
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 10 May 2024
Sec. Breast Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1291034
Background: Neuroendocrine breast carcinoma (NECB) is a rare, special histologic type of breast cancer. There are some small sample studies on the clinical outcomes of NECB patients, which are worthy of further discussion.
Methods: We conducted a retrospective case-control study of clinical characteristics and outcomes among patients with primary NECB versus invasive carcinoma of no special type (NST) between November 2004 and November 2017 in the Peking Union Medical College Hospital, Beijing. NST patients were strictly matched 1:4 during the same period based on the TNM stage. Statistical comparisons were performed to determine the differences in survival between NST and NECB patients and to identify clinical factors that correlate with prognosis.
Results: A total of 121 participants affected by primary NECB were included in our analysis from November 2004 to November 2017. Elderly persons (>60 years of age) were more likely to have primary NECB than young persons (p=0.001). In addition, primary NECB patients had significantly higher odds of having tumors 2-5 cm (36.5%) and >5 cm (6.1%) in size than NST patients. Despite a significant difference in tumor size, the proportion of patients with lymph node metastases showed no difference between the two groups (p=0.021). In addition, the rate of patients with ER-negative tumors in the NECB group (4.2%) was significantly lower than that in the primary NST group (29.8%). Significant differences were noted in the PR-negative (13.3% versus 36.6%, P<0.001) and HER2-negative (90.5% versus 76.4%, P=0.001) expression statuses among these patients. Of 121 primary NECB patients, 11 (9.1%) experienced relapses during the follow-up period. We found that tumor size was an independent risk factor for relapse. For hormone receptors on tumor cells, ER-positive breast cancer patients had significantly lower odds of relapse than receptor-negative patients.
Conclusions: Our data demonstrate no significant difference in mortality and relapse between the primary NECB and NST groups. The tumor size in the primary NECB group was significantly larger than that in the NST group. In addition, the absence of ER independently increased the relapse rate for breast carcinoma patients.
Breast cancer is the most frequently diagnosed malignancy and is a leading cause of cancer deaths in females worldwide. This form of cancer represents 12% of all new incident cancer cases and one-quarter of all cancers in women (1). In past decades, significant progress has been achieved in diagnosis and therapeutic strategies engaged in breast cancer management (2). Unfortunately, poor prognosis in patients affected by breast cancer remains of great concern among the population of underdeveloped areas due to diagnostic delay (3). More efforts are needed to achieve health equity to reduce the mortality associated with breast cancer in women.
According to tumor location, size, histology, and grade (4), significant diversity is noted in differentiation and proliferative activity across subgroups, mirroring its aggressiveness and prognosis (5). The most frequently diagnosed forms of breast cancer include invasive carcinoma of no special type, without tissue of origin(NST), constituting 80%–90% of all cases (5). In addition, NECB was first recognized in 1963 and is a rare special histologic type of breast cancer (6, 7). This special tumor differs in pathogenesis from others that have similar morphologic and phenotypic characteristics to digestive and pulmonary neuroendocrine tumors (7, 8). Therefore, NECB patients may have a different disease trajectory than those with other breast tumors, which triggers whether these patients represent a heterogeneous group of disease entities with different outcomes (9, 10). Unfortunately, its reported prevalence ranged from 0.1% to 15%, depending on the study series (7). Several studies have documented breast NECB studies with survival associations, but information on the clinical outcomes of patients with NECB is still lacking and needs further exploration (8, 11). More insights into NECB are required to guide treatment decision-making and optimize the design of clinical trials.
To address this concern, we conducted a retrospective study of clinical characteristics and outcomes among patients with primary NECB versus NST in the Peking Union Medical College Hospital, Beijing. Our objectives were to determine the differences in survival between these two groups and to identify clinical factors that correlate with prognosis.
