- 1Department of Pathology, Wannan Medical College First Affiliated Hospital, Yijishan Hospital, Wuhu, Anhui, China
- 2Department of Pathology, Wannan Medical College, Wuhu, Anhui, China
Background: Angiomyolipoma with epithelial cysts (AMLEC) is an extremely rare subtype of kidney angiomyolipoma that contains epithelial-lined cysts. The most distinctive immunohistochemical feature of AMLEC is its immunoreactivity with melanocytic markers. AMLEC also has a distinct histological structure, which aids in its pathological diagnosis. To date 27 cases of AMLEC have been reported in 11 case series. However, the molecular biology underlying the pathogenesis of AMLEC remains unexplored.
Case report: A 30-year-old female was diagnosed with AMLEC and underwent partial nephrectomy. Histologically, the cross-section of cystic tissue revealed a multilocular appearance, with some cysts containing thrombus-like material, and the wall thickness was approximately 0.2 ~ 0.3 cm. Additionally, the compact subepithelial cellular stroma showed strong and diffuse nuclear labeling for estrogen receptor, progesterone receptor, and CD10, as well as HMB45 and Melan A, which are markers of melanocytic differentiation. Furthermore, using a DNA targeted sequencing panel with next-generation sequencing, we identified a nonsense mutation in TSC Complex Subunit 2 (TSC2) gene, resulting in the formation of a premature termination codon. Moreover, the mutated genes found to be enriched in the PI3K-AKT pathway. The patient in this case had a favorable postoperative follow-up at 3 months.
Conclusion: To the best of our knowledge, this study represents the first analysis of genotype mutations in AMLEC, providing valuable insights for future clinical practice. These findings have significant potential in guiding the understanding and management of AMLEC, paving the way for further research and advancements in the field.
1 Introduction
Renal angiomyolipoma (AML) is one of the most common benign neoplasms, typically composed of mature adipose tissue, blood vessels, and smooth muscle. In clinical practice, AMLs can be easily identified through abdominal imaging (1). However, there are same AMLs that have minimal fat, known as “fat-poor AMLs”. Among them, angiomyolipoma with epithelial cysts (AMLEC) is an exceptionally rare subtype of fat-poor AML. Histologically, AMLEC is characterized by the presence of epithelial-lined cysts and a thick exterior wall consisting of poorly formed fascicles of smooth muscle and dysplastic blood vessels with thick walls (2). Immunohistochemical imaging of AMLECs typically exhibits distinct features and shows positive reactivity for melanocytic markers, estrogen and progesterone receptors, such as HMB45, Melan A, ER, PR, CD10 and WT-1. AMLECs may manifest either incidentally or with associated symptoms commonly including flank pain and hematuria. While the majority of AMLEC lesions are benign, there is a rare possibility of cystic lesions being malignant, necessitating surgical excision.
To best of our knowledge, there is limited information about the imaging findings of AMLEC available. To date, only a total of 27 cases have been reported in 11 published case reports (3–14). Furthermore, there are only two reports that present the immunohistochemical and imaging characteristics of a combination of AMLEC and EAML another variant of AML (6, 15). Notably, there have been no reports of progression or metastasis during the indolent clinical course of AMLEC. However, the molecular biology underlying the pathogenesis of AMLEC remains unaddressed in the literature.
TSC2 (TSC Complex Subunit 2) is a crucial tumor suppressor gene responsible for encoding the growth inhibitory protein tuberin. Tuberin interacts with hamartin to form the TSC protein complex, which plays a vital role in regulating cell growth. Specifically, this TSC protein complex exerts a negative control on the signaling pathway of mammalian target of rapamycin complex 1 (mTORC1), a key regulator of anabolic cell growth. It is worth noting that mutations in this gene have been associated with tuberous sclerosis and lymphangioleiomyomatosis (16). To date, there have been no reports have linked TSC2 mutations to AMLEC.
So, in this study, we not only reported the histological and immunohistochemical features of AMLEC, but also identified a nonsense mutation in TSC2 using a DNA targeted sequencing panel with next-generation sequencin (NGS). Furthermore, we observed multiple mutation types in a total of 20 genes, and KEGG analysis indicated enrichment of the PI3K-AKT pathway. Notably, the TSC2 nonsense mutation discovered in this study has the potential to be a clinically significant variant that could confer sensitivity to everolimus.
