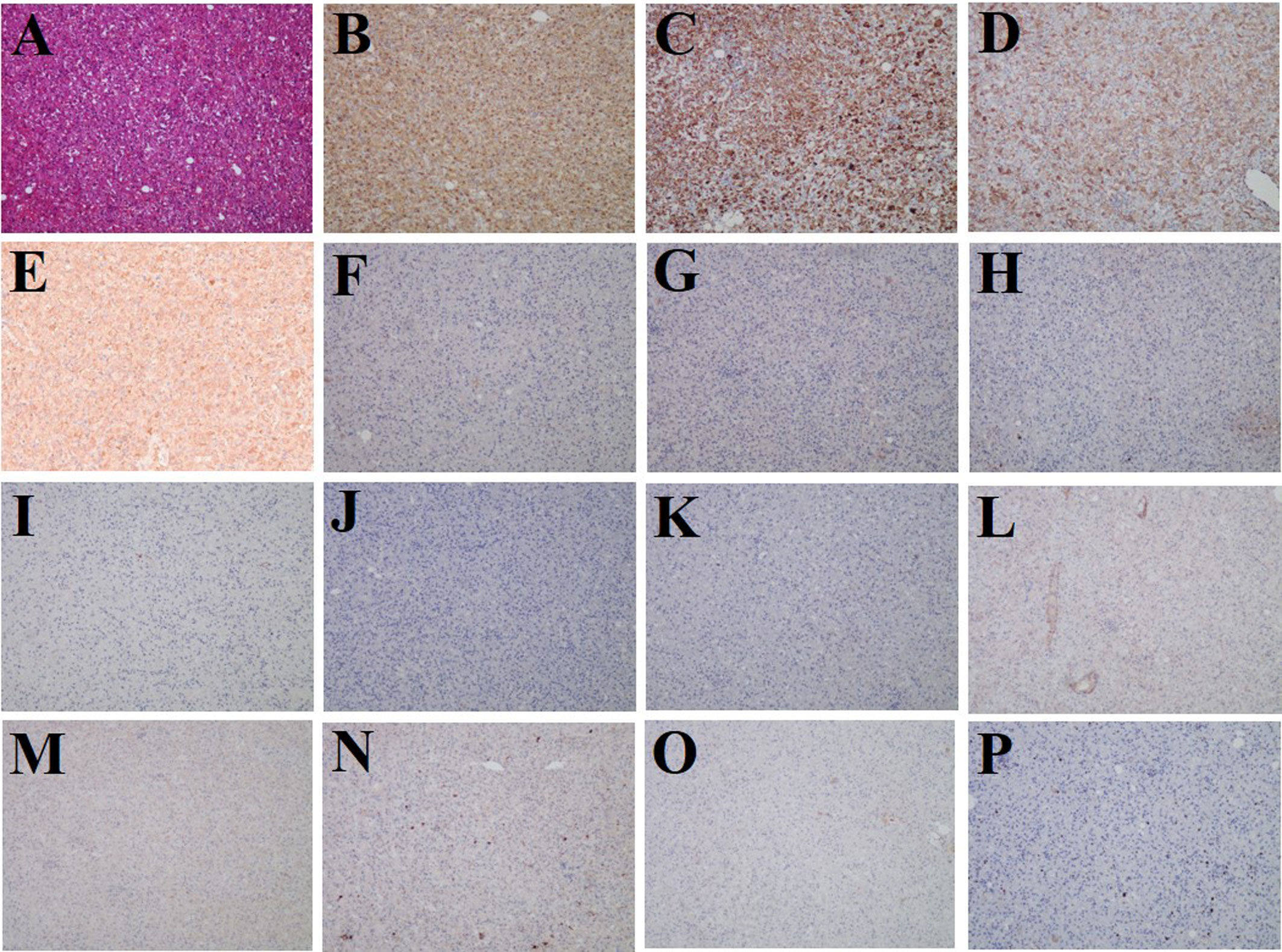
Figure 3 Histological and immunostaining analysis of hepatic PEComa. (A) Histological examination using hematoxylin and eosin (HE) staining revealed hepatic PEComa. Immunohistochemical markers HMB45 (B) and Melan-A (C) for melanocytic, SMA (D) for smooth muscle, and FAP (E) exhibited positive expression. Conversely, immunohistochemical markers glypican-3 (F), hepatocyte (G), arginase-1 (H), and AFP (I) for HCC, CK19 (J) and CK7 (K) for intrahepatic cholangiocarcinoma, desmin (L) for muscle tissue, SOX-10 (M) and S-100 (N) for nerve and melanoma, and PCK (O) for broad-spectrum epithelial tumors demonstrated negative expression. (P) the Ki-67 LI was merely 3%, indicating a low proliferation rate and benign biological characteristics.