A series of 131 primary NECB cases were retrieved from the electronic records of the Peking Union Medical College Hospital between November 2004 and November 2017. Two pathologists reviewed representative histological slides to confirm the diagnosis. Inclusion criteria included (1) patients who had a first cancer diagnosis of primary NECB, (2) metastases from the GI tract NECBs were excluded, and (3) patients who completed the follow-up in our hospital. The primary NECB tumors were diagnosed following the guidelines of the World Health Organization (12). They were characterized by densely packed hyperchromatic cells with scant cytoplasm, streaming, and crush artifacts. Either chromogranin A (CgA) or synaptophysin (Syn) expression was observed in up to 50% of tumor cells. In addition, the hormone estrogen receptor (ER) and progesterone receptor (PR) were highly expressed, but human epidermal growth factor 2 (HER2) was negative according to ISH results. To assess the potential impact on the survival of primary NECB patients, NST patients were strictly matched 1:4 during the same period based on the TNM stage. One hundred thirty-one cases were retrieved from the medical records and re-evaluated. Ten cases were excluded after the re-evaluation, and 121 cases were finally included in this study. The flowchart of the study was shown in Figure 1. This study was approved by the Ethics Committee of Peking Union Medical College Hospital. The institutional review board approved a waiver of patient informed consent because of the anonymization of patient data and presentation of no more than minimal risk of harm to patient subjects.
The electronic medical record system documented morbidity, treatment, and care over time. Demographic and clinical variables were collected from electronic medical records to compare the primary NECB and NST groups, including sex, age, place of residence, and comorbidities. In addition, the histological findings were also retrieved from electronic medical records, including morphology and expression levels of neuroendocrine and hormone markers. A cutoff of 1% expression or greater was used to define ER and PR positivity (13). For neuroendocrine markers, CgA, Syn, and neuron-specific enolase (NSE) were considered positive if at least 50% of tumor cells demonstrated expression of each marker (14). According to the previous guidelines, HER2 was scored using both percent positive and intensity, and only tumors expressing HER2 in ≥30% of cells at 3+ intensity were considered positive. The Ki-67 expression was defined as the percentage of tumor cells with nuclear Ki-67 staining. Based on the 2013 St. Gallen consensus standard (15), the patients affected by breast cancer were divided into four subtypes. In addition, internationally recognized TNM staging systems were used to classify malignant tumors. The immunohistochemical staining of tumor cells is shown in Figure 2.