2 Case presentation
The patient is a 30-year-old female who presented with discomfort and pain in the right flank area. A lump was discovered during physical examination specifically located between the liver and right kidney in the abdomen. The patient had no personal or family history of chronic diseases such as hypertension, diabetes, or coronary heart disease. Additionally, there was no history of renal masses, renal cell carcinoma, angiomyolipomas, tuberous sclerosis, or any similar conditions in the patient or her family. The physical examination revealed a negative Murphy’s sign for the gallbladder, and the spleen was not palpable. There were no apparent signs of edema in both lower limbs, and the nervous system appeared normal.
The abdominal computed tomography (CT) scan, which included both plain and contrast enhancement, identified a cystic density lesion measuring 63 mm × 39 mm in the right kidney, which was classified as Bosniak III type. On January 12th, 2023, the patient underwent a right partial nephrectomy. The treatment route followed by the patient is summarized in Figure 1.
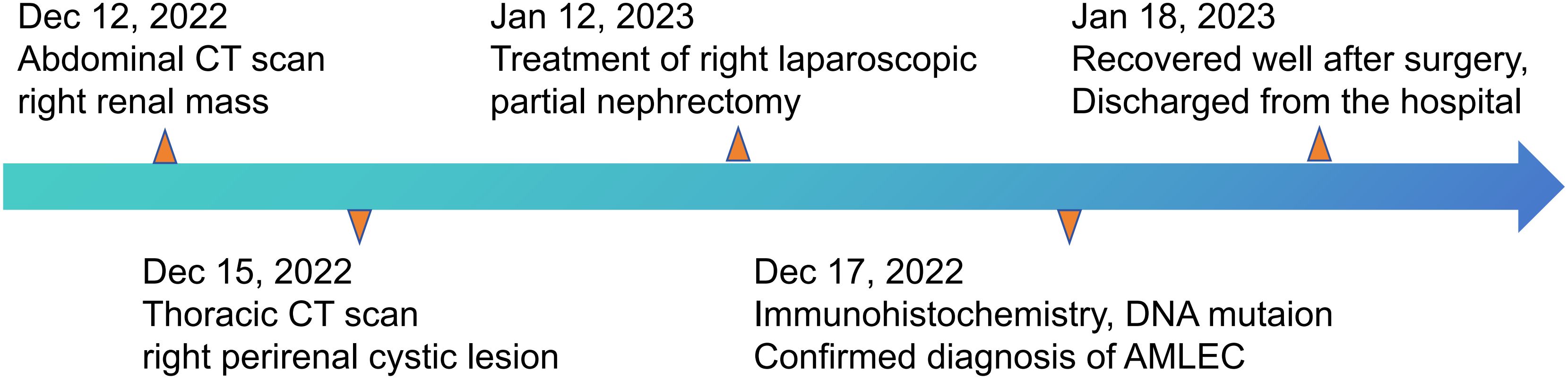
Figure 1 The clinical course schedule of this patient showing the treatment route received by the patient.
The border of the cyst was still clear, and it protruded outward locally (Figure 2A). Subsequently, the patient was transferred to the urology department for further diagnosis and treatment. Upon admission, the patient was diagnosed with a “right renal occupying lesion”. A gray-yellow-gray-white cystic tissue measuring approximately 6.0 cm × 4.5 cm × 2.8 cm was submitted for histopathological and Immunohistochemical examination. Histopathologically, the sliced specimen revealed a multilocular cyst with some cysts containing thrombus-like material. The cyst wall measured approximately 0.2cm-0.3cm in thickness (Figure 2B). Immunohistochemically, the epithelial cells lining the cyst wall showed diffuse expressed of AE1/AE3 and PAX-8 (Figure 3), suggesting that the cysts may have resulted from cystic dilatation of collecting ducts that invaginated into the tumor. The compact subepithelial cellular stroma exhibited strong and diffuse nuclear labeling for estrogen receptor (ER), progesterone receptor (PR), and cytoplasmic labeling for CD10. However, labeling for ER and PR was patchy in the smooth muscle-like cells. Additionally, HMB45 and Melan A (Figure 3), markers of melanocytic differentiation, were most intense and concentrated in the subepithelial cells, while HMB45 showed patchy labeling in the exterior muscle-predominant AML component. Meanwhile, SMA and Desmin were specifically labeled in smooth muscle-like cells. S-100 protein did not label any of the three components of the tumor, except for patchy labeling of subepithelial cells. The tumor exhibited a low proliferative index, with Ki67 (Figure 3) labeling less than 1% of neoplastic cells. Furthermore, CA IX, CD117 (c-kit), CD34 (endothelial markers), and inhibin-a did not label any of the three components of the tumor. Based on all these findings, we diagnosed the predominantly cystic tumor as AMLEC, accompanied by vascular smooth muscle lipoma and an epidermal cyst.
