
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 08 January 2024
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1326676
This article is part of the Research TopicMechanism and Treatment for Pancreatic Cancer MetastasesView all 7 articles
 Etienne Gouton1
Etienne Gouton1 Marine Gilabert1,2†
Marine Gilabert1,2† Simon Launay1
Simon Launay1 Elika Loir1
Elika Loir1 Marguerite Tyran3
Marguerite Tyran3 Philippe Rochigneux1
Philippe Rochigneux1 Olivier Turrini4,5
Olivier Turrini4,5 Jonathan Garnier4
Jonathan Garnier4 Emmanuel Mitry1
Emmanuel Mitry1 Brice Chanez1*‡
Brice Chanez1*‡Background: Brain metastases (BM) are rare in pancreatic ductal adenocarcinoma (PDAC) and little data exists concerning these patients and their outcomes.
Aim: We aimed to analyze the management, practices, and outcomes of patients presenting BM from PDAC both in our institution and in all cases reported in the literature.
Methods: We conducted a retrospective, monocentric analysis using a data mining tool (ConSoRe) to identify all patients diagnosed with PDAC and BM in our comprehensive cancer center (Paoli-Calmettes Institute), from July 1997 to June 2022 (cohort 1). Simultaneously, we reviewed and pooled the case reports and case series of patients with PDAC and BM in the literature (cohort 2). The clinical characteristics of patients in each cohort were described and survival analyses were performed using the Kaplan-Meier method.
Results: In cohort 1, 19 patients (0.3%) with PDAC and BM were identified with a median age of 69 years (range: 39-81). Most patients had metastatic disease (74%), including 21% with BM, at diagnosis. Lung metastases were present in 58% of patients. 68% of patients had neurological symptoms and 68% were treated by focal treatment (surgery: 21%, radiotherapy: 42%, Gamma Knife radiosurgery: 5%). In cohort 2, among the 61 PDAC patients with BM described in the literature, 59% had metastatic disease, including 13% with BM at diagnosis. Lung metastases were present in 36% of patient and BM treatments included: surgery (36%), radiotherapy (36%), radiosurgery (3%), or no local treatment (25%). After the pancreatic cancer diagnosis, the median time to develop BM was 7.8 months (range: 0.0-73.9) in cohort 1 and 17.0 months (range: 0.0-64.0) in cohort 2. Median overall survival (OS) in patients of cohort 1 and cohort 2 was 2.9 months (95% CI [1.7,4.0]) and 12.5 months (95% CI [7.5,17.5]), respectively.
Conclusion: BM are very uncommon in PDAC and seem to occur more often in younger patients with lung metastases and more indolent disease. BM are associated with poor prognosis and neurosurgery offers the best outcomes and should be considered when feasible.
With new efficient drugs lacking and a dismal prognosis, pancreatic ductal adenocarcinoma (PDAC) is estimated to become the second leading cause of cancer death by 2030 (1, 2). Currently, the majority of PDACs are diagnosed at a metastatic stage (mPDAC) with the most common metastatic sites being the liver (76%), lung (20%), lymph node (9.4%), and bone (6.8%) (3). Brain metastases (BM) are the most common intracranial tumors (4, 5) and occur mainly in lung cancer (19.9%), melanoma (6.9%), kidney cancer (6.5%), and breast cancer (5.1%) (6). They are associated with poor prognosis and are fatal in 18% to 50% of cases (7, 8). Regarding gastrointestinal (GI) cancers, the incidence of BM depends mainly on the primary tumor (9). Barnholtz-Sloan et al. revealed that colorectal cancer was the most common GI cancer to cause BM, with an incidence of approximatively 1.8% (5). Specific biological features have been identified for these GI-derived BM, such as HER2 overexpression or the impairment of DNA repair systems, e.g., defects in homologous repair (HRD) or mismatch repair (MMRd) (10, 11).
Regarding patients with PDAC, the incidence of BM is even less, occurring in only 0.25% to 0.6% of cases (3, 9, 12), probably as a consequence of either the unsually reported neurological symptoms in that disease or the short survival time of PDAC patients. Furthermore, brain imaging is not routinely recommended for the diagnosis of PDAC, thus some patients may have undetected asymptomatic BM. This hypothesis is supported by post mortem examinations of patients with PDAC, which reported a BM rate of 7.9% (13).
Therefore, little is known about the characteristics and therapeutic management of these patients as only case reports or small series have been reported thus far. In our retrospective study, we aimed to describe the management of all patients with BM from metastatic PDAC that were treated at the Paoli-Calmettes Institute, in Marseille, France (cohort 1). We then performed a comprehensive literature review and described the characteristics and clinical outcomes of a population pooled from these reported cases (cohort 2).
A single-center retrospective analysis was conducted at the Paoli-Calmettes Institute (IPC, Marseille, France) to identify all patients diagnosed with both PDAC and BM during their cancer history. Said patients were identified via the clinical data mining interface ConSoRe (Continum Soin Recherche)—a tool for semantic search associated with a clinical data warehouse (French ConSoRe project)—using the following keywords: “pancreas” as primary tumor and “brain” as metastases location (14). Between July 1997 and June 2022, all patients ≥ 18 years presenting brain metastasis during the evolution of PDAC were recorded. Patients with pancreatic neuroendocrine tumors and patients with leptomeningeal disease without parenchymal tumors were excluded. All medical files of patients selected by ConSoRe were manually reviewed.
For each patient, clinical, biological, and radiological treatment data and outcomes were collected retrospectively after obtaining informed consent. This study was approved by our institution’s Institutional Review Board as n° PDAC-MC-IPC 2022-035 and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the institution’s human research committee.