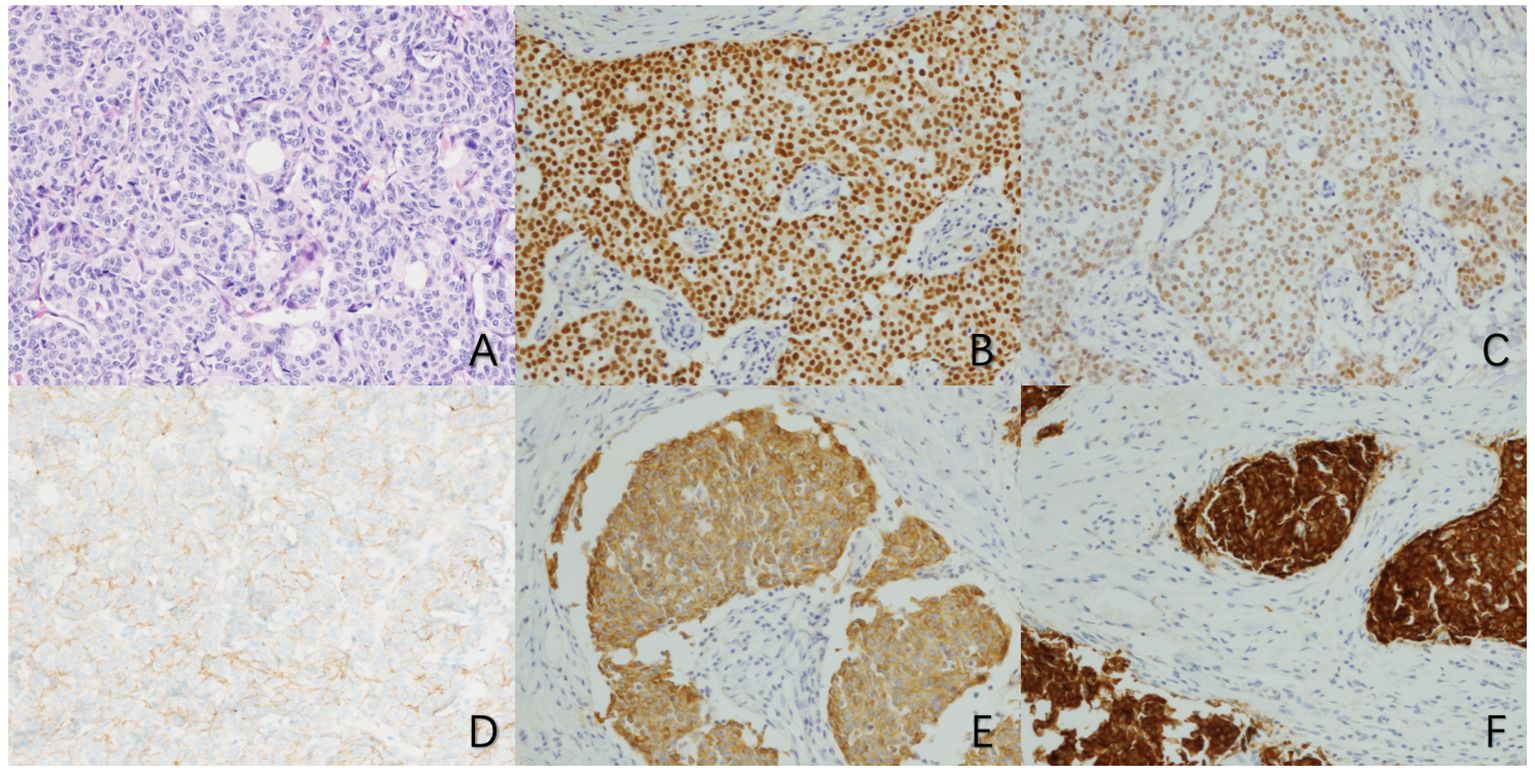
Figure 2 Microscopic manifestations of primary neuroendocrine carcinoma of the breast (×100) (A) HE staining shows a large number of cancer cells with tubular and trabecular structures. (B) Immunohistochemical staining for ER showed strong positivity in tumor cells. (C) Immunohistochemical staining for PR showed moderate positivity in tumor cells. (D) Immunohistochemical staining for HER2 showed no significant staining of tumor cells. (E) Immunohistochemical staining for CgA showed diffuse positive staining in tumor cells. (F) Immunohistochemical staining for Syn showed diffuse positive staining in tumor cells).
The tumor subtypes were classified as Luminal A (ER and PR positive, HER2 negative, ‘low’ Ki-67, and a ‘low’ recurrence risk based on multi-gene-expression assay results if available), Luminal B (‘Luminal B-like (HER2 negative)’: ER positive, HER2 negative, and at least one of the following: ‘high’ Ki-67, ‘negative or low’ PR, or ‘high’ recurrence risk based on multi-gene-expression assay if available. ‘Luminal B-like (HER2 positive)’: ER positive, HER2 over-expressed or amplified with any Ki-67, and any PR), HER2+ (Hormone receptor-negative and HER2-positive), and triple negative (TN) (Negative ER, PR, and HER2) according to St. Gallen’s Guide in 2013 (16).
The anonymization of patient data was conducted prior to analysis. SPSS version 20.0 (IBM Corp, Armonk, NY) was used for the statistical calculations. Numbers and proportions of cases and various demographic and clinical characteristics were tabulated. The mean and standard deviation (SD) are presented for normally distributed continuous variables, while the median and interquartile range (IQR) present nonnormally distributed continuous variables. As requested, statistical comparisons were performed using the Student’s t-test, Chi-square test, and Fisher’s exact test. A survival curve was constructed with the Kaplan–Meier method. Two-sided tests with P<0.05 were considered statistically significant.
We reviewed the consecutive pathology findings for 4492 women with breast cancers diagnosed between November 2004 to November 2017 in our hospital. A total of 121 patients were diagnosed with primary NECB based on histopathology results. The clinical characteristics of the included patients are shown in Table 1.
Of these, 78 patients had stage I or II disease, 22 had stage III or IV disease, and 21 patients could not be staged. 67 patients (55.4%) received mastectomy + axillary lymph node dissection and 16 patients (13.2%) received mastectomy + sentinel lymph node biopsy. 7 patients (5.8%) received lumpectomy + axillary lymph node dissection and 15 patients (12.4%) received lumpectomy + sentinel lymph node biopsy. 16 patients (13.2%) received lumpectomy without axillary stage due to the old age.