3 Discussion
PEComa, an uncommon mesenchymal tumor deriving from perivascular epithelioid cells, demonstrates a significant gender-related disparity in the incidence rates, with women being affected nearly four times more frequently than men (9, 10). Nevertheless, it is crucial to recognize that these data might be susceptible to bias due to the limited sample size, potential geographical and population variations, and the rarity of the disease itself. Based on the distinct distribution of PEC across tissues, PEComa primarily encompasses AML, CCST, LAMs, and PEComa NOS in other tissue sites. Likewise, the diverse distribution of PEC in tissues gives rise to some variations in clinical manifestations, pathological alterations, and differential diagnosis for each type. Intra-abdominal PEComa may present with palpable masses that cause pain due to compression of adjacent tissues or nerves (11). Cutaneous PEComa may cause bleeding symptoms (12), while lung involvement can lead to coughing and respiratory difficulties (13). PEComa occurring in the liver is considered to be very rare. According to reference, more than 200 cases of hepatic PEComa have been reported, but most of them were the type of hepatic AML (14). While AML predominantly affects the kidneys, it can also manifest in extrarenal organs, including the liver. Hepatic AML primarily consists of fat, blood vessels, and PEC, and if PEC largely predominates, it can be defined as a type of PEComa called hepatic epithelioid AML (HEAML). A recent study conducted in 2023 identified an additional 113 cases of HEAML as a distinct subtype of PEComa (15). With the increasing clinical understanding of PEComa, it becomes evident that the existing cases may not precisely reflect the actual incidence of hepatic PEComa. Hepatic PEComa typically does not experience any discomfort but sometimes exhibits some nonspecific symptoms, such as dull upper right abdominal pain, abdominal bloating, nausea, vomiting, and diarrhea related to the gastrointestinal system (16, 17). As in our case, the hepatic PEComa was accidentally found at a medical checkup with no initial clinical manifestation. It is interesting to note that the patient had been using oral contraceptives for several years. Prior research established a notable association between oral contraceptives and liver tumors, particularly HA (18), which can lead to complications such as malignant transformation to HCC. Furthermore, HA primarily affects women of childbearing age who are taking oral contraception (19). However, cases of hepatic PEComa with a medication use history of oral contraceptives have been rarely reported.
The molecular mechanisms underlying PEComa remain elusive; however, the TSC1-TSC2/mTOR signaling pathway has been identified as a significant molecular pathway implicated in tumorigenesis by promoting the dysfunction of TSC1-TSC2, with particular emphasis on TSC2 mutations that activate mTOR. A comprehensive molecular analysis revealed TSC2 mutations in eight out of 13 cases of PEComa (62%). Excluding TFE3 fusion cases increased the proportion of PEComa cases with TSC2 mutations to 80% (eight out of 10 cases) (20). Another molecular mechanism driving PEComa development involves TFE3 fusion. Among studies investigating the impact of TFE3-related molecular pathways on PEComa, TFE3 fusion was detected in nine out of 38 cases (24%) of PEComa (20). SFPQ/PSF emerged as the most commonly recurring gene in PEComa cases with TFE3 rearrangement (21). Notably, no occurrence of TSC2 mutations was observed in the presence of TFE3 rearranged PEComa, highlighting a high degree of mutual exclusion between TFE3 fusion and TSC2 mutation (20, 22).
The preoperative diagnosis of PEComa by imaging is challenging. In a study by Chen et al., the radiologic findings of hepatic PEComa were analyzed in seven cases (23). Among four of the patients who underwent contrast-enhanced scans, three showed enhancement during the arterial phase, but imaging manifestations during the venous and delayed phases varied. In our case, the hepatic lesion demonstrated distinct arterial phase enhancement followed by gradual clearance in the venous and delayed phases (Figure 1). The presence of a rich arterial blood supply and relatively regular morphology of the liver mass should be distinguished from other liver masses, such as HCC, HA, and focal nodular hyperplasia (FNH) (15). While PEComa often contains fat, this particular hepatic PEComa is primarily composed of epithelioid cells, with no detectable fat component observed, which also indicates the subtype of HEAML. The imaging feature of fast-in and fast-out enhancement exhibited by this hepatic PEComa makes it challenging to differentiate it from HCC. In the case of most HA, the lesion may show faint enhancement in the arterial phase and lack gradual clearance, while some atypical HA cases may also display the imaging features of fast-in and fast-out enhancement. Especially when considering the history of using oral contraceptives, the case can be easily misdiagnosed as HA. FNH, on the other hand, often presents a scar in the center of the mass without enhancement in the arterial phase. The imaging features of hepatic PEComa are diverse and could be mimicked by other liver masses. Therefore, the preoperative diagnosis of hepatic PEComa poses a challenge.