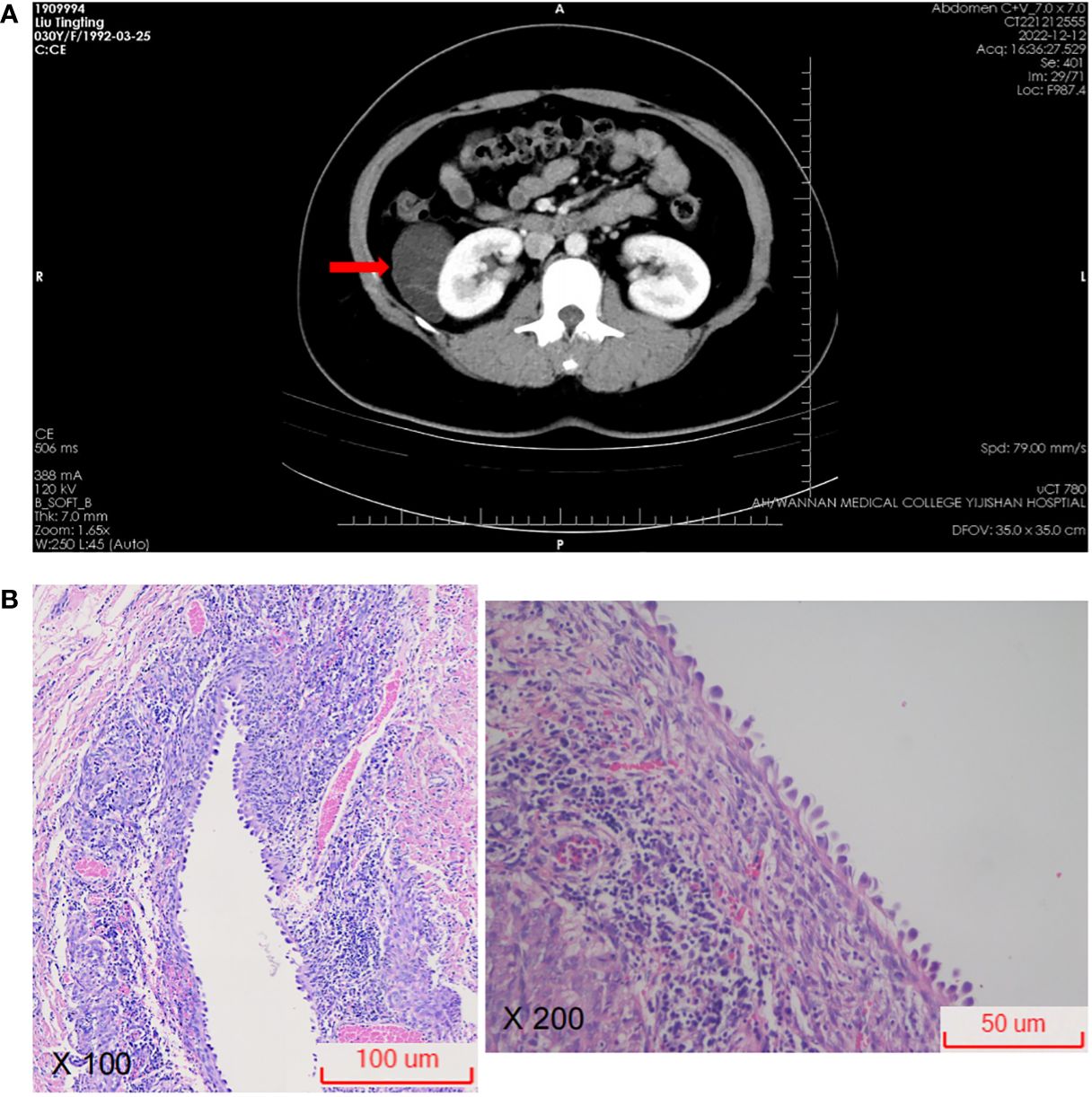
Figure 2 Radiological and microscopic findings. (A) On computed tomography, a cystic density lesion measuring 63 mm × 39 mm was found at the upper part of the right kidney (red arrow). (B) Photomicrograph showing epithelial cysts lined by cuboidal to hobnailed cells (H&E100X, H&E200X).