A medical literature review was performed via the Pubmed and Google Scholar databases until June 2022, using the following keywords: “pancreatic adenocarcinoma”, “pancreatic neoplasms”, “pancreatic cancer”, “PDAC”, “brain metastasis”, “brain metastases”, “brain tumor”, and “CNS tumor”. Case reports, case series, and abstracts in English were considered. These articles’ references were also reviewed in order to locate additional cases. Reports of lesions discovered post-mortem were excluded. Clinical, biological, and radiological patient characteristics, as well as all available survival data, were also collected.
The primary objective of our study was to describe the characteristics and outcomes of patients with PDAC and BM from cohort 1 and compare these to those of the patients in cohort 2. Descriptive statistics were used to analyze patient characteristics. Overall survival (OS) was defined as the time from diagnosis of PDAC (pOS) or BM (bmOS) to death from any cause, censored at the date of last follow-up. Survival analyses were estimated via the Kaplan-Meier method, and differences between treatment groups in each cohort were tested via the log-rank test. We did not perform any statistical comparison between the two cohorts due to missing data in cohort 2 and great heterogeneity between both cohorts in terms of patient characteristics and treatments. Thus, we reported data from cohort 1 and cohort 2 in a side-by-side method. Statistical analyses were reviewed by our institution’s Biomedical Statistics Department. Statistical analyses were performed on IBM SPSS Statistics software, version 26.0.0.0 (IBM SPSS Inc., Chicago, IL, USA). A p-value<0.05 was considered as statistically significant.
Among the 6,113 patients diagnosed with PDAC at our institution between July 1997 and June 2022, we identified 19 patients with brain metastases (0.3%). Their characteristics are listed in Table 1. The median age at BM diagnosis was 69 years (range: 39-81) and primary tumors were located in the head (63%), tail (26%), or body (11%) of the pancreas. Two patients were tested for germline BRCA status, and one had a germline BRCA1 mutation. Cancer genome sequencing was performed in only two patients: one patient with an MSH6 mutation and one patient with a KRAS G12V mutation. Most patients were initially diagnosed with metastatic PDAC (74%). Prior to BM diagnosis, patients had received a median of one line of systemic chemotherapy (range: 0-3) and 37% had received at least two lines of chemotherapy.
Symptoms resulted from BM in 13 patients (68%) (Table 2). The most common neurological symptoms were confusion (26%) and symptoms of intracranial hypertension including headaches (21%) and motor disorders (16%). Notably, 37% had a single brain metastasis and 21% had more than 4, located exclusively in the supratentorial area in 74% (Table 2). Pathological analysis of BM was available in four patients (21%), with confirmation of the primary pancreatic origin. At BM diagnosis, extracranial disease was stable in 32% and progressive in 21% of patients. One patient had a persistent complete extracranial response.
Four patients (21%) underwent surgery for BM (Table 2), among which three received postoperative radiotherapy. Exclusive radiotherapy was performed in 42% of patients with whole-brain radiotherapy (WBRT) in six patients (32%) and standard or stereotactic radiotherapy in two patients (11%). One patient (5%) received stereotactic radiosurgery and 1 patient had missing data (5%). Five patients (26%) did not receive any local treatment for BM.
Based on our literature review, 24 articles were found, including 61 patients (Supplementary Table S1, Supplementary Materials) (15–38). The pooled patient characteristics are described in Table 1. Patients of cohort 2 were younger at BM diagnosis than those in cohort 1 (58 years vs 69 years, respectively). Among six patients with known germline BRCA status in cohort 2, three patients had a germline BRCA1 (n=1) or BRCA2 (n=2) mutation, all reported by Jordan et al. (29). One additional patient displayed a somatic BRCA2 mutation in both the primary pancreatic tumor and BM (38). At initial diagnosis, 25 patients (41%) had localized PDAC. Patients had received a median of two prior lines of chemotherapy (range: 0-3). The most common metastatic sites were the liver (54%), lung (36%), lymph nodes (20%), and peritoneum (11%).
Regarding BM, the most frequent symptoms were those of intracranial hypertension including headaches (41%), motor alteration (28%), and visual impairment (13%). Patients were less often confused in cohort 2 than in cohort 1 (5% vs 26%, respectively). Cystic lesions constituted BM in 16% of patients and BM were localized mainly in the supratentorial area (61%), infratentorial area (10%), or both (26%). Of note, 16% of patients had discordant evolution between intra and extracranial lesions; 11% and 5% had complete and partial extracranial responses at BM diagnosis, respectively.
In cohort 2, BM treatments comprised surgery (36%), radiotherapy (36%, of which 26% and 8% received WBRT and standard/stereotactic radiotherapy, respectively), radiosurgery (3%), and no local treatment (25%). Among the 22 surgery patients, 13 (59%) received postoperative irradiation of the tumor bed.
The median time to BM diagnosis after PDAC diagnosis in cohorts 1 and 2 was 7.8 months (range: 0.0-73.9) and 17.0 months (range: 0.0-64.0), respectively (Table 3). At the end date of follow-up (July 4th, 2022), all patients from cohort 1 had died. Figure 1 illustrates OS for both cohorts. After BM surgery, patients in cohorts 1 and 2 experienced a bmOS of 6.3 months (95% CI [3.4, 9.3]) and 36.0 months (95% CI [NR, NR]) respectively (Figures 2A, B). After surgery, the median time to recurrence was 4.1 months (range: 3.9-4.3) and 8 months (range: 4.0-12.0) in cohorts 1 and 2, respectively (Table 3). Despite recurrences, five patients (from both cohorts 1 and 2) still benefited from surgery, as they displayed an overall median bmOS of 21.7 months (95% CI [0.0, 44.7]).