More than half of the patients(66/121, 54.5%) received chemotherapy to treat primary breast cancer. Most patients received adjuvant chemotherapy; the interval between surgery and adjuvant chemotherapy was 2-4 weeks. Two patients received neoadjuvant chemotherapy. HER2-negative patients received doxorubicin/epirubicin + cyclophosphamide, docetaxel + cyclophosphamide, paclitaxel/docetaxel + doxorubicin, or docetaxel + doxorubicin/epirubicin + cyclophosphamide, and HER2-positive patients received docetaxel + cyclophosphamide + trastuzumab and doxorubicin + cyclophosphamide followed by docetaxel + trastuzumab or trastuzumab+pertuzumab. Regimens included doxorubicin/epirubicin 50/75 mg/m2, cyclophosphamide 500 mg/m2, paclitaxel/docetaxel 175/75 mg/m2 every 3 weeks, trastuzumab 8 mg/kg IV on day 1 followed by 6 mg/kg every 3 weeks, and pertuzumab 840 mg for the first dose followed by 420 mg every 3 weeks. The duration of anti HER2 therapy was one year. The above methods and doses of radiotherapy and chemotherapy are also applicable to IDC group.
Fifty patients received adjuvant radiotherapy to treat primary breast cancer. For breast conserving therapy, whole-breast irradiation was delivered via opposed tangential fields using a regimen of 50Gy in 2Gy daily fractions with 6Mv-X rays from a linear accelerator. Invasive disease was treated with a boost of 10Gy in 5 fractions to the tumor bed and 1-2 cm margins. Regional nodal irradiation included the lower part of the ipsilateral axillary LN in all cases and the upper part of the ipsilateral axillary LN when there were metastases to the LNs in the axilla. Postmastectomy, 45-50Gy at 2Gy/fx with 6Mv-X rays was delivered from a linear accelerator to a target volume that included the chest wall and supraclavicular fossa. High risk patients were treated with an electron boost to bring the scar dose to 60-66Gy.
The majority of patients (109/121, 90.1%) received endocrine therapy to treat primary breast cancer, including tamoxifen, 10 mg twice a day or 20 mg once a day; letrozole, 2.5 mg once a day; anastrozole, 1 mg once a day; exemestane, 25 mg once a day; or a goserelin acetate 3.6mg depot implanted subcutaneously very 4 weeks. The duration of adjuvant endocrine therapy was 5 years for most patients, and 5-10 years for the patients with high risk of recurrence.
In the primary NECB group, the main types were luminal A and B, accounting for 26.4% and 64.5%, respectively. TN subtype accounted for 3.3%, and there was no Her2 subtype among these patients. In the NST group, the proportion of subtypes tended to be similar to that reported in the literature(luminal A 21.1%, luminal B 52.5%, Her2 10.5% and TN 13.6%). There were statistical differences in the subtypes between primary NECB and NST (P < 0.05) (17).
One hundred twenty-one participants affected by primary NECB were included in our analysis from November 2004 to November 2017, while 484 NST patients were matched based on the TNM stage as a control group during the same period. The detailed comparison of demographic and clinical characteristics is summarized in Table 1. The median ages of patients with primary NECB and NST were 54.0 (41.0-65.0) and 51.0 (44.0-58.0), respectively. The distribution of the two groups showed significant differences stratified into various age groups. We found that elderly persons (>60 years of age) were more likely to have primary NECB than young persons (p=0.001). In addition, primary NECB patients had significantly higher odds of having tumors 2-5 cm (36.5%) and >5 cm (6.1%) in size than NST patients. Despite a significant difference in tumor size, the proportion of patients with lymph node metastases showed no difference between the two groups (p=0.021). In addition, there was a significant difference in ER, PR, and HER2 expression between the primary NECB and NST groups. The rate of patients with ER-negative tumors in the primary NECB group (4.2%) was significantly lower than that in the NST group (29.8%)(p<0.001). Significant differences were noted in the PR-negative (13.3% versus 36.6%, P<0.001) and HER2-negative (90.5% versus 76.4%, P=0.001) expression statuses among these patients. We observed no significant difference in relapse between the two groups.
Of 121 primary NECB patients, 10 (10.7%) experienced relapses during the follow-up period. We further analyzed the risk factors associated with relapse in primary NECB patients. As summarized in Table 2, tumor size was an independent risk factor for relapse. The relapse rate in tumors of 2-5 cm (17.4%) was significantly higher than that in tumors less than 2 cm (aOR: 2.206, 95% CI: 1.244-3.912). For hormone receptors on tumor cells, we found that ER-positive breast cancer patients had significantly lower odds of relapse than ER-negative patients (aOR 0.235, 95% CI 0.135-0.410). Similarly, patients with lymph node metastasis had a higher risk for relapse than those without (OR 2.371, 95% CI 1.325-4.244).
