- 1The Department of Obstetrics and Gynecology, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Zhengzhou Key Laboratory of Endometrial Disease Prevention and Treatment, Zhengzhou Science and Technology Bureau, Zhengzhou, China
Ovarian mature teratoma represents a benign ovarian tumor, while ovarian yolk sac tumor (YST, endodermal sinus tumor) is a rare malignant tumor predominantly affecting young women, often associated with a grim prognosis post-metastasis. Both ovarian mature teratoma and ovarian YST are germ cell tumors. There are few studies on the correlation between ovarian YST and mature teratoma. Recurrence or malignant transformation may occur following the surgical intervention for ovarian mature teratoma. However, the occurrence of YST subsequent to such procedures is notably rare. In this investigation, we reported a case involving a 24-year-old unmarried woman with both mature ovarian teratoma and YST within a brief 1-year interval. Regular reexamination protocols facilitated the early-stage detection of YST. The patient underwent surgical treatment, chemotherapy, and measures to preserve ovarian function, resulting in a favorable prognosis. Our primary purpose is to distill clinical insights from the diagnostic and therapeutic journey of this patient. Our purpose is to enhance medical professionals’ awareness that YST may be secondary to mature teratoma. Additionally, we underscore the critical importance of routine postoperative surveillance for ovarian mature teratoma, emphasizing its pivotal role in early malignant tumor detection—a factor paramount to the prognosis of patients.
Introduction
Approximately 90% of ovarian cancers manifest as epithelial cell types, presenting a diverse array of histological variations. Conversely, the remaining 10% comprise non-epithelial ovarian cancers, which include a majority of germ cell tumors, sex cord-stromal tumors, and a subset of exceedingly rare entities such as small cell carcinomas. Ovary germ cell tumors are the most common ovarian neoplasms in women until 30 years of age, originating from germ cells. With the exception of certain tissue types, such as mature teratoma, the majority of ovarian germ cell tumors are malignant and are often diagnosed at the early stage (60%–70%) (1). Ovarian mature teratomas are the most common ovarian germ cell tumor (2), accounting for 10%–20% of ovarian tumors (3). Typically, these teratomas measure 5–10 cm, with only approximately 9% exceeding 15 cm (2). Malignant ovarian germ cell tumors (MOGCTs) are thought to originate from primordial germ cells, featuring inherited or somatic acquired alterations (4). MOGCTs accounts for 5% of ovarian germ cell tumors (5), principally in the teenage years. The pathogenesis of malignant ovarian germ cell tumors remains elusive, potentially related to genetic or environmental factors. Surgery or surgery combined with chemoradiotherapy can significantly improve the prognosis (6). Among MOGCTs, YST accounts for 14%–20% of ovarian malignant germ cell tumors (7), and the incidence is approximately 0.048/100,000 (8). YST is highly malignant and easy to early metastasize and relapse. YSTs often present challenges in treatment due to chemotherapy resistance upon recurrence. We have conducted extensive literature reviews. There are many studies on the secondary occurrence of YST in immature teratoma (9–12).
However, the occurrence of YST subsequent to mature teratoma surgery is rare. In the literature that we searched, there were no documented instances of secondary YST after ovarian mature teratoma surgery. We report a case of giant ovarian YST only 1 year after surgery for mature ovarian teratoma, accompanied by a comprehensive review of pertinent literature. The diagnosis and treatment of this patient are meticulously summarized and analyzed. The patient underwent both surgery and chemotherapy, with a favorable prognosis attributed to the early detection of the YST.
Case presentation
The patient, a 24-year-old unmarried woman with no sexual history, sought medical attention at our hospital in August 2019 following the discovery of a pelvic cyst during a physical examination. 3D transrectal ultrasonography showed a mixed echo measuring 128 mm × 111 mm × 109 mm at the right anterior quadrant of the uterus. Before the operation, pelvic magnetic resonance imaging (MRI) indicated a large solid cystic mixed-signal mass in the pelvis, exhibiting well-defined boundaries and measuring a maximum cross-section of 158.56 mm × 83.74 mm × 112.54 mm. The mass exhibited closely proximity to the right ovary. The right ovarian mature teratoma was removed by conducting a single-hole laparoscopic operation at our hospital. During the operation, no significant abnormalities were found in the left ovary and bilateral fallopian tubes. The postoperative pathology was mature cystic teratoma. The patient recovered well from the operation.