PET/CT imaging might play a role in distinguishing between benign and malignant PEComa. Previous studies identified that positive 18F-FDG uptake was predictive of an aggressive disease. Malignant PEComa tends to display high radioactive uptake on 18F-FDG PET/CT; however, benign PEComa is commonly non-FDG avid or with low FDG uptake (24). In addition, the use of FDG PET/CT in liver cancer is challenging because of the degree of heterogeneity of the tumor. As a tumor stroma imaging agent, 68Ga-FAPI PET/CT offers enhanced imaging effectiveness when compared to 18F-FDG in various tumors, particularly those with low 18F-FDG uptake or tumors located within tissues or organs exhibiting high physiological uptake of 18F-FDG (25, 26). In a case of renal malignant PEComa reported by Zhang et al., 68Ga-FAPI demonstrated superior radioactive uptake compared to 18F-FDG in that girl (27). We presented the initial instance of benign hepatic PEComa revealed on 68Ga-FAPI PET/CT, demonstrating greater tumor-to-background contrast compared to 18F-FDG PET/CT in visualizing the tumor (Figure 2). Intense uptake of 68Ga-FAPI was found in various sarcomas, possibly owing to the abundant interstitial constituents in these neoplasms (28, 29). Based on the fact that PEComa is one type of mesenchymal tumor, we performed further immunohistochemical analysis to validate the heightened expression of FAP in this neoplasm (Figure 3E). Furthermore, in contrast to 18F-FDG PET, 68Ga-FAPI PET demonstrates remarkable image contrast accompanied by minimal background activity across the entirety of the body, which enables the detection of aggressive PEComa with multiorgan involvement. Accurate evaluation of organ involvement is crucial for predicting the prognosis and making therapeutic decisions in patients with PEComa. As indicated by our case, 18F-FDG and 68Ga-FAPI dual-tracer PET/CT imaging may play a significant role in the detection, differentiation, and whole-body evaluation of hepatic PEComa. Additionally, the high expression of FAP in PEComa lesions suggests that the use of FAP-targeted radiotherapy for aggressive PEComa is promising; this has already been shown in other types of tumors (30).
The definitive diagnosis of hepatic PEComa primarily relies on pathological examination. A fine needle aspiration biopsy is commonly employed for diagnosis; however, limited tissue availability often poses challenges in achieving accurate results or may lead to false negatives (31). In our case, liver segment resection was performed, and the excised specimen was meticulously examined under a microscope. Recent guidelines from the World Health Organization (WHO) highlight that PEComa is a unique type of mesenchymal tumor characterized by specific smooth muscle and melanocytic markers, notably SMA, Melan-A, and HMB-45 (32). Therefore, in addition to the cellular morphological features, immunohistochemistry plays a crucial role in the differential diagnosis of hepatic PEComa. In this liver mass, intense staining of HMB45, Melan-A, and SMA was observed, which was consistent with the diagnosis of hepatic PEComa (Figures 3B–D). Immunohistochemistry was performed to differentiate liver tumors, and glypican-3, hepatocyte, arginase-1, and AFP were used for HCC (Figures 3F–I), CK19 and CK7 for intrahepatic cholangiocarcinoma (Figures 3J, K), desmin for muscle tissue (Figure 3L), SOX-10 and S-100 for nerve and melanoma (Figures 3M, N), and PCK for broad-spectrum epithelial tumors (Figure 3O). All these markers showed negative staining. Additionally, the low proliferation rate of Ki-67 LI (3%) indicated benign biological characteristics (Figure 3P).