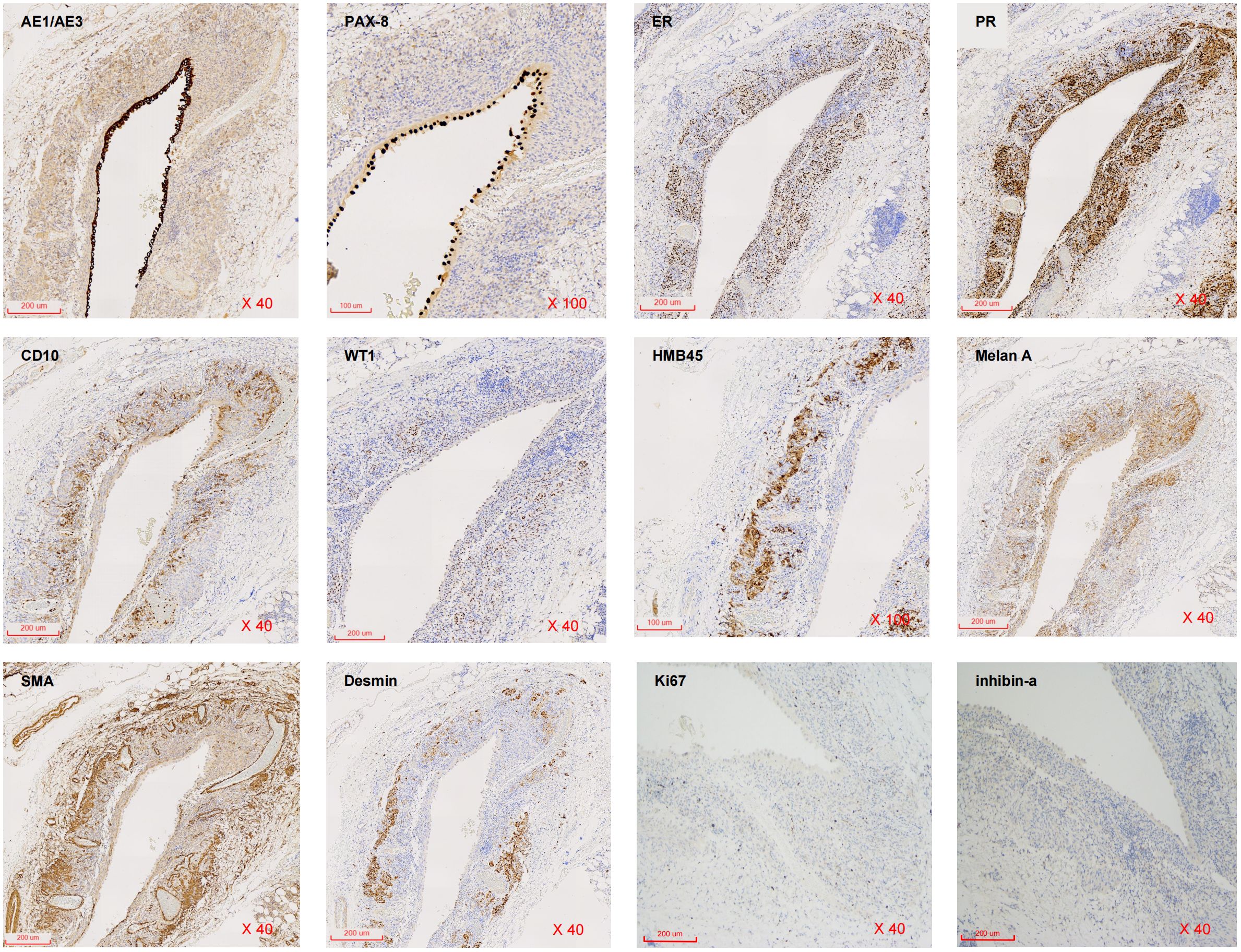
Figure 3 Immunohistochemical findings. Immunohistochemistry for AE1/AE3, PAX-8, ER, PR, CD10, WT1, HMB45, Melan A, SMA, Desmin, Ki67 and inhibin-a expression in AMLEC lesions (from right to light, from top to below) (H&E40X).