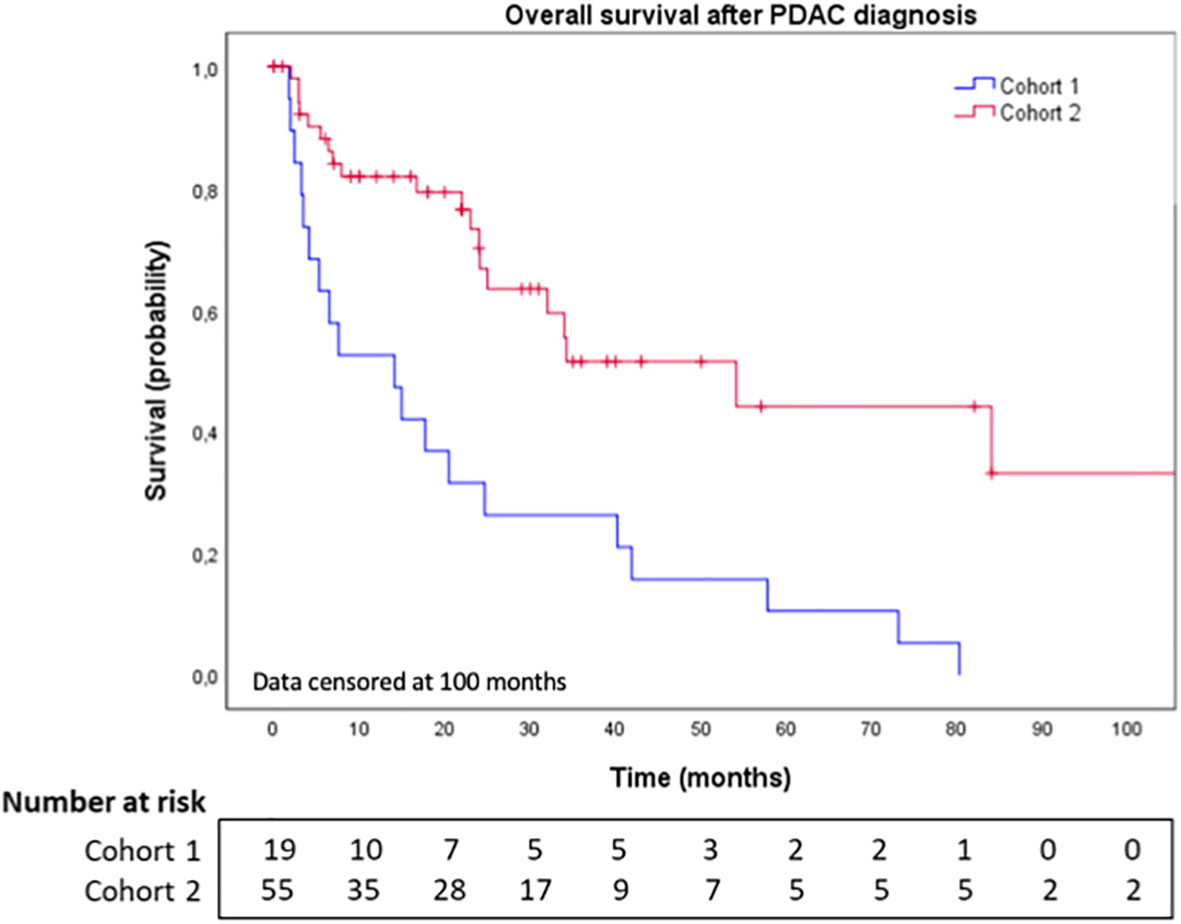
Figure 1 Overall survival after brain metastases diagnosis in cohort 1 and cohort 2. BM: Brain metastases. Data censored at 100 months.
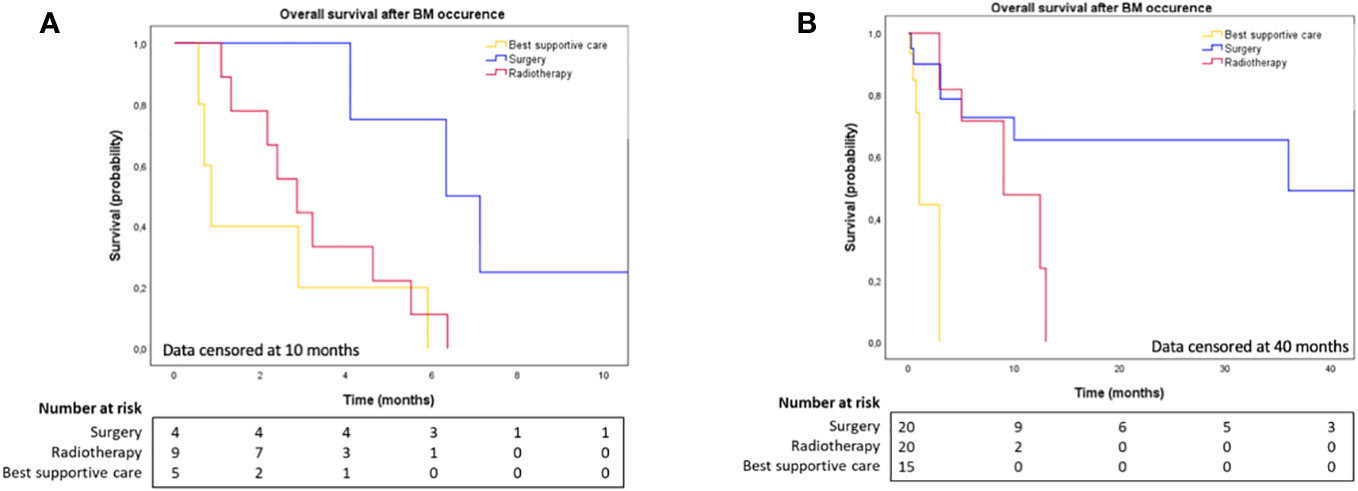
Figure 2 (A) Overall survival after brain metastases diagnosis in cohort 1, according to BM local treatment. Data censored at 10 months. (B) Overall survival after brain metastases diagnosis in cohort 2, according to BM local treatment. Data censored at 40 months.