NECB is a rare type of breast cancer, and many cases remain undiagnosed due to its rarity and heterogeneity (7). In this study, we described and analyzed clinical characteristics and prognosis in the largest number of primary NECB patients from China. Our data demonstrated no significant difference in mortality and relapse between the primary NECB and NST groups. Consistent with our observation, several previous studies confirmed that patients afflicted with primary NECB had similar prognoses and clinical presentations compared with other breast carcinomas (6, 18). However, conflicting results were noted in a population-based study from the Surveillance, Epidemiology, and End Results (SEER) database, which indicated that primary NECB was associated with worse long-term outcomes (19). In addition, a few studies have revealed that primary NECB is a nonaggressive breast carcinoma type with a better prognosis (20, 21). These contradictory results might be explained by the limited number of cases reported in each cohort and varying inclusion criteria from the WHO definitions for identifying primary NECB. In addition, routine physical examinations have been widely conducted in recent years in China and are helpful for the early identification of breast cancer patients. Thus, we speculate that diagnosing these breast carcinomas at an early stage may be another possible explanation for the comparative outcomes between the two groups. Consistent with our hypothesis, most of our primary NECB participants were classified as early T1-2 stage, which significantly contributed to the low relapse rate.
Despite no difference in prognosis between primary NECB and NST patients, we observed that the tumor size in the primary NECB group was significantly larger than that in the NST group. These findings are consistent with previous data that primary NECB presented with larger tumor size and high histological grade (22), which may reflect the faster intrinsic growth rate of primary NECB than NST. A previous study revealed that HER2- breast tumors often display higher proliferation rates than HER+ tumors (23). In our cohort, higher proportions of patients with ER-positive and HER2-negative breast cancer were noted in the primary NECB group than in the NST group. Thus, it is probable that the subtypes of HER2- breast cancer have an enhanced proliferation rate to achieve a larger tumor size at the time of diagnosis. Tumor size reflects the number of cancer cells and is also a predictor of outcome (23). Although we found no significant difference in relapse rate between the two groups due to the high potential for early diagnosis, our results imply a higher risk of poor clinical outcomes, including metastasis and short-term survival for patients with fast-growing primary NECB cancer.
A previous study by Wang and colleagues confirmed that primary NECB disease is more commonly diagnosed in older women in or above their sixth decade of life (19). Similar results were also reported in a retrospective analysis from China, demonstrating that primary NECB patients seemed to be older than the onset of the other tumor subtype (24). We also found that the proportion of patients with primary NECB aged >60 years was higher than that of the IDC group; however, approximately one-fifth of female patients were aged < 40 years. The diverse distribution of patients across age subgroups between primary NECB and NST indicates the difference in cancer pathogenesis. Specifically, hormone levels may play an essential role in the occurrence of primary NECB tumors, considering that this type of cell tends to express hormone receptors and lacks HER-2 (7, 25).
Due to the lack of an established standard treatment protocol, the treatment of primary NECB is consistent with that for other conventional types of invasive breast carcinomas. Based on our findings, routine therapies provided sufficient efficacy for the treatment of this rare breast cancer compared with other subtypes. The subsequent quantification of risk factors found that the absence of ER independently increased the relapse rate for breast carcinoma patients. Consistent with our findings, serial studies of breast carcinomas revealed that patients with ER-negative breast cancer had poor clinical outcomes (24, 26). More attention should be given to the follow-up of these patients at high risk of relapse. Previous studies on the prognostic significance of neuroendocrine differentiation in NECB have yielded contrary results due to different diagnostic criteria and the limited number of cases. In this study, we found that NECB tends to be a luminal-like type. There were only a small number of TN subtype patients and no patients with HER2 subtype in the queue. Despite all this, most recent studies have reported poorer clinical outcomes for NEBC compared with typical breast carcinomas (27, 28).
