- 1Helmsley Cancer Center, Shaare Zedek Medical Center, Jerusalem, Israel
- 2Institute of Oncology, Samson Assuta Ashdod University Hospital, Ashdod, Israel
- 3Ben-Gurion University of the Negev, Be’er Sheva, Israel
- 4Thoracic Surgery Department, Shaare Zedek Medical Center, Jerusalem, Israel
Background: Over the past decade, progress in the diagnosis and treatment of Non-Small Cell Lung Cancer (NSCLC) has led to the identification of many targeted mutations. This has enhanced PFS and OS in both advanced and early-stage NSCLC. The current standard of care for stage III NSCLC varies, and it may combine chemotherapy with either immunotherapy or radiotherapy. This study evaluated the role of induction targeted therapies in patients with driver mutations and inoperable NSCLC.
Methods: This is a single-center, retrospective study assessing the efficacy of targeted therapy in resectable stage III NSCLC patients who are EGFR or ALK-positive, using patient records, PET-CT, brain MRI staging, and mediastinal lymph node evaluation.
Results: Between January 2020 and February 2024, we identified four patients with either EML4-ALK fusions (2/4) or EGFR mutations (2/4) who underwent treatment with brigatinib or osimertinib before surgery. All patients experienced clinical benefits. Of the two patients with ALK fusion, one responded almost completely, while the other exhibited a notable partial response. Among the patients with EGFR mutations, one had a complete response and the other displayed a significant partial response. All four patients subsequently underwent lobectomy surgical resection.
Conclusions: This case series highlights the potential of targeted therapies for resectable NSCLC in the neoadjuvant setting. Further research is required to confirm their benefits, assess their safety and efficacy, and determine optimal timing and sequencing.
Introduction
Lung cancer, the most common form of cancer globally, has the highest mortality rate among all cancers. Smoking is the primary risk factor. Lung cancer is broadly classified into two types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC, which makes up around 85% of cases, includes subtypes such as adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (1). Technological advancements and immunohistochemical techniques have enabled personalized treatments based on specific driver mutations in individual tumors, providing new hope for lung cancer patients (2).
Patients who undergo surgical resection are still at a high risk of relapse. To address this concern, adjuvant and neoadjuvant chemotherapy have been studied extensively and have shown promising results in improving disease-free survival (DFS) and overall survival (OS) (3–5). In addition to chemotherapy, recent trials have explored the potential benefits of adjuvant immunotherapy and targeted therapy in patients with early-stage disease. One notable study is a phase III trial that compared adjuvant atezolizumab to the standard of care (SOC) in patients with resected stage II or III disease and PD-L1 expression of 1% or greater. The results of this trial demonstrated a significant improvement in DFS for patients with PD-L1 >1% and OS, particularly for those with high PD-L1 expression (>50%) (6, 7). Another important trial investigated the use of adjuvant pembrolizumab versus placebo in patients with stage IB-III, regardless of tumor proportion score PD-L1 expression. This study also revealed a notable enhancement in DFS (8).
