- Department of Anesthesiology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Background: Dexmedetomidine (DEX) has been widely applied in the anesthesia and sedation of patients with oncological diseases. However, the potential effect of DEX on tumor metastasis remains contradictory. This study follows up on patients who received intraoperative DEX during laparoscopic resection of colorectal cancer as part of a previous clinical trial, examining their outcomes 5 years later.
Methods: Between June 2015 and December 2015, 60 patients undergoing laparoscopic colorectal resection were randomly assigned to the DEX and control groups. The DEX group received an initial loading dose of 1μ/kg before surgery, followed by a continuous infusion of 0.3μg/kg/h during the operation and the Control group received an equivalent volume of saline. A 5-year follow-up analysis was conducted to evaluate the overall survival, disease-free survival, and tumor recurrence.
Results: The follow-up analysis included 55 of the 60 patients. The DEX group included 28 patients, while the control group included 27 patients. Baseline characteristics were comparable between the two groups, except for vascular and/or neural invasion of the tumor in the DEX group (9/28 vs. 0/27, p = 0.002). We did not observe a statistically significant benefit but rather a trend toward an increase in overall survival and disease-free survival in the DEX group, 1-year overall survival (96.4% vs. 88.9%, p = 0.282), 2-year overall survival (89.3% vs. 74.1%, p = 0.144), 3-year overall survival (89.3% vs. 70.4%, p = 0.08), and 5-year overall survival (78.6% vs. 59.3%, p = 0.121). The total rates of mortality and recurrence between the two groups were comparable (8/28 vs. 11/27, p = 0.343).
Conclusion: Administration of DEX during laparoscopic resection of colorectal cancer had a nonsignificant trend toward improved overall survival and disease-free survival.
Clinical Trial Registration: http://www.chictr.org.cn/, identifier ChiCTRIOR-15006518.
Introduction
Surgical resections are the major treatment for most solid tumors and are associated with patients’ long-term functionality and quality of life. Perioperative treatment has shown great potential for influencing postoperative outcomes of cancer patients. For instance, intraoperative local anesthetic infusion would increase cancer-specific mortality in colon resections (1), and propofol-based total intravenous anesthesia was associated with better overall survival compared to volatile anesthesia in oncological patients (2). However, the effect of different anesthesia methods and anesthetics on the long-term prognosis of oncological patients remains controversial (3–5).
In recent years, dexmedetomidine (DEX), a highly selective alpha2 adrenoceptor agonist, has been widely applied in clinical anesthesia settings, including in oncological patients (6–8). However, whether DEX is reasonably used in tumor resections remains controversial. Some recent investigations suggested that DEX could promote tumor cell proliferation (9–11), metastasis, and migration in vitro (12, 13), and even decrease the overall postoperative survival in oncological patients who underwent lung resections (14), whereas others found that DEX would attenuate tumor cell metastasis and progression in the perioperative period (15–17). Regarding these controversial reports, there is still a notable lack of high-quality clinical studies to clarify the effects of DEX on the long-term prognosis of cancer patients.
In a previous study, we examined the immediate effects of administering DEX during elective laparoscopic resection of colorectal cancer. The findings indicated that DEX improved postoperative gastrointestinal motility function and resulted in more stable hemodynamics throughout the surgery (18). In the current study, we conducted a 5-year follow-up analysis of the same cohort to investigate the impact of intraoperative DEX on long-term survival and tumor recurrence following laparoscopic resection of colorectal cancer.
Methods
The present study was carried out in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Third Affiliated Hospital of Sun Yat-Sen University (approval number: [2015]02-95-02). The study was registered on the Chinese Clinical Trial Registry (www.chictr.org) on June 7, 2015 (registration number: ChiCTRIOR-15006518). The trial protocol, design, and short-term outcomes of the randomized double-blind clinical trial have been reported previously (18).
A total of 60 patients undergoing elective laparoscopic colorectal resection at the institution (The Third Affiliated Hospital, Sun Yat-Sen University, China) between June 2015 and December 2015 were randomly assigned to the DEX group and the control group. All patients were operated on under the same general anesthesia protocol as described previously (18). All surgical procedures were performed by the same surgical group. In the DEX group, a loading dose of DEX (1 μg/kg) was given before induction for 10 min, followed by continuous intraoperative infusion (0.3 μg/kg/h). The patients in the control group were given the same volume of saline instead. Patients who met the following criteria were excluded in our previous research: gastrointestinal motility disorder; abdominal surgery history; bradyarrhythmia including sick sinus syndrome, sinus bradycardia or atrioventricular block; long-term administration of sedatives; psychiatric or neurologic comorbidity; hepatic or renal dysfunction; or distant metastasis.
A follow-up analysis of postoperative mortality and tumor recurrence was conducted in November 2021. Medical records were extracted from the hospital information system (HIS), and telephone follow-ups were utilized to access patient information. Patients who had benign lesions, non-malignant polyps, or Stage IV metastatic disease were not included in the follow-up analysis. Survival rate was calculated from the date of surgery until the date of death resulting from any cause. The duration of disease-free survival was measured from the date of surgery to the date of recurrence or death due to any cause. All-cause mortality was defined as death by any cause, while cancer-specific mortality was defined as death due to metastatic progression. The types of recurrence were classified as locoregional or distant. The duration between the date of surgery and the date of recurrence was defined as the time to recurrence. Patients with no evidence of recurrence at the time of death were censored on the date of patients’ death, while patients who remained alive at the time of analysis were censored at the end date of the follow-up period.