Currently, there is no consensus or universally accepted criteria for the differential diagnosis between benign and malignant PEComa. According to Folpe et al., malignant PEComas typically exhibit certain features, including a tumor size larger than 5 cm, elevated nuclear grade, increased cellular density, a mitotic rate surpassing 1/50 high-power fields, necrosis, infiltration into the adjacent normal parenchyma, and invasion of blood vessels (33). The presence of two or more of these characteristics usually indicates a malignant PEComa. However, none of these criteria were met in this specific case. Surgery remains the primary treatment option for PEComa. However, for patients with advanced metastasis, there is currently no established, effective medical approach. Some studies suggest that oral mTOR inhibitors may have varying degrees of effectiveness in treating advanced-stage PEComa patients, while a few cases show no significant treatment response (34–36). A retrospective study indicated that mTOR inhibitor therapy exhibits a higher objective response rate and longer disease progression-free survival in comparison to chemotherapy and VEGFR inhibitor therapy (37). Nonetheless, further research, particularly prospective cohort studies, is still required to provide effective treatment options for patients with advanced PEComa.
4 Conclusion
The authors present a patient with hepatic PEComa evaluated by MRI, integrated 18F-FDG, and 68Ga-FAPI PET/CT. Although the preoperative diagnosis of PEComa is challenging, the possibility of PEComa should be kept as one of the differentials in interpreting imaging studies in patients with hepatic mass. In this case report, we present the first 68Ga-FAPI PET/CT observations of benign hepatic PEComa, unveiling a higher tumor-to-liver ratio in contrast to 18F-FDG PET/CT. 18F-FDG and 68Ga-FAPI dual-tracer PET/CT imaging can have a substantial impact on the detection, differentiation, and whole-body evaluation of hepatic PEComa. The underlying mechanism of radiotracer uptake of 68Ga-FAPI and its prospective implications for the diagnosis and therapeutic interventions for hepatic PEComa necessitate further exploration.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DZ: Data curation, Funding acquisition, Writing – original draft, Project administration. SS: Resources, Writing – review & editing. DW: Resources, Writing – review & editing. DK: Data curation, Writing – review & editing. SC: Data curation, Writing – review & editing. JZ: Data curation, Writing – review & editing. SZ: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Natural Science Foundation of Hubei Province in China, No. 2022CFB206; the National Natural Science Foundation of China (No. 82202215, 82302246, 82001871).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maebayashi T, Abe K, Aizawa T, Sakaguchi M, Ishibashi N, Abe O, et al. Improving recognition of hepatic perivascular epithelioid cell tumor: case report and literature review. World J Gastroenterol (2015) 21(17):5432–41. doi: 10.3748/wjg.v21.i17.5432
2. Bonetti F, Pea M, Martignoni G, Zamboni G. Pec and sugar. Am J Surg Pathol (1992) 16(3):307–08. doi: 10.1097/00000478-199203000-00013
3. Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E. et al. Clear cell “sugar” tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol (1996) 20(6):722–30. doi: 10.1097/00000478-199606000-00010
4. O’Malley ME, Chawla TP, Lavelle LP, Cleary S, Fischer S. Primary perivascular epithelioid cell tumors of the liver: ct/mri findings and clinical outcomes. Abdom Radiol (Ny) (2017) 42(6):1705–12. doi: 10.1007/s00261-017-1074-y
5. Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, et al. Clinical activity of mtor inhibition with sirolimus in Malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mtorc1 in tumors. J Clin Oncol (2010) 28(5):835–40. doi: 10.1200/JCO.2009.25.2981