In October 2020, a routine physical examination revealed a left adnexa cyst in the patient with a maximum diameter of 3 cm. Then, the color ultrasound at our hospital identified an echoless area measuring approximately 35 mm × 14 mm in the left accessory region. By January 2021, a follow-up color ultrasound of our hospital exhibited a cystic mass with a range of 104 mm × 105 mm × 68mm in the left ovary, with a high echo approximately 41 mm × 33 mm in the mass. The level of α-fetoprotein (AFP) was 72.20 IU/mL. A pelvic MRI showed a vast solid cystic mass in the pelvis, featuring clumpy solid components within the capsule. The mass measured approximately 112 mm × 78 mm × 128 mm and exerted compression on the adjacent uterus, bowel duct, and the right oviduct and ovary, encapsulated in its entirety.
Because the patient had not yet married and had not given birth, both the patient and her parents expressed a strong desire to preserve her fertility function as much as possible. Considering the potential malignancy of the ovarian tumor, we discussed fertility preservation for the patient and made a plan with the Department of Pathology and the Reproductive Center before the operation. Ultrasound showed that the dominant follicle was in the right ovary, with an average number of follicles observed in both ovaries. The patient’s Anti-Müllerian Hormone (AMH) level measured 34.04 pmol/L, indicating a robust reserve function in both ovaries.
We performed an exploratory laparotomy, during which the left ovary exhibited a significant enlargement, measuring approximately 12 cm × 12 cm × 10 cm, with a smooth surface. The appearance of bilateral fallopian tubes and the right ovary was normal. There was approximately 50 ml of dark red bloody effusion in the pelvic cavity, with no abnormalities noted in the peritoneal of the pelvic abdominal cavity. A thorough examination of various surfaces, including the liver, spleen, ligaments, diaphragm, large omentum, and intestinal tubes, revealed no apparent abnormalities. A total of approximately 1,000 mL of dark red translucent liquid was aspirated from the left ovarian cyst, and the left ovarian cysts were completely excised. The yellow irregular meat-like tissue, approximately 4 cm × 4 cm, was identified in the ovarian cyst cavity. The intraoperative pathological analysis confirmed the presence of an ovarian YST. After consulting with the patient’s parent, the left fallopian tube ovary and large omentum were removed, and a multi-point biopsy on the pelvic peritoneum was conducted. The right ovarian cortex, approximately 1 cm × 1 cm, was excised for cryopreservation of the ovarian tissue. Simultaneously, the reproductive physician cryopreserved the oocytes from the left ovary.
The postoperative pathological examination was a yolk cystic tumor. The postoperative diagnosis was a stage IC left ovarian yolk sac tumor. After surgery, she underwent chemotherapy with a combination of bleomycin, etoposide, and cisplatin for four cycles. Gonadotropin-releasing hormone agonist (GnRH-a) was administered to protect her ovarian function throughout chemotherapy with a total of four administrations, spaced at 28-day intervals. AFP was normal after the first cycle of chemotherapy. She resumed menstruating more than 3 months after the last chemotherapy treatment. There has been no recurrence of the disease for 33 months follow-up to now.