In patients that underwent BM surgery, the median age at BM diagnosis was 61 years in both cohorts. The median time from PDAC diagnosis to BM diagnosis was 21.7 months (range: 0-36.0) and 17.0 months (range: 0-64) in cohorts 1 and 2, respectively. In cohort 1, neurosurgery was performed in a median time of 6.5 days after BM diagnosis and the median OS after surgery was 5.9 months (95% CI [3.7, 8.2]). Because of missing data, analysis of BM surgery was not conducted in cohort 2, only survival analyses from BM diagnosis.
Three patients (75%) in cohort 1 and 12 patients (55%) in cohort 2 had metastatic PDAC at initial diagnosis, including BM for four of them (one in cohort 1, three in cohort 2). In cohort 1, one patient had an extracranial complete response after chemotherapy and BM were the only metastatic recurrence. Other patients had lung (50%), liver (50%), lymph nodes (25%), and bone (25%) metastases. In cohort 2, 10 patients (45%) had no extracranial disease and the most common metastatic sites were the liver (23%), lymph nodes (9%), and lung (5%).
There were no major postoperative complications in either cohort; only one patient from cohort 1 had a transitory aphasia that resolved rapidly. Postoperative irradiation of the tumor bed was performed in 16 patients (62%): three patients (75%) in cohort 1 and 13 patients (59%) in cohort 2.
A 57-year male patient in cohort 1 was diagnosed in October 2015 with a lesion in the head of the pancreas and synchronous multiple hepatic metastases. The patient had a family history of cancer (grandmother with pancreatic cancer, mother with breast cancer) and a germline BRCA1 mutation was identified. The patient achieved a complete response after the first line of a FOLFIRINOX regimen followed by maintenance therapy with capecitabine.
In October 2018, the patient presented with headache without neurological impairment. Although a CT-scan indicated a persistent extracranial complete response, brain MRI revealed a > 5cm left anterior frontal tumor in contact with the left optic nerve, perilesional edema, and compression of the left lateral ventricle (Figure 3A). The patient underwent complete surgical resection of the BM in November 2018 (Figure 3B), reporting a CK7 positive, CK20 and TTF-1 negative adenocarcinoma consistent with the primary pancreatic origin. Postoperative stereotactic irradiation of the tumor bed was performed. Due to the absence of extra and intracranial residual disease, simple monitoring was proposed.
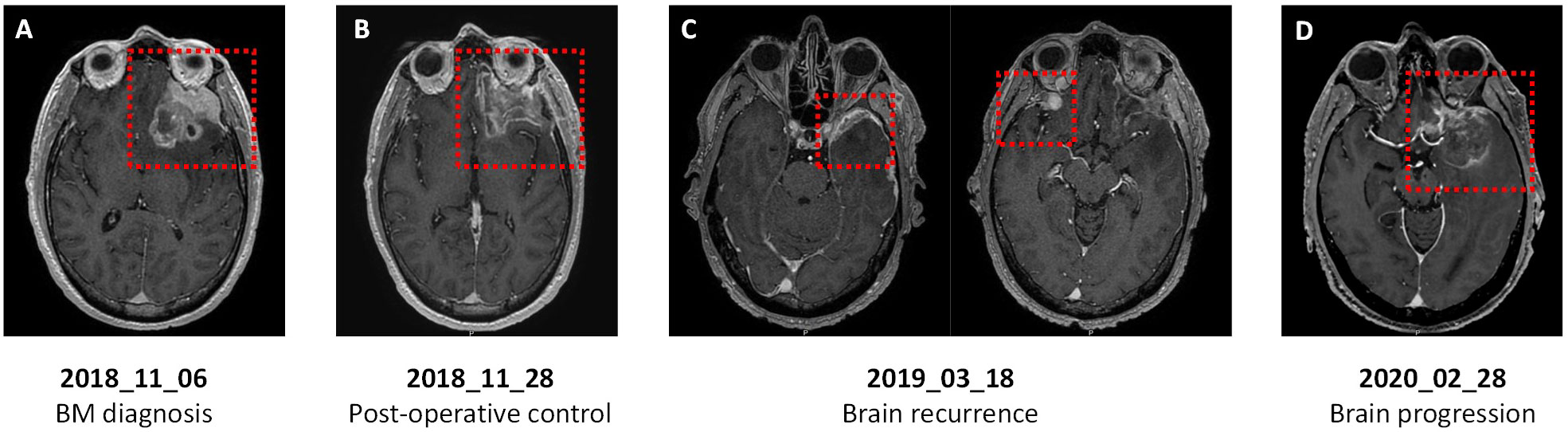
Figure 3 Axial T1-weighted brain magnetic resonance imaging (MRI) of a patient with PDAC and germline BRCA1 mutation. (A) At BM diagnosis: large left anterior frontal tumor in contact with the left optic nerve and perilesional edema. (B) After surgery: left fronto-orbital and dorsolateral ablation site without intraparenchymal enhancement. (C) At first brain recurrence: left frontal nodular meningeal tissue lesion (left) and new right inferior frontal enhanced nodular lesion with meningeal implantation (right). (D) At second brain recurrence: large left temporopolar and frontobasal lesion with significant perilesional edema.