6. Baba T, Kawano T, Saito Y, Onishi S, Yamada K, Yamada W, et al. Malignant perivascular epithelioid cell neoplasm in the liver: report of a pediatric case. Surg Case Rep (2021) 7(1):212. doi: 10.1186/s40792-021-01300-w
7. Tanaka Y, Ijiri R, Kato K, Kato Y, Misugi K, Nakatani Y, et al. Hmb-45/melan-a and smooth muscle actin-positive clear-cell epithelioid tumor arising in the ligamentum teres hepatis: additional example of clear cell ‘sugar’ tumors. Am J Surg Pathol (2000) 24(9):1295–99. doi: 10.1097/00000478-200009000-00015
8. Anderson WJ, Kojc N, Fletcher C, Hornick JL. Micronodular pecomas of the appendix. Histopathology (2021) 78(7):1047–50. doi: 10.1111/his.14341
9. Demyashkin GA, Zaborskii IN. [New approach to diagnosis, immunophenotypic verification and prognostic prediction for renal angiomyolipoma]. Urologiia (2018) 1):35–41. doi: 10.18565/urology.2018.1.35-41
10. Cai X, Sun S, Deng Y, Liu J, Pan S. Hepatic epithelioid angiomyolipoma is scattered and unsuitable for surgery: a case report. J Int Med Res (2023) 51(2):655751711. doi: 10.1177/03000605231154657
11. Mete O, van der Kwast TH. Epithelioid angiomyolipoma: a morphologically distinct variant that mimics a variety of intra-abdominal neoplasms. Arch Pathol Lab Med (2011) 135(5):665–70. doi: 10.5858/2009-0637-RSR.1
12. Cornell G, Jiang B, Ghaferi J. A rare case of primary cutaneous Malignant perivascular epithelioid cell tumor and review of the literature. J Cutan Pathol (2023) 50(4):301–05. doi: 10.1111/cup.14330
13. Cong CV, Anh TT, Ly TT, Duc NM. Pulmonary lymphangioleiomyomatosis (lam): a literature overview and case report. Radiol Case Rep (2022) 17(5):1646–55. doi: 10.1016/j.radcr.2022.02.075
14. Yang X, Wang Q, Zhou X, Zhou H, Jia W, Hu C, et al. Retrospective analysis of hepatic perivascular epithelioid cell tumour (pecoma) in a single centre for clinical diagnosis and treatment clinical diagnosis and treatment of hepatic pecoma. Med (Baltimore) (2022) 101(25):e29506. doi: 10.1097/MD.0000000000029506
15. Junhao L, Hongxia Z, Jiajun G, Ahmad I, Shanshan G, Jianke L, et al. Hepatic epithelioid angiomyolipoma: magnetic resonance imaging characteristics. Abdom Radiol (Ny) (2023) 48(3):913–24. doi: 10.1007/s00261-023-03818-z
16. Abhirup B, Kaushal K, Sanket M, Ganesh N. Malignant hepatic perivascular epithelioid cell tumor (pecoma) - case report and a brief review. J Egypt Natl Canc Inst (2015) 27(4):239–42. doi: 10.1016/j.jnci.2015.05.004
17. De la Sancha C, Khan S, Alruwaii F, Cramer H, Saxena R. Hepatic angiomyolipoma with predominant epithelioid component: diagnostic clues on aspiration and core needle biopsies. Diagn Cytopathol (2021) 49(7):E238–41. doi: 10.1002/dc.24688
18. Lammert C, Toal E, Mathur K, Khungar V, House M, Roberts LR, et al. Large hepatic adenomas and hepatic adenomatosis: a multicenter study of risk factors, interventions, and complications. Am J Gastroenterol (2022) 117(7):1089–96. doi: 10.14309/ajg.0000000000001743
19. Beaufrere A, Paradis V. Hepatocellular adenomas: review of pathological and molecular features. Hum Pathol (2021) 112:128–37. doi: 10.1016/j.humpath.2020.11.016
20. Agaram NP, Sung YS, Zhang L, Chen CL, Chen HW, Singer S, et al. Dichotomy of genetic abnormalities in pecomas with therapeutic implications. Am J Surg Pathol (2015) 39(6):813–25. doi: 10.1097/PAS.0000000000000389
21. Rao Q, Shen Q, Xia QY, Wang ZY, Liu B, Shi SS, et al. Psf/sfpq is a very common gene fusion partner in tfe3 rearrangement-associated perivascular epithelioid cell tumors (pecomas) and melanotic xp11 translocation renal cancers: clinicopathologic, immunohistochemical, and molecular characteristics suggesting classification as a distinct entity. Am J Surg Pathol (2015) 39(9):1181–96. doi: 10.1097/PAS.0000000000000502
22. Akumalla S, Madison R, Lin DI, Schrock AB, Yakirevich E, Rosenzweig M, et al. Characterization of clinical cases of Malignant pecoma via comprehensive genomic profiling of dna and rna. Oncology (2020) 98(12):905–12. doi: 10.1159/000510241