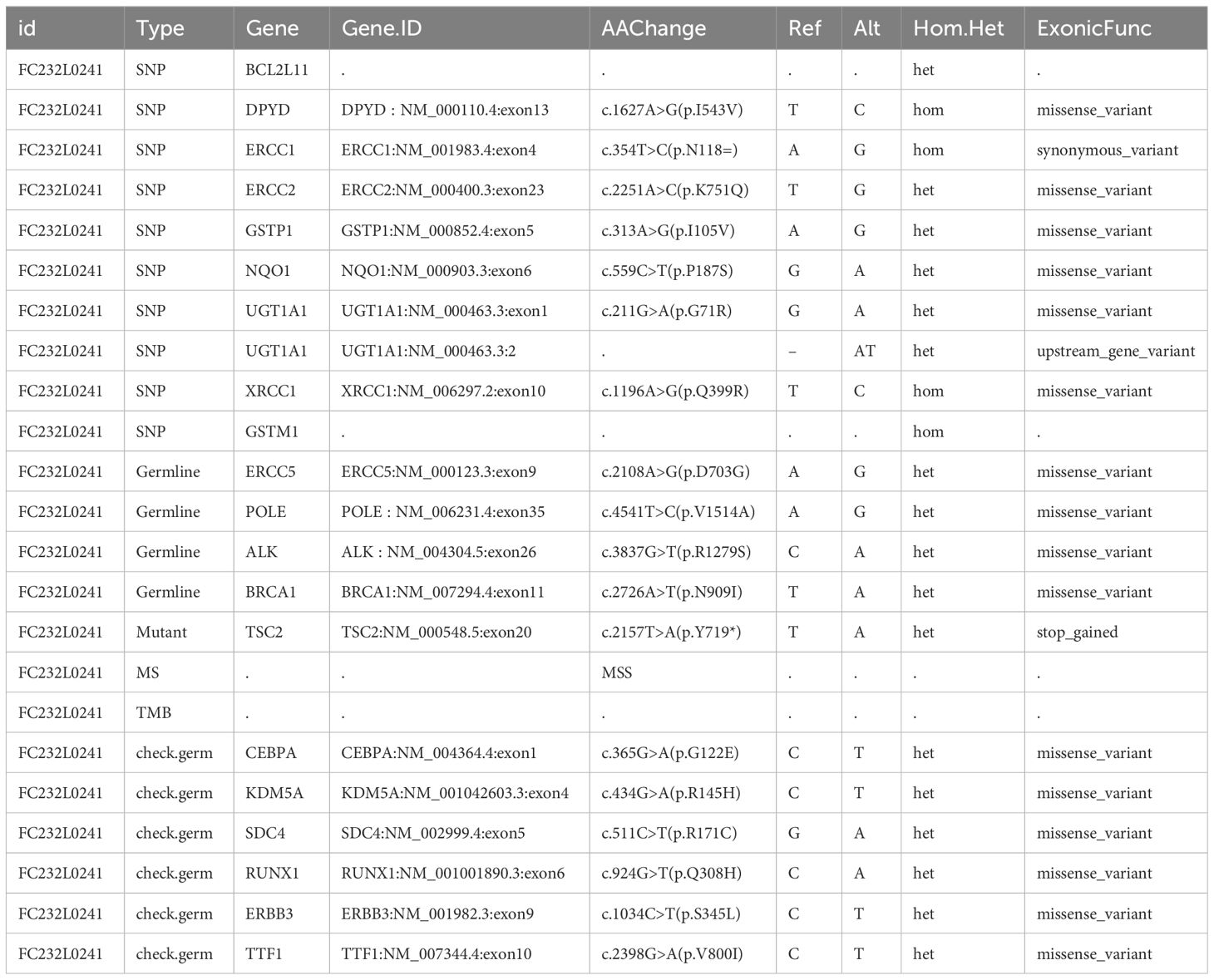
Simultaneously, the patient’s tumor biospecimen and circulating tumor DNA in plasma (ctDNA) nearby the tumor were sent to Geneseeq Technology Inc. for NGS analysis. The NGS test covered approximately 1.53 Mb base pairs, including exons of 437 genes, fusion-related introns, variable splicing regions, and specific microsatellite (MS) loci. The test results provided information on point mutations, small fragment insertion and deletion mutations, gene fusion and copy number variations, microsatellite (MS) analysis results, and tumor mutation burden (TMB) information within the coverage range.