Finally, the ADAURA trial, a phase III trial comparing adjuvant osimertinib to SOC, demonstrated an improvement in DFS and OS for patients with EGFR mutant NSCLC (9, 10). Furthermore, the Phase III ALINA trial also showed an improvement in DFS with the addition of adjuvant alectinib (11). These results, along with those from other ongoing trials, highlight the integration of immunotherapy and targeted therapies in the treatment approach for patients with surgically resected NSCLC. As a result, the FDA and EMA have granted approvals for specific populations.
In the neoadjuvant setting, a phase III trial comparing chemotherapy and nivolumab with chemotherapy alone demonstrated an improvement in the rate of pathological complete response and event-free survival in patients with stage IB-IIIA disease (12). Neoadjuvant trials have explored new endpoints, such as major and complete pathological response, which could potentially serve as surrogate endpoints in future trials. We recently published a Phase II trial focusing on neoadjuvant Osimertinib in Stage III EGFR-positive NSCLC, followed by definitive radiation and/or surgery. The trial showed a high response rate of 95.2% with excellent safety, as well as a nearly 50% reduction in the radiation field (13). In light of this, we present four patients who received neoadjuvant targeted therapies for potentially resectable stage III NSCLC with oncogenic driver mutations (EGFR or ALK), with the goal of determining their efficacy in this setting.
Methods
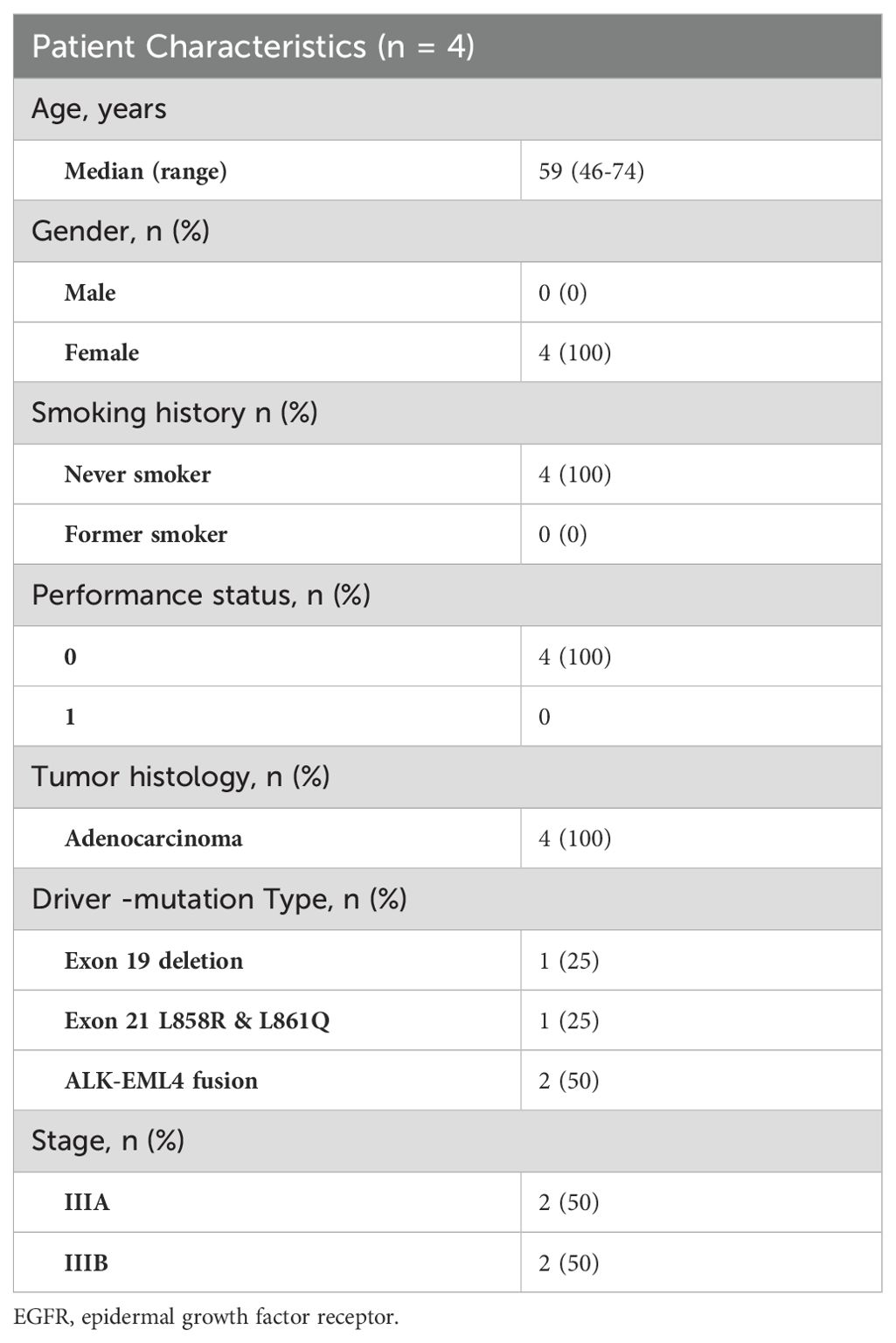
This document pertains to a single-center, retrospective, observational study aimed at assessing the efficacy of neoadjuvant targeted therapy in patients with potentially resectable NSCLC harboring EGFR or ALK-positive mutations. Data were extracted from patient records, including PET-CT scans, brain MRI for baseline tumor staging (according to the AJCC 8th edition), and pathological evaluation of mediastinal lymph nodes. Eligible patients demonstrated normal organ function, adequate pulmonary function, and an Eastern Cooperative Oncology Group performance status score of zero. Driver mutations were confirmed through next-generation sequencing.
Among the cohort, two patients had ALK fusions and two had EGFR mutations, all of whom received targeted tyrosine kinase inhibitor (TKI) therapies. Patients with ALK fusion genes were treated with brigatinib at a daily dosage of 180mg, while those with EGFR mutations received osimertinib at a daily dosage of 80mg. It is important to note that the off-label use of treatment in these cases was conducted as part of a local scientific project.
PET-CT scans and brain MRIs were utilized to evaluate treatment efficacy. Following induction of targeted therapy, all responsive patients underwent surgery, after which pathological response was assessed.
Case presentation
Case 1
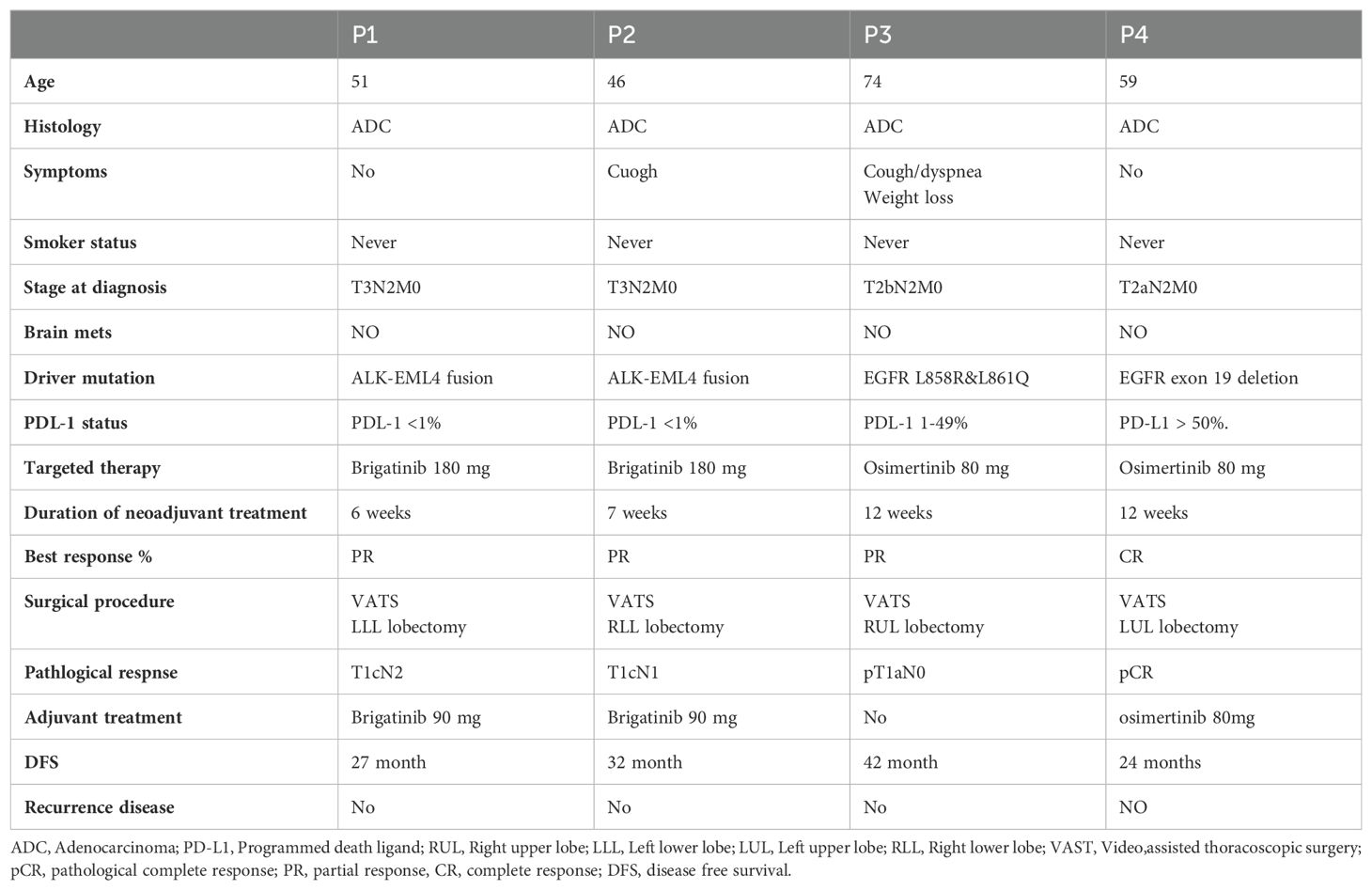
In August 2021, a 51-year-old non-smoking female underwent a routine imaging exam which revealed the presence of a 5 cm mass in the left lower lobe. This mass was diagnosed as adenocarcinoma of lung origin through a CT-guided biopsy. Further testing using PET-CT showed significant fluorodeoxyglucose (FDG) uptake in the left lower lobe and moderate uptake in the mediastinal lymph nodes on the same side, indicating the absence of distant metastasis (Figure 1). Brain MRI results were negative for intracranial metastasis.