23. Chen W, Liu Y, Zhuang Y, Peng J, Huang F, Zhang S. Hepatic perivascular epithelioid cell neoplasm: a clinical and pathological experience in diagnosis and treatment. Mol Clin Oncol (2017) 6(4):487–93. doi: 10.3892/mco.2017.1168
24. Sun L, Sun X, Li Y, Xing L. The role of (18)f-fdg pet/ct imaging in patient with Malignant pecoma treated with mtor inhibitor. Onco Targets Ther (2015) 8:1967–70. doi: 10.2147/OTT.S85444
25. Zhao L, Chen J, Pang Y, Fu K, Shang Q, Wu H, et al. Fibroblast activation protein-based theranostics in cancer research: a state-of-the-art review. Theranostics (2022) 12(4):1557–69. doi: 10.7150/thno.69475
26. Zhang J, He Q, Jiang S, Li M, Xue H, Zhang D, et al. [(18)f]fapi pet/ct in the evaluation of focal liver lesions with [(18)f]fdg non-avidity. Eur J Nucl Med Mol Imaging (2023) 50(3):937–50. doi: 10.1007/s00259-022-06022-1
27. Zhang Z, Yu Y, Zhang L, Cheng C, Zuo C. 18 f-fdg and 68 ga-fapi-04 in the evaluation of aggressive perivascular epithelioid cell tumor. Clin Nucl Med (2022) 47(10):897–99. doi: 10.1097/RLU.0000000000004249
28. Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. (68)ga-fapi pet/ct: tracer uptake in 28 different kinds of cancer. J Nucl Med (2019) 60(6):801–05. doi: 10.2967/jnumed.119.227967
29. Zhou X, Wang S, Zhu H, Yang Z, Li N. Imaging superiority of [(68)ga]-fapi-04 over [(18)f]-fdg pet/ct in alveolar soft part sarcoma (asps). Eur J Nucl Med Mol Imaging (2021) 48(11):3741–42. doi: 10.1007/s00259-021-05388-y
30. Baum RP, Schuchardt C, Singh A, Chantadisai M, Robiller FC, Zhang J, et al. Feasibility, biodistribution, and preliminary dosimetry in peptide-targeted radionuclide therapy of diverse adenocarcinomas using (177)lu-fap-2286: first-in-humans results. J Nucl Med (2022) 63(3):415–23. doi: 10.2967/jnumed.120.259192
31. Xie L, Jessurun J, Manivel JC, Pambuccian SE. Hepatic epithelioid angiomyolipoma with trabecular growth pattern: a mimic of hepatocellular carcinoma on fine needle aspiration cytology. Diagn Cytopathol (2012) 40(7):639–50. doi: 10.1002/dc.21703
32. Coindre JM. [New who classification of tumours of soft tissue and bone]. Ann Pathol (2012) 32(5 Suppl):S115–16. doi: 10.1016/j.annpat.2012.07.006
33. Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol (2005) 29(12):1558–75. doi: 10.1097/01.pas.0000173232.22117.37
34. Bergamo F, Maruzzo M, Basso U, Montesco MC, Zagonel V, Gringeri E, et al. Neoadjuvant sirolimus for a large hepatic perivascular epithelioid cell tumor (pecoma). World J Surg Oncol (2014) 12:46. doi: 10.1186/1477-7819-12-46
35. Yates DH. Mtor treatment in lymphangioleiomyomatosis: the role of everolimus. Expert Rev Respir Med (2016) 10(3):249–60. doi: 10.1586/17476348.2016.1148603
36. Fabbroni C, Sbaraglia M, Sanfilippo R. Medical treatment of advanced Malignant perivascular epithelioid cell tumors. Curr Opin Oncol (2020) 32(4):301–06. doi: 10.1097/CCO.0000000000000649
Keywords: PEComa, melanocytic, smooth muscle, 18F-FDG, 68Ga-FAPI
Citation: Zhu D, Song S, Wang D, Kuang D, Cheng S, Zhou J and Zou S (2024) Hepatic perivascular epithelioid cell tumor resembling hepatic adenoma and hepatocellular carcinoma on preoperative imaging: a case report. Front. Oncol. 14:1292313. doi: 10.3389/fonc.2024.1292313
Received: 26 September 2023; Accepted: 10 January 2024;
Published: 01 February 2024.
Edited by:
Georgios S. Limouris, National and Kapodistrian University of Athens, GreeceReviewed by:
Emina Talakic, Medical University of Graz, AustriaRashmi T Samdani, MedStar Georgetown University Hospital, United States
Copyright © 2024 Zhu, Song, Wang, Kuang, Cheng, Zhou and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sijuan Zou, em91c2lqdWFuMTI2QHRqaC50am11LmVkdS5jbg==
 Dongling Zhu
Dongling Zhu Shuang Song1
Shuang Song1 Dong Kuang
Dong Kuang Siyuan Cheng
Siyuan Cheng Sijuan Zou
Sijuan Zou