In the AMLEC tumor sample, a low TMB of 1.0 mutation per megabase (Mb) was observed, and no germline mutations were found when compared with ctDNA. Mutations were identified in a total of 20 genes, as detailed in Table 1, with additional information available in Supplementary Table S1. Notably, no mutations were identified in four mismatch repair-related genes: MLH1, MSH2, MSH6, and PMS2. Additionally, the analysis revealed 10 polymorphic mutations associated with drug metabolism enzymes. Specifically, a nonsense mutation of c.2157T>A was detected in the 20th exon of the TSC2 gene. This mutation resulted in the substitution of the 719th amino acid from tyrosine to a stop codon, leading to premature termination of protein synthesis and the production of a truncated protein. The presence of mutations in these 20 genes suggests enrichment in pathways related to platinum drug resistance, PI3K-Akt signaling pathway, and nucleotide excision repair. These findings provide potential implications for targeted therapies and insights into the molecular mechanisms underlying the development and progression of AMLEC (Supplementary Figure S1).
3 Discussion
AML is a well-known and frequently encountered neoplasm characterized by a triphasic composition of adipose tissue, thick-walled vessels, and epithelioid smooth muscular. However, there are instances where AMLs exhibit insufficient amounts of fat, posing challenges in their detection using unenhanced CT scans. In such cases, misdiagnosed as renal cell carcinoma may occur. AMLEC, a rare variant of AML, presents a particular diagnostic challenge due to its minimal fat content and the presence of epithelial cysts. Unlike typical AMLs, AMLECs exhibit both solid and cystic areas, accompanied by an epithelial component. Therefore, when evaluating cystic renal neoplasms in adults, it is crucial to consider AMLEC as a potential differential diagnosis. It is worth noting that AMLECs are less frequently associated with tuberous sclerosis compared to conventional AMLs (5, 17).
Currently, there are no specific tumor markers or imaging characteristics that can definitively diagnose AMLEC prior to surgery. The accurate diagnosis of AMLEC relies solely on histopathological and immunohistochemical findings. When considering the differential diagnosis, AMLEC stands out as distinct from most adult cystic renal tumors. It is crucial to rule out other conditions such as mixed epithelial and stromal tumor (MEST), cystic nephroma, multilocular cystic renal cell carcinomas, and cystic partially differentiated nephroblastoma. It is worth noting that AMLEC and MEST share some morphological similarities, including cysts lined by cuboidal or hobnail epithelia and smooth muscle walls. Additionally, both AMLEC and MEST exhibit immunoreactivity for ER, PR, and CD10 in their stromal cells. However, the presence of dysplastic features in the vessels of AMLEC, which are absent in MEST, can serve as an important distinguishing factor. Furthermore, the presence of melanocytic markers HMB45 and Melan A is a significant positive indicator for AMLEC, while MEST does not exhibit immunoreactivity for these markers.
The clinical features of AMLEC do not appear to differ significantly from classic AMLs. AMLs are generally considered to be benign and exhibit an indolent clinical course. Based on reported cases of AMLECs, all patients underwent either partial nephrectomy (including our case) or radical nephrectomy. Only one case resulted in the patient’s death, which was unrelated to AMLEC but due to another underlying disease. Additionally, there have been two reported cases of a combination of AMLEC and EAML, which were also confirmed based on immunohistochemical and imaging studies (6, 15). It is crucial to establish appropriate timing and frequency for follow-up examinations, but chemotherapy or radiation therapy is unnecessary for AMLEC (15).
We detected a nonsense mutation of p.Y719* in the 20th exon of the TSC2 gene, which caused a stop codon and the production of a truncated protein. It is reported that AML with multiple lesions is closely associated with tuberous sclerosis complex (TSC), which shares the same name as the gene responsible for its development. However, the sporadic solo lesion AMLEC has not been reported to have any known relationship with mutations in the TSC2 genes. The presence of this truncated protein could potentially lead to the inactivation of the TSC2 protein. Consequently, it may result in a reduction in the hydrolysis of downstream RHEB molecules in the mTOR pathway, leading to an accumulation of RHEB GTP levels. This, in return, activates the mTOR pathway, ultimately contributing to tumor development by enhancing the cell’s anti-apoptotic ability. Moreover, this mutation may also increase the sensitivity to mTOR inhibitors (18). Similarly, mutations in the TSC2 gene can lead to the development of TSC by causing hyperactivation of the mTOR pathway. In recent years, the mTOR inhibitor everolimus has been approved as an adjunctive therapy for TSC-associated partial seizures (19). It is reasonable to propose that everolimus, as mTOR inhibitor, holds promising clinical significance as an adjunct therapy for AMLEC.