Figure 1. Case 1: A 51-year-old female with NSCLC-adenocarcinoma and EML4–ALK fusion. (A) Chest CT shows a 5cm mass in the left lower lobe, classified as T3N2M0. (B) PET-CT scan indicates no metastasis. (C, D) Follow-up CT chest after 6 weeks of treatment with brigatinib 180mg daily.
According to the American Joint Committee on Cancer (AJCC) 8th Edition, the patient’s condition was classified as T3N2M0. To address the patient’s condition, the multidisciplinary team decided to initiate neoadjuvant treatment with brigatinib, followed by surgery. After six weeks of treatment, a chest CT showed a partial response with significant tumor shrinkage. Subsequently, the patient underwent left lower lobectomy and mediastinal lymph node dissection. The pathology report indicated a pathological response of pT1cN2 and negative Spread through air spaces (STAS). Currently, the patient is 27 months post-surgery and is undergoing adjuvant treatment with a daily dose of 90mg of brigatinib. Recent PET-CT scan and brain MRI results showed no evidence of disease, as summarized in Table 1.
Case 2
A 46-year-old nonsmoking female presented with a suspicious mass on a chest x-ray while hospitalized with SARS-CoV-2 in February 2021. A chest CT scan revealed a 5.5 cm mass involving the costophrenic angle in the right lower lobe. A subsequent PET-CT scan revealed high FDG uptake in the right lower lobe and moderate FDG uptake in the ipsilateral mediastinal lymph nodes but no distant metastasis (Figure 2). An MRI of the brain revealed no evidence of intracranial metastasis. Adenocarcinoma of the lung was confirmed by CT-guided biopsy and tissue next-generation sequencing revealed an EML4-ALK fusion rearrangement. The patient was classified as T3N2M0. The patient began neoadjuvant brigatinib, but experienced side effects such as fever and weakness, resulting in a 50% reduction in dosage from 180mg to 90mg, which was maintained for 7 weeks. Based on a follow-up chest CT, the patient showed a partial response to treatment, with 60% remarkable tumor shrinkage. The patient had a right lower lobectomy and mediastinal lymph node dissection (pT1cN1 pathological response, STAS negative). Following surgery, the patient received adjuvant brigatinib 90 mg once daily for 32 months, with no evidence of disease detected on PET-CT. An MRI also revealed no brain metastases, as summarized in Table 1.
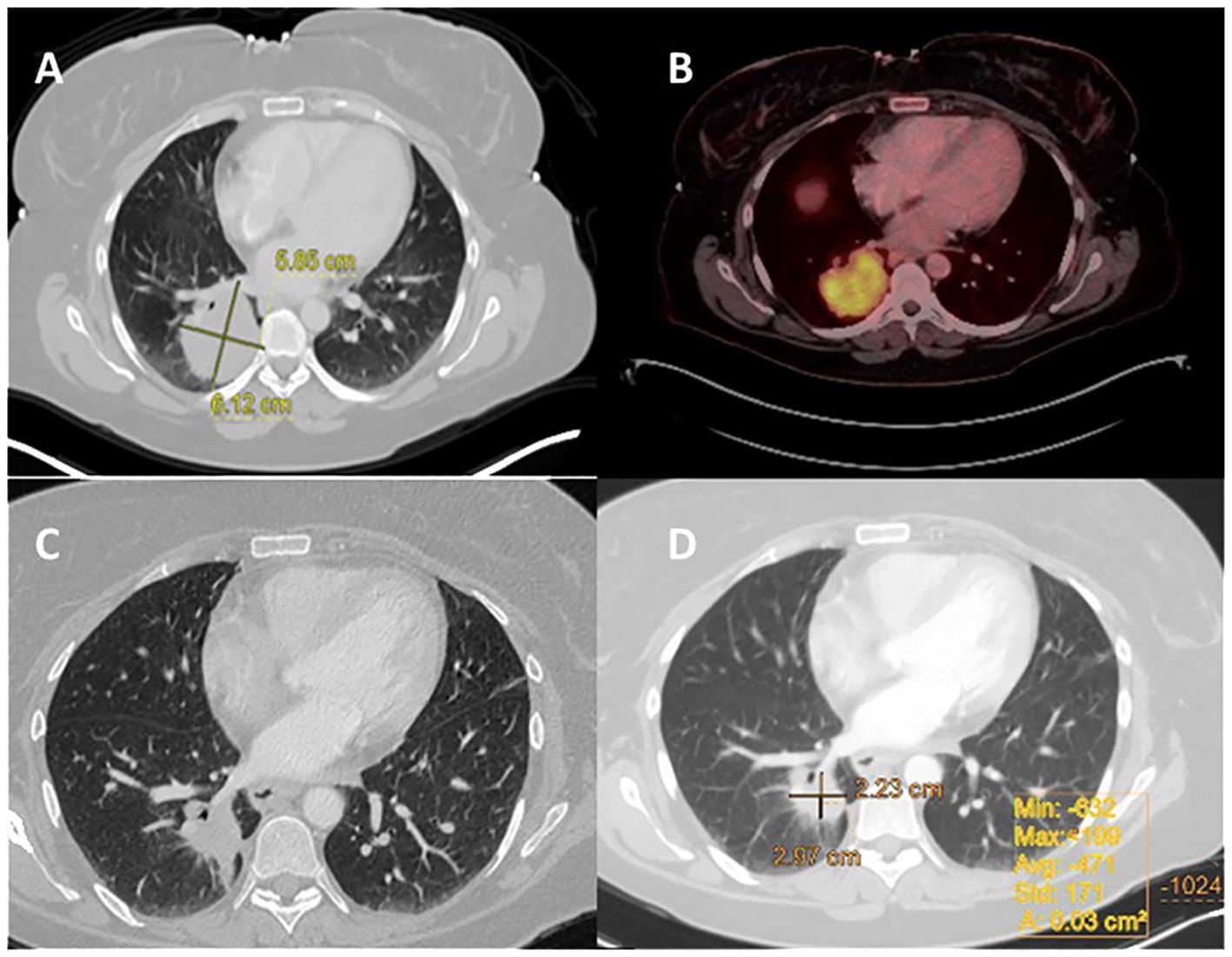
Figure 2. Case 2: A 46-year-old female with NSCLC-adenocarcinoma and EML4-ALK Fusion. (A) Chest CT shows a right lower lobe (RLL) mass measuring 5.5 cm, with a staging of T3N0M0. (B) PET CT shows FDG uptake in the RLL mass measuring 5.5 cm, without metastasis. (C, D) After 6 weeks of treatment with brigatinib 180 mg daily, CT chest was performed.