We also acknowledge several apparent limitations to this study. First, despite the enrollment of all primary NECB patients throughout the study period, the small number of patients associated with its low prevalence limits further analysis of risk factors for relapse in primary NECB patients. Second, the detailed treatment regimens were not considered in our analysis. Finally, our survival prognostic analysis of primary NECB patients was partially biased due to the low mortality of this study cohort. Despite these limitations, our study extends our knowledge about this rare breast cancer subtype.
In conclusion, our data demonstrate no significant difference in mortality and relapse between the primary NECB and NST groups. The tumor size in the primary NECB group was significantly larger than that in the NST group, and the primary NECB patients seemed to be older than the onset of the other tumor subtype. In addition, the absence of ER independently increased the relapse rate for breast carcinoma patients. Further clinical study is required to perform a prognostic analysis of the survival of primary NECB patients through long-term large-sample follow-up.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by peking union medical college hospital ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LP: Resources, Writing – original draft. MM: Software, Writing – original draft. DZ: Data curation, Resources, Writing – original draft. JZ: Data curation, Investigation, Methodology, Writing – original draft. QS: Supervision, Writing – review & editing. FM: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–386. doi: 10.1002/ijc.29210
2. Waks AG, Winer EP. Breast cancer treatment: A review. JAMA. (2019) 321:288–300. doi: 10.1001/jama.2018.19323
3. Abdullah N, Mohamed N. Influence of cultural practices on breast cancer risks, stage at presentation and outcome in a multi-ethnic developing country. Oncol Lett. (2021) 22:806. doi: 10.3892/ol
4. Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression. J Pathol. (2011) 223:307–17. doi: 10.1002/path.2808
5. Shyyan R, Masood S, Badwe RA, Errico KM, Liberman L, Ozmen V, et al. Breast cancer in limited-resource countries: diagnosis and pathology. Breast J. (2006) 12 Suppl 1:S27–37. doi: 10.1111/j.1075-122X.2006.00201.x
6. Sapino A, Papotti M, Righi L, Cassoni P, Chiusa L, Bussolati G. Clinical significance of neuroendocrine carcinoma of the breast. Ann Oncol. (2001) 12 Suppl 2:S115–117. doi: 10.1023/A:1012417903068
7. Rovera F, Lavazza M, La Rosa S, Fachinetti A, Chiappa C, Marelli M, et al. Neuroendocrine breast cancer: retrospective analysis of 96 patients and review of literature. Int J Surg. (2013) 11 Suppl 1:S79–83. doi: 10.1016/S1743-9191(13)60023-0
8. Bogina G, Munari E, Brunelli M, Bortesi L, Marconi M, Sommaggio M, et al. Neuroendocrine differentiation in breast carcinoma: clinicopathological features and outcome. Histopathology. (2016) 68:422–32. doi: 10.1111/his.12766
9. Karihtala P, Porvari K, Roininen N, Voutilainen S, Mattson J, Heikkilä P, et al. Comparison of the mutational profiles of neuroendocrine breast tumours, invasive ductal carcinomas and pancreatic neuroendocrine carcinomas. Oncogenesis. (2022) 11:53. doi: 10.1038/s41389-022-00427-1
10. Pareja F, Vahdatinia M, Marchio C, Lee SSK, Da Cruz Paula A, Derakhshan F, et al. Neuroendocrine tumours of the breast: a genomic comparison with mucinous breast cancers and neuroendocrine tumours of other anatomic sites. J Clin Pathol. (2022) 75:10–7. doi: 10.1136/jclinpath-2020-207052
11. Marchiò C, Geyer FC, Ng CK, Piscuoglio S, De Filippo MR, Cupo M, et al. The genetic landscape of breast carcinomas with neuroendocrine differentiation. J Pathol. (2017) 241:405–19. doi: 10.1002/path.4837
12. Pareja F, D'alfonso TM. Neuroendocrine neoplasms of the breast: A review focused on the updated World Health Organization (WHO) 5th Edition morphologic classification. Breast J. (2020) 26:1160–7. doi: 10.1111/tbj.13863
13. Subik K, Lee JF, Baxter L, Strzepek T, Costello D, Crowley P, et al. The expression patterns of ER, PR, HER2, CK5/6, EGFR, ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer (Auckl). (2010) 4:35–41. doi: 10.1177/117822341000400004
14. Tsang JY, Tse GM. Breast cancer with neuroendocrine differentiation: an update based on the latest WHO classification. Mod Pathol. (2021) 34:1062–73.. doi: 10.1038/s41379-021-00736-7
15. Gnant M, Harbeck N, Thomssen C. St. Gallen 2011: summary of the consensus discussion. Breast Care (Basel). (2011) 6:136–41. doi: 10.1159/000328054
16. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. (2013) 24:2206–23. doi: 10.1093/annonc/mdt303
17. Orrantia-Borunda E, Anchondo-Nuñez P, Acuña-Aguilar LE, Gómez-Valles FO, Ramírez-Valdespino CA. "Subtypes of Breast Cancer,". In: Mayrovitz HN, editor. Breast Cancer. Exon Publications, Brisbane (AU (2022).