4 Conclusion
In this study, we present a rare case of AMLEC in a 30-year-old female patient. AMLEC is an extremely rare benign kidney tumor, and the main treatment options are partial nephrectomy (as in our case) or radical nephrectomy. AMLECs typically appear as radiographically and grossly cystic renal lesions, emphasizing the importance of distinguishing them from other cystic renal neoplasms. A notable aspect of this case is the presence of three distinct morphological patterns and three different immune phenotypes. While AMLEC may resemble MEST, its most distinctive feature is its immunoreactivity not only to ER, PR, and CD10, but also to melanocytic markers such as Melan-A and HMB45. Understanding the characteristics of this disease will help differentiate predominantly cystic renal tumors. Currently, there is no literature or clinical reports specifically addressing the relationship between TSC2 nonsense mutation and AMLEC, apart from this case. However, given the potential implications of TSC2 mutations in the pathogenesis of AMLEC, further investigation is necessary to assess its role as a potential target for diagnosis and treatment in future clinical research.
There were some limitations in our study. Firstly, we only had one AMLEC case, which is a small sample size for AMLEC treatment. In the future, we plan to increase the sample size to obtain more generalizable ultrasound features that can provide references for the diagnosis and treatment of AMLEC. Secondly, the TSC2 nonsense mutation identified in this case may be a specific mutation, and its clinical significance as an adjunct therapy for AMLEC needs further exploration. Additionally, more cases are needed to verify this mutation and its therapeutic effect.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://ngdc.cncb.ac.cn/search/?dbId=hra&q=PRJCA018538, PRJCA018538.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Wannan Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LL: Data curation, Funding acquisition, Resources, Writing – original draft, Writing – review & editing. HS: Data curation, Investigation, Writing – original draft, Writing – review & editing. GM: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. NJ: Data curation, Investigation, Formal analysis, Writing – original draft. JL: Data curation, Supervision, Conceptualization, Project administration, Writing – original draft. WG: Data curation, Investigation, Supervision, Validation, Writing – original draft. YL: Formal analysis, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Foundation of Wannan medical college and Foundation of Wannan Medical College First Affiliated Hospital (WK2021ZF13, WK2021F27 and YR202107) as well as Wannan Medical College Yijishan Hospital (WK2023ZQNZ54).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1274953/full#supplementary-material
Supplementary Figure 1 | KEGG pathways enriched by 20 genes with mutations as shown in Table 1.
References
1. Lane BR, Aydin H, Danforth TL, Zhou M, Remer EM, Novick AC, et al. Clinical correlates of renal angiomyolipoma subtypes in 209 patients: classic, fat poor, tuberous sclerosis associated and epithelioid. J Urol. (2008) 180:836–43. doi: 10.1016/j.juro.2008.05.041
2. LeRoy MA, Rao P. Angiomyolipoma with epithelial cysts. Arch Pathol Lab Med. (2016) 140:594–7. doi: 10.5858/arpa.2015-0170-RS
3. Armah HB, Yin M, Rao UN, Parwani AV. Angiomyolipoma with epithelial cysts (AMLEC): a rare but distinct variant of angiomyolipoma. Diagn Pathol. (2007) 2:11. doi: 10.1186/1746-1596-2-11
4. Davis CJ, Barton JH, Sesterhenn IA. Cystic angiomyolipoma of the kidney: a clinicopathologic description of 11 cases. Mod Pathol. (2006) 19:669–74. doi: 10.1038/modpathol.3800572