Case 3
In January 2020, a 74-year-old female non-smoker was diagnosed with a 3.5 cm mass in the right upper lobe during a routine imaging examination. A PET-CT scan revealed high FDG uptake in the right upper lobe and moderate FDG uptake in the ipsilateral mediastinal lymph nodes, without distant metastasis (Figure 3). Brain MRI showed no intracranial metastasis. A CT-guided biopsy revealed lung adenocarcinoma. Tissue next-generation sequencing showed an EGFR L858R and L861Q mutations. According to the AJCC 8th Edition guidelines, the patient was staged as T2bN2M0. The patient was treated with osimertinib for 12 weeks, demonstrating a partial response to treatment of 80% on chest CT. In August 2020, the patient underwent right upper lobectomy and mediastinal lymph node dissection (pT1aN0 pathological response, STAS negative). Following recovery from surgery, no adjuvant therapy was taken. After 42 months of follow-up, there was no evidence of disease on PET-CT or brain MRI, as summarized in Table 1.
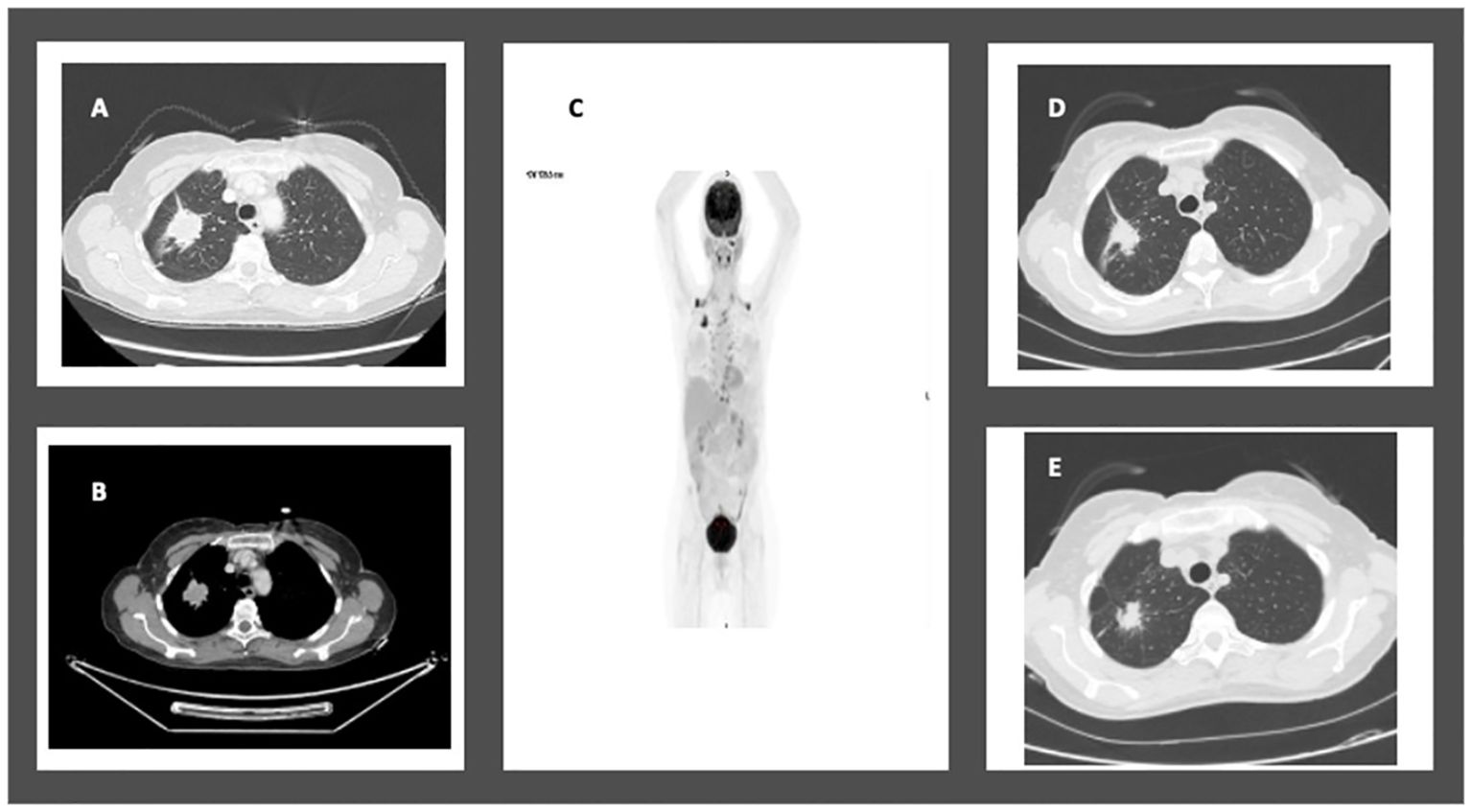
Figure 3. Case 4: A 74-year-old female with NSCLC-adenocarcinoma and EGFR exon 21 L858R mutation. (A, B) Chest CT showing a 3.5cm mass in the right upper lobe. (C) RUL mass and moderate FDG uptake in the ipsilateral mediastinal lymph nodes. (D, E) CT chest after 12 weeks of treatment with Osimertinib 80mg daily showing partial response.
Case 4
A 59-year-old former smoker was diagnosed with a 3.7 cm mass in her left lower lung lobe during a routine imaging exam in September 2021. A PET-CT scan revealed high FDG uptake in the left upper lobe and moderate FDG uptake in both the ipsilateral and contralateral mediastinal lymph nodes, with no distant metastasis (Figure 4). No intracranial metastasis was detected on a brain MRI.