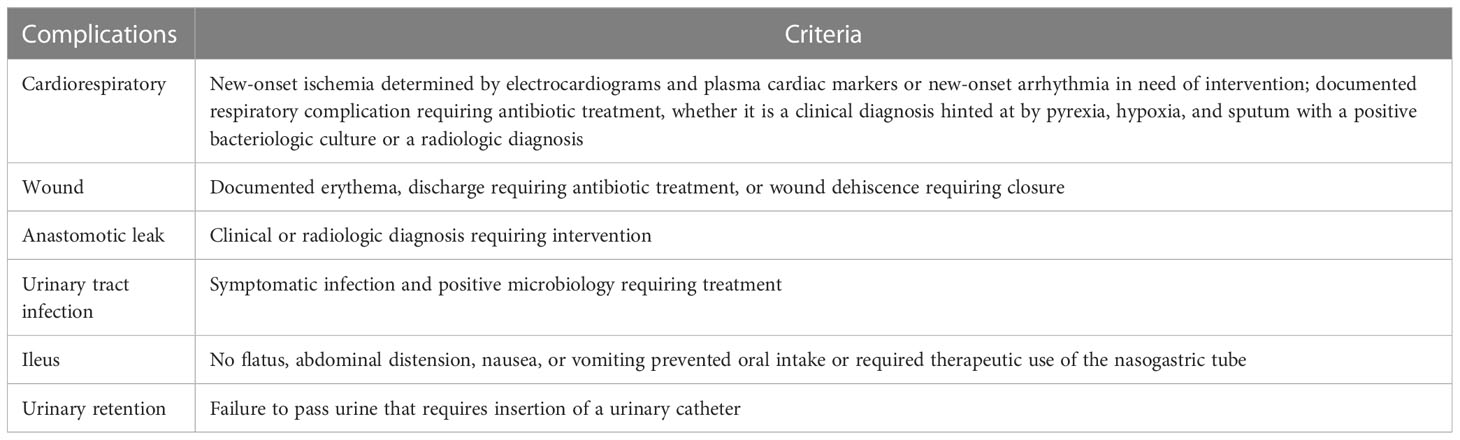
Baseline characteristics compared between the two groups included age, gender, body mass index (BMI), American Society of Anesthesiologists Physical Status Classification (ASA grade), type of operation, American Joint Committee on Cancer (AJCC) stage, tumor pathology, and adjuvant chemotherapy treatment. To ensure that recorded postoperative complications up to 30 days after surgery were comparable in both groups, specific complications were defined according to the criteria shown in Table 1 (19). The Clavien-Dindo classification system (20) was used to grade postoperative complications. If a patient experienced multiple complications, the highest grade was considered for analysis.
Statistical analysis
Statistical analysis was conducted using SPSS 19.0 software (SPSS Inc., Chicago, IL). One-sample Kolmogorov-Smirnov test was performed to assess the normality of the quantitative data. Mean ± standard deviation (SD) was used to describe quantitative variables that followed a normal distribution, and the T-test was utilized to compare the differences between groups. Categorical data or data without normal distribution were presented as median (interquartile range) or counts and compared by Fisher’s exact test for categorical variables or otherwise by Mann–Whitney U test. Survival differences between groups were assessed by Kaplan-Meier curves and analyzed using the Mantel-Cox test. Statistical significance was defined a priori as a p-value < 0.05.