The patient relapsed in March 2019 with local meningeal recurrence at the surgical site and a left temporal lesion (Figure 3C). These lesions were treated with stereotactic irradiation, then systemic therapy with FOLFOX was resumed for a new extracranial adrenal lesion. A partial response was achieved until February 2020, when a new brain tumor of the left frontotemporal lesion was identified (Figure 3D). The patient underwent a second brain surgery in March 2020 and chemotherapy was pursued. After three additional cycles, hepatic and peritoneal progression was detected, and the patient died from PDAC-related complications in August 2020, 58 months after initial PDAC diagnosis and 22 months after first BM occurrence.
Our study evaluated clinical characteristics and outcomes of patients with BM from PDAC using a single institution cohort of 19 patients and a pooled analysis of the literature review and aimed to provide a global overview of this rare and dreadful form of metastatic cancer.
Here, we confirm that BM are uncommon in pancreatic cancer, as previously reported` in the SEER PDAC database with BM occurring in only 0.6% of cases (90 out of 13,233) (3), or as reported by Jordan et al. with 0.4% of BM out of 5,824 mPDAC cases between 2000 and 2016 (29). In our study, 0.3% of patients with mPDAC disease were diagnosed with BM over a 25-year time period. However, the incidence of BM may be underestimated as suggested by post-mortem reports (13, 39), mainly due to the poor prognosis associated with extracranial disease; patients do not have “time” to develop neurological symptoms and therefore BM remain asymptomatic and unknown. Furthermore, due to the low incidence of BM in pancreatic cancer, routine brain imaging is only recommended by international guidelines if symptoms occur in the initial evaluation of pancreatic cancer (40, 41).
In our study, 58% and 36% of patients in cohorts 1 and cohort 2 had lung metastases, respectively. Such incidence is higher compared to the 20% rates published in epidemiological studies of mPDAC (3). Conversely, patients in our study had less frequent liver metastases (58% in cohort 1 and 54% in cohort 2) than usual in PDAC patients without BM (76%) (3). Interestingly, it has been demonstrated that lung and liver metastases in PDAC are associated with better and worse survival, respectively (3, 42). Hence, a more indolent disease in patients with lung metastases and no liver metastases could have more time to develop BM. Sasaki et al. also suggested that lung metastases could be a risk factor for the development of BM in patients with PDAC (31). Moreover, the patients in our study were younger than the usual median age of 71 years old at PDAC diagnosis (43), with a median age of 69 years and 58 years in cohorts 1 and 2, respectively. Data analysis of over 126,066 patients with pancreatic cancer from the SEER database revealed that younger patients (< 40 years) had significantly better OS than older patients aged 40-60 years (HR = 1.86; 95% CI [1.72, 2.01]; p<0.0001), 60-80 years (HR = 2.22; 95% CI [2.05, 2.40]; p<0.0001), and over 80 years (HR = 3.30; 95% CI [3.05, 3.57]; p<0.0001) (44). Altogether, these data highlight a patient profile of those more susceptible to developing BM, such as young patients with lung metastases and more likely indolent disease.
Additionally, a BRCA mutation (germline or somatic) was identified in 5 patients in the overall population of both cohorts, representing approximatively half of patients with an available BRCA status. This is a relatively high incidence considering that very few patients had genomic data available in our study. In mPDAC, a BRCA mutation is associated with an improved response to platinum-based chemotherapy and better survival (45–47). Interestingly, BRCA mutations have been associated with an increased risk of BM in both ovarian and breast cancer (48, 49). While further study is required in mPDAC, BRCA mutations may lead to increased BM development in PDAC. We also reported the case of a patient with a germline BRCA1-mutated PDAC who achieved a 3-year complete response to chemotherapy though BM occurred—and was surgically removed—and who also achieved an extracranial response that persisted for four years, indicating an indolent and chemotherapy-sensitive BRCA-mutated disease.
The management of BM is variable according to the European Association of Neuro-Oncology (EANO) guidelines (50). Surgery should be considered for a limited number of metastases (one to three), especially in cases of large (≥ 3 cm), symptomatic, or complicated (edema, necrosis, posterior fossa location with obstructive hydrocephalus) BM. Stereotactic radiosurgery should be offered to patients with either a limited number of BM (one to four) or a higher number (five to ten) but with a total tumor volume < 15 ml. The utilization of WBRT as an option for multiple BM is widespread yet currently disputed due to significant neurocognitive toxicities (51, 52). Nevertheless, best supportive cares remain a valid option for patients with poor ECOG performance status.
Our results suggest that in PDAC, BM surgery was both well-tolerated with no postoperative issues and associated with improved survival compared to radiotherapy or BSC (Figure 2). This finding is consistent with previous reports of several patients with BM from PDAC who experienced improved survival after neurosurgery. For example, Lemke et al. reported two cases of patients initially treated for localized PDAC who developed a single metachronous brain metastasis. Both had BM surgery, and no recurrence or other systemic metastases occurred during follow-up. The survival time of these two patients were 136 months and 74 month, and they were still alive when the case report was published (22). This emphasizes the importance of surgery in brain oligometastases. One of our patients from cohort 1, with a BRCA1 mutation, underwent surgery for a single BM and achieved a prolonged survival of 21.7 months after the first BM resection. Overall, brain surgery appears feasible in selected patients and can provide improved survival.