5. Fine SW, Reuter VE, Epstein JI, Argani P. Angiomyolipoma with epithelial cysts (AMLEC): a distinct cystic variant of angiomyolipoma. Am J Surg Pathol. (2006) 30:593–9. doi: 10.1097/01.pas.0000194298.19839.b4
6. Filho Jdo E, Meneses de Amorim D, Sweet GM, Rodrigues de Freitas LA, Athanazio PR, Athanazio DA. Renal epithelioid angiomyolipoma with epithelial cysts: demonstration of Melan A and HMB45 positivity in the cystic epithelial lining. Ann Diagn Pathol. (2012) 16:397–401. doi: 10.1016/j.anndiagpath.2011.03.004
7. Gorin MA, Rowe SP, Allaf ME, Argani P. Angiomyolipoma with epithelial cysts: Add one to the differential of cystic renal lesions. Int J Urol. (2015) 22:1081–2. doi: 10.1111/iju.12908
8. Mikami S, Oya M, Mukai M. Angiomyolipoma with epithelial cysts of the kidney in a man. Pathol Int. (2008) 58:664–7. doi: 10.1111/j.1440-1827.2008.02287.x
9. Karafin M, Parwani AV, Netto GJ, Illei PB, Epstein JI, Ladanyi M, et al. Diffuse expression of PAX2 and PAX8 in the cystic epithelium of mixed epithelial stromal tumor, angiomyolipoma with epithelial cysts, and primary renal synovial sarcoma: evidence supporting renal tubular differentiation. Am J Surg Pathol. (2011) 35:1264–73. doi: 10.1097/PAS.0b013e31822539a1
10. Rosenkrantz AB, Hecht EM, Taneja SS, Melamed J. Angiomyolipoma with epithelial cysts: mimic of renal cell carcinoma. Clin Imaging. (2010) 34:65–8. doi: 10.1016/j.clinimag.2009.04.026
11. Varshney B, Vishwajeet V, Madduri V, Chaudhary GR, Elhence PA. Renal angiomyolipoma with epithelial cyst. Autops Case Rep. (2021) 11:e2021308. doi: 10.4322/acr.2021.308
12. Wei J, Li Y, Wen Y, Li L, Zhang R. Renal angiomyolipoma with epithelial cysts: a rare entity and review of literature. Int J Clin Exp Pathol. (2015) 8:11760–5.
13. Wood A, Young F, O'Donnell M. Angiomyolipoma with epithelial cysts masquerading as a cystic renal cell carcinoma. Curr Urol. (2017) 9:209–11. doi: 10.1159/000447142
14. Yeo DM, Chung D, Kim T, Lee I, Hahn S, Lee J. CT and MRI findings of angiomyolipoma with epithelial cysts of the kidney: A case report. J Korean Soc Radiol. (2010) 63:173. doi: 10.3348/jksr.2010.63.2.173
15. Lee SH, Lee JS, Kim JJ, Ko SY, Lee KR, Hwang IK, et al. Renal epithelioid angiomyolipoma with epithelial cysts mimicking cystic renal cell carcinoma: A case report of combination of two rare entities. J Korean Soc Radiol. (2022) 83:1109–15. doi: 10.3348/jksr.2021.0172
16. Caliò A, Brunelli M, Gobbo S, Pedron S, Segala D, Argani P, et al. Stimulator of interferon genes (STING) immunohistochemical expression in the spectrum of perivascular epithelioid cell (PEC) lesions of the kidney. Pathology. (2021) 53:579–85. doi: 10.1016/j.pathol.2020.09.025
17. Cheng L, Gu J, Eble JN, Bostwick DG, Younger C, MacLennan GT, et al. Molecular genetic evidence for different clonal origin of components of human renal angiomyolipomas. Am J Surg Pathol. (2001) 25:1231–6. doi: 10.1097/00000478-200110000-00002
18. Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, et al. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. (2003) 112:1223–33. doi: 10.1172/jci17222
Keywords: case report, angiomyolipoma, AMLEC, TSC2, Pi3k-akt
Citation: Song H, Mao G, Jiao N, Li J, Gao W, Liu Y and Lu L (2024) TSC2 nonsense mutation in angiomyolipoma with epithelial cysts: a case report and literature review. Front. Oncol. 14:1274953. doi: 10.3389/fonc.2024.1274953
Received: 16 August 2023; Accepted: 11 March 2024;
Published: 25 March 2024.
Edited by:
Ronald M. Bukowski, Cleveland Clinic, United StatesReviewed by:
Poonam Elhence, All India Institute of Medical Sciences Jodhpur, IndiaHuan Deng, Fourth Affiliated Hospital of Nanchang University, China
Copyright © 2024 Song, Mao, Jiao, Li, Gao, Liu and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linming Lu, MTk5NjAwMTBAd25tYy5lZHUuY24=
†These authors have contributed equally to this work
 Hong Song1†
Hong Song1† Yinhua Liu
Yinhua Liu Linming Lu
Linming Lu