Results
Out of the 60 patients, 55 from the previous randomized clinical trial were included in the follow-up analysis. Five subjects were excluded from the analysis because of metastatic tumor at the time of operation. In total, 28 patients received intraoperative DEX, while 27 received the same dose of saline.
Baseline characteristics of the study population
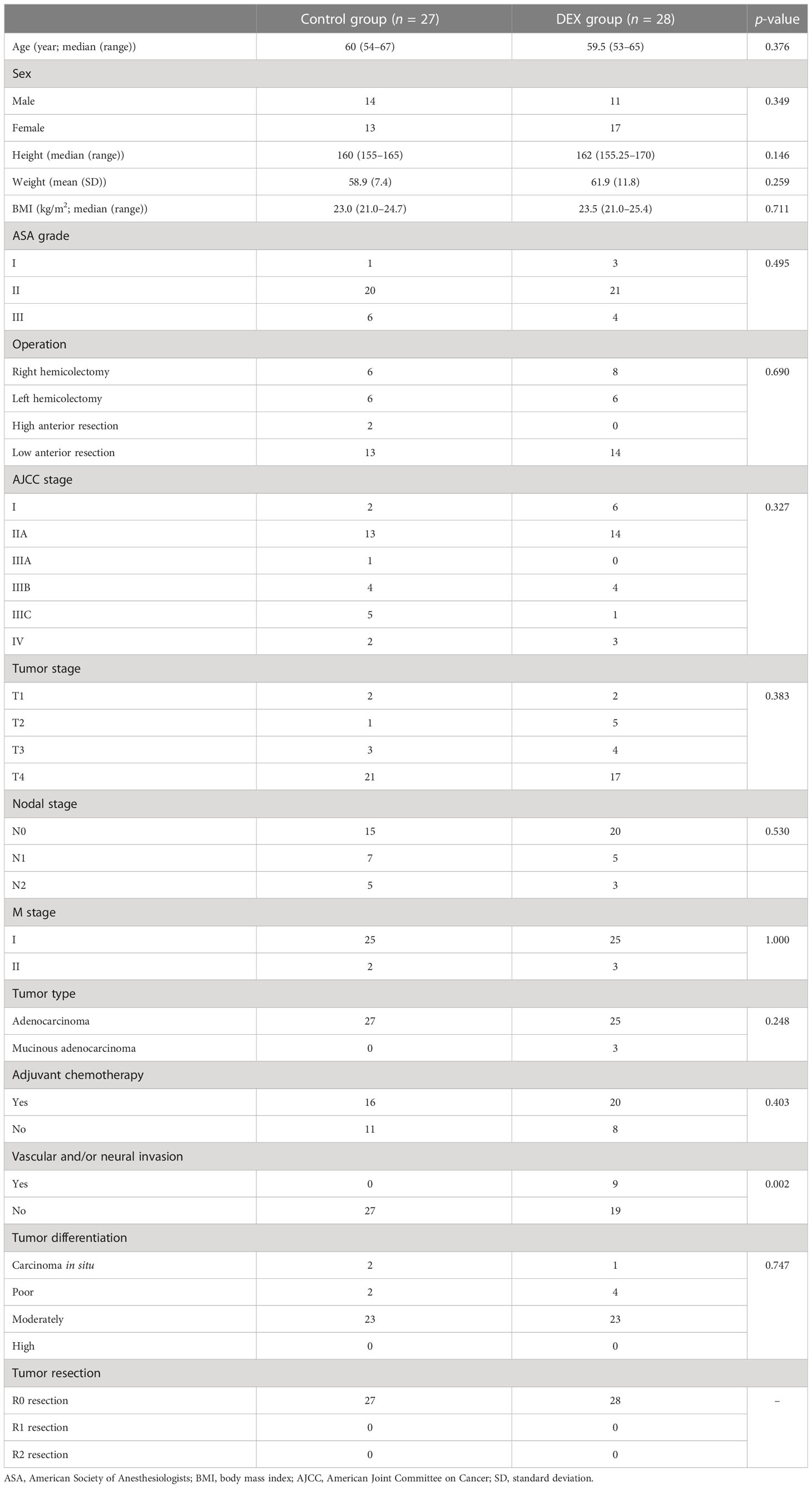
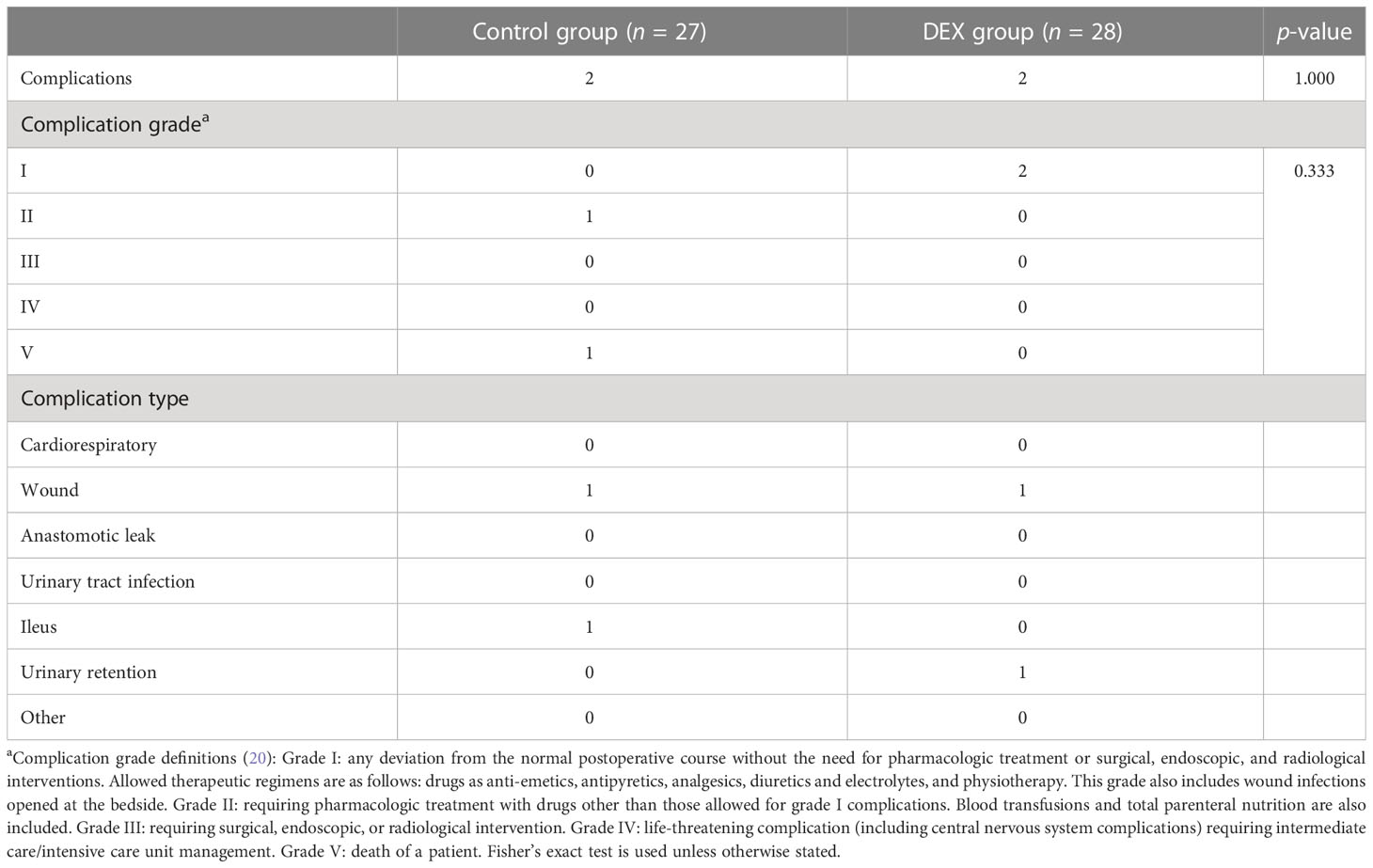
Baseline characteristics between the two groups are listed in Table 2. Demographic characteristics were comparable between the two groups in age, gender, height, weight, BMI, ASA grade, operation type, tumor stage, and adjuvant chemotherapy treatment. The majority tumor type was adenocarcinoma at stages II or III. All patients underwent R0 resection. Tumor differentiation between the two groups was comparable. However, there was a significant difference between the two groups in vascular and/or neural invasion of the tumor, with more patients in the DEX group having vascular and/or neural invasion of the tumor (9/28 vs. 0/27, p = 0.002). There were no significant differences in either the grade or type of postoperative complications observed between the groups (Table 3).