While the central nervous system’s (CNS) involvement is uncommon in PDAC, other rare neurological locations have been reported. Choroidal metastases are also an exceptional event, as Shah et al. reported an incidence of 0.4% (53). Leptomeningeal metastases related to pancreatic cancer have also been reported, to our knowledge, in only 12 patients (54). One of these, reported by Rao et al., was also included in our review because of the association of multiple supratentorial and cerebellar hemispheric lesions (24). Although patients with isolated carcinomatous meningitis were not included in our study, one patient with leptomeningeal metastasis associated with BM was identified in our institutional database. In this patient, BM were diagnosed 39 months after the initial diagnosis of PDAC and the patient died only 2 weeks after the BM diagnosis.
Our study has several limitations. First, it is a retrospective, single-center study, with all the associated biases. Moreover, due to large amounts of missing data and great heterogeneity in reported cases, which would have caused biases, we did not perform any statistical comparison between the two cohorts. However, a large difference in survival appears to exist between the two cohorts (bmOS: 2.9 months in cohort 1 vs 12.5 months in cohort 2) that may be explained by i) the selective bias associated with case reports where usually the more favorable cases are published and ii) greater access in our center to brain imagery and neurosurgeons that could enlarge treatment indications. Moreover, in the largest study published investigating BM from PDAC, Jordan et al. (29) described 25 patients with a median bmOS of 1.9 months. Their study highlights the importance of analyses based on unselected patient cohorts like our cohort 1. Another limitation is the lack of molecular profiling available for most patients (cohort 1 and cohort 2) that limits the analysis of specific genomic profiles for BM from PDAC. However, BRCA mutations could be frequently associated to BM and brain CT-scan or MRI could be considered in that population when neurological symptoms or at disease progression. Certain data on genomic status in PDAC with BM describe the frequent alteration of KRAS, which is usual in PDAC (29), as well as a case of an ALK rearrangement–positive PDAC with BM that was sensitive to the ALK tyrosine-kinase inhibitor crizotinib after chemotherapy resistance and was also responsive to alectinib after BM diagnosis (36).
In conclusion, the occurrence of BM remains a very rare event in pancreatic cancer. A profile of patients susceptible to developing BM could be drawn from our results, mostly in indolent and slowly progressive disease (younger patients, lung metastases, BRCA mutations). However, additional studies are required to further explore molecular and/or genetic specificities. Improved survival in patients who underwent brain surgery was also clearly shown and thus, when feasible, should be proposed as the most personalized treatment.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by IRB _ Paoli Calmettes Institute. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
EG: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. MG: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing, Visualization. SL: Writing – review & editing, Data curation, Investigation. EL: Data curation, Investigation, Writing – review & editing. MT: Data curation, Writing – review & editing. PR: Data curation, Writing – review & editing, Formal Analysis, Methodology. OT: Writing – review & editing. JG: Data curation, Writing – review & editing. EM: Data curation, Formal Analysis, Writing – review & editing. BC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1326676/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res (2014) 74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155
3. Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A, et al. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: A Surveillance Epidemiology and End Results database analysis. World J Gastroenterol (2017) 23:1872. doi: 10.3748/wjg.v23.i10.1872
4. Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer (2002) 94:2698–705. doi: 10.1002/cncr.10541
5. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol (2004) 22:2865–72. doi: 10.1200/JCO.2004.12.149
6. Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res (2012) 32:4655–62.
7. Hall WA, Djalilian HR, Nussbaum ES, Cho KH. Long-term survival with metastatic cancer to the brain. Med Oncol Northwood Lond Engl (2000) 17:279–86. doi: 10.1007/BF02782192
8. Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer (1981) 48:384–94. doi: 10.1002/1097-0142(19810715)48:2<384::aid-cncr2820480227>3.0.co;2-8
9. Lemke J, Scheele J, Kapapa T, von Karstedt S, Wirtz C, Henne-Bruns D, et al. Brain metastases in gastrointestinal cancers: is there a role for surgery? Int J Mol Sci (2014) 15:16816–30. doi: 10.3390/ijms150916816
10. Mitra D, Clark JW, Shih HA, Oh KS, Brastianos PK, Wo JY, et al. Enrichment of HER2 amplification in brain metastases from primary gastrointestinal Malignancies. Oncologist (2019) 24:193–201. doi: 10.1634/theoncologist.2018-0152
11. Sun J, Wang C, Zhang Y, Xu L, Fang W, Zhu Y, et al. Genomic signatures reveal DNA damage response deficiency in colorectal cancer brain metastases. Nat Commun (2019) 10:3190. doi: 10.1038/s41467-019-10987-3
12. He C, Huang X, Zhang Y, Lin X, Li S. The impact of different metastatic patterns on survival in patients with pancreatic cancer. Pancreatology (2021) 21:556–63. doi: 10.1016/j.pan.2021.01.014
13. Lee YT, Tatter D. Carcinoma of the pancreas and periampullary structures. Pattern metastasis at autopsy Arch Pathol Lab Med (1984) 108:584–7.