Discussion
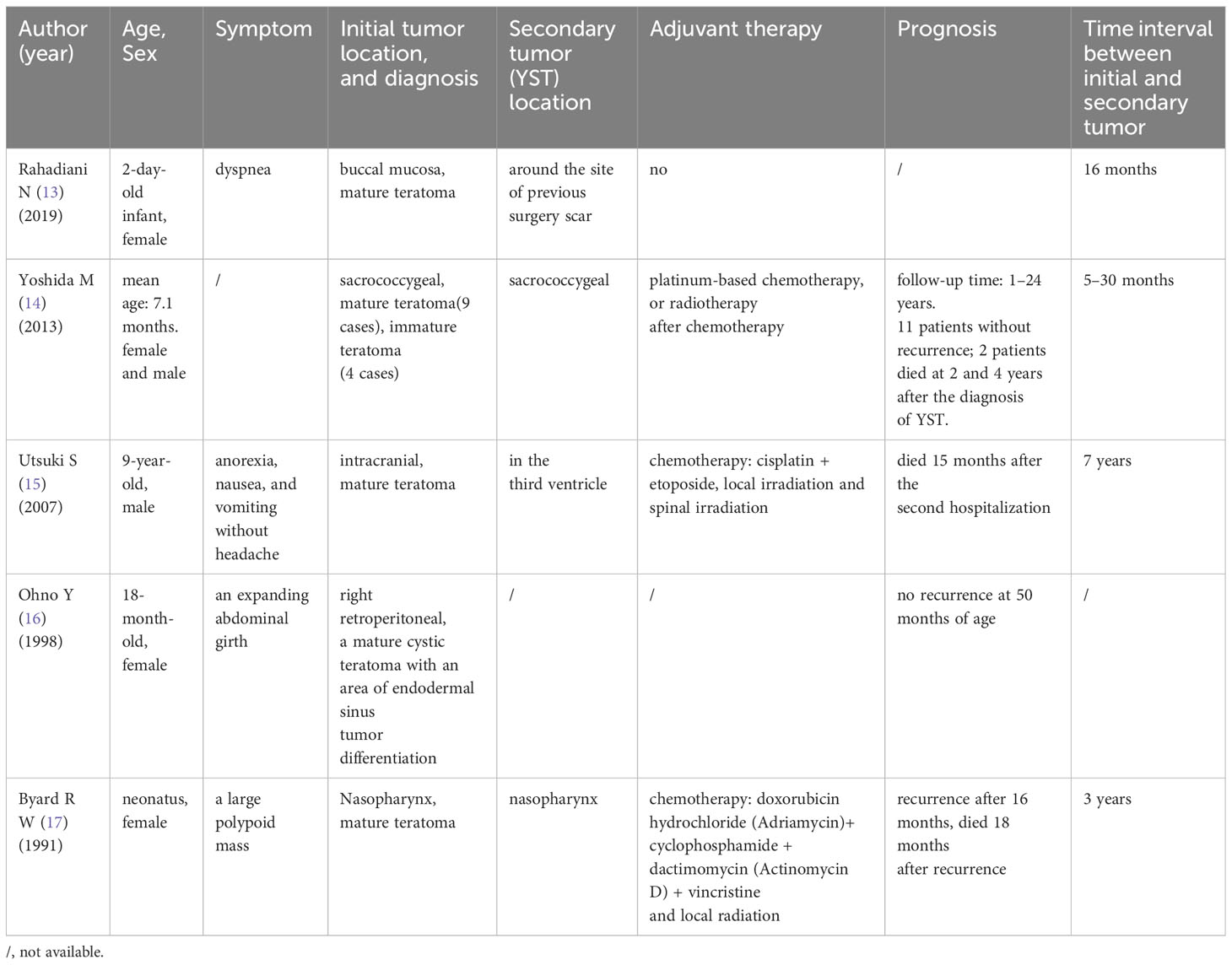
The occurrence of ovarian YST after the operation of ovarian mature teratoma is exceptionally rare. In this case, the patient sequentially developed ovarian mature teratoma and ovarian YST within only a 1-year interval. It has been documented that some non-ovarian YSTs are secondary to teratoma. We conducted extensive literature searches to gather clinical characteristics, summarizing them in Table 1. Yoshida’s study revealed that the rate of YST developing after sacrococcygeal teratoma in children was 5.4%, while there was no statistically significant difference in the incidence of secondary to mature teratoma or immature teratoma (5.2% vs. 6.4%) (14). The possible mechanisms of recurrent YST after the surgery of mature teratoma are outlined below. First, yolk sac tumor is considered as malignant transformation of teratoma (18, 19), and mature teratoma is malignant prelesion (16). The possibility of subsequent malignant development exists even for mature teratoma (17). Second, YST lesions may be microscopic and often cannot be positive for AFP, so they are easily ignored (20). Some researchers believe the YST develops from undetected small yolk sac lesions in the original teratoma (18, 21). Both views are considered to be forms of teratoma recurrence, regardless of the presence or absence of YST lesions in the primary teratoma. YST has a variety of histological patterns, and it is difficult to identify specific subtypes. Therefore, when the pathological specimen is a mature teratoma, it is necessary to thoroughly sample the tumor and carefully examine the pathological section to identify the malignant tumor components (22, 23). Third, Yoshida et al. hold a different perspective, suggesting that teratoma and secondary YST are metachronous multifocal germ cell tumors, which are the presence of multiple de novo tumors arising in different sites after long intervals, rather than the transformation of residual teratoma (14). AFP level plays a crucial role in the early diagnosis of YST and monitoring treatment effect (24). Even in postmenopausal patients with ovarian tumors, AFP is recommended to be tested for early detection of ovarian malignant germ cell tumors (25). It is recommended that patients with sacrococcygeal teratoma should be tested for AFP every 3 months for 3 years after surgery to facilitate early detection of YST (14). Activation and malignant transformation of mature teratoma may occur after 7 years (15). Serum AFP concentration above 100 ng/dL almost always indicates the presence of a YST focus. However, the detection of AFP is not emphasized in the clinical periodic review of ovarian mature teratoma (20). In this case, if AFP was detected at the same time when the left ovarian tumor volume was 3 cm, the yolk sac tumor would possibly be detected earlier. While the existence of ovarian YST alongside the previous ovarian mature teratoma may be a random occurrence, further research is warranted on the correlation between mature ovarian teratoma and YST.