Primary and secondary outcomes
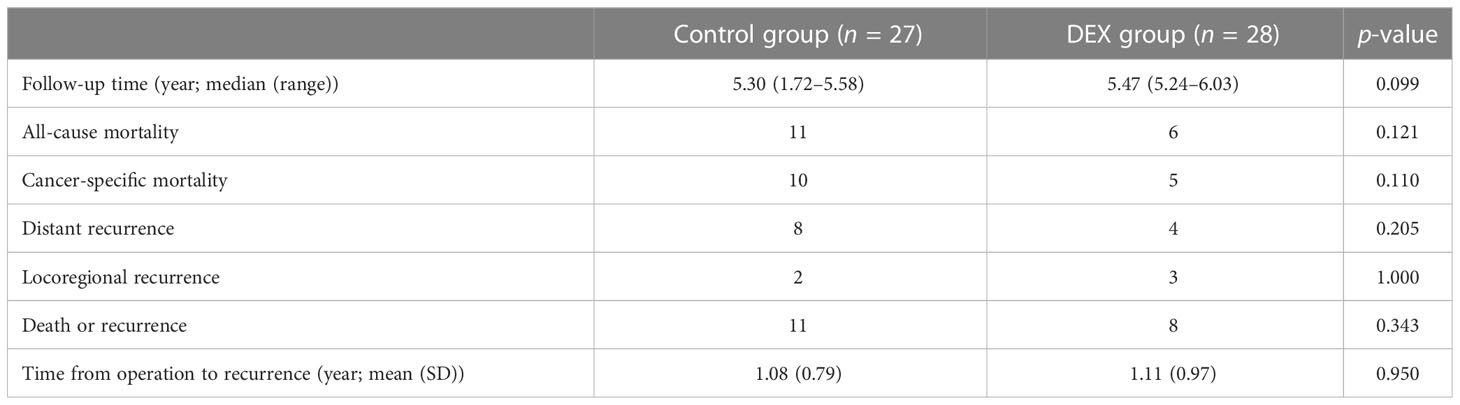
By the time of analysis, the median duration of the follow-up was 5.3 years (1.72–5.58 years) in the control group and 5.47 years (5.24–6.03 years) in the DEX Group (Table 4). The primary outcome, the overall survival, is shown in Figure 1. The study did not demonstrate a statistically significant benefit for overall survival in 5 years, but rather a trend towards an increase in survival of the DEX group, which was demonstrated by relatively higher 1-year overall survival (96.4% vs. 88.9%, p = 0.282), 2-year overall survival (89.3% vs. 74.1%, p = 0.144), 3-year overall survival (89.3% vs. 70.4%, p = 0.08), and 5-year overall survival (78.6% vs. 59.3%, p = 0.121). Similarly, there was also a nonsignificant trend towards improved disease-free survival in DEX group in 1 (85.7% vs. 77.8%, p = 0.446), 2 (78.6% vs. 66.7%, p = 0.322), 3 (75.0% vs. 59.3%, p = 0.214), and 5 years (71.4% vs. 59.3%, p = 0.343).