14. Bertucci F, Le Corroller-Soriano A-G, Monneur A, Fluzin S, Viens P, Maraninchi D, et al. Santé numérique et « cancer hors les murs », Big Data et intelligence artificielle. Bull Cancer (Paris) (2020) 107:102–12. doi: 10.1016/j.bulcan.2019.07.006
15. Kuratsu J, Murakami M, Uemura S, Ushio Y. Brain and skull metastases of hepatic or pancreatic cancer. Neurol Med Chir (Tokyo) (1990) 30:476–82. doi: 10.2176/nmc.30.476
16. Park K-S, Kim M, Park S-H, Lee K-W. Nervous system involvement by pancreatic cancer. J Neurooncol (2003) 63:313–6. doi: 10.1023/A:1024337020884
17. El Kamar FG, Jindal K, Grossbard ML, Mizrachi HH, Kozuch PS. Pancreatic carcinoma with brain metastases: case report and literature review. Dig Liver Dis (2004) 36:355–60. doi: 10.1016/j.dld.2003.10.019
18. Caricato M, Borzomati D, Ausania F, Garberini A, Rabitti C, Tonini G, et al. Cerebellar metastasis from pancreatic adenocarcinoma. Pancreatology (2006) 6:306–8. doi: 10.1159/000092693
19. MarepaIly R, Micheals D, Sloan A, Hatfield J, Adsay V, Joyrich R, et al. Octreotide uptake in intracranial metastasis of pancreatic ductal adenocarcinoma origin in a patient with a prolonged clinical course. Dig Dis Sci (2009) 54:188–90. doi: 10.1007/s10620-008-0531-4
20. Matsumura T, Ohzato H, Yamamoto T, Ota K, Mabuchi E, Miwa H, et al. A case of postoperative brain metastasis originated from pancreatic cancer which was successfully treated by resection and postoperative irradiation. Gan To Kagaku Ryoho (2009) 36:2433–5.
21. Zaanan A, Lequoy M, Landi B, Lievre A, Franco D, Taieb J. Brain metastases from pancreatic adenocarcinoma. Case Rep (2009) 2009:bcr0820080620–bcr0820080620. doi: 10.1136/bcr.08.2008.0620
22. Lemke J, Barth TFE, Juchems M, Kapapa T, Henne-Bruns D, Kornmann M. Long-term survival following resection of brain metastases from pancreatic cancer. Anticancer Res (2011) 31:4599–603.
23. Chiang K-C, Yu C-C, Chen J-R, Huang Y-T, Huang C-C, Yeh C-N, et al. Oncocytic-type intraductal papillary mucinous neoplasm (IPMN)-derived invasive oncocytic pancreatic carcinoma with brain metastasis - a case report. World J Surg Oncol (2012) 10:138. doi: 10.1186/1477-7819-10-138
24. Rao R, Sadashiv SK, Goday S, Monga D. An extremely rare case of pancreatic cancer presenting with leptomeningeal carcinomatosis and synchronous intraparenchymal brain metastasis. Gastrointest Cancer Res GCR (2013) 6:90–2.
25. Rajappa P, Margetis K, Wernicke G, Ginter P, Cope W, Sherr DL, et al. Stereotactic radiosurgery plays a critical role in enhancing long-term survival in a patient with pancreatic cancer metastatic to the brain. Anticancer Res (2013) 33:3899–903.
26. Kumar A, Dagar M, Herman J, Iacobuzio-Donahue C, Laheru D. CNS involvement in pancreatic adenocarcinoma: a report of eight cases from the johns hopkins hospital and review of literature. J Gastrointest Cancer (2015) 46:5–8. doi: 10.1007/s12029-014-9667-y
27. Matsumoto H, Yoshida Y. Brain metastasis from pancreatic cancer: A case report and literature review. Asian J Neurosurg (2015) 10:35. doi: 10.4103/1793-5482.151507
28. Tardivo V, Vincitorio F, Monticelli M, Bertero L, Zenga F, Ducati A, et al. Double cystic brain metastasis in a patient with stable pancreatic intraductal papillary mucinous neoplasm. Br J Neurosurg (2018) 35(2):236–40. doi: 10.1080/02688697.2018.1451824
29. Jordan EJ, Lowery MA, Basturk O, Allen PJ, Yu KH, Tabar V, et al. Brain metastases in pancreatic ductal adenocarcinoma: assessment of molecular genotype–phenotype features—An entity with an increasing incidence? Clin Colorectal Cancer (2018) 17:e315–21. doi: 10.1016/j.clcc.2018.01.009
30. Lee SJ, Cho CM, Jung MK, Cho SH, Kim GC, Bae HI. Pancreatic cancer with brain metastases: case report with literature review. Korean J Pancreas Biliary Tract (2018) 23:65–70. doi: 10.15279/kpba.2018.23.2.65
31. Sasaki T, Sato T, Nakai Y, Sasahira N, Isayama H, Koike K. Brain metastasis in pancreatic cancer: Two case reports. Med (Baltimore) (2019) 98:e14227. doi: 10.1097/MD.0000000000014227
32. Matsuo S, Amano T, Kawauchi S, Nakamizo A. Multiple brain metastases from pancreatic adenocarcinoma manifesting with simultaneous intratumoral hemorrhages. World Neurosurg (2019) 123:221–5. doi: 10.1016/j.wneu.2018.12.036
33. Luu AM, Künzli B, Hoehn P, Munding J, Lukas C, Uhl W, et al. Prognostic value and impact of cerebral metastases in pancreatic cancer. Acta Chir Belg (2020) 120:30–4. doi: 10.1080/00015458.2018.1549379
34. Dalal K, Khrizman P, Chaaya A. Isolated brain metastasis from pancreatic cancer in lynch syndrome. Am J Gastroenterol (2019) 114:S697–7. doi: 10.14309/01.ajg.0000594536.59856.1f
35. Oka Y, Takano S, Kouchi Y, Furukawa K, Takayashiki T, Kuboki S, et al. Simultaneous brain and lung metastases of pancreatic ductal adenocarcinoma after curative pancreatectomy: a case report and literature review. BMC Gastroenterol (2021) 21:9. doi: 10.1186/s12876-020-01587-3