After surgery, mature ovarian teratoma may recur in the ipsilateral or contralateral ovaries. The intermediate and long-term recurrence rate in one study was 4.2%. The risk of recurrence increases if mature ovarian teratoma is bilateral, multiple, above 8 cm in diameter, with bone and central nervous system components (26, 27). Various tissue components of mature teratoma will have a secondary malignant transformation potentiality, a phenomenon more common in postmenopausal women. The malignant transformation rate is approximately 0.17%–2%, with 80% developing into squamous cell carcinoma, carrying a poor prognosis (28). Overexpression of p53, incomplete tumor resection, and tumor grade are risk factors for ovarian teratoma recurrence (29). In this case, the right ovarian mature teratoma was removed in 2019. The tumor was sizable, and the operation was performed using transumbilical single-incision laparoscopy, heightening the risk of ovarian cyst rupture (30). After the rupture of the tumor capsule wall, even a large amount of irrigation for the abdominal cavity could not avoid the residue of the contents, which increased the possibility of secondary malignant tumors (31). For giant teratoma, pathological examination is particularly challenging for the detection of malignant tumor components. Therefore, intraoperative rupture of tumor components should be minimized even for benign ovarian tumors to avoid tumor residue.
YST is highly malignant, prone to metastasize in the early stage, and carries a poor prognosis upon recurrence. Due to the absence of specific diagnostic markers, it is difficult to diagnose before surgery. In this case, the patient underwent regular color ultrasound after the operation of the ovarian mature teratoma. She promptly consulted a doctor when the ovarian cyst was detected so that the yolk sac tumor could be detected at an early stage without metastasis. In this case, ultrasound examination at an interval of 3 months showed that the ovarian tumor increased rapidly, threefold the ovarian tumor’s initial volume. According to imaging examination and clinical characteristics, the possibility of malignancy could not be ruled out. For patients with ovarian tumors with rapid growth or large tumor volume in a short period, it may be caused by internal tumor bleeding or tissue necrosis (28), and physicians should be vigilant about whether it is a malignant tumor.
The preferred treatment option is surgery combined with chemotherapy for YST. As we know, high-grade serous ovarian cancer (HGSOC), constituting 75% of epithelial ovarian cancers, is highly chemosensitive, primarily characterized by uniform TP53 mutants (32). While ovarian YSTs rarely exhibit TP53 mutation (33), unlike HGSOC, they still demonstrate sensitivity to chemotherapy. Therefore, ovarian YST is sensitive to chemotherapy. Postoperative chemotherapy is recommended for all stages of ovarian YST to improve prognosis (34), and fertility preservation surgery is feasible regardless of stage. The thoroughness of initial surgical treatment and postoperative chemotherapy are independent risk factors for progression-free survival (35). Various factors, such as the malignant tumor itself, ovarian surgery, pelvic surgery, hyperthermic intraperitoneal chemotherapy, and chemoradiotherapy, may lead to a reduction in ovarian reserve function (36, 37). For MOGCTs, the fertility-sparing comprehensive surgical staging includes the excision of the affected unilateral salpingo-oophorectomy, preservation of the uterus and the contralateral ovary, or preservation of one or both normal ovarian tissues and uterus if both ovaries are involved, in addition to biopsy or excision of the omentum, and excision of lymph nodes depending on age and stage. Considering that YST is more common in children and young women, fertility-sparing surgery to preserve and protect fertility is particularly important for patients’ quality of life in the future (38, 39). For unilateral malignant germ cell tumors, even in an advanced stage, fertility preservation surgery can be performed (40). The patient was 24 years old and unmarried in the case, and corresponding measures were taken to protect ovarian function before, during, and after surgery.