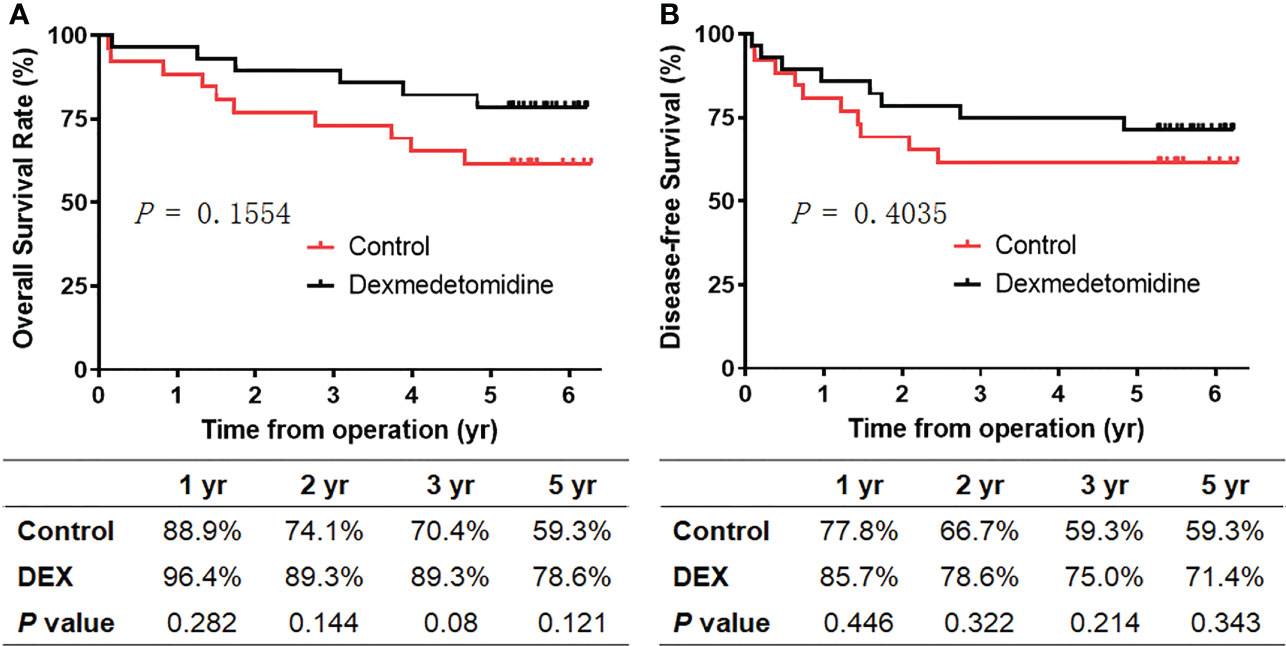
Figure 1 Survival of patients between the two groups. (A) Overall survival. (B) Disease-free survival. Kaplan–Meier curves showing overall survival and disease-free survival for patients receiving intraoperative dexmedetomidine (black line) or saline (red line).
Consistently, the all-cause mortality (6/28 vs. 11/27, p = 0.121) and cancer-specific mortality (5/28 vs. 10/27, p = 0.110) in the DEX group were relatively lower during the follow‐up period, though there were no significant differences (Table 4). Meanwhile, compared with the control group, there was a trend toward a lower rate of tumor distant recurrence in the DEX group (4/28 vs. 8/27, p = 0.205). The total rates of mortality and recurrence between the two groups were comparable (8/28 vs. 11/27, p = 0.343), as well as the rate of locoregional recurrence (3/28 vs. 2/27, p = 1.000). Moreover, there was no significant difference in the time from operation to recurrence between the two groups (1.08 (0.79) years vs.1.11 (0.97) years, p = 0.95).
Discussion
This study tried to analyze the follow-up of the patients involved in a previously published randomized controlled trial who were operated on for colorectal cancer and who had DEX during the surgical procedure. We compared the long-term outcomes of patients who had DEX vs. those who had saline instead, after 5 years of follow-up. The results showed a nonsignificant trend toward improved overall survival and disease-free survival in the DEX group compared with the control group. The total rates of mortality and cancer recurrence between the two groups were comparable. However, the postoperative pathological results showed a significant difference in vascular and/or neural invasion of the tumor, there were more patients having vascular and/or neural invasion of the tumor in the DEX group. Patients receiving DEX had relatively lower all-cause mortality, cancer-specific mortality, and rate of distant recurrence, though not statistically different. However, the sample of the study was too small to get such results and conclusions. It would be more significant to wait and add more patients.
Being one of the most effective treatments for most solid tumors, surgical resection has been reported to potentially promote tumor metastases by different mechanisms, including the increased risks of micro-metastasis and the formation of new metastatic foci when shedding tumor lesions. Stress-related immunity suppression, the trauma-related release of growth factors to facilitate tumor cell proliferation, attenuated inhibition of angiogenesis after primary tumor removal, and the complex effect of anesthetics have also been reported to be involved (2, 21–24). The introduction of Enhanced Recovery After Surgery (ERAS) has prompted an increased focus among anesthesiologists on the impact of perioperative interventions on the long-term prognosis of cancer patients (25). There is growing evidence suggesting that perioperative care and different anesthetics can influence long-term oncological outcomes (26). For instance, it was suggested that patients who received propofol and sevoflurane in general anesthesia were associated with better overall survival than those who received desflurane alone (2). Although DEX has been shown to promote tumorigenesis in neurogliomas and lung carcinomas, breast cancer, and colon cancers (12, 27), others suggested that DEX could lower the tumor weight and tumor burden in xenograft mice with ovarian cancer (28), and repressed esophageal cancer cell proliferation in vivo (29). Despite the controversial in vivo results, the effect of DEX on long-term survival and tumor recurrence after laparoscopic resection of colorectal cancer has not been evaluated in the clinical setting.
Being a widely applied anesthesia adjuvant drug, administration of DEX has appeared to be associated with lower mortality in cardiac surgery and demonstrated a trend toward reduced cardiac complications in non-cardiac surgery (30–32). In a previous study conducted by our team, it was demonstrated that administering DEX during the intraoperative period improved the recovery of gastrointestinal motility function following laparoscopic resection of colorectal cancer (18). Vascular and neural infiltrations are known to be ominous prognostic factors in the tumor. The presence of vascular and/or neural invasion is associated with worse 5-year cancer-specific survival and worse 5-year overall survival in stages III and IV patients (33, 34). Although more patients in the DEX group had neurovascular invasion, there was no significant difference between the two groups in survival and mortality. Surprisingly, it presented a trend toward an increase in overall survival and disease-free survival in the DEX group. The study suggested that intraoperative administration of DEX may have potential benefits for the long-term prognosis of patients undergoing laparoscopic resection of colorectal cancer, which is consistent with the results of its recent application in uterine cancer surgery (35), but contradictory to what is biologically plausible based on some in vivo evidence (27, 36).