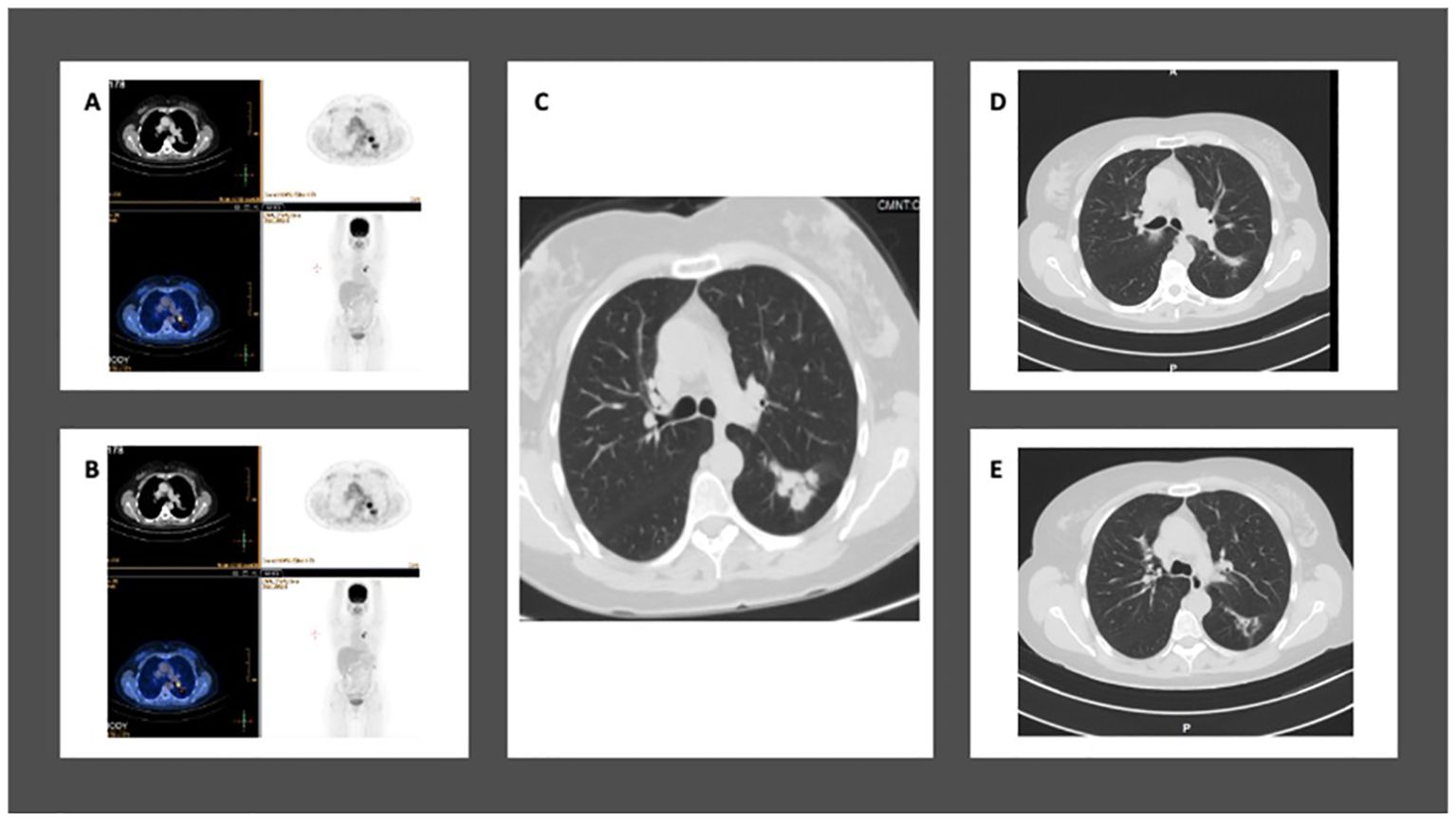
Figure 4. Case 5: A 59-year-old female with NSCLC-adenocarcinoma and EGFR exon 19 deletion. (A, B) PET-CT shows a 3.5 cm mass in the left lower lobe with mediastinal lymph nodes, but without distant metastasis. The staging is T2aN2M0. (C) Chest CT shows a mass in the left lower lobe measuring 3.5 cm. (D, E) After 12 weeks of treatment with Osimertinib at a daily dose of 80 mg, CT chest shows complete response.
A CT-guided biopsy confirmed the mass to be an adenocarcinoma of lung origin. Tissue next generation sequencing revealed an EGFR exon 19 deletion. The patient was classified as T2aN2M0.
The patient started treatment with Osimertinib, taking an 80 mg dose daily for 12 weeks. This resulted in a radiological complete response on the PET-CT. In February 2022, she underwent a resection of the left upper lobe, achieving a pathological complete response. She continued with adjuvant Osimertinib treatment. After 24 months of follow-up, there is no sign of metastasis on her PET-CT and brain MRI, as summarized in Table 1.