For women with fertility requirements, multidisciplinary consultation can be conducted before surgery to reduce the misdiagnosis rate and avoid over-treatment. Hormone levels and color ultrasound can determine initial ovarian reserve function. During the operation, immature oocytes can be extracted from the ovary for cryopreservation (41), and ovarian cortex cryopreservation can be performed (42). Frozen ovarian tissues can be used to isolate follicles or for transplantation. Resuscitation transplantation of cryopreserved ovarian tissue can increase autologous hormone levels and the probability of natural pregnancy. However, there may be a potential risk of malignant tumor cells implantation during transplantation, although this risk is low in other malignant tumors except in leukemia patients (43). For post-pubertal patients and patients with delayed chemoradiotherapy, it is feasible to obtain mature oocytes by promoting ovulation after surgery. Immature oocyte cryopreservation can be performed in preadolescent girls and patients with hormone-sensitive tumors (44).
Given the numerous previous studies on the protection of ovarian function by GnRH-a, the patient in this case received GnRH-a to protect ovarian function before chemotherapy. The GnRH-a inhibits ovarian function, prevents ovarian follicle recruitment, and prevents follicle growth and ovulation. The GnRH-a suppresses the secretion of endogenous gonadotropic hormone and follicle-stimulating hormone (FSH) levels and puts the follicle cells in a dormant state (45, 46). Therefore, reducing the sensitivity of follicles to chemotherapy drugs can minimize the destruction of follicles induced by chemotherapy and reduce the accumulation of chemotherapy drugs in ovarian tissue to reduce the damage to the ovary during chemotherapy. However, the role of GnRH-a in protecting ovarian function in patients with malignant tumors remains controversial (47–49). In this case, appropriate measures were taken to protect the patient’s fertility before, during, and after the operation. The diagnostic and treatment process serves as a valuable learning experience for clinicians.
Conclusion
The genomic landscape and pathogenesis of ovarian YST remain elusive, posing challenges to study the diagnosis, treatment, and prognosis of the disease. The relationship between the occurrence of YST and ovarian mature teratoma cannot be determined. Because YST may be secondary to teratoma, patients with ovarian tumor after treatment of ovarian teratoma need to be vigilant about the possibility of YST. Attention should be paid to whether laparoscopic surgery increases the residual tumor lesions, especially single-incision laparoscopic surgery. Clinical surgeons are urged to minimize the exposure of tumor contents to the abdominal cavity during mature teratoma operations. Both physicians and patients should prioritize adherence to medical advice and engage in regular postoperative follow-up examinations, even for mature teratoma. These measures are paramount for the timely detection of ovarian malignant tumors, emphasizing the collaborative role of medical professionals and patients in ensuring comprehensive postoperative care.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
SL: Data curation, Investigation, Writing – original draft. JP: Data curation, Formal analysis, Writing – review & editing. YZ: Data curation, Investigation, Writing – review & editing. DL: Data curation, Writing – review & editing. LL: Conceptualization, Supervision, Writing – review & editing. MN: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Saani I, Raj N, Sood R, Ansari S, Mandviwala HA, Sanchez E, et al. Clinical challenges in the management of Malignant ovarian germ cell tumours. Int J Environ Res Public Health (2023) 20(12):6089. doi: 10.3390/ijerph20126089