The contradictory findings could potentially be attributed to variations in the study subjects. It has been suggested that DEX may inhibit the hypothalamic-pituitary-adrenal (HPA) axis and reduce sympathetic activation (37). Surgical stress has been reported to activate the HPA axis and sympathoadrenal responses, which promote the expression of adrenoreceptors on T cells (38, 39), facilitate T cells to differentiate from Th1 into Th2 cells, thus altering the balance between the two subtypes, and result in inhibition of immune function (40, 41). Increasing evidence confirmed that administration with DEX was associated with improved postoperative immunosuppression, as reflected by the increased CD4+:CD8+ ratio and Th1:Th2 ratio (42, 43), and the results were also confirmed in the patients with colorectal cancer (38, 44).
Notably, we found the incidence of postoperative complications within 30 days after surgery in our study to be lower than in other reports (1, 26). We think this may be attributed to the superb technical skills of our gastrointestinal surgical team (45), who are devoted to applying total mesorectal excision with preservation of Denonvilliers’ fascia (iTME) in laparoscopic colorectal resection, which has shown to improve postoperative urogenital function (46).
This study has several limitations. Firstly, given that the initial randomized controlled trial was designed to detect postoperative intestinal function, The primary objective of the original study was not to assess long-term survival and cancer recurrence rates. Consequently, the sample size was limited, and the conclusions that can be drawn from this follow-up study are of restricted scope. As such, it should be noted that this study is exploratory in nature and serves to generate hypotheses for further investigation. A retrospective cohort study enrolling more patients who underwent laparoscopic resection of colorectal cancer could be conducted in the near future to confirm the current hypothesis. However, the inclusion and exclusion criteria, the dosage of dexmedetomidine, and the difference in surgical and anesthesia groups are all confounding factors that are difficult to control. Thus, it was difficult for us to expand the sample size for this study. A further multicenter randomized controlled study with a larger sample size would help to confirm the effects of dexmedetomidine on all-cause mortality and recurrence among patients who undergo laparoscopic resection for colorectal cancer. Secondly, we did not collect detailed information on the mediation and surgery history of the patients, and whether the patients in the control group also received DEX during the 5-year follow-up period was unclear; this might be another confounder. Despite its limitations, the initial randomized controlled trial design has enhanced the analysis in this study by ensuring subject randomization, which creates equivalent groups and minimizes the chance of significant confounding variables.
In summary, administration of DEX during laparoscopic resection of colorectal cancer had a nonsignificant trend towards improved overall survival and disease-free survival. The small sample size may limit statistically positive findings in the study. Studies with larger sample sizes should be developed to verify the results.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Third Affiliated Hospital of Sun Yat-Sen University (approval number: [2015]02-95-02). The patients/participants provided their written informed consent to participate in this study.
Author contributions
JH: data collection, data analysis, and write-up of the manuscript. CG and XX: data collection, analysis, and interpretation. LC: study design, data analysis, and interpretation. YZ, XL, YL and XZ: data collection and critical review of the manuscript. PH, SZ, and CC: study conception, study design, data analysis, and interpretation and critical review of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported partly by the National Natural Science Foundation of China (Grant No. 82102297), the Natural Science Foundation of Guangdong Province (Grant Nos. 2021A1515011827, 2022A1515012603, and 2018A0303130224), and the Young Talent Support Project of the Guangzhou Association for Science and Technology (Grant No. QT20220101257).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. MacFater WS, Xia W, Barazanchi AWH, MacFater HS, Lightfoot N, Svirskis D, et al. Association between perioperative intraperitoneal local anaesthetic infusion and long-term survival and cancer recurrence after colectomy: Follow-up analysis of a previous randomized controlled trial. ANZ J Surg (2020) 90(5):802–6. doi: 10.1111/ans.15753
2. Chang CY, Wu MY, Chien YJ, Su IM, Wang SC, Kao MC. Anesthesia and long-term oncological outcomes: A systematic review and meta-analysis. Anesth Analg (2021) 132(3):623–34. doi: 10.1213/ane.0000000000005237
3. Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: A retrospective analysis. Anesthesiology. (2016) 124(1):69–79. doi: 10.1097/aln.0000000000000936