Results
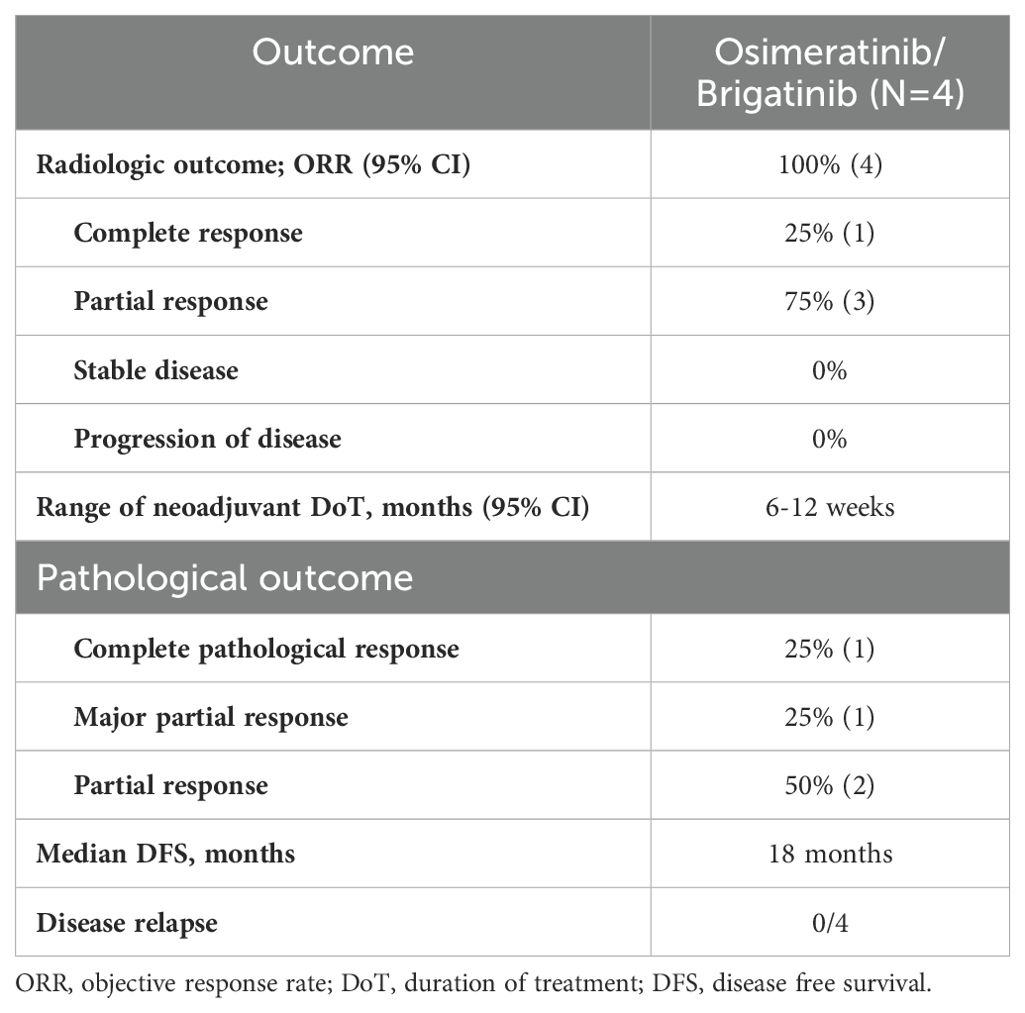
Between January 2020 and February 2024, four enrolled participants received targeted therapy. All patients had been diagnosed with adenocarcinoma, with two Stage IIIA patients and two Stage IIIB patients. The participants characteristics shown in Tables 1, 2. Representative radiologic and pathological responses after 6 to 12 weeks of brigatinib or osimertinib are shown in Table 3.
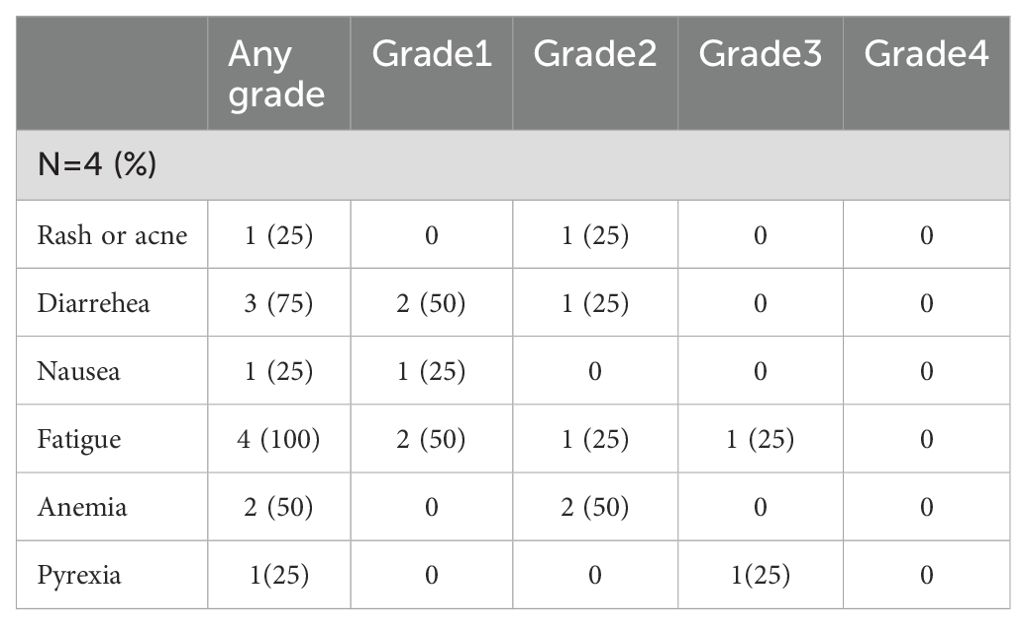
Among the two patients who had an ALK fusion, one showed a radiological response of 90%, while the other showed a partial response of 60%. The first EGFR patient had a partial radiological response rate of about 80%, while the second patient had a complete radiological response. During neoadjuvant therapy, only one patient experienced grade 3 side effects (fever and weakness) that necessitated a dose reduction, as summarized in Table 4.
All the patients underwent lobectomy resection. After surgery, one patient had a major pathological response (MPR), another patient had a complete pathological response, and the other two had a partial pathological response. The patients underwent postoperative follow-up using PET-CT and brain MRI every four months. All patients showed no evidence of disease. The treatment regimen was tolerable, and no new adverse events related to the targeted therapies osimertinib and brigatinib were reported, shown in Table 4.
Discussion
The efficacy of the respective targeted therapies has been confirmed for patients with metastatic NSCLC (14, 15). These confirmatory trials suggest that these treatments prolong the progression free survival and overall survival compared to chemotherapy alone or the combination of chemo-immunotherapy (16, 17). The emergence of next-generation TKIs has ignited significant interest among researchers, with encouraging signs of sustained enhancements in disease-free survival rates observed across various intervals, as demonstrated in trials such as ADAURA with osimertinib. Furthermore, these advancements have led to improved overall survival outcomes in the adjuvant treatment of EGFR-positive NSCLC (9, 10). Notably, the recent ALINA trial revealed that adjuvant alectinib, a second-generation ALK-TKI, significantly enhanced disease-free survival compared to platinum-based chemotherapy among patients with resected ALK-positive NSCLC of stage IB, II, or IIIA (18). The use of neoadjuvant targeted therapy in NSCLC remains an important topic for study as there are many advantages of administering molecular treatment with targeting molecules before planned definitive surgery to patients with non-metastatic disease (3, 12).