36. Ou K, Liu X, Li W, Yang Y, Ying J, Yang L. ALK rearrangement–positive pancreatic cancer with brain metastasis has remarkable response to ALK inhibitors: A case report. Front Oncol (2021) 11:724815. doi: 10.3389/fonc.2021.724815
37. Papadimitriou K, Kiss-Bodolay D, Hedjoudje A, Millan DS, Simonin A, Fournier J-Y, et al. Late metachronous cerebral metastasis of pancreatic adenocarcinoma of the tail of the pancreas: a case report. J Med Case Rep (2022) 16:144. doi: 10.1186/s13256-022-03314-w
38. Utsunomiya T, Funamizu N, Ozaki E, Tamura K, Sakamoto K, Ogawa K, et al. A case of radical resection for brain metastases of pancreatic cancer after curative chemotherapy for para-aortic lymph node metastases. Surg Case Rep (2022) 8:108. doi: 10.1186/s40792-022-01461-2
39. Yamada K, Miura M, Miyayama H, Sakashita N, Kochi M, Ushio Y. Brain metastases from asymptomatic adenocarcinoma of the pancreas. Surg Neurol (2002) 58:332–6. doi: 10.1016/S0090-3019(02)00805-4
40. Sohal DPS, Mangu PB, Khorana AA, Shah MA, Philip PA, O’Reilly EM, et al. Metastatic pancreatic cancer: american society of clinical oncology clinical practice guideline. J Clin Oncol (2016) 34:2784–96. doi: 10.1200/JCO.2016.67.1412
41. Ducreux M, Cuhna A, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2015) 26:v56–68. doi: 10.1093/annonc/mdv295
42. Decoster C, Gilabert M, Autret A, Turrini O, Oziel-, Poizat F, et al. Heterogeneity of metastatic pancreatic adenocarcinoma: Lung metastasis show better prognosis than liver metastasis—a case control study. Oncotarget (2016) 7:45649–55. doi: 10.18632/oncotarget.9861
43. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med (2014) 371:1039–49. doi: 10.1056/NEJMra1404198
44. Wang H, Liu J, Xia G, Lei S, Huang X, Huang X. Survival of pancreatic cancer patients is negatively correlated with age at diagnosis: a population-based retrospective study. Sci Rep (2020) 10:7048. doi: 10.1038/s41598-020-64068-3
45. Devico Marciano N, Kroening G, Dayyani F, Zell JA, Lee F-C, Cho M, et al. BRCA-mutated pancreatic cancer: from discovery to novel treatment paradigms. Cancers (2022) 14:2453. doi: 10.3390/cancers14102453
46. O’Reilly EM, Lee JW, Zalupski M, Capanu M, Park J, Golan T, et al. Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. J Clin Oncol (2020) 38:1378–88. doi: 10.1200/JCO.19.02931
47. Goldstein JB, Zhao L, Wang X, Ghelman Y, Overman MJ, Javle MM, et al. Germline DNA sequencing reveals novel mutations predictive of overall survival in a cohort of patients with pancreatic cancer. Clin Cancer Res (2020) 26:1385–94. doi: 10.1158/1078-0432.CCR-19-0224
48. Ratner E, Bala M, Louie-Gao M, Aydin E, Hazard S, Brastianos PK. Increased risk of brain metastases in ovarian cancer patients with BRCA mutations. Gynecol Oncol (2019) 153:568–73. doi: 10.1016/j.ygyno.2019.03.004
49. Zavitsanos PJ, Wazer DE, Hepel JT, Wang Y, Singh K, Leonard KL. BRCA1 mutations associated with increased risk of brain metastases in breast cancer: A 1:2 matched-pair analysis. Am J Clin Oncol (2018) 41:1252–6. doi: 10.1097/COC.0000000000000466
50. Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, et al. EANO–ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol (2021) 32(11):1332–47. doi: 10.1016/j.annonc.2021.07.016
51. Robin TP, Rusthoven CG. Strategies to preserve cognition in patients with brain metastases: A review. Front Oncol (2018) 8:415. doi: 10.3389/fonc.2018.00415
52. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA (2016) 316:401. doi: 10.1001/jama.2016.9839
53. Shah SU, Shields CL, Bianciotto CG, Shields JA. Pancreatic cancer metastasis to choroid. Ophthalmology (2011) 118:1483–1483.e4. doi: 10.1016/j.ophtha.2011.02.004
Keywords: pancreatic cancer, brain metastases, brain surgery, machine learning, data mining, big data, literature review
Citation: Gouton E, Gilabert M, Launay S, Loir E, Tyran M, Rochigneux P, Turrini O, Garnier J, Mitry E and Chanez B (2024) Management and outcomes of brain metastases from pancreatic adenocarcinoma: a pooled analysis and literature review. Front. Oncol. 13:1326676. doi: 10.3389/fonc.2023.1326676
Received: 23 October 2023; Accepted: 11 December 2023;
Published: 08 January 2024.
Edited by:
Dong Wu, Beckman Research Institute, City of Hope, United StatesReviewed by:
Stephen Bowden, Oregon Health and Science University, United StatesCopyright © 2024 Gouton, Gilabert, Launay, Loir, Tyran, Rochigneux, Turrini, Garnier, Mitry and Chanez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brice Chanez, Y2hhbmV6YkBpcGMudW5pY2FuY2VyLmZy
†Deceased
‡ORCID: Brice Chanez, orcid.org/0000-0003-4688-8765
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.