4. Abdallah FW, Wijeysundera DN. Anaesthetic interventions and long-term tumour recurrence. Lancet (2019) 394(10211):1781–2. doi: 10.1016/s0140-6736(19)32314-1
5. Lai R, Peng Z, Chen D, Wang X, Xing W, Zeng W, et al. The effects of anesthetic technique on cancer recurrence in percutaneous radiofrequency ablation of small hepatocellular carcinoma. Anesth Analg (2012) 114(2):290–6. doi: 10.1213/ANE.0b013e318239c2e3
6. Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs (2000) 59(2):263–8. doi: 10.2165/00003495-200059020-00012
7. Blaudszun G, Lysakowski C, Elia N, Tramèr MR. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: Systematic review and meta-analysis of randomized controlled trials. Anesthesiology (2012) 116(6):1312–22. doi: 10.1097/ALN.0b013e31825681cb
8. Deutsch E, Tobias JD. Hemodynamic and respiratory changes following dexmedetomidine administration during general anesthesia: Sevoflurane vs desflurane. Paediatr Anaesth (2007) 17(5):438–44. doi: 10.1111/j.1460-9592.2006.02139.x
9. Bruzzone A, Piñero CP, Rojas P, Romanato M, Gass H, Lanari C, et al. α(2)-adrenoceptors enhance cell proliferation and mammary tumor growth acting through both the stroma and the tumor cells. Curr Cancer Drug Targets (2011) 11(6):763–74. doi: 10.2174/156800911796191051
10. Dahmani S, Rouelle D, Gressens P, Mantz J. Effects of dexmedetomidine on hippocampal focal adhesion kinase tyrosine phosphorylation in physiologic and ischemic conditions. Anesthesiology (2005) 103(5):969–77. doi: 10.1097/00000542-200511000-00011
11. Chen HY, Li GH, Tan GC, Liang H, Lai XH, Huang Q, et al. Dexmedetomidine enhances hypoxia-induced cancer cell progression. Exp Ther Med (2019) 18(6):4820–8. doi: 10.3892/etm.2019.8136
12. Chi M, Shi X, Huo X, Wu X, Zhang P, Wang G. Dexmedetomidine promotes breast cancer cell migration through Rab11-mediated secretion of exosomal TMPRSS2. Ann Transl Med (2020) 8(8):531. doi: 10.21037/atm.2020.04.28
13. Lavon H, Matzner P, Benbenishty A, Sorski L, Rossene E, Haldar R, et al. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth (2018) 120(1):188–96. doi: 10.1016/j.bja.2017.11.004
14. Cata JP, Singh V, Lee BM, Villarreal J, Mehran JR, Yu J, et al. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol (2017) 33(3):317–23. doi: 10.4103/joacp.JOACP_299_16
15. Cai QH, Tang Y, Fan SH, Zhang ZF, Li H, Huang SQ, et al. In vivo effects of dexmedetomidine on immune function and tumor growth in rats with ovarian cancer through inhibiting the p38MAPK/NF-κB signaling pathway. BioMed Pharmacother. (2017) 95:1830–7. doi: 10.1016/j.biopha.2017.09.086
16. Zheng L, Zhao J, Zheng L, Jing S, Wang X. Effect of dexmedetomidine on perioperative stress response and immune function in patients with tumors. Technol Cancer Res Treat (2020) 19:1533033820977542. doi: 10.1177/1533033820977542
17. Huang L, Qin C, Wang L, Zhang T, Li J. Effects of dexmedetomidine on immune response in patients undergoing radical and reconstructive surgery for oral cancer. Oncol Lett (2021) 21(2):106. doi: 10.3892/ol.2020.12367
18. Chen C, Huang P, Lai L, Luo C, Ge M, Hei Z, et al. Dexmedetomidine improves gastrointestinal motility after laparoscopic resection of colorectal cancer: A randomized clinical trial. Med (Baltimore) (2016) 95(29):e4295. doi: 10.1097/MD.0000000000004295
19. Zargar-Shoshtari K, Connolly AB, Israel LH, Hill AG. Fast-track surgery may reduce complications following major colonic surgery. Dis Colon Rectum (2008) 51(11):1633–40. doi: 10.1007/s10350-008-9386-1
20. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg (2009) 250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2
21. Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer (1999) 80(6):880–8. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y
22. Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol (2003) 10(8):972–92. doi: 10.1245/aso.2003.02.007
23. Tsuchiya Y, Sawada S, Yoshioka I, Ohashi Y, Matsuo M, Harimaya Y, et al. Increased surgical stress promotes tumor metastasis. Surgery (2003) 133(5):547–55. doi: 10.1067/msy.2003.141
24. van der Bij GJ, Oosterling SJ, Beelen RH, Meijer S, Coffey JC, van Egmond M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg (2009) 249(5):727–34. doi: 10.1097/SLA.0b013e3181a3ddbd
25. Chen C, Li X, Luo G, Zhou S, Hei Z. Realising the full potential of anaesthesiology to promote enhanced recovery after surgery programmes in China. Br J Anaesth. (2021) 126(5):e157–9. doi: 10.1016/j.bja.2021.01.016
26. Singh PP, Lemanu DP, Taylor MH, Hill AG. Association between preoperative glucocorticoids and long-term survival and cancer recurrence after colectomy: Follow-up analysis of a previous randomized controlled trial. Br J Anaesth (2014) 113 Suppl 1:i68–73. doi: 10.1093/bja/aet577
27. Wang C, Datoo T, Zhao H, Wu L, Date A, Jiang C, et al. Midazolam and dexmedetomidine affect neuroglioma and lung carcinoma cell biology In vitro and in vivo. Anesthesiology (2018) 129(5):1000–14. doi: 10.1097/aln.0000000000002401