18. Miremadi A, Pinder SE, Lee AH, Bell JA, Paish EC, Wencyk P, et al. Neuroendocrine differentiation and prognosis in breast adenocarcinoma. Histopathology. (2002) 40:215–22. doi: 10.1046/j.1365-2559.2002.01336.x
19. Wang J, Wei B, Albarracin CT, Hu J, Abraham SC, Wu Y. Invasive neuroendocrine carcinoma of the breast: a population-based study from the surveillance, epidemiology and end results (SEER) database. BMC Cancer. (2014) 14:147. doi: 10.1186/1471-2407-14-147
20. Zekioglu O, Erhan Y, Ciris M, Bayramoglu H. Neuroendocrine differentiated carcinomas of the breast: a distinct entity. Breast. (2003) 12:251–7. doi: 10.1016/S0960-9776(03)00059-6
21. Tse GM, Ma TK, Chu WC, Lam WW, Poon CS, Chan WC. Neuroendocrine differentiation in pure type mammary mucinous carcinoma is associated with favorable histologic and immunohistochemical parameters. Mod Pathol. (2004) 17:568–72. doi: 10.1038/modpathol.3800092
22. Inno A, Bogina G, Turazza M, Bortesi L, Duranti S, Massocco A, et al. Neuroendocrine carcinoma of the breast: current evidence and future perspectives. Oncologist. (2016) 21:28–32. doi: 10.1634/theoncologist.2015-0309
23. Foulkes WD, Reis-Filho JS, Narod SA. Tumor size and survival in breast cancer–a reappraisal. Nat Rev Clin Oncol. (2010) 7:348–53. doi: 10.1038/nrclinonc.2010.39
24. Li Y, Du F, Zhu W, Xu B. Neuroendocrine carcinoma of the breast: a review of 126 cases in China. Chin J Cancer. (2017) 36:45. doi: 10.1186/s40880-017-0211-x
25. Adams RW, Dyson P, Barthelmes L. Neuroendocrine breast tumours: breast cancer or neuroendocrine cancer presenting in the breast? Breast. (2014) 23:120–7. doi: 10.1016/j.breast.2013.11.005
26. Wapnir IL, Price KN, Anderson SJ, Robidoux A, Martín M, Nortier JWR, et al. Efficacy of chemotherapy for ER-negative and ER-positive isolated locoregional recurrence of breast cancer: final analysis of the CALOR trial. J Clin Oncol. (2018) 36:1073–9. doi: 10.1200/JCO.2017.76.5719
27. Trevisi E, La Salvia A, Daniele L, Brizzi MP, De Rosa G, Scagliotti GV, et al. Neuroendocrine breast carcinoma: a rare but challenging entity. Med Oncol. (2020) 37:70. doi: 10.1007/s12032-020-01396-4
Keywords: neuroendocrine breast carcinoma, invasive carcinoma of no special type, clinical characteristics, case-control study, prognosis
Citation: Peng L, Ma M, Zhao D, Zhao J, Sun Q and Mao F (2024) Comparison of clinical characteristics and outcomes in primary neuroendocrine breast carcinoma versus invasive ductal carcinoma. Front. Oncol. 14:1291034. doi: 10.3389/fonc.2024.1291034
Received: 08 September 2023; Accepted: 22 April 2024;
Published: 10 May 2024.
Edited by:
Julio de la Torre, Comillas Pontifical University, SpainReviewed by:
Giuseppe Angelico, Agostino Gemelli University Polyclinic (IRCCS), ItalyCopyright © 2024 Peng, Ma, Zhao, Zhao, Sun and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Mao, c2Jka3MyMDIxQHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.