2. Kim MJ, Kim NY, Lee DY, Yoon BK, Choi D. Clinical characteristics of ovarian teratoma: age-focused retrospective analysis of 580 cases. Am J Obstet Gynecol (2011) 205(1):32.e31–34. doi: 10.1016/j.ajog.2011.02.044
3. Matz MH. Benign cystic teratomas of the ovary. A review. Obstet Gynecol Surv (1961) 16:591–605. doi: 10.1097/00006254-196110000-00001
4. Kraggerud SM, Hoei-Hansen CE, Alagaratnam S, Skotheim RI, Abeler VM, Rajpert-De Meyts E, et al. Molecular characteristics of Malignant ovarian germ cell tumors and comparison with testicular counterparts: implications for pathogenesis. Endocr Rev (2013) 34(3):339–76. doi: 10.1210/er.2012-1045
5. Sagae S, Kudo R. Surgery for germ cell tumors. Semin Surg Oncol (2000) 19(1):76–81. doi: 10.1002/1098-2388(200007/08)19:1<76::aid-ssu12>3.0.co;2-b
6. Weinberg LE, Lurain JR, Singh DK, Schink JC. Survival and reproductive outcomes in women treated for Malignant ovarian germ cell tumors. Gynecol Oncol (2011) 121(2):285–9. doi: 10.1016/j.ygyno.2011.01.003
7. Shah JP, Kumar S, Bryant CS, Ali-Fehmi R, Malone JM Jr., Deppe G, et al. A population-based analysis of 788 cases of yolk sac tumors: A comparison of males and females. Int J Cancer (2008) 123(11):2671–5. doi: 10.1002/ijc.23792
8. van Leeuwen MT, Gurney H, Turner JJ, Turner SL, Pearson SA, Laaksonen MA, et al. Patterns and trends in the incidence of paediatric and adult germ cell tumours in Australia, 1982-2011. Cancer Epidemiol (2016) 43:15–21. doi: 10.1016/j.canep.2016.05.006
9. De Backer A, Madern GC, Hakvoort-Cammel FG, Haentjens P, Oosterhuis JW, Hazebroek FW. Study of the factors associated with recurrence in children with sacrococcygeal teratoma. J Pediatr Surg (2006) 41(1):173–81. doi: 10.1016/j.jpedsurg.2005.10.022
10. Ukiyama E, Endo M, Yoshida F, Tezuka T, Kudo K, Sato S, et al. Recurrent yolk sac tumor following resection of a neonatal immature gastric teratoma. Pediatr Surg Int (2005) 21(7):585–8. doi: 10.1007/s00383-005-1404-y
11. Gilcrease MZ, Brandt ML, Hawkins EP. Yolk sac tumor identified at autopsy after surgical excision of immature sacrococcygeal teratoma. J Pediatr Surg (1995) 30(6):875–7. doi: 10.1016/0022-3468(95)90770-x
12. Morinaga S, Nomori H, Kobayashi R, Atsumi Y. Well-differentiated adenocarcinoma arising from mature cystic teratoma of the mediastinum (teratoma with Malignant transformation). Report of a surgical case. Am J Clin Pathol (1994) 101(4):531–4. doi: 10.1093/ajcp/101.4.531
13. Rahadiani N, Krisnuhoni E, Stephanie M, Handjari DR. Extragonadal yolk sac tumor following congenital buccal mature cystic teratoma. J Oral Maxillofac Pathol (2019) 23(Suppl 1):49–53. doi: 10.4103/jomfp.JOMFP_127_18
14. Yoshida M, Matsuoka K, Nakazawa A, Yoshida M, Inoue T, Kishimoto H, et al. Sacrococcygeal yolk sac tumor developing after teratoma: a clinicopathological study of pediatric sacrococcygeal germ cell tumors and a proposal of the pathogenesis of sacrococcygeal yolk sac tumors. J Pediatr Surg (2013) 48(4):776–81. doi: 10.1016/j.jpedsurg.2012.08.028
15. Utsuki S, Oka H, Sagiuchi T, Shimizu S, Suzuki S, Fujii K. Malignant transformation of intracranial mature teratoma to yolk sac tumor after late relapse. Case report. J Neurosurg (2007) 106(6):1067–9. doi: 10.3171/jns.2007.106.6.1067
16. Ohno Y, Kanematsu T. An endodermal sinus tumor arising from a mature cystic teratoma in the retroperitoneum in a child: is a mature teratoma a premalignant condition? Hum Pathol (1998) 29(10):1167–9. doi: 10.1016/s0046-8177(98)90432-4
17. Byard RW, Smith CR, Chan HS. Endodermal sinus tumor of the nasopharynx and previous mature congenital teratoma. Pediatr Pathol (1991) 11(2):297–302. doi: 10.3109/15513819109064766