28. Shin S, Kim KJ, Hwang HJ, Noh S, Oh JE, Yoo YC. Immunomodulatory effects of perioperative dexmedetomidine in ovarian cancer: An In vitro and xenograft mouse model study. Front Oncol (2021) 11:722743. doi: 10.3389/fonc.2021.722743
29. Hu Y, Qiu LL, Zhao ZF, Long YX, Yang T. Dexmedetomidine represses proliferation and promotes apoptosis of esophageal cancer cells by regulating c-myc gene expression via the ERK signaling pathway. Eur Rev Med Pharmacol Sci (2021) 25(2):950–6. doi: 10.26355/eurrev_202101_24664
30. Ji F, Li Z, Young N, Moore P, Liu H. Perioperative dexmedetomidine improves mortality in patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth (2014) 28(2):267–73. doi: 10.1053/j.jvca.2013.06.022
31. Ji F, Li Z, Nguyen H, Young N, Shi P, Fleming N, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation (2013) 127(15):1576–84. doi: 10.1161/CIRCULATIONAHA.112.000936
32. Biccard BM, Goga S, de Beurs J. Dexmedetomidine and cardiac protection for non-cardiac surgery: A meta-analysis of randomised controlled trials. Anaesthesia. (2008) 63(1):4–14. doi: 10.1111/j.1365-2044.2007.05306.x
33. Knijn N, van Exsel UEM, de Noo ME, Nagtegaal ID. The value of intramural vascular invasion in colorectal cancer - a systematic review and meta-analysis. Histopathology (2018) 72(5):721–8. doi: 10.1111/his.13404
34. Gibson KM, Chan C, Chapuis PH, Dent OF, Bokey L. Mural and extramural venous invasion and prognosis in colorectal cancer. Dis Colon Rectum. (2014) 57(8):916–26. doi: 10.1097/dcr.0000000000000162
35. Cho JS, Seon K, Kim MY, Kim SW, Yoo YC. Effects of perioperative dexmedetomidine on immunomodulation in uterine cancer surgery: A randomized, controlled trial. Front Oncol (2021) 11:749003. doi: 10.3389/fonc.2021.749003
36. Su X, Fan Y, Yang L, Huang J, Qiao F, Fang Y, et al. Dexmedetomidine expands monocytic myeloid-derived suppressor cells and promotes tumour metastasis after lung cancer surgery. J Transl Med (2018) 16(1):347. doi: 10.1186/s12967-018-1727-9
37. Mantz J, Josserand J, Hamada S. Dexmedetomidine: New insights. Eur J Anaesthesiol. (2011) 28(1):3–6. doi: 10.1097/EJA.0b013e32833e266d
38. Scanzano A, Cosentino M. Adrenergic regulation of innate immunity: A review. Front Pharmacol (2015) 6:171. doi: 10.3389/fphar.2015.00171
39. Wang K, Wu M, Xu J, Wu C, Zhang B, Wang G, et al. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: Systematic review and meta-analysis. Br J Anaesth (2019) 123(6):777–94. doi: 10.1016/j.bja.2019.07.027
40. Irwin M. Stress-induced immune suppression: Role of brain corticotropin releasing hormone and autonomic nervous system mechanisms. Adv Neuroimmunol (1994) 4(1):29–47. doi: 10.1016/s0960-5428(06)80188-9
41. Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA. Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora's box? Mol Med (2008) 14(3-4):195–204. doi: 10.2119/2007-00105
42. Wang K, Li C. Effects of dexmedetomidine on inflammatory factors, T lymphocyte subsets and expression of NF-κB in peripheral blood mononuclear cells in patients receiving radical surgery of colon carcinoma. Oncol Lett (2018) 15(5):7153–7. doi: 10.3892/ol.2018.8205
43. Yang XH, Bai Q, Lv MM, Fu HG, Dong TL, Zhou Z. Effect of dexmedetomidine on immune function of patients undergoing radical mastectomy: A double blind and placebo control study. Eur Rev Med Pharmacol Sci (2017) 21(5):1112–6.
44. Tang Y, Liu J, Huang X, Ding H, Tan S, Zhu Y. Effect of dexmedetomidine-assisted intravenous inhalation combined anesthesia on cerebral oxygen metabolism and serum Th1/Th2 level in elderly colorectal cancer patients. Front Surg (2021) 8:832646. doi: 10.3389/fsurg.2021.832646
45. Wei B, Zheng Z, Fang J, Xiao J, Han F, Huang M, et al. Effect of denonvilliers' fascia preservation versus resection during laparoscopic total mesorectal excision on postoperative urogenital function of Male rectal cancer patients: Initial results of Chinese PUF-01 randomized clinical trial. Ann Surg (2021) 274(6):e473–80. doi: 10.1097/sla.0000000000004591
Keywords: colorectal resection, dexmedetomidine, recurrence, survival, colorectal cancer
Citation: Hu J, Gong C, Xiao X, Chen L, Zhang Y, Li X, Li Y, Zang X, Huang P, Zhou S and Chen C (2023) Association between intraoperative dexmedetomidine and all-cause mortality and recurrence after laparoscopic resection of colorectal cancer: Follow-up analysis of a previous randomized controlled trial. Front. Oncol. 13:906514. doi: 10.3389/fonc.2023.906514
Received: 28 March 2022; Accepted: 17 March 2023;
Published: 30 March 2023.
Edited by:
Suren Soghomonyan, Wexner Medical Center, The Ohio State University, United StatesReviewed by:
Martin Hoffmann, Asklepios Paulinen Clinic Wiesbaden, GermanyIrene Scalera, University of Bari Medical School, Italy
Copyright © 2023 Hu, Gong, Xiao, Chen, Zhang, Li, Li, Zang, Huang, Zhou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaojin Chen, Y2hlbmNoajI4QG1haS5zeXN1LmVkdS5jbg==; Shaoli Zhou, MTM2MTAyNzIzMDhAMTM5LmNvbQ==; Pinjie Huang, aHBqaWVAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jingping Hu†
Jingping Hu† Shaoli Zhou
Shaoli Zhou Chaojin Chen
Chaojin Chen