The exploration of neoadjuvant targeted therapy in NSCLC represents a pivotal area of investigation, offering several advantages, especially for patients with non-metastatic disease. Early-stage NSCLC management has seen notable progress, with studies indicating that neoadjuvant chemotherapy presents a viable alternative to adjuvant chemotherapy, leading to a substantial reduction in the relative risk of death, along with significant improvements in overall survival and time-to-distant recurrence (19, 20). Specifically, for stage IIIA (N2) NSCLC, several randomized-controlled trials and meta-analyses have shown a significant survival advantage with neoadjuvant chemotherapy. Preoperative chemoradiotherapy increases the proportion of complete resections (75% vs 60%), while also increasing the rate of mediastinal downstaging (46% vs. 29%, P=0.02) and pathological responses (60% vs. 20%, P=0.0001) (21). However, both treatment strategies appear to be effective.
Recent studies have reported encouraging outcomes of neoadjuvant chemo-immunotherapy in early-stage NSCLC, surpassing previous benchmarks set by neoadjuvant chemotherapy or chemoradiation alone (22). However, the role of immunotherapy in patients with oncogenic drivers remains under scrutiny, particularly due to observed low response rates in advanced disease (23, 24).
Approximately 15% of NSCLC cases present with locoregional N2 disease (stage IIIA). The optimal treatment strategies for patients with N2 disease, as well as the criteria for defining resectability, remain subjects of ongoing debate in thoracic oncology (25). While there is still controversy surrounding the definition of resectability, the management of patients with ‘unresectable’ N2 disease is more clear-cut. The current standard of care involves concurrent chemo-radiotherapy followed by maintenance therapy with durvalumab if there is no evidence of disease progression post-induction treatment, as demonstrated in the PACIFIC trial (21, 26). For patients with potentially resectable stage IIIA (N2) disease, various trimodal approaches, including surgery, perioperative chemotherapy, and radiotherapy, are being explored by multidisciplinary thoracic teams, particularly in cases where a microscopically margin-negative resection is anticipated. Significantly, several recently published phase III trials have assessed the efficacy of perioperative chemo-immunotherapy, encompassing resectable N2 diseases, demonstrating promising improvements in event-free survival and pathological complete response (27–29). Notably, the KEYNOTE 671 trial exhibited enhancements in overall survival (27). However, it is crucial to acknowledge that these trials included a limited number of patients with EGFR or ALK fusion mutations, rendering it challenging to draw definitive conclusions based on these findings.
The principal advantage of targeted therapy lies in its ability to commence treatment promptly, facilitating the reduction of micro-metastatic disease burden and potentially rendering tumors more amenable to surgery, particularly in cases of lymph node involvement or unresectable disease (30). Our case series underscores the effectiveness and reliability of targeted therapy as a perioperative treatment option for stage III NSCLC patients harboring EGFR mutations or ALK fusion. Promisingly, our findings revealed a median objective response rate of 100%, with no disease progression observed during the presurgical interval and no significant adverse events reported. Larger-scale studies are warranted to validate these findings across a broader patient population.
Conclusion
This case series provides insights into the potential benefits of targeted therapies for locally advanced non-small cell lung cancer (NSCLC) in the neoadjuvant setting. The findings suggest that the use of targeted therapies in this context could be a promising approach to improve treatment outcomes for NSCLC patients.
However, while these results are certainly encouraging, more research is necessary to fully establish the role of targeted therapies in the neoadjuvant setting for NSCLC. For example, further studies are needed to verify the effectiveness and safety of these treatments, and to develop a better understanding of the optimal timing and sequencing of such therapies.
Overall, the findings of this case series underscore the importance of ongoing research into new and innovative therapeutic approaches for NSCLC and suggest that targeted therapies may have a key role to play in improving outcomes for patients with this challenging disease.
Limitations
This case study has some limitations that should be taken into consideration. Firstly, the number of patients included in the study is relatively small, which may limit the generalizability of the results. Secondly, the follow-up period for these patients is relatively short. Thirdly, it is important to acknowledge that in actual clinical settings, postoperative patients who receive adjuvant treatments cannot be controlled compulsorily.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
WK: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. BK: Formal analysis, Data collection, Conceptualization, Writing – original draft. BG: Data analysis, Data collection, Conceptualization, Visualization, Writing – original draft. NE: Formal analysis, Conceptualization, Writing – original draft. AI: Formal analysis, Data collection, Conceptualization, Writing – original draft. DF: Formal analysis, Data Conceptualization, Visualization, Writing – review & editing. NP: Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization, Writing – review & editing. LR: Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors sincerely thank the patient and their family for their cooperation and trust throughout the treatment process. We also extend our appreciation to Sabri El-said and Areen A. Remilah for their significant contributions to the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kian W, Zemel M, Levitas D, Alguayn W, Remilah AA, Rahman NA, et al. Lung cancer screening: a critical appraisal. Curr Opin Oncol. (2022) 34:36–43. doi: 10.1097/CCO.0000000000000801
2. Guan X, Qin T, Qi T. Precision medicine in lung cancer theranostics: paving the way from traditional technology to advance era. Cancer Control. (2022) 29. doi: 10.1177/10732748221077351
3. Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE collaborative group. J Clin Oncol. (2008) 26:3552–9. doi: 10.1200/JCO.2007.13.9030
4. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. (2012) 13:239–46. doi: 10.1016/S1470-2045(11)70393-X
5. Subramanian MP, Puri V. Neoadjuvant vs. adjuvant chemotherapy in locally advanced non-small cell lung cancer—is timing everything? J Thorac Dis. (2019) 11:5674. doi: 10.21037/JTD.2019.12.40
6. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. (2021) 398:1344–57. doi: 10.1016/S0140-6736(21)02098-5
7. Felip E, Altorki N, Zhou C, Vallières E, Martínez-Martí A, Rittmeyer A, et al. Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase III trial. Ann Oncol. (2023) 0. doi: 10.1016/j.annonc.2023.07.001
8. O’Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. (2022) 23:1274–86. doi: 10.1016/S1470-2045(22)00518-6
9. Wu Y-L, Tsuboi M, He J, et al. Osimertinib in resected EGFR -mutated non–small-cell lung cancer. N Engl J Med. (2020) 383:1711–23. doi: 10.1056/NEJMOA2027071
10. Herbst RS, Tsuboi M, John T, Kato T, Majem M, Grohé C, et al. Overall survival analysis from the ADAURA trial of adjuvant osimertinib in patients with resected EGFR-mutated (EGFRm) stage IB–IIIA non-small cell lung cancer (NSCLC). J Clin Oncol. (2023) 41(17_suppl):LBA3–3. doi: 10.1200/JCO.2023.41.17_SUPPL.LBA3
11. ALINA study of alectinib meets primary DFS end point in ALK+ NSCLC. Available online at: https://www.targetedonc.com/view/alina-study-of-alectinib-meets-primary-dfs-end-point-in-alk-nsclc (Accessed February 10, 2024).
12. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. (2022) 386:1973–85. doi: 10.1056/NEJMOA2202170
13. Peled N, Roisman LC, Levison E, Dudnik J, Chernomordikov E, Heching N, et al. Neoadjuvant osimertinib followed by sequential definitive radiation therapy and/or surgery in stage III epidermal growth factor receptor-mutant non-small cell lung cancer: an open-label, single-arm, phase 2 study. Int J Radiat Oncol Biol Phys. (2023) 117. doi: 10.1016/J.IJROBP.2023.03.042
14. Shaw AT, Kim D-W, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK -positive lung cancer. N Engl J Med. (2013) 368:2385–94. doi: 10.1056/NEJMOA1214886
15. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. (2010) 362:2380–8. doi: 10.1056/NEJMOA0909530
16. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BCG, Jhanelle EO, Yuichiro Z, et al. Overall survival with osimertinib in untreated, EGFR -mutated advanced NSCLC. N Engl J Med. (2020) 382:41–50. doi: 10.1056/NEJMoa1913662
17. Camidge DR, Kim HR, Ahn M-J, Yang JC, Han JY, Lee JS, et al. Brigatinib versus crizotinib in ALK -positive non–small-cell lung cancer. N Engl J Med. (2018) 379:2027–39. doi: 10.1056/NEJMOA1810171
18. Wu Y-L, Dziadziuszko R, Ahn JS, Barlesi F, Nishio M, Lee DH, et al. Alectinib in resected ALK -positive non–small-cell lung cancer. N Engl J Med. (2024) 390:1265–76. doi: 10.1056/NEJMOA2310532
19. Shukla N, Hanna N. Neoadjuvant and adjuvant immunotherapy in early-stage non-small cell lung cancer. Lung Cancer Targets Ther. (2021) 12:51. doi: 10.2147/LCTT.S277717
20. Reyes R, Reguart N. Neoadjuvant treatment of stage IIIA-N2 in EGFR-Mutant/ALK-rearranged non-small cell lung cancer. Transl Lung Cancer Res. (2021) 10:607. doi: 10.21037/TLCR-20-780
21. Sher DJ. Neoadjuvant chemoradiotherapy for stage III non-small cell lung cancer. Front Oncol. (2017) 7:281. doi: 10.3389/FONC.2017.00281
22. Shao L, Lou G. Neoadjuvant immunotherapy in non-small cell lung cancer: a narrative review on mechanisms, efficacy and safety. J Thorac Dis. (2022) 14:3565–74. doi: 10.21037/JTD-22-1192/COIF
23. Upfront atezolizumab plus bevacizumab and chemo shows no significant PFS benefit in NSCLC. Available online at: https://www.targetedonc.com/view/upfront-atezolizumab-plus-bevacizumab-and-chemo-shows-no-significant-pfs-benefit-in-nsclc (Accessed September 25, 2023).
24. Pembro plus chemo fails to show efficacy benefit in TKI-resistant, EGFR-mutated, metastatic nonsquamous NSCLC. Available online at: https://dailynews.ascopubs.org/do/pembro-plus-chemo-fails-show-efficacy-benefit-tki-resistant-egfr–mutated-metastatic (Accessed September 25, 2023).
25. Provencio-Pulla M, Nadal E, Larriba JLG, Martinez-Marti A, Bernabé R, Bosch-Barrera J, et al. Nivolumab + chemotherapy versus chemotherapy as neoadjuvant treatment for resectable stage IIIA NSCLC: Primary endpoint results of pathological complete response (pCR) from phase II NADIM II trial. J Clin Oncol. (2022) 40(16_suppl):8501–1. doi: 10.1200/JCO.2022.40.16_SUPPL.8501
26. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. (2022), JCO2101308. doi: 10.1200/JCO.21.01308
27. Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, et al. Perioperative pembrolizumab for early-stage non–small-cell lung cancer. N Engl J Med. (2023) 389:491–503. doi: 10.1056/NEJMOA2302983
28. Heymach JV, Harpole D, Mitsudomi T, Taube JM, Galffy G, Hochmair M, et al. Perioperative durvalumab for resectable non–small-cell lung cancer. N Engl J Med. (2023) 389:1672–84. doi: 10.1056/NEJMOA2304875
29. Perioperative nivolumab plus chemotherapy improves event-free survival in resectable non–small cell lung cancer - the ASCO post. Available online at: https://ascopost.com/issues/december-10-2023-supplement-esmo-highlights/perioperative-nivolumab-plus-chemotherapy-improves-event-free-survival-in-resectable-non-small-cell-lung-cancer/ (Accessed May 14, 2024).
30. Peled N, Roisman LC, Levison E, Dudnik J, Chernomordikov E, Heching N, et al. Neoadjuvant osimertinib followed by sequential definitive radiotherapy and/or surgery in stage III EGFR-mutant NSCLC: An open-label, single-arm, phase II study. Int J Radiat Oncol Biol Phys. (2023). doi: 10.1016/J.IJROBP.2023.03.042
Keywords: brigatinib, osimertinib, neoadjuvant, ALK, EGFR, lung cancer
Citation: Kian W, Krayim B, Giles B, Elkiaan NA, Idris A, Fink D, Peled N and Roisman LC (2024) Case report: The effect of induction targeted therapies in stage III driver mutants non-small cell lung cancer. Front. Oncol. 14:1286116. doi: 10.3389/fonc.2024.1286116
Received: 30 August 2023; Accepted: 10 June 2024;
Published: 13 November 2024.
Edited by:
Aakash Desai, University of Alabama at Birmingham, United StatesReviewed by:
Magdalena Knetki-Wróblewska, Maria Sklodowska-Curie National Research Institute of Oncology, PolandXiaofei Wang, Tennessee State University, United States
Copyright © 2024 Kian, Krayim, Giles, Elkiaan, Idris, Fink, Peled and Roisman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nir Peled, bmlycEBzem1jLm9yZy5pbA==
†These authors have contributed equally to this work and share first authorship
 Waleed Kian
Waleed Kian Belal Krayim1†
Belal Krayim1†