- 1Department of Medicine, Division of Gastroenterology and Hepatology, University of Maryland School of Medicine, Baltimore, MD, United States
- 2The Institute for Genome Sciences, University of Maryland School of Medicine, Baltimore, MD, United States
- 3Medical Service, Veterans Affairs Maryland Healthcare System, Baltimore, MD, United States
- 4Marlene and Stewart Greenebaum Cancer Center, University of Maryland Medical Center, Baltimore, MD, United States
- 5Department of Biochemistry and Molecular Biology, University of Maryland School of Medicine, Baltimore, MD, United States
Colorectal cancer (CRC) remains a major cause of morbidity and mortality. Therapeutic approaches for advanced CRC are limited and rarely provide long-term benefit. Enzymes comprising the 24-member matrix metalloproteinase (MMP) family of zinc- and calcium-dependent endopeptidases are key players in extracellular matrix degradation, a requirement for colon tumor expansion, invasion, and metastasis; hence, MMPs are an important research focus. Compared to sporadic CRC, less is known regarding the molecular mechanisms and the role of MMPs in the development and progression of colitis-associated cancer (CAC) − CRC on a background of chronic inflammatory bowel disease (IBD) − primarily ulcerative colitis and Crohn’s disease. Hence, the potential of MMPs as biomarkers and therapeutic targets for CAC is uncertain. Our goal was to review data regarding the role of MMPs in the development and progression of CAC. We sought to identify promising prognostic and therapeutic opportunities and novel lines of investigation. A key observation is that since MMPs may be more active in early phases of CAC, using MMPs as biomarkers of advancing neoplasia and as potential therapeutic targets for adjuvant therapy in those with advanced stage primary CAC rather than overt metastases may yield more favorable outcomes.
1 Introduction
Enzymes comprising the 24-member matrix metalloproteinase (MMP) family of zinc- and calcium-dependent endopeptidases play key roles in cancer progression by degrading the extracellular matrix, an obstacle to tumor expansion, invasion, and metastasis. Notably, differential induction of specific MMPs has been reported depending on the cancer type examined and how the neoplastic cells are stimulated. For example, more than a decade ago, our research team reported that stimulating human colon cancer cells with muscarinic receptor agonists resulted in robust, selective induction of MMP-1, -7, and -10 (1). Subsequent work indicated that blocking muscarinic receptor activation or the activity of MMP-1, whose expression correlates with advanced colon cancer stage, tumor metastasis, and reduced survival (2–4), abolished acetylcholine-induced colon cancer cell invasion (5). Moreover, we showed that muscarinic receptor agonist-induced MMP-1 expression is mediated by potentiating crosstalk between different post-muscarinic receptor signaling cascades (6).
Despite the great interest in understanding the role MMPs play in the progression of sporadic colorectal cancer (CRC), relatively little attention has been paid to their role in colitis-associated colon cancer (CAC). This area of investigation is also relevant to our focus on the role of muscarinic receptors and ligands in CRC (7–9) – our work and that of others has demonstrated that muscarinic receptor activation may play an important role in the progression of inflammatory bowel disease (IBD, primarily Crohn’s disease and ulcerative colitis (UC)) (10, 11). Moreover, current work highlights the unique molecular differences between CAC compared to non-colitis-associated CRC (12), and the strong involvement of MMPs in regulating colitis and intestinal permeability (13, 14). Hence, understanding the precise role that MMPs play in the genesis and progression of CAC, and how these differ from their roles in sporadic CRC, may reveal novel mechanistic insights, but more importantly, identify MMPs as promising biomarkers of CAC progression and therapeutic targets.
For these reasons, in this review, we aim to provide a thorough appraisal of MMP’s functions, their role in the progression from chronic colitis to CAC, and an overview of the application of MMP inhibitors in clinical therapy. We searched the literature for English language publications between 1962 and June 30, 2023, using keywords relevant to this search (IBD, inflammation, matrix metalloproteinases, colitis-associated cancer, extracellular matrix, dysplasia, biomarkers), alone and in combination, in the following public databases: PubMed, ScienceDirect, and BioMed Central. Included publications were reviewed critically, and the results summarized in the following text and tables. We believe the results of this approach yielded important information that can be used to develop preliminary insights, but perhaps more importantly, identify areas worthy of further basic and clinical investigation.
2 Matrix metalloproteinases
2.1 Subgroups of matrix metalloproteinases
The extracellular matrix (ECM), comprised of fibers constructed from collagen, elastin, laminin, fibronectin, proteoglycans, glycoproteins, and polysaccharides, serves as a meshwork for tissue development and maintenance, and plays a key role in cell migration and adhesion (15). Matrix metalloproteinases (MMPs) represent a 24-member family of zinc and calcium endopeptidases that degrade structural macromolecules within the ECM. Non-ECM substrates for MMPs include proenzymes and proinflammatory cytokines, chemokines, and bacteria (16). MMPs are numbered from 1 to 28; MMP-4, -5, and -6, subsequently recognized to be MMP-1, -2, and -3, respectively, are omitted, and MMP-18 was likewise later recognized to be MMP-19 (17–19). The first MMP was isolated and characterized in 1962 in a tadpole tail (20) and 26 years later, human fibroblast stromelysin and type IV collagenase were discovered and found to possess a wider range of substrate specificities (21, 22). Interest within the scientific community continued to evolve as additional MMPs were discovered and their role in disease, particularly cancer, became apparent. The late 20th century and early 21st century witnessed important advances in the characterization of MMPs, followed by investigation into their roles as potential biomarkers and therapeutic targets. This is especially due to advances in research tools, evolving from protein isolation and purification to more advanced tools, e.g., gene cloning, transgenic mice, immunofluorescence microscopy, and single cell RNA expression profiling.
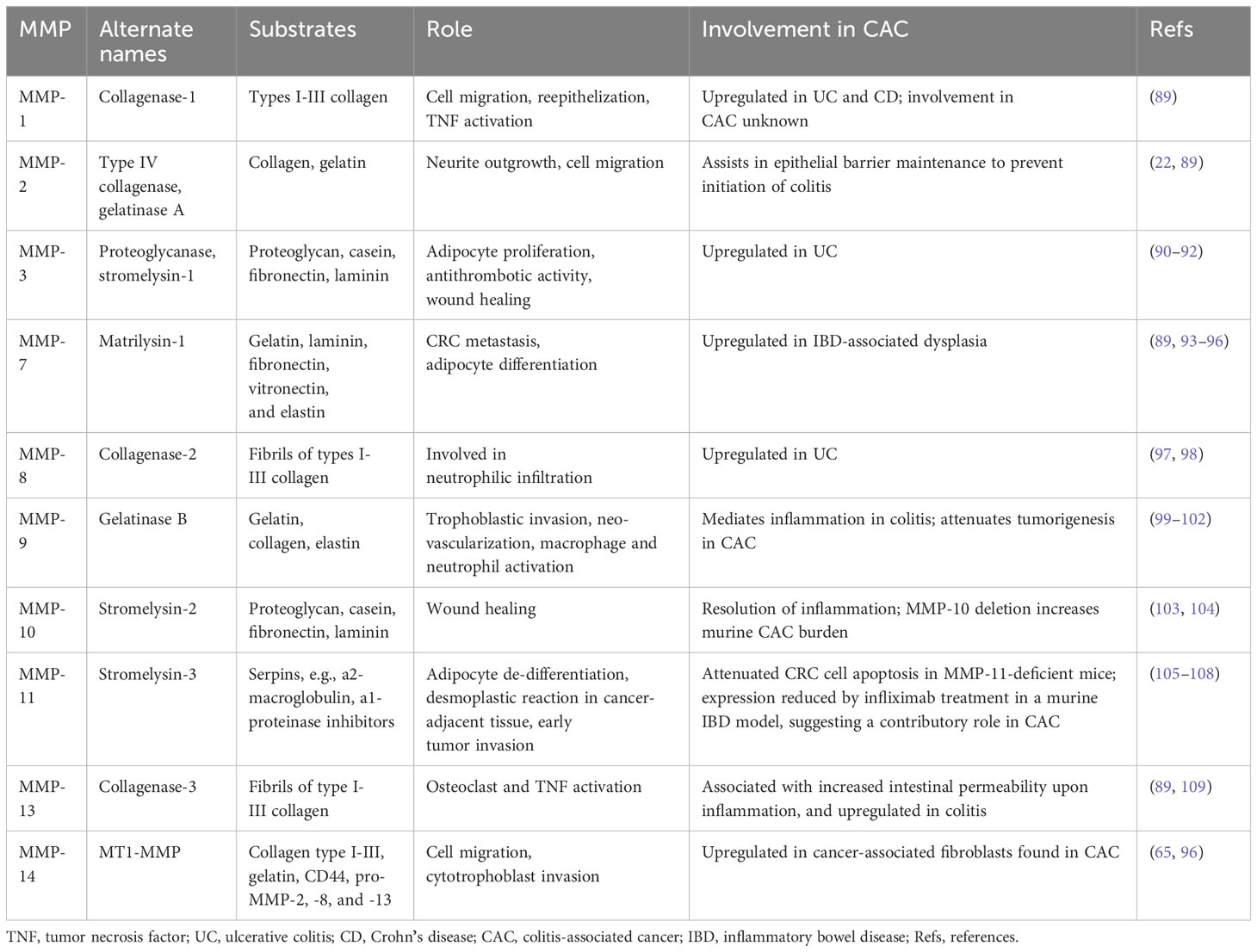
MMPs are classified as collagenases, gelatinases, stromelysins, matrilysins, metalloelastase, enamelysin, epilysin, and membrane-type MMPs. The major difference between these enzymes is domain organization and substrate specificity, illustrated in Figure 1. As anticipated from a family of proteins, MMP structural similarity is high. MMPs share three basic domains: the N-terminus domain, pro-domain near the active site, and catalytic domain. Upon activation, the N-terminus and pro-domain are cleaved. Whereas matrilysins (MMP-7 and -26) have this basic organization, collagenases (MMP-1, -8, -13), stromelysins (MMP-3, -10), metalloelastase (MMP-12), enamelysin (MMP-20), and MMP-22 and -27 have an additional hemopexin-like domain close to the C- terminus, which binds and helps to orient the substrate correctly. MMPs differ broadly in substrate specificity; collagenases cleave peptide bonds present in collagen, stromelysins cleave a wider range of substrates (proteoglycan, gelatin, fibronectin, lamin, and collagen), metalloelastases degrade elastin, and, as its name implies, enamelysin modulates enamel formation. Gelatinases (MMP-2, -9) have fibronectin repeats around the catalytic site and primarily target gelatin and type IV collagen. Membrane-bound MMPs possess either an additional transmembrane peptide domain (MMP-14, -15, -16, -24), or glycophosphatidylinositol (GPI)-anchoring domain (MMP-17, -25) and have a basic amino acid motif at the C-terminus, that is recognized and cleaved for activation by proprotein convertases. Notably, MMP-23 contains a type II transmembrane domain and, instead of the hemopexin domain common to other MMPs, possesses a small toxin-like domain and immunoglobulin-like cell adhesion molecule domain that informs its unique properties in modulating voltage-gated potassium activity and interactions with components of the ECM and signaling molecules (23, 24). An overview of the MMP substrates and physiological functions unrelated to CAC development are included in Table 1.
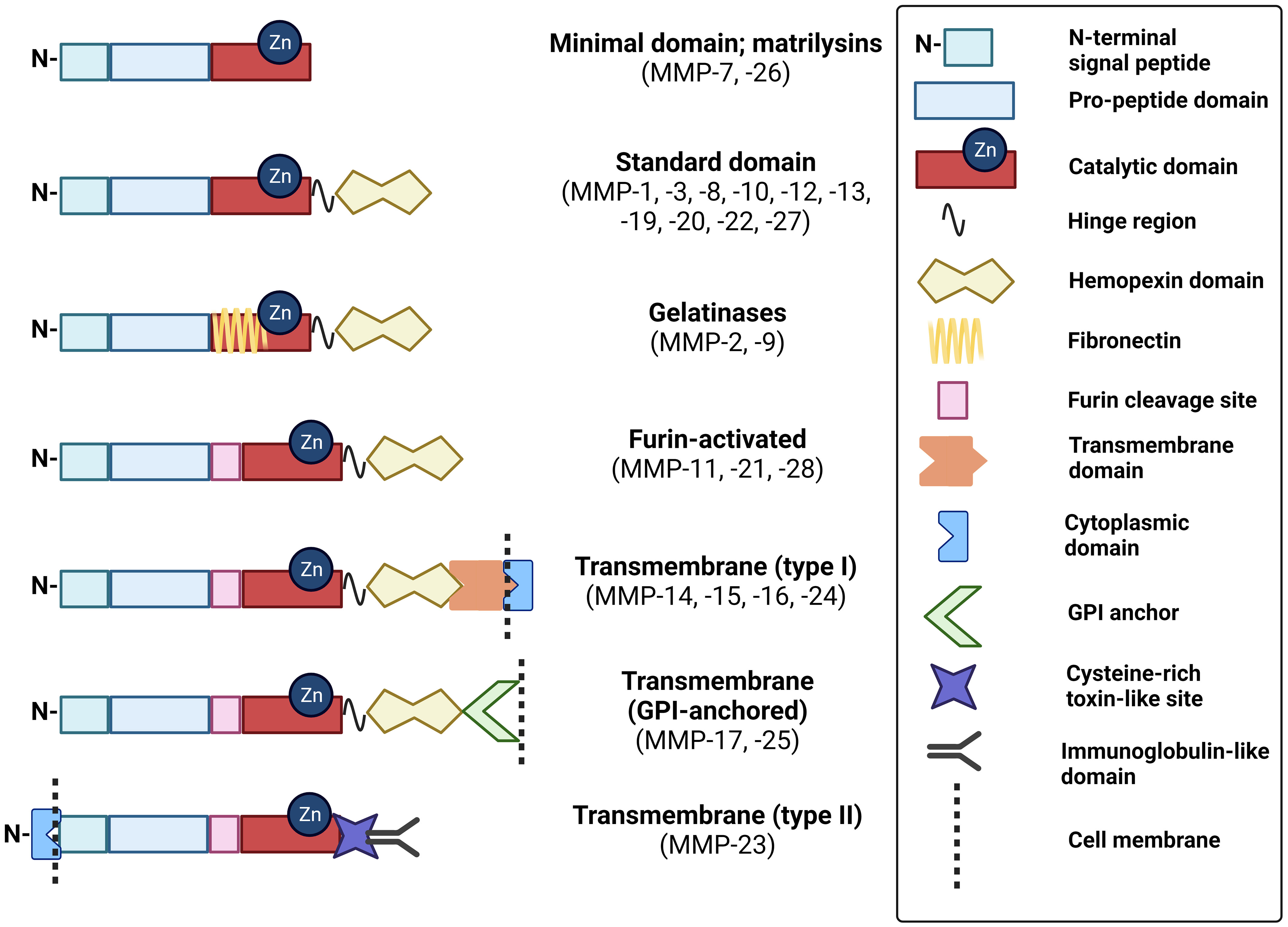
Figure 1 Schematic of MMP domain structures. These schematics of MMP subtypes illustrate the relative distribution of key domains that modulate their structure and function. GPI, glycophosphatidylinositol. Created with BioRender.com.
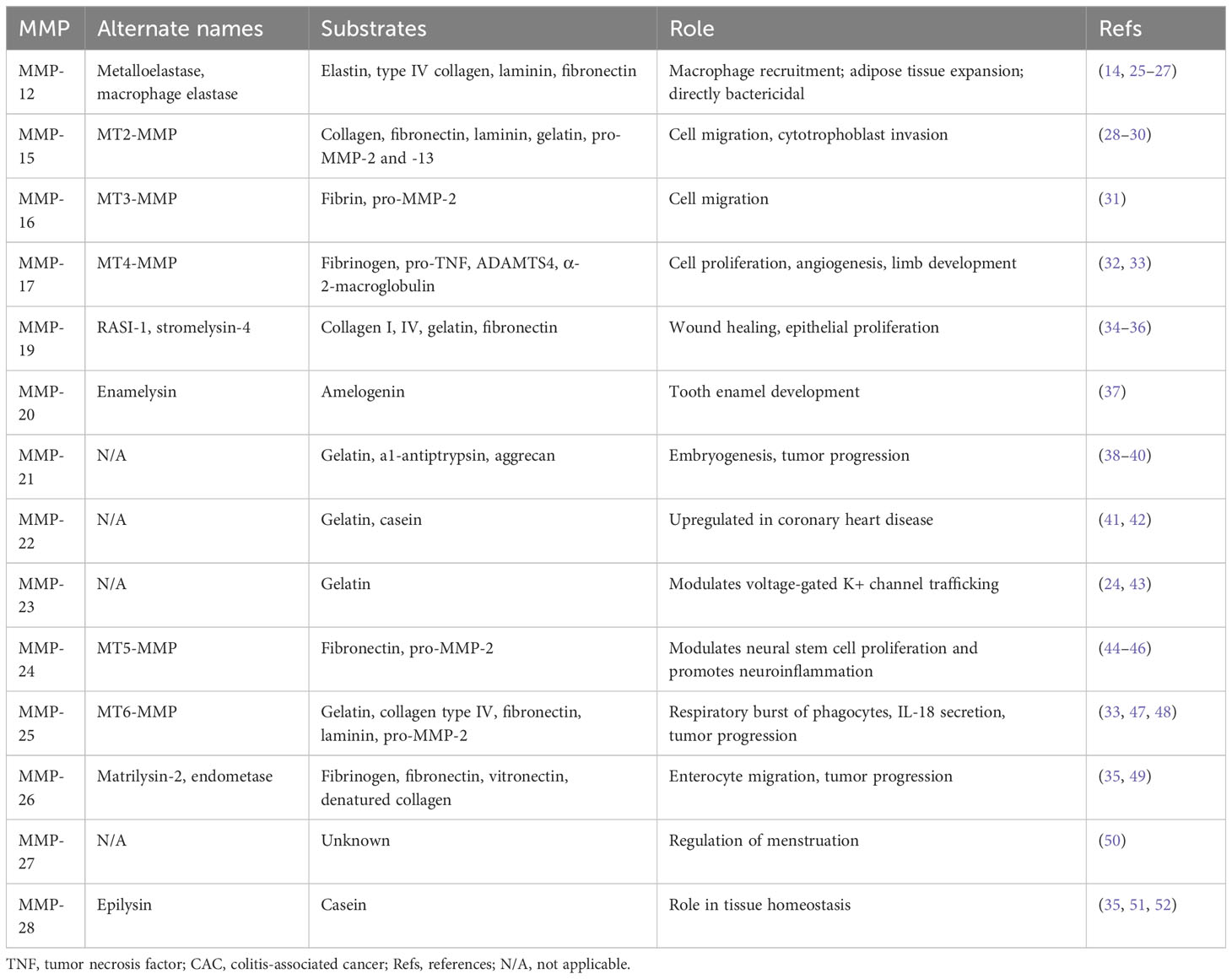
Table 1 Overview of MMP substrates and physiological functions with currently unknown roles in the development of CAC.
Due to their involvement in a multitude of biological processes, dysregulated MMP expression or function can broadly impact health and disease. MMP dysregulation is evident in fibrotic diseases, vasculopathies, and tissue degradation that promote cancer progression, ulceration, rheumatoid arthritis, osteoarthritis, periodontal diseases, among a host of other conditions. For example, poor wound healing is characterized by increased MMP-9 expression, with significantly higher levels detected in unhealed diabetic foot ulcers (53). As a consequence of their roles in degrading the pericellular matrix, activating other MMPs, and stimulating epidermal growth factor (EGF) receptor (EGFR)-dependent cell motility and growth, many MMPs, including collagenases, gelatinases, matrilysin, metalloelastase, and membrane-bound MMPs, promote neoplasia – e.g., cellular invasion and metastasis (23). By modulating chemokine and cytokine activity and bacterial clearance, MMPs are involved in initiating acute inflammatory responses after tissue injury. Nonetheless, MMPs can have conflicting functions that either attenuate or augment inflammation. For example, although MMP-7 expressed in non-inflamed epithelium modulates homeostasis, it can also act on syndecan-1 after its release by endothelial cells, to establish a local post-injury chemokine gradient that activates FAS ligand to trigger apoptosis (54, 55).
2.2 Cellular regulation of matrix metalloproteinase activity
Because they play such key roles in modulating cell functions, MMP activity must be tightly regulated at the transcriptional, post-transcriptional, and proteomic levels (Figure 2). MMPs are produced and secreted by many different cell types, including dermal fibroblasts, osteoblasts, and endothelial and inflammatory cells. Typically located peri- or extracellularly, both secreted and membrane-bound MMPs can also localize to intracellular sites (56). MMP secretion can be stimulated by cytokines, including proinflammatory cytokines (e.g., interleukins and interferons), growth factors (e.g., EGF and acetylcholine), and physiochemical agents (e.g., heat) (15, 57). Particular cell types evidence signal-dependent activation and repression of MMP gene transcription by processes involving mitogen-activated protein kinase (MAPK)-, nuclear factor-kB (NF-kB)-, and Smad-dependent pathways. Specific transcription factors such as AP-1, PEA3, Sp1, Tcf/Lef-1, and NF-kB serve as cis-acting elements that can bind proximal to the MMP promoter to induce expression. In 1987, AP-1 was the first inducer implicated in MMP-1 expression (58).
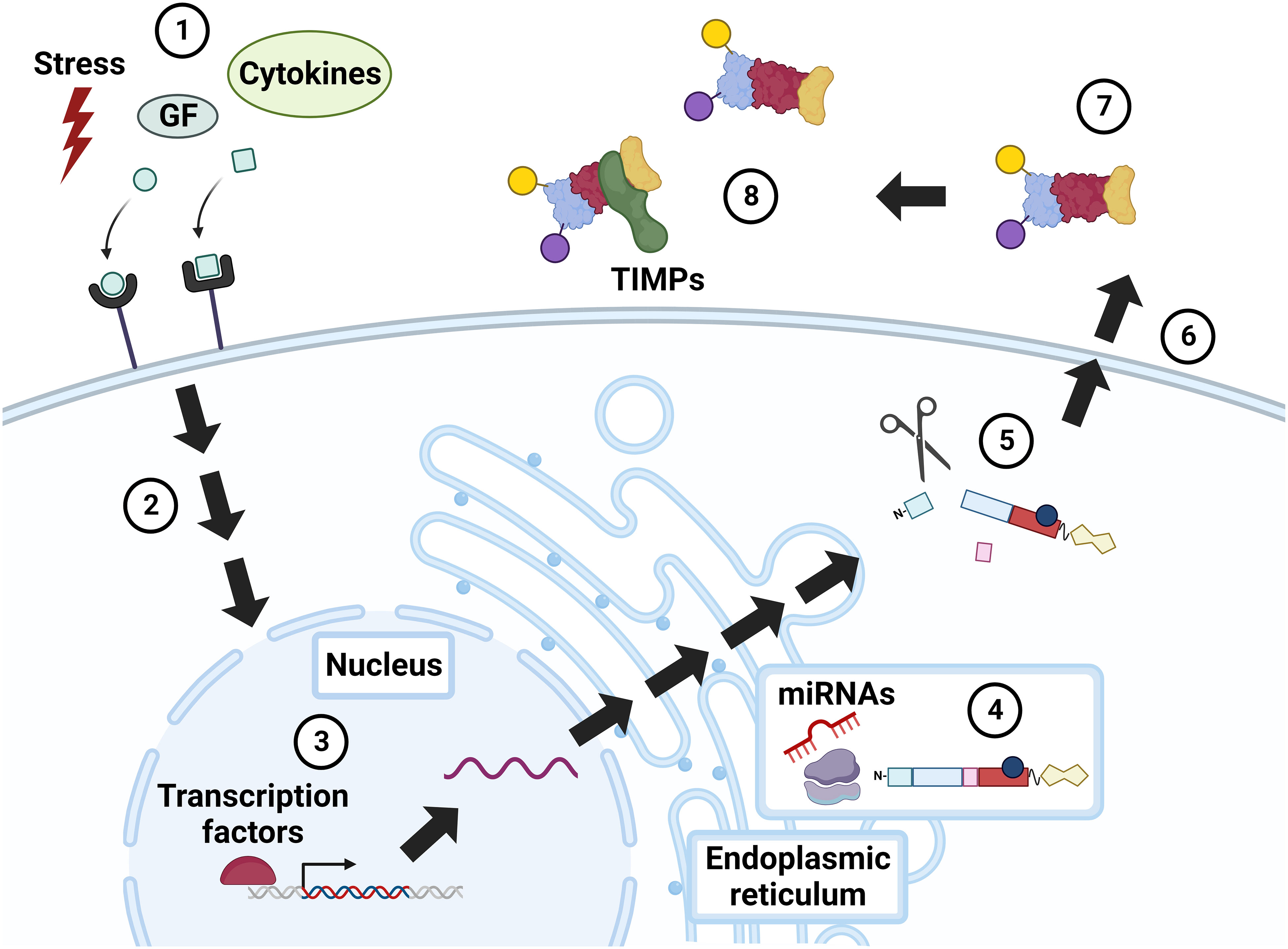
Figure 2 Regulation of MMP activity. Constitutive MMP expression is augmented in response to external stimuli, e.g., physiochemical stress, growth factors (GF), and cytokines (1), that incite intracellular signaling via mitogen activated protein kinase (MAPK), NF-kB, Smad, and others (2). MMP expression is further modulated by transcription factors, notably AP-1 (3). MMP RNA is translated into a pre-propeptide (4), a process that can be up- and down-regulated by microRNAs (miRNAs). Cleavage of the N-terminal signal peptide in the endoplasmic reticulum (ER) yields the pro-MMP zymogen, which may undergo intracellular (e.g., cleavage by furin) and extracellular (e.g., cleavage by plasmin) modification (5). Cellular release of MMPs is also regulated by stress, growth factors, and cytokines (6). Extracellular MMPs are activated by post-translational modifications (7), or by the action of other, membrane-bound MMPs. MMP activity is also modulated by complexes formed with inhibitors, i.e., TIMPs and α2-macroglobulin (8). GF, growth factors; TIMP, tissue inhibitor of metalloproteinase. Created with BioRender.com.
MMPs are regulated post transcriptionally by micro(mi)RNAs, a family of short non-coding RNAs that modify gene expression. Dysregulated miRNA activity can alter cancer progression by sustaining proliferative signaling or enhancing invasion and metastasis (59). For example, Wu et al. showed that the tumor suppressor p53 can induce miR-34a expression and, thereby, downregulate MMP-1 and MMP-9, and attenuate cell migration and invasion (60). Sun et al. demonstrated that increased miR-21 expression in serum from patients with CRC and in colon cancer cell lines correlated with increased MMP-2, -9, and -11 expression (61). Other miRNA-MMP-linked interactions impacting CRC progression have been studied, including miR-139-MMP-2, miR-146a-MMP-16 (62, 63).
After transcription, MMPs are synthesized as pre-proenzymes. Following removal of the N-terminal signal sequence during translation, each MMP exists in three forms: the inactive secreted form or zymogen known as pro-MMP, active MMP, and inhibitor-complexed MMP (64, 65). Pro-MMPs are secreted and activated by proteolytic removal of the pro-domain by other MMPs, particularly membrane-type MMPs, serine proteases, plasmin, or furin. Activation via serine proteases can be regulated by inhibition of plasma proteinase inhibitors, such as α1-proteinase (66). Once activated, MMPs possess catalytic activity until they bind to two major types of endogenous inhibitors, α2-macroglobulin and tissue inhibitor of matrix metalloproteinases (TIMPs). α2-macroglobulin, a protease inhibitor abundant in plasma, binds to MMPs extracellularly, allowing for their removal by receptor-mediated endocytosis (67). In turn, TIMPs, a family of four inhibitors (numbered 1-4), regulate processes mediated by MMPs and ADAMs (A Disintegrin And Metalloproteinase), another family of zinc-dependent peptidases related to MMPs (68). Dysregulation of the MMP : TIMP ratio can upregulate active MMP expression and ECM damage (64). Recent data show that TIMPs also modulate biological processes independent of MMP and ADAM activity, adding additional complexity to their impact on tumor proliferation and/or inhibition (69). TIMP-1 preferentially inhibits MMP-1, -3, -7, and -9, and has stimulatory cellular properties (68, 70). TIMP-2 traditionally inhibits MMP-2 and -9, but, in aggressive cancers, TIMP-2 uniquely binds and activates pro-MMP-2 and MT1-MMP to stimulate MAPK/ERK signaling (68, 71). Recently, Li et al. identified elevated TIMP-2 expression in the serum of patients with CRC resistant to 5-fluorouracil (5-FU), a possible biomarker of 5-FU resistance (72). TIMP-3 preferentially inhibits MMP-2 and MMP-9. In mice, TIMP-3 deficiency leads to maladaptive ECM remodeling, cardiomyocyte hypertrophy, and cardiac dysfunction (73). Though less studied, TIMP-4 appears to inhibit MMP-26 preferentially as well as contribute to the proteolysis of a cell surface fatty acid transporter, underlying its role in intestinal lipid absorption (74, 75).
3 Colitis-associated colon cancer
3.1 Relationships between IBD and colon cancer
IBD is associated with a two- to three-fold increased risk of CRC, a major cause of IBD mortality and need for colectomy (76). The frequency rate of CAC is 1.78% among IBD patients (2.1% for ulcerative colitis and 1.5% for Crohn’s disease), while the rate of CRC is 1.23% in the general population (77). Notably, in line with the decreasing mortality from CRC in the United States, the rates of CAC incidence and mortality also appear to be declining. The latter is likely impacted by the development of a wide array of novel therapeutic modalities targeting the inflammatory cascade, e.g., biologicals targeting tumor necrosis factor and the immune response.
Key mechanistic differences that distinguish CAC from sporadic CRC raise important questions regarding the need for a distinct approach to prevention, surveillance, and management. The driving force behind CAC is chronic inflammation, which amongst other consequences, promotes DNA oxidative damage that may alter the expression and function of genes identified as tumor promotors and suppressors (12). The risk factors most often associated with CAC reflect the influence of chronic inflammation, including the anatomical extent of disease, the severity of histological injury, and the cumulative inflammatory burden (78).
While the same molecular pathways that contribute to the development of CRC are involved in CAC, including chromosomal and microsatellite instability, the sequence of common gene alterations differs considerably. In contrast to the canonical CRC adenoma-carcinoma sequence, in CAC, p53 mutation is an early event, occurring even before the development of dysplasia (79) (Figure 3). APC mutation, an early event in CRC, occurs later in the development of CAC (79). The presence of similar burden of mutations in non-dysplastic mucosa adjacent to CAC suggests a primed field effect, which may explain high rates of synchronous and metachronous dysplasia in the same region (80, 81). These differences may contribute to the difficulty detecting epithelial dysplasia using colonoscopy in those with IBD. While most dysplastic colonic lesions in IBD are visible on high-definition white light endoscopic evaluation, their flatter morphology can have a more subtle appearance than classical adenomatous polyps that precede the development of CRC. Using tools like dye-based and virtual chromoendoscopy can augment the ability to detect dysplasia in CAC (82). Additionally, in IBD, the rapid and recurrent development of dysplasia in primed precancerous fields requires repeated surveillance colonoscopies at shorter intervals than those recommended for screening for sporadic CRC (83). The implementation of such dysplasia surveillance protocols in IBD is also thought to be a key contributor to the decreasing rates of CAC.
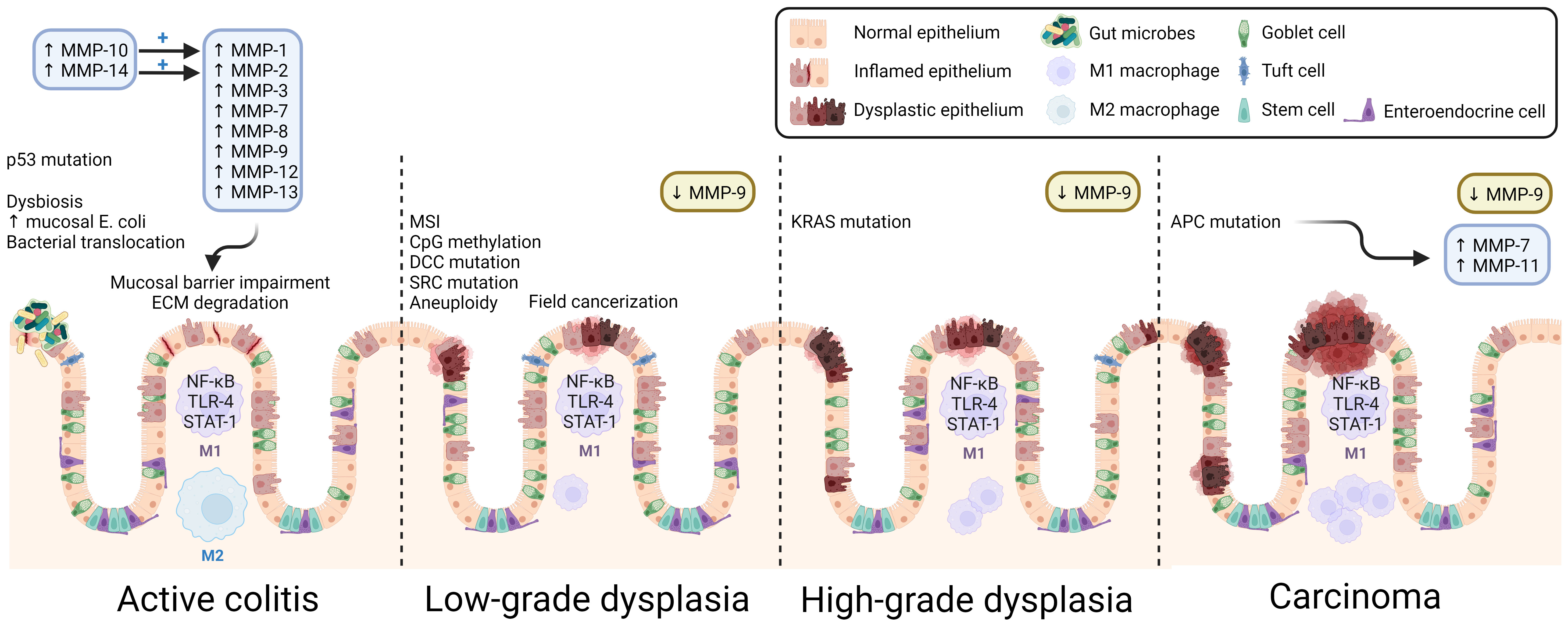
Figure 3 Overview of CAC pathogenesis. Many MMPs are over-expressed, and their activity upregulated in active colitis. MMP-induced mucosal damage, inflammatory dysbiosis, early p53 mutation, and other results of DNA damage promote dysplastic changes. Because the inflammatory insult is widespread, CAC exhibits field cancerization, and multiple malignant foci form in the affected tissue. As dysplasia progresses, the population of colonic macrophages shifts toward the pro-inflammatory M1 macrophage subtype. Despite its membrane-degrading effects in colitis, MMP-9 is consistently found to be downregulated in CAC and acts as a tumor suppressor. ECM, extracellular matrix; MSI, microsatellite instability. Created with BioRender.com.
In IBD, concomitant primary sclerosing cholangitis (PSC) greatly augments CAC risk. Compared to those with IBD alone, the additional diagnosis of PSC confers an additional three- to five-fold increased risk of developing CAC. The mechanisms leading to this increased risk have not yet been clarified but may differ from those underlying CAC in IBD alone. Proposed factors include altered bile acid metabolism, intestinal or biliary dysbiosis, and systemic immunological dysfunction (84). It is both interesting and puzzling that colonic inflammation itself appears to be less influential, as those with concomitant IBD and PSC who develop CAC often have quiescent clinical, endoscopic, and histologic disease.
Given the many mechanistic differences between CAC and sporadic CRC, as well as the relative paucity of research on the treatment of CAC compared to that of sporadic CRC, CAC presents many challenges to treatment. Management of CAC includes surgical intervention with administration of adjuvant chemotherapy, like treatment of CRC (85, 86). Unfortunately, patients with metastatic CAC fare more poorly than age- and tumor type-matched patients with metastatic sporadic CRC, even when treated with first-line chemotherapy regimens such as FOLFOX or FOLFIRI (87). This underscores the need for improved early disease detection, and for the development of therapies that target not only the carcinogenic milieu of CRC but also CAC’s hallmark inflammatory dysregulation (85). Even the type of underlying IBD may influence disease outcome in CAC, adding additional facets to consider in the search for meaningful CAC biomarkers. For example, individuals with Crohn’s disease had more advanced CAC stage at diagnosis than those with UC, leading to poorer survival outcomes for Crohn’s-associated colorectal cancer (88).
3.2 Roles of matrix metalloproteinases in CAC pathogenesis
MMPs are involved in myriad cellular and extracellular processes, including epithelial proliferation, ECM homeostasis, angiogenesis, and response to inflammation. As such, they are a topic of interest in cancer research, particularly CRC research. There is a growing body of literature regarding the roles of MMPs in the relatively more enigmatic CAC, and in pre-cancerous IBD (Table 2). Given clear differences in the pathogenesis and clinical features of CAC, compared to non-inflammatory CRC, in the following paragraphs, we summarize the current literature on MMPs in CAC, an underdeveloped focus of investigation.
Unlike sporadic colon cancer, CAC arises from chronic inflammation-related DNA damage, with distinct mechanisms and a significant role for immune dysregulation (12, 110). It is not surprising that increasing IBD duration and severity pose progressively increased CAC risk (111). During acute intestinal inflammation, several MMPs are upregulated, most prominently MMP-1, -8, -9, -10, -12, and -13; persistent overactivity of MMPs impairs ECM integrity, which itself can exacerbate IBD, heralding chronic inflammation and pre-cancerous DNA damage (Figure 1) (112). Biopsies of IBD-related intestinal epithelial damage (ulcers) reveal increased gene and protein expression of MMP-1, -2, -3, -7, and -9, as well as MMP-10, and -14 that activate MMP-1 and -2 (113, 114). In vitro studies revealed that MMP-9 derived from intestinal epithelial cells (but not from neutrophils) plays a key role in the pathogenesis of colitis by inhibiting epithelial cell-ECM interaction and wound healing; MMP-9 knockout mice are more resistant to various types of induced colitis (115, 116).
The roles of MMP-9 in IBD and in CAC are of particular interest. Although MMP-9 appears to facilitate pro-inflammatory signaling in non-cancerous or pre-cancerous IBD, its actions may oppose tumorigenesis in the setting of CAC. In a recent study highlighting the applicability of big data methods to such questions, investigators used a transcriptomic approach to find thatMmp9 and Mmp3 were overexpressed in colitis, but not in CAC (13). Using different approaches, others reported increased MMP-9 expression in CAC tissue samples (99, 117). Decades of investigation by Garg and colleagues uncovered potential mechanisms whereby MMP-9 regulates CAC by promoting caspase-3-dependent apoptosis, restraining cell proliferation, and limiting DNA damage by reactive oxygen species (ROS) (118, 119). MMP-9 most likely mediates enterocyte apoptosis and proliferation by activating Notch-1, a transcription factor which gatekeeps the differentiation of goblet cells and, in turn, activates the tumor suppressor p53 (120). In multiple models, inhibiting this pathway leads to increased tumor number and grade. Both in vivo and in vitro, MMP-9 was found to be involved in mismatch repair and amelioration of oxidative stress in CAC (119). Thus, although MMP-9 promotes inflammation in non-cancerous IBD via ECM degradation, by a different mechanism, it acts as a tumor suppressor in CAC. Furthermore, Timp1, which encodes a TIMP that preferentially inhibits MMP-1, -3, -7, and -9, is overexpressed in colitis and CAC, suggesting tight regulation of MMP activity in both disorders (13).
Other MMPs have been studied in the context of CAC, however their roles remain largely obscure. Compared to ulcerative colitis without dysplasia, MMP-7 expression is increased at the crypt bases of CAC tissue and its expression is more widespread in high-grade compared to low-grade dysplasia (93). It is possible that increased MMP-7 expression in CAC may result from the loss of APC, which occurs relatively later in the progression from inflammation-related dysplasia to CAC. APC loss results in nuclear translocation of β-catenin and activation of MMP-7 transcription via TCF-4, like the mechanism underlying MMP-7 overexpression in sporadic CRC (121). In a transcriptomics study, the genes encoding MMP-7 and MMP-13 were overexpressed in CAC but not in colitis. In contrast to pre-omics era MMP expression data, this difference might be attributable to the analysis of a different tissue type (13, 113). Additional studies using in vivo models of colitis and CAC found that the expression signature of colitis (i.e., Mmp3 and Mmp9) versus that of CAC (i.e., Mmp7 and Mmp13) could predict the development of CAC in mice with dextran sulfate sodium (DSS)-induced colitis (13).
Koller et al. found that MMP-10-deficient mice were more susceptible to DSS-induced colitis, a common experimental model approximating UC, and their disease was more severe and refractory to resolution. Moreover, MMP-10-deficient mice had a higher burden of inflammation-associated colonic dysplasia, suggesting that MMP-10 may protect against both colonic inflammation and CAC (103).
MMP-11 mRNA expression is also upregulated in CAC compared to normal tissue, and the positive correlation between MMP-11 and β-catenin expression in intestinal crypts suggests the mechanism may be similar to that for MMP-7, i.e., APC loss in CAC (117). Interestingly, in mice, treatment with the proton pump inhibitor omeprazole prevented experimental CAC, with concomitant decreases in MMP-11, MMP-9, and MMP-14 (aka MT1-MMP) and colon inflammatory markers. While attempting to induce experimental CAC, the same group found similar decreases in MMP-11 and -9 expression and activity in mice treated with infliximab, an anti-TNF-α medication used in IBD (105). Little else is known about the role of MMP-11 in CAC.
3.3 MMPs as potential biomarkers
The dynamic levels of selective MMP expression in inflammation and disease progression, including CRC, make them attractive biomarker candidates. Different methods of analysis such as ELISA, zymography, mass spectrometry, and cytology are used to detect MMPs in plasma, serum, urine, and tissue samples (122, 123). Incidentally, it is important to pay attention to sample collection – differences in MMP concentration values are reported depending on sample type (e.g., higher in serum compared to plasma) (124).
The utility of MMPs to mark disease status was studied in different contexts, including COVID-19 infection, cardiovascular disease, IBD, and cancer. In sera from 156 patients hospitalized with COVID-19 and respective controls, Gelzo et al. detected a significant increase in MMP-3 in early infection and increased MMP-9 levels throughout the course of disease (125). In a prospective study of 1127 patients with coronary artery disease, Blankenberg et al. reported an increase in plasma MMP-9 levels, deeming it a possible predictor of cardiovascular mortality (126). Regarding inflammation, Yoblecovitch et al. noted that serum MMP-9 levels predicted a clinical flare in patients with quiescent Crohn’s disease (127). Lastly, in CRC, elevated levels of MMP-2, MMP-9, and MMP-13 were observed in plasma and cancer biopsy samples (128–130). Together, these proofs of principle suggest that specific MMPs can be used to gauge disease status and intestinal inflammation, thus highlighting their potential use as clinical biomarkers.
4 Clinical therapies targeting MMPs in CAC
4.1 Approaches to targeting MMPs
MMPs are increasingly of interest as therapeutic targets for several disorders, including complications of diabetes (e.g., diabetic retinopathy (131) and foot ulcers (132)), and ischemic heart injury (133). Both natural (i.e., TIMPs) and synthetic MMP inhibitors have been pursued as therapeutic agents. In early studies, TIMPs were of great interest as natural inhibitors, particularly given their aberrant expression in multiple cancer types (134). Unfortunately, the lack of effective and selective delivery methods and increased TIMP levels in several cancers complicate their use. TIMP size and structure make tissue penetration and targeted delivery difficult, and once administered, they are prone to rapid degradation. Further, there is an inverse correlation between survival and TIMP levels. Although the rise in TIMP levels is likely due to a concurrent rise in MMP levels, their failure to impact tumor progression suggests that exogenous TIMPs are unlikely to have therapeutic efficacy (135), even though they may synergize with synthetic MMP inhibitors (MMPIs) (136). Neovastat, a compound derived from shark cartilage, targets MMP-2 while also blocking VEGF signaling. Neovastat has only been explored for non-GI metastatic cancers (e.g., breast, prostate, kidney, and lung (137)), but its dual mechanism of action increases the likelihood of success beyond phase III clinical trials. Combined with its excellent safety profile, these features suggest Neovastat may be an attractive candidate to target cancers in which MMP-2 is an important player (138).
Synthetic MMPIs are generally categorized as broad-spectrum peptidomimetics, non-peptidomimetics, tetracycline derivatives, and bisphosphonates (139). Peptidomimetics are collagen-mimicking pseudopeptides that gained traction as potential inhibitors, though they have not yet succeeded in phase III clinical trials. The archetype, Marimastat, efficaciously targets MMP-1, -3, -7, and -9 and dose-dependently reduced carcinoembryonic antigen (CEA) levels in recipients with CRC (140). Although Marimastat’s excellent oral bioavailability and success in phase III trials were favorable, the lack of extended overall survival and continued tumor progression diminished enthusiasm (139). Synthetic MMPIs may provide benefit when used in combination with other therapies; for example, TIMP-2 synergistically enhances the effects of Marimastat (136).
Synthetic non-peptidomimetics, described in more detail below, provide an alternative therapeutic avenue. Their development resulted from an attempt to increase oral bioavailability and improve pharmacokinetic properties of peptidomimetic compounds (141). Many are specific to MMP-2, -3, and -9, such as Prinomostat’s targeting of MMP-2, -3, -9, -13, and -14 and Tanomastat’s targeting of MMP-2, -3, and -9 (142). Tetracycline derivatives, such as Periostat and Metastat, inhibit both the production and secretion of MMP-2 and -9 (143, 144), however, because of their toxicity at therapeutic doses, greater tissue specificity is required for successful drug development.
Another approach utilizes miRNAs to modify the action of MMPs indirectly. Multiple potential points of intervention exist, including at the level of MMP secretion, enzymatic activity, and expression. An example pursued in sporadic CRC is miR-34a, a miRNA downregulated in several digestive cancers that transcriptionally targets p53 (60). Treatment of colon cancer cells with miR-34a reduced MMP-1 and -9 protein levels and decreased proliferation and invasion in vitro (60). However, targeted delivery of miRNAs remains challenging. A potential strategy is to target tissues that exhibit increased MMP activity using polyplexes of miRNA and MMP-responsive polyethylene glycol (PEG) shields, which can deshield in the presence of high MMP levels. miR-34a has been successfully polyplexed to MMP-2-responsive PEGylated polyethyleneimine (PEI), a complex in which PEG shields PEI, but can be cleaved by MMP-2. Compared to treatment with non-PEGylated PEI and miRNA, application of this MMP-cleavable system increases cellular co-uptake of the PEGylated PEI and miRNA, and improves metrics of anti-cancer activity in vitro and in vivo (145, 146).
4.2 Synthetic MMP inhibitors
The 1990s witnessed great interest in the therapeutic potential of synthetic MMPIs for cancer. Theoretically, synthetic MMPIs offered a narrower spectrum of MMP inhibition compared to their predecessors, and might therefore target specific MMPs and avoid off-target toxicity. The earlier synthetic MMPIs were typically small molecule inhibitors which targeted the MMP active site using zinc binding groups on a peptide backbone (147). Aside from poor MMP selectivity, low efficacy and musculoskeletal side effects prevented these small molecule inhibitors, including Batimastat and Marimastat, from advancing through clinical trials for CRC (148, 149). Later, the use of transition state analogs and of targeting multiple MMP structural features, e.g., the variable S1’, S2, and S3 subsites and exosite domains, improved substrate selectivity (148, 150, 151).
In the early 2010s, another avenue in MMPI drug development emerged: allosteric regulation. In a proof of principle, Udi et al. developed two branched amphiphilic molecules capable of binding to “hidden regulatory sites” unique to MMP-12 and MMP-14, thereby disrupting the geometric conformation of these MMPs and their function, without the need for a less specific zinc binding group (152). Soon thereafter, synthetic allosteric inhibitors and inhibitory antibodies were developed to target MMPs implicated in disease. In a murine model, AB0041, an antibody which noncompetitively inhibits MMP-9 with high specificity, attenuated colon cancer xenograft growth and metastasis (153). The humanized form of AB0041, GS-5745 (Andecaliximab), had equivalent potency and selectivity and completed Phase I clinical trials for UC and advanced solid tumors, including CRC, but has not been studied in CAC (153–155). Notably, Andecaliximab is the only MMP-targeting monoclonal antibody that has undergone clinical trials (149). As noted by others, MMPs may be more active in the pre- and peri-metastatic phases of disease. Hence, studying MMPIs only in advanced (i.e., metastatic) cancer may miss interventional opportunities; perhaps including early-stage cancers in MMPI trials would yield more favorable results (156).
4.3 Challenges in clinical application
Promising avenues for the use of MMP inhibitors in CRC treatment are well established, but general challenges in their clinical use warrant discussion. As discussed in section 2, the development of specific MMP inhibitors was complicated by the hard-to-define role certain MMPs had in inflammatory disease progression, along with general substrate redundancy and high structural homology (147). Broad spectrum MMPI use encountered challenges such as low bioavailability, a poor metabolic profile, and, as in the case of Marimastat, limiting musculoskeletal side effects (157). Administration of non-pepdomimetic compounds also led to variable effects in cell proliferation and adverse reactions. For example, in phase III clinical trials for non-small lung cancer, determining the dosage and timing of Tanomastat administration proved problematic (158). Tetracycline derivatives such as Metastat, trialed for advanced solid tumors, were associated with severe toxicity, including photosensitivity skin reactions, showing that greater tissue selectivity is required (159).
Over time, more selective MMPIs were developed via NMR and X-ray crystallography methods to identify less conserved binding sites, such as cavities formed specific to individual enzymatic structures (158). As novel MMPIs progress through clinical trials, fewer challenges pertaining to delivery or heterogeneous effects are noted. For example, in a randomized phase I/II study in psoriasis, the adverse effects of Neovastast, used to inhibit MMP-2 and VEGF, were mainly nausea and diarrhea (160). As observed in phase Ib and phase II/III clinical studies for ulcerative colitis and rheumatoid arthritis, selective MMP-9 inhibition using the monoclonal antibody Andecaliximab also has a generally safer side-effect profile. In these trials, test subjects exhibited modest adverse events requiring only observation or minimal interventions (161, 162). As we consider challenges in their therapeutic use, in addition to efficacy and safety concerns, the high cost of monoclonal antibodies and similar advanced approaches should be considered.
5 Conclusions and future directions
As a consequence of chronic inflammation, colitis increases the risk of developing CAC, underscoring the importance of understanding the pathology of chronic inflammation-induced CRC (163). Although previously thought to participate only in ECM degradation, MMPs are now recognized to be directly involved in the development and sustenance of inflammatory states, including those that underlie both Crohn’s and ulcerative colitis. Yet, despite advances reviewed here, large gaps in knowledge exist regarding the role MMPs play in both CAC and the chronic inflammation that precedes and promotes CAC. There is a concordant lack of information regarding the potential utility of MMP inhibitors to treat these disorders.
The upregulation of specific MMPs, like MMP-1, -2, -3, and -7, in intestinal inflammation and IBD implicates not only their contribution to the development of CAC but also their potential as biomarkers to identify increasing dysplasia and incipient neoplasia. In contrast, other MMPs, like MMP-10, appear to be protective, auguring a more favorable prognosis. Some, like MMP-9, play seemingly context-dependent dual pro-inflammatory and anti-tumorigenic roles. Thus, prospectively testing the value of individual MMPs or a panel of MMPs in blood and tissue samples to gauge their value as biomarkers for dysplasia and incipient neoplasia appears to be a worthy avenue of investigation.
This comprehensive review of MMP involvement in CAC pathogenesis and potential therapeutic approaches targeting MMPs, emphasizes the need for continuing research focused on unraveling the pathways whereby different MMPs exert their actions. For example, further work is needed to unravel the pathways that modulate selective MMP upregulation after muscarinic agonism in colon cancer, how this interplay contributes to an invasive phenotype, and its importance for the development and progression of CAC. Another potential avenue is to use this information to develop a panel of MMPs that can be tested in primary tumors to gauge the potential for advancing disease, the need for adjuvant therapy, and the likelihood of response to conventional chemotherapeutic regimens. Lastly, continuing attention must be devoted to exploring the growing therapeutic applications of MMP inhibitors, particularly as they relate to the management of IBD and CAC in those with an MMP profile predictive of aggressive disease.
Author contributions
NS: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. AS: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. MA: Data curation, Writing – original draft, Writing – review & editing. SP: Data curation, Writing – original draft. J-PR: Conceptualization, Data curation, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the United States (U.S.) National Institutes of Health grant number T32 DK067872 (J-PR) and the U.S. Department of Veteran Affairs Biomedical Laboratory Research and Development Program, VA Merit grant number BX002129 (J-PR).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The contents do not represent the views of the U.S. Government or the U.S. Department of Veterans Affairs.
References
1. Xie G, Cheng K, Shant J, Raufman JP. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am J Physiol Gastrointest Liver Physiol (2009) 296(4):G755–63. doi: 10.1152/ajpgi.90519.2008
2. Murray GI, Duncan ME, O’Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med (1996) 2(4):461–2. doi: 10.1038/nm0496-461
3. Sunami E, Tsuno N, Osada T, Saito S, Kitayama J, Tomozawa S, et al. MMP-1 is a prognostic marker for hematogenous metastasis of colorectal cancer. Oncologist (2000) 5(2):108–14. doi: 10.1634/theoncologist.5-2-108
4. Baker EA, Bergin FG, Leaper DJ. Matrix metalloproteinases, their tissue inhibitors and colorectal cancer staging. Br J Surg (2000) 87(9):1215–21. doi: 10.1046/j.1365-2168.2000.01531.x
5. Raufman JP, Cheng K, Saxena N, Chahdi A, Belo A, Khurana S, et al. Muscarinic receptor agonists stimulate matrix metalloproteinase 1-dependent invasion of human colon cancer cells. Biochem Biophys Res Commun (2011) 415(2):319–24. doi: 10.1016/j.bbrc.2011.10.052
6. Said AH, Hu S, Abutaleb A, Watkins T, Cheng K, Chahdi A, et al. Interacting post-muscarinic receptor signaling pathways potentiate matrix metalloproteinase-1 expression and invasion of human colon cancer cells. Biochem J (2017) 474(5):647–65. doi: 10.1042/BCJ20160704
7. Schledwitz A, Sundel MH, Alizadeh M, Hu S, Xie G, Raufman JP. Differential actions of muscarinic receptor subtypes in gastric, pancreatic, and colon cancer. Int J Mol Sci (2021) 22(23):13153. doi: 10.3390/ijms222313153
8. Tolaymat M, Sundel MH, Alizadeh M, Xie G, Raufman JP. Potential role for combined subtype-selective targeting of M(1) and M(3) muscarinic receptors in gastrointestinal and liver diseases. Front Pharmacol (2021) 12:786105. doi: 10.3389/fphar.2021.786105
9. Raufman JP, Samimi R, Shah N, Khurana S, Shant J, Drachenberg C, et al. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res (2008) 68(10):3573–8. doi: 10.1158/0008-5472.CAN-07-6810
10. McLean LP, Smith A, Cheung L, Urban JF Jr., Sun R, Grinchuk V, et al. Type 3 muscarinic receptors contribute to intestinal mucosal homeostasis and clearance of Nippostrongylus brasiliensis through induction of TH2 cytokines. Am J Physiol Gastrointest Liver Physiol (2016) 311(1):G130–41. doi: 10.1152/ajpgi.00461.2014
11. McLean LP, Smith A, Cheung L, Sun R, Grinchuk V, Vanuytsel T, et al. Type 3 muscarinic receptors contribute to clearance of citrobacter rodentium. Inflammation Bowel Dis (2015) 21(8):1860–71. doi: 10.1097/MIB.0000000000000408
12. Shah SC, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterol (2022) 162(3):715–30.e3. doi: 10.1053/j.gastro.2021.10.035
13. Markov AV, Savin IA, Zenkova MA, Sen’kova AV. Identification of novel core genes involved in Malignant transformation of inflamed colon tissue using a computational biology approach and verification in murine models. Int J Mol Sci (2023) 24(5):4311. doi: 10.3390/ijms24054311
14. Nighot M, Ganapathy AS, Saha K, Suchanec E, Castillo EF, Gregory A, et al. Matrix metalloproteinase MMP-12 promotes macrophage transmigration across intestinal epithelial tight junctions and increases severity of experimental colitis. J Crohns Colitis (2021) 15(10):1751–65. doi: 10.1093/ecco-jcc/jjab064
15. Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci (2017) 147:1–73. doi: 10.1016/bs.pmbts.2017.02.005
16. Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol (2004) 4(8):617–29. doi: 10.1038/nri1418
17. Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A (1962) 48(6):1014–22. doi: 10.1073/pnas.48.6.1014
18. Overall CM, Sodek J. Initial characterization of a neutral metalloproteinase, active on native 3/4-collagen fragments, synthesized by ROS 17/2.8 osteoblastic cells, periodontal fibroblasts, and identified in gingival crevicular fluid. J Dent Res (1987) 66(7):1271–82. doi: 10.1177/00220345870660071201
19. Somerville RP, Oblander SA, Apte SS. Matrix metalloproteinases: old dogs with new tricks. Genome Biol (2003) 4(6):216. doi: 10.1186/gb-2003-4-6-216
20. Nagai Y, Lapiere CM, Gross J. Tadpole collagenase. Preparation and purification. Biochemistry (1966) 5(10):3123–30. doi: 10.1021/bi00874a007
21. Wilhelm SM, Collier IE, Kronberger A, Eisen AZ, Marmer BL, Grant GA, et al. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci U S A (1987) 84(19):6725–9. doi: 10.1073/pnas.84.19.6725
22. Collier IE, Wilhelm SM, Eisen AZ, Marmer BL, Grant GA, Seltzer JL, et al. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem (1988) 263(14):6579–87. doi: 10.1016/S0021-9258(18)68680-6
23. Itoh Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol (2015) 44-46:207–23. doi: 10.1016/j.matbio.2015.03.004
24. Galea CA, Nguyen HM, George Chandy K, Smith BJ, Norton RS. Domain structure and function of matrix metalloprotease 23 (MMP23): role in potassium channel trafficking. Cell Mol Life Sci (2014) 71(7):1191–210. doi: 10.1007/s00018-013-1431-0
25. Jiang L, Yang M, He S, Li Z, Li H, Niu T, et al. MMP12 knockout prevents weight and muscle loss in tumor-bearing mice. BMC Cancer (2021) 21(1):1297. doi: 10.1186/s12885-021-09004-y
26. Pezeshkian Z, Nobili S, Peyravian N, Shojaee B, Nazari H, Soleimani H, et al. Insights into the role of matrix metalloproteinases in precancerous conditions and in colorectal cancer. Cancers (Basel) (2021) 13(24):6226. doi: 10.3390/cancers13246226
27. Dong Z, Kumar R, Yang X, Fidler IJ. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell (1997) 88(6):801–10. doi: 10.1016/s0092-8674(00)81926-1
28. Majali-Martinez A, Hoch D, Tam-Amersdorfer C, Pollheimer J, Glasner A, Ghaffari-Tabrizi-Wizsy N, et al. Matrix metalloproteinase 15 plays a pivotal role in human first trimester cytotrophoblast invasion and is not altered by maternal obesity. FASEB J (2020) 34(8):10720–30. doi: 10.1096/fj.202000773R
29. Ito E, Yana I, Fujita C, Irifune A, Takeda M, Madachi A, et al. The role of MT2-MMP in cancer progression. Biochem Biophys Res Commun (2010) 393(2):222–7. doi: 10.1016/j.bbrc.2010.01.105
30. English WR, Puente XS, Freije JM, Knauper V, Amour A, Merryweather A, et al. Membrane type 4 matrix metalloproteinase (MMP17) has tumor necrosis factor-alpha convertase activity but does not activate pro-MMP2. J Biol Chem (2000) 275(19):14046–55. doi: 10.1074/jbc.275.19.14046
31. Tatti O, Arjama M, Ranki A, Weiss SJ, Keski-Oja J, Lehti K. Membrane-type-3 matrix metalloproteinase (MT3-MMP) functions as a matrix composition-dependent effector of melanoma cell invasion. PloS One (2011) 6(12):e28325. doi: 10.1371/journal.pone.0028325
32. Yip C, Foidart P, Noël A, Sounni NE. MT4-MMP: the GPI-anchored membrane-type matrix metalloprotease with multiple functions in diseases. Int J Mol Sci (2019) 20(2):354. doi: 10.3390/ijms20020354
33. Sohail A, Sun Q, Zhao H, Bernardo MM, Cho JA, Fridman R. MT4-(MMP17) and MT6-MMP (MMP25), A unique set of membrane-anchored matrix metalloproteinases: properties and expression in cancer. Cancer Metastasis Rev (2008) 27(2):289–302. doi: 10.1007/s10555-008-9129-8
34. Yu G, Kovkarova-Naumovski E, Jara P, Parwani A, Kass D, Ruiz V, et al. Matrix metalloproteinase-19 is a key regulator of lung fibrosis in mice and humans. Am J Respir Crit Care Med (2012) 186(8):752–62. doi: 10.1164/rccm.201202-0302OC
35. Bister VO, Salmela MT, Karjalainen-Lindsberg ML, Uria J, Lohi J, Puolakkainen P, et al. Differential expression of three matrix metalloproteinases, MMP-19, MMP-26, and MMP-28, in normal and inflamed intestine and colon cancer. Dig Dis Sci (2004) 49(4):653–61. doi: 10.1023/B:DDAS.0000026314.12474.17
36. Brauer R, Tureckova J, Kanchev I, Khoylou M, Skarda J, Prochazka J, et al. MMP-19 deficiency causes aggravation of colitis due to defects in innate immune cell function. Mucosal Immunol (2016) 9(4):974–85. doi: 10.1038/mi.2015.117
37. Llano E, Pendás AM, Knäuper V, Sorsa T, Salo T, Salido E, et al. Identification and structural and functional characterization of human enamelysin (MMP-20). Biochemistry (1997) 36(49):15101–8. doi: 10.1021/bi972120y
38. Ahokas K, Lohi J, Illman SA, Llano E, Elomaa O, Impola U, et al. Matrix metalloproteinase-21 is expressed epithelially during development and in cancer and is up-regulated by transforming growth factor-beta1 in keratinocytes. Lab Invest (2003) 83(12):1887–99. doi: 10.1097/01.LAB.0000106721.86126.39
39. Zakiyanov O, Kalousová M, Zima T, Tesař V. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in kidney disease. Adv Clin Chem (2021) 105:141–212. doi: 10.1016/bs.acc.2021.02.003
40. Snoek-van Beurden PA, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques (2005) 38(1):73–83. doi: 10.2144/05381RV01
41. Jin DY, Liu CL, Tang JN, Zhu ZZ, Xuan XX, Zhu XD, et al. Interleukin-18, matrix metalloproteinase-22 and -29 are independent risk factors of human coronary heart disease. J Zhejiang Univ Sci B (2017) 18(8):685–95. doi: 10.1631/jzus.B1700073
42. Yang M, Kurkinen M. Cloning and characterization of a novel matrix metalloproteinase (MMP), CMMP, from chicken embryo fibroblasts. CMMP, Xenopus XMMP, and human MMP19 have a conserved unique cysteine in the catalytic domain. J Biol Chem (1998) 273(28):17893–900. doi: 10.1074/jbc.273.28.17893
43. Fonseca-Camarillo G, Furuzawa-Carballeda J, Martínez-Benitez B, Barreto-Zuñiga R, Yamamoto-Furusho JK. Increased expression of extracellular matrix metalloproteinase inducer (EMMPRIN) and MMP10, MMP23 in inflammatory bowel disease: Cross-sectional study. Scand J Immunol (2021) 93(1):e12962. doi: 10.1111/sji.12962
44. Horozoglu C, Ozdes T, Erginel T, Erginel Unaltuna N. Expression of MMP-15 and MMP-24 in atherosclerotic and nonatherosclerotic coronary arteries. Metalloproteinases Med (2014) 1:15–20. doi: 10.2147/MNM.S68778
45. Baranger K, Marchalant Y, Bonnet AE, Crouzin N, Carrete A, Paumier JM, et al. MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer’s disease. Cell Mol Life Sci (2016) 73(1):217–36. doi: 10.1007/s00018-015-1992-1
46. Pilat D, Paumier JM, García-González L, Louis L, Stephan D, Manrique C, et al. MT5-MMP promotes neuroinflammation, neuronal excitability and Aβ production in primary neuron/astrocyte cultures from the 5xFAD mouse model of Alzheimer’s disease. J Neuroinflammation (2022) 19(1):65. doi: 10.1186/s12974-022-02407-z
47. Sun Q, Weber CR, Sohail A, Bernardo MM, Toth M, Zhao H, et al. MMP25 (MT6-MMP) is highly expressed in human colon cancer, promotes tumor growth, and exhibits unique biochemical properties. J Biol Chem (2007) 282(30):21998–2010. doi: 10.1074/jbc.M701737200
48. Fortin CF, Sohail A, Sun Q, McDonald PP, Fridman R, Fülöp T. MT6-MMP is present in lipid rafts and faces inward in living human PMNs but translocates to the cell surface during neutrophil apoptosis. Int Immunol (2010) 22(8):637–49. doi: 10.1093/intimm/dxq048
49. Marchenko GN, Ratnikov BI, Rozanov DV, Godzik A, Deryugina EI, Strongin AY. Characterization of matrix metalloproteinase-26, a novel metalloproteinase widely expressed in cancer cells of epithelial origin. Biochem J (2001) 356(Pt 3):705–18. doi: 10.1042/bj3560705
50. Cominelli A, Gaide Chevronnay HP, Lemoine P, Courtoy PJ, Marbaix E, Henriet P. Matrix metalloproteinase-27 is expressed in CD163+/CD206+ M2 macrophages in the cycling human endometrium and in superficial endometriotic lesions. Mol Hum Reprod (2014) 20(8):767–75. doi: 10.1093/molehr/gau034
51. Rodgers UR, Kevorkian L, Surridge AK, Waters JG, Swingler TE, Culley K, et al. Expression and function of matrix metalloproteinase (MMP)-28. Matrix Biol (2009) 28(5):263–72. doi: 10.1016/j.matbio.2009.04.006
52. Rath T, Roderfeld M, Halwe JM, Tschuschner A, Roeb E, Graf J. Cellular sources of MMP-7, MMP-13 and MMP-28 in ulcerative colitis. Scand J Gastroenterol (2010) 45(10):1186–96. doi: 10.3109/00365521.2010.499961
53. Liu Y, Min D, Bolton T, Nubé V, Twigg SM, Yue DK, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care (2009) 32(1):117–9. doi: 10.2337/dc08-0763
54. Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, López-Boado YS, Stratman JL, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science (1999) 286(5437):113–7. doi: 10.1126/science.286.5437.113
55. Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol (1999) 9(24):1441–7. doi: 10.1016/S0960-9822(00)80113-X
56. Mannello F, Medda V. Nuclear localization of matrix metalloproteinases. Prog Histochem Cytochem (2012) 47(1):27–58. doi: 10.1016/j.proghi.2011.12.002
57. Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol (2007) 211(1):19–26. doi: 10.1002/jcp.20948
58. Angel P, Baumann I, Stein B, Delius H, Rahmsdorf HJ, Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5’-flanking region. Mol Cell Biol (1987) 7(6):2256–66. doi: 10.1128/mcb.7.6.2256-2266.1987
59. Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther (2016) 1:15004. doi: 10.1038/sigtrans.2015.4
60. Wu J, Wu G, Lv L, Ren YF, Zhang XJ, Xue YF, et al. MicroRNA-34a inhibits migration and invasion of colon cancer cells via targeting to Fra-1. Carcinogenesis (2012) 33(3):519–28. doi: 10.1093/carcin/bgr304
61. Sun LH, Tian D, Yang ZC, Li JL. Exosomal miR-21 promotes proliferation, invasion and therapy resistance of colon adenocarcinoma cells through its target PDCD4. Sci Rep (2020) 10(1):8271. doi: 10.1038/s41598-020-65207-6
62. Shen K, Liang Q, Xu K, Cui D, Jiang L, Yin P, et al. MiR-139 inhibits invasion and metastasis of colorectal cancer by targeting the type I insulin-like growth factor receptor. Biochem Pharmacol (2012) 84(3):320–30. doi: 10.1016/j.bcp.2012.04.017
63. Astarci E, Erson-Bensan AE, Banerjee S. Matrix metalloprotease 16 expression is downregulated by microRNA-146a in spontaneously differentiating Caco-2 cells. Dev Growth Differ (2012) 54(2):216–26. doi: 10.1111/j.1440-169X.2011.01324.x
64. Nguyen TT, Mobashery S, Chang M. Roles of matrix metalloproteinases in cutaneous wound healing. In: Vlad Adrian A, editor. Wound Healing. Rijeka: IntechOpen (2016).
65. Laronha H, Caldeira J. Structure and function of human matrix metalloproteinases. Cells (2020) 9(5):1076. doi: 10.3390/cells9051076
66. Chellaiah MA, Ma T. Membrane localization of membrane type 1 matrix metalloproteinase by CD44 regulates the activation of pro-matrix metalloproteinase 9 in osteoclasts. BioMed Res Int (2013) 2013:302392. doi: 10.1155/2013/302392
67. Vandooren J, Itoh Y. Alpha-2-macroglobulin in inflammation, immunity and infections. Front Immunol (2021) 12:803244. doi: 10.3389/fimmu.2021.803244
68. Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol (2010) 20(3):161–8. doi: 10.1016/j.semcancer.2010.05.002
69. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta (2010) 1803(1):55–71. doi: 10.1016/j.bbamcr.2010.01.003
70. Ries C. Cytokine functions of TIMP-1. Cell Mol Life Sci (2014) 71(4):659–72. doi: 10.1007/s00018-013-1457-3
71. Park KS, Kim SJ, Kim KH, Kim JC. Clinical characteristics of TIMP2, MMP2, and MMP9 gene polymorphisms in colorectal cancer. J Gastroenterol Hepatol (2011) 26(2):391–7. doi: 10.1111/j.1440-1746.2010.06504.x
72. Li Y, Xu C, Zhu R, Shen L, Hu G, Tao K, et al. TIMP-2 as a predictive biomarker in 5-Fu-resistant colorectal cancer. J Cancer Res Clin Oncol (2023) 149:7235–46. doi: 10.1007/s00432-023-04670-w
73. Fedak PW, Smookler DS, Kassiri Z, Ohno N, Leco KJ, Verma S, et al. TIMP-3 deficiency leads to dilated cardiomyopathy. Circulation (2004) 110(16):2401–9. doi: 10.1161/01.CIR.0000134959.83967.2D
74. Melendez-Zajgla J, Del Pozo L, Ceballos G, Maldonado V. Tissue inhibitor of metalloproteinases-4. road less traveled Mol Cancer (2008) 7:85. doi: 10.1186/1476-4598-7-85
75. Sakamuri S, Watts R, Takawale A, Wang X, Hernandez-Anzaldo S, Bahitham W, et al. Absence of Tissue Inhibitor of Metalloproteinase-4 (TIMP4) ameliorates high fat diet-induced obesity in mice due to defective lipid absorption. Sci Rep (2017) 7(1):6210. doi: 10.1038/s41598-017-05951-4
76. Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflammation Bowel Dis (2013) 19(4):789–99. doi: 10.1097/MIB.0b013e31828029c0
77. Abu-Freha N, Cohen B, Gordon M, Weissmann S, Kestenbaum EH, Vosko S, et al. Colorectal cancer among inflammatory bowel disease patients: risk factors and prevalence compared to the general population. Front Med (2023) 10. doi: 10.3389/fmed.2023.1225616
78. Wijnands AM, de Jong ME, Lutgens M, Hoentjen F, Elias SG, Oldenburg B. Prognostic factors for advanced colorectal neoplasia in inflammatory bowel disease: systematic review and meta-analysis. Gastroenterol (2021) 160(5):1584–98. doi: 10.1053/j.gastro.2020.12.036
79. Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med (2015) 372(15):1441–52. doi: 10.1056/NEJMra1403718
80. Baker AM, Cross W, Curtius K, Al Bakir I, Choi CR, Davis HL, et al. Evolutionary history of human colitis-associated colorectal cancer. Gut (2019) 68(6):985–95. doi: 10.1136/gutjnl-2018-316191
81. Choi CR, Bakir IA, Hart AL, Graham TA. Clonal evolution of colorectal cancer in IBD. Nat Rev Gastroenterol Hepatol (2017) 14(4):218–29. doi: 10.1038/nrgastro.2017.1
82. Alexandersson B, Hamad Y, Andreasson A, Rubio CA, Ando Y, Tanaka K, et al. High-definition chromoendoscopy superior to high-definition white-light endoscopy in surveillance of inflammatory bowel diseases in a randomized trial. Clin Gastroenterol Hepatol (2020) 18(9):2101–7. doi: 10.1016/j.cgh.2020.04.049
83. Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc (2015) 81(3):489–501.e26. doi: 10.1053/j.gastro.2015.01.031
84. Shah SC, Ten Hove JR, Castaneda D, Palmela C, Mooiweer E, Colombel JF, et al. High risk of advanced colorectal neoplasia in patients with primary sclerosing cholangitis associated with inflammatory bowel disease. Clin Gastroenterol Hepatol (2018) 16(7):1106–13.e3. doi: 10.1016/j.cgh.2018.01.023
85. Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res (2011) 4(2):53–61.
87. Yaeger R, Paroder V, Bates DDB, Capanu M, Chou J, Tang L, et al. Systemic chemotherapy for metastatic colitis-associated cancer has a worse outcome than sporadic colorectal cancer: matched case cohort analysis. Clin Colorectal Cancer (2020) 19(4):e151–e6. doi: 10.1016/j.clcc.2020.02.008
88. Vetter LE, Merkel S, Bénard A, Krautz C, Brunner M, Mittelstädt A, et al. Colorectal cancer in Crohn’s colitis is associated with advanced tumor invasion and a poorer survival compared with ulcerative colitis: a retrospective dual-center study. Int J Colorectal Dis (2021) 36(1):141–50. doi: 10.1007/s00384-020-03726-4
89. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res (2006) 69(3):562–73. doi: 10.1016/j.cardiores.2005.12.002
90. Chin JR, Murphy G, Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem (1985) 260(22):12367–76.
91. Wan J, Zhang G, Li X, Qiu X, Ouyang J, Dai J, et al. Matrix metalloproteinase 3: A promoting and destabilizing factor in the pathogenesis of disease and cell differentiation. Front Physiol (2021) 12:663978. doi: 10.3389/fphys.2021.663978
92. Pan Z, Lin H, Fu Y, Zeng F, Gu F, Niu G, et al. Identification of gene signatures associated with ulcerative colitis and the association with immune infiltrates in colon cancer. Front Immunol (2023) 14:1086898. doi: 10.3389/fimmu.2023.1086898
93. Newell KJ, Matrisian LM, Driman DK. Matrilysin (matrix metalloproteinase-7) expression in ulcerative colitis-related tumorigenesis. Mol Carcinog (2002) 34(2):59–63. doi: 10.1002/mc.10049
94. Adachi Y, Yamamoto H, Itoh F, Hinoda Y, Okada Y, Imai K. Contribution of matrilysin (MMP-7) to the metastatic pathway of human colorectal cancers. Gut (1999) 45(2):252–8. doi: 10.1136/gut.45.2.252
95. Shahnazari M, Afshar S, Emami MH, Amini R, Jalali A. Novel biomarkers for neoplastic progression from ulcerative colitis to colorectal cancer: a systems biology approach. Sci Rep (2023) 13(1):3413. doi: 10.1038/s41598-023-29344-y
96. Altadill A, Eiro N, González LO, Andicoechea A, Fernández-Francos S, Rodrigo L, et al. Relationship between metalloprotease-7 and -14 and tissue inhibitor of metalloprotease 1 expression by mucosal stromal cells and colorectal cancer development in inflammatory bowel disease. Biomedicines (2021) 9(5):495. doi: 10.3390/biomedicines9050495
97. Juurikka K, Butler GS, Salo T, Nyberg P, Åström P. The role of MMP8 in cancer: A systematic review. Int J Mol Sci (2019) 20(18):4506. doi: 10.3390/ijms20184506
98. Koelink PJ, Overbeek SA, Braber S, Morgan ME, Henricks PA, Abdul Roda M, et al. Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease. Gut (2014) 63(4):578–87. doi: 10.1136/gutjnl-2012-303252
99. Garg P, Sarma D, Jeppsson S, Patel NR, Gewirtz AT, Merlin D, et al. Matrix metalloproteinase-9 functions as a tumor suppressor in colitis-associated cancer. Cancer Res (2010) 70(2):792–801. doi: 10.1158/0008-5472.CAN-09-3166
100. Huang H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: recent advances. Sensors (Basel) (2018) 18(10):3249. doi: 10.3390/s18103249
101. Cañete-Soler R, Gui YH, Linask KK, Muschel RJ. Developmental expression of MMP-9 (gelatinase B) mRNA in mouse embryos. Dev Dyn (1995) 204(1):30–40. doi: 10.1002/aja.1002040105
102. Shang K, Bai YP, Wang C, Wang Z, Gu HY, Du X, et al. Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PloS One (2012) 7(12):e51848. doi: 10.1371/journal.pone.0051848
103. Koller FL, Dozier EA, Nam KT, Swee M, Birkland TP, Parks WC, et al. Lack of MMP10 exacerbates experimental colitis and promotes development of inflammation-associated colonic dysplasia. Lab Invest (2012) 92(12):1749–59. doi: 10.1038/labinvest.2012.141
104. Derkacz A, Olczyk P, Olczyk K, Komosinska-Vassev K. The role of extracellular matrix components in inflammatory bowel diseases. J Clin Med (2021) 10(5):1122. doi: 10.3390/jcm10051122
105. Kim YJ, Hong KS, Chung JW, Kim JH, Hahm KB. Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res (Phila) (2010) 3(10):1314–33. doi: 10.1158/1940-6207.CAPR-09-0272
106. Meyer BS, Rademann J. Extra- and intracellular imaging of human matrix metalloprotease 11 (hMMP-11) with a cell-penetrating FRET substrate. J Biol Chem (2012) 287(45):37857–67. doi: 10.1074/jbc.M112.371500
107. Ma B, Ran R, Liao HY, Zhang HH. The paradoxical role of matrix metalloproteinase-11 in cancer. BioMed Pharmacother (2021) 141:111899. doi: 10.1016/j.biopha.2021.111899
108. Zhang X, Huang S, Guo J, Zhou L, You L, Zhang T, et al. Insights into the distinct roles of MMP-11 in tumor biology and future therapeutics (Review). Int J Oncol (2016) 48(5):1783–93. doi: 10.3892/ijo.2016.3400
109. Vandenbroucke RE, Dejonckheere E, Van Hauwermeiren F, Lodens S, De Rycke R, Van Wonterghem E, et al. Matrix metalloproteinase 13 modulates intestinal epithelial barrier integrity in inflammatory diseases by activating TNF. EMBO Mol Med (2013) 5(7):1000–16. doi: 10.1002/emmm.201202100
110. Rajamäki K, Taira A, Katainen R, Välimäki N, Kuosmanen A, Plaketti RM, et al. Genetic and epigenetic characteristics of inflammatory bowel disease-associated colorectal cancer. Gastroenterol (2021) 161(2):592–607. doi: 10.1053/j.gastro.2021.04.042
111. Luo C, Zhang H. The role of proinflammatory pathways in the pathogenesis of colitis-associated colorectal cancer. Mediators Inflamm (2017) 2017:5126048. doi: 10.1155/2017/5126048
112. O’Shea NR, Smith AM. Matrix metalloproteases role in bowel inflammation and inflammatory bowel disease: an up to date review. Inflammation Bowel Dis (2014) 20(12):2379–93. doi: 10.1097/MIB.0000000000000163
113. Matsuno K, Adachi Y, Yamamoto H, Goto A, Arimura Y, Endo T, et al. The expression of matrix metalloproteinase matrilysin indicates the degree of inflammation in ulcerative colitis. J Gastroenterol (2003) 38(4):348–54. doi: 10.1007/s005350300062
114. von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut (2000) 47(1):63–73. doi: 10.1136/gut.47.1.63
115. Castaneda FE, Walia B, Vijay-Kumar M, Patel NR, Roser S, Kolachala VL, et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterol (2005) 129(6):1991–2008. doi: 10.1053/j.gastro.2005.09.017
116. Santana A, Medina C, Paz-Cabrera MC, Díaz-Gonzalez F, Farré E, Salas A, et al. Attenuation of dextran sodium sulphate induced colitis in matrix metalloproteinase-9 deficient mice. World J Gastroenterol (2006) 12(40):6464–72. doi: 10.3748/wjg.v12.i40.6464
117. Kim YJ, Lee JS, Hong KS, Chung JW, Kim JH, Hahm KB. Novel application of proton pump inhibitor for the prevention of colitis-induced colorectal carcinogenesis beyond acid suppression. Cancer Prev Res (Phila) (2010) 3(8):963–74. doi: 10.1158/1940-6207.CAPR-10-0033
118. Walter L, Harper C, Garg P. Role of matrix metalloproteinases in inflammation/colitis-associated colon cancer. Immuno-Gastroenterol (2013) 2:22–8. doi: 10.7178/ig.29
119. Walter L, Canup B, Pujada A, Bui TA, Arbasi B, Laroui H, et al. Matrix metalloproteinase 9 (MMP9) limits reactive oxygen species (ROS) accumulation and DNA damage in colitis-associated cancer. Cell Death Dis (2020) 11(9):767. doi: 10.1038/s41419-020-02959-z
120. Garg P, Jeppsson S, Dalmasso G, Ghaleb AM, McConnell BB, Yang VW, et al. Notch1 regulates the effects of matrix metalloproteinase-9 on colitis-associated cancer in mice. Gastroenterol (2011) 141(4):1381–92. doi: 10.1053/j.gastro.2011.06.056
121. Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol (1999) 155(4):1033–8. doi: 10.1016/S0002-9440(10)65204-2
122. Lopez-Avila V, Spencer JV. Methods for detection of matrix metalloproteinases as biomarkers in cardiovascular disease. Clin Med Cardiol (2008) 2:CMC.S484. doi: 10.4137/CMC.S484
123. Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol (2009) 27(31):5287–97. doi: 10.1200/JCO.2009.23.5556
124. Mannello F. Effects of blood collection methods on gelatin zymography of matrix metalloproteinases. Clin Chem (2003) 49(2):339–40. doi: 10.1373/49.2.339
125. Gelzo M, Cacciapuoti S, Pinchera B, De Rosa A, Cernera G, Scialò F, et al. Matrix metalloproteinases (MMP) 3 and 9 as biomarkers of severity in COVID-19 patients. Sci Rep (2022) 12(1):1212. doi: 10.1038/s41598-021-04677-8
126. Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation (2003) 107(12):1579–85. doi: 10.1161/01.CIR.0000058700.41738.12
127. Yablecovitch D, Kopylov U, Lahat A, Amitai MM, Klang E, Ben-Ami Shor D, et al. Serum MMP-9: a novel biomarker for prediction of clinical relapse in patients with quiescent Crohn’s disease, a post hoc analysis. Therap Adv Gastroenterol (2019) 12:1756284819881590. doi: 10.1177/1756284819881590
128. Hilska M, Roberts PJ, Collan YU, Laine VJ, Kössi J, Hirsimäki P, et al. Prognostic significance of matrix metalloproteinases-1, -2, -7 and -13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in colorectal cancer. Int J Cancer (2007) 121(4):714–23. doi: 10.1002/ijc.22747
129. Tutton MG, George ML, Eccles SA, Burton S, Swift RI, Abulafi AM. Use of plasma MMP-2 and MMP-9 levels as a surrogate for tumour expression in colorectal cancer patients. Int J Cancer (2003) 107(4):541–50. doi: 10.1002/ijc.11436
130. Leeman MF, McKay JA, Murray GI. Matrix metalloproteinase 13 activity is associated with poor prognosis in colorectal cancer. J Clin Pathol (2002) 55(10):758–62. doi: 10.1136/jcp.55.10.758
131. Drankowska J, Kos M, Kościuk A, Marzęda P, Boguszewska-Czubara A, Tylus M, et al. MMP targeting in the battle for vision: Recent developments and future prospects in the treatment of diabetic retinopathy. Life Sci (2019) 229:149–56. doi: 10.1016/j.lfs.2019.05.038
132. Jones JI, Nguyen TT, Peng Z, Chang M. Targeting MMP-9 in diabetic foot ulcers. Pharm (Basel) (2019) 12(2):79. doi: 10.3390/ph12020079
133. Hughes BG, Schulz R. Targeting MMP-2 to treat ischemic heart injury. Basic Res Cardiol (2014) 109(4):424. doi: 10.1007/s00395-014-0424-y
134. Soloway PD, Alexander CM, Werb Z, Jaenisch R. Targeted mutagenesis of Timp-1 reveals that lung tumor invasion is influenced by Timp-1 genotype of the tumor but not by that of the host. Oncogene (1996) 13(11):2307–14.
135. Mannello F, Tonti G, Papa S. Matrix metalloproteinase inhibitors as anticancer therapeutics. Curr Cancer Drug Targets (2005) 5(4):285–98. doi: 10.2174/1568009054064615
136. Toth M, Bernardo MM, Gervasi DC, Soloway PD, Wang Z, Bigg HF, et al. Tissue inhibitor of metalloproteinase (TIMP)-2 acts synergistically with synthetic matrix metalloproteinase (MMP) inhibitors but not with TIMP-4 to enhance the (Membrane type 1)-MMP-dependent activation of pro-MMP-2. J Biol Chem (2000) 275(52):41415–23. doi: 10.1074/jbc.M006871200
137. Mirunalini S, Maruthanila VL. The impact of bioactive compounds derived from marine fish on cancer. Anticancer Agents Med Chem (2022) 22(15):2757–65. doi: 10.2174/1871520622666220330142442
138. Mannello F. Natural bio-drugs as matrix metalloproteinase inhibitors: new perspectives on the horizon? Recent Pat Anticancer Drug Discovery (2006) 1(1):91–103. doi: 10.2174/157489206775246421
139. Li X, Wu JF. Recent developments in patent anti-cancer agents targeting the matrix metalloproteinases (MMPs). Recent Pat Anticancer Drug Discovery (2010) 5(2):109–41. doi: 10.2174/157489210790936234
140. Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene (2000) 19(56):6642–50. doi: 10.1038/sj.onc.1204097
141. Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst (2001) 93(3):178–93. doi: 10.1093/jnci/93.3.178
142. Tu G, Xu W, Huang H, Li S. Progress in the development of matrix metalloproteinase inhibitors. Curr Med Chem (2008) 15(14):1388–95. doi: 10.2174/092986708784567680
143. Duivenvoorden WC, Hirte HW, Singh G. Use of tetracycline as an inhibitor of matrix metalloproteinase activity secreted by human bone-metastasizing cancer cells. Invasion Metastasis (1997) 17(6):312–22.
144. Giavazzi R, Taraboletti G. Preclinical development of metalloproteasis inhibitors in cancer therapy. Crit Rev Oncol Hematol (2001) 37(1):53–60. doi: 10.1016/S1040-8428(00)00096-2
145. Yao Q, Kou L, Tu Y, Zhu L. MMP-responsive ‘Smart’ Drug delivery and tumor targeting. Trends Pharmacol Sci (2018) 39(8):766–81. doi: 10.1016/j.tips.2018.06.003
146. Zeng Y, Zhou Z, Fan M, Gong T, Zhang Z, Sun X. PEGylated cationic vectors containing a protease-sensitive peptide as a miRNA delivery system for treating breast cancer. Mol Pharm (2017) 14(1):81–92. doi: 10.1021/acs.molpharmaceut.6b00726
147. Levin M, Udi Y, Solomonov I, Sagi I. Next generation matrix metalloproteinase inhibitors - Novel strategies bring new prospects. Biochim Biophys Acta Mol Cell Res (2017) 1864(11 Pt A):1927–39. doi: 10.1016/j.bbamcr.2017.06.009
148. Pirard B. Insight into the structural determinants for selective inhibition of matrix metalloproteinases. Drug Discovery Today (2007) 12(15-16):640–6. doi: 10.1016/j.drudis.2007.06.003
149. Laronha H, Carpinteiro I, Portugal J, Azul A, Polido M, Petrova KT, et al. Challenges matrix metalloproteinases inhibition. Biomol (2020) 10(5):717. doi: 10.3390/biom10050717
150. Overall CM, Kleifeld O. Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Br J Cancer (2006) 94(7):941–6. doi: 10.1038/sj.bjc.6603043
151. Devel L, Czarny B, Beau F, Georgiadis D, Stura E, Dive V. Third generation of matrix metalloprotease inhibitors: Gain in selectivity by targeting the depth of the S1’ cavity. Biochimie (2010) 92(11):1501–8. doi: 10.1016/j.biochi.2010.07.017
152. Udi Y, Fragai M, Grossman M, Mitternacht S, Arad-Yellin R, Calderone V, et al. Unraveling hidden regulatory sites in structurally homologous metalloproteases. J Mol Biol (2013) 425(13):2330–46. doi: 10.1016/j.jmb.2013.04.009
153. Marshall DC, Lyman SK, McCauley S, Kovalenko M, Spangler R, Liu C, et al. Selective allosteric inhibition of MMP9 is efficacious in preclinical models of ulcerative colitis and colorectal cancer. PloS One (2015) 10(5):e0127063. doi: 10.1371/journal.pone.0127063
154. Bhandari BR, Fogel R, Onken J, Yen EH, Kanwar B, Subramanian GM, et al. Safety and efficacy of GS-5745 an anti-matrix metalloproteinase 9 (MMP) monoclonal antibody in patients with moderately to severely active ulcerative colitis