18. Derikx JP, De Backer A, van de Schoot L, Aronson DC, de Langen ZJ, van den Hoonaard TL, et al. Factors associated with recurrence and metastasis in sacrococcygeal teratoma. Br J Surg (2006) 93(12):1543–8. doi: 10.1002/bjs.5379
19. Rescorla FJ, Sawin RS, Coran AG, Dillon PW, Azizkhan RG. Long-term outcome for infants and children with sacrococcygeal teratoma: a report from the Childrens Cancer Group. J Pediatr Surg (1998) 33(2):171–6. doi: 10.1016/s0022-3468(98)90426-2
20. Heifetz SA, Cushing B, Giller R, Shuster JJ, Stolar CJ, Vinocur CD, et al. Immature teratomas in children: pathologic considerations: a report from the combined Pediatric Oncology Group/Children’s Cancer Group. Am J Surg Pathol (1998) 22(9):1115–24. doi: 10.1097/00000478-199809000-00011
21. Kops AL, Hulsker CC, Fiocco M, Zsiros J, Mavinkurve-Groothuis AMC, Looijenga LH, et al. Malignant recurrence after mature Sacrococcygeal teratoma: A meta-analysis and review of the literature. Crit Rev Oncol Hematol (2020) 156:103140. doi: 10.1016/j.critrevonc.2020.103140
22. Atwi D, Kamal M, Quinton M, Hassell LA. Malignant transformation of mature cystic teratoma of the ovary. J Obstet Gynaecol Res (2022) 48(12):3068–76. doi: 10.1111/jog.15409
23. Young RH, Ulbright TM, Policarpio-Nicolas ML. Yolk sac tumor with a prominent polyvesicular vitelline pattern: a report of three cases. Am J Surg Pathol (2013) 37(3):393–8. doi: 10.1097/PAS.0b013e31827dcc2b
24. Talerman A, Haije WG, Baggerman L. Serum alphafetoprotein (AFP) in patients with germ cell tumors of the gonads and extragonadal sites: correlation between endodermal sinus (yolk sac) tumor and raised serum AFP. Cancer (1980) 46(2):380–5. doi: 10.1002/1097-0142(19800715)46:2<380::aid-cncr2820460228>3.0.co;2-u
25. Boussios S, Attygalle A, Hazell S, Moschetta M, McLachlan J, Okines A, et al. Malignant ovarian germ cell tumors in postmenopausal patients: the royal marsden experience and literature review. Anticancer Res (2015) 35(12):6713–22.
26. Harada M, Osuga Y, Fujimoto A, Fujimoto A, Fujii T, Yano T, et al. Predictive factors for recurrence of ovarian mature cystic teratomas after surgical excision. Eur J Obstet Gynecol Reprod Biol (2013) 171(2):325–8. doi: 10.1016/j.ejogrb.2013.09.004
27. Yoshikata R, Yamamoto T, Kobayashi M, Ota H. Immunohistochemical characteristics of mature ovarian cystic teratomas in patients with postoperative recurrence. Int J Gynecol Pathol (2006) 25(1):95–100. doi: 10.1097/01.pgp.0000172082.17805.6c
28. Hackethal A, Brueggmann D, Bohlmann MK, Franke FE, Tinneberg HR, Münstedt K. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol (2008) 9(12):1173–80. doi: 10.1016/s1470-2045(08)70306-1
29. Łuczak J, Bagłaj M, Dryjański P. What recent primary studies tell us about ovarian teratomas in children: a scoping review. Cancer Metastasis Rev (2020) 39(1):321–9. doi: 10.1007/s10555-020-09844-3
30. Childress KJ, Santos XM, Perez-Milicua G, Hakim J, Adeyemi-Fowode O, Bercaw-Pratt JL, et al. Intraoperative rupture of ovarian dermoid cysts in the pediatric and adolescent population: should this change your surgical management? J Pediatr Adolesc Gynecol (2017) 30(6):636–40. doi: 10.1016/j.jpag.2017.03.139
31. Mayer C, Miller DM, Ehlen TG. Peritoneal implantation of squamous cell carcinoma following rupture of a dermoid cyst during laparoscopic removal. Gynecol Oncol (2002) 84(1):180–3. doi: 10.1006/gyno.2001.6484
32. Pavlidis N, Rassy E, Vermorken JB, Assi T, Kattan J, Boussios S, et al. The outcome of patients with serous papillary peritoneal cancer, fallopian tube cancer, and epithelial ovarian cancer by treatment eras: 27 years data from the SEER registry. Cancer Epidemiol (2021) 75:102045. doi: 10.1016/j.canep.2021.102045
33. Zong X, Zhang Y, Peng X, Cao D, Yu M, Wang J, et al. Analysis of the genomic landscape of yolk sac tumors reveals mechanisms of evolution and chemoresistance. Nat Commun (2021) 12(1):3579. doi: 10.1038/s41467-021-23681-0
34. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw (2019) 17(8):896–909. doi: 10.6004/jnccn.2019.0039
35. Wang X, Ma Z, Li Y. Ovarian yolk sac tumor: the experience of a regional cancer center. Int J Gynecol Cancer (2016) 26(5):884–91. doi: 10.1097/igc.0000000000000704
36. Szymanska KJ, Tan X, Oktay K. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol Hum Reprod (2020) 26(8):553–66. doi: 10.1093/molehr/gaaa043
37. Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod (2003) 18(1):117–21. doi: 10.1093/humrep/deg016
38. Boussios S, Moschetta M, Zarkavelis G, Papadaki A, Kefas A, Tatsi K. Ovarian sex-cord stromal tumours and small cell tumours: Pathological, genetic and management aspects. Crit Rev Oncol Hematol (2017) 120:43–51. doi: 10.1016/j.critrevonc.2017.10.007
39. Morrison A, Nasioudis D. Reproductive outcomes following fertility-sparing surgery for Malignant ovarian germ cell tumors: A systematic review of the literature. Gynecol Oncol (2020) 158(2):476–83. doi: 10.1016/j.ygyno.2020.05.036
40. Low JJ, Perrin LC, Crandon AJ, Hacker NF. Conservative surgery to preserve ovarian function in patients with Malignant ovarian germ cell tumors. A review of 74 cases. Cancer (2000) 89(2):391–8. doi: 10.1002/1097-0142(20000715)89:2<391::AID-CNCR26>3.0.CO;2-V
41. Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med (2005) 353(3):318–21. doi: 10.1056/NEJMc055237
42. Fisch B, Abir R. Female fertility preservation: past, present and future. Reproduction (2018) 156(1):F11–27. doi: 10.1530/rep-17-0483
43. Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet (2013) 30(1):11–24. doi: 10.1007/s10815-012-9912-x
44. Del-Pozo-Lérida S, Salvador C, Martínez-Soler F, Tortosa A, Perucho M, Giménez-Bonafé P. Preservation of fertility in patients with cancer (Review). Oncol Rep (2019) 41(5):2607–14. doi: 10.3892/or.2019.7063
45. Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist (2007) 12(9):1044–54. doi: 10.1634/theoncologist.12-9-1044
46. Blumenfeld Z, Avivi I, Eckman A, Epelbaum R, Rowe JM, Dann EJ. Gonadotropin-releasing hormone agonist decreases chemotherapy-induced gonadotoxicity and premature ovarian failure in young female patients with Hodgkin lymphoma. Fertil Steril (2008) 89(1):166–73. doi: 10.1016/j.fertnstert.2007.02.010
47. Choi MC, Chung YS, Lee JW, Kwon BS, Park BK, Kim SI, et al. Feasibility and efficacy of gonadotropin-releasing hormone agonists for the prevention of chemotherapy-induced ovarian insufficiency in patients with Malignant ovarian germ cell tumours (KGOG 3048R). Eur J Cancer (2020) 133:56–65. doi: 10.1016/j.ejca.2020.03.030
48. Wang SSY, Loong H, Chung JPW, Yeo W. Preservation of fertility in premenopausal patients with breast cancer. Hong Kong Med J (2020) 26(3):216–26. doi: 10.12809/hkmj198268
Keywords: ovary, teratoma, yolk sac tumor, case report, literature review
Citation: Li S, Peng J, Zhang Y, Liu D, Li L and Nai M (2024) Subsequent ovarian yolk sac tumor after operation of ovarian mature teratoma: a case report and review of the literature. Front. Oncol. 13:1327724. doi: 10.3389/fonc.2023.1327724
Received: 25 October 2023; Accepted: 29 December 2023;
Published: 17 January 2024.
Edited by:
Mignon Van Gent, Amsterdam University Medical Center, NetherlandsReviewed by:
Stergios Boussios, Canterbury Christ Church University, United KingdomNektarios Koufopoulos, University General Hospital Attikon, Greece
Copyright © 2024 Li, Peng, Zhang, Liu, Li and Nai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Li, YmVpbGVpbGlAMTYzLmNvbQ==; Manman Nai, MTM1MjMwNDYzNTJAMTYzLmNvbQ==
†These authors have contributed equally to this work and share last authorship
 Shuqing Li
Shuqing Li Juan Peng1,2
Juan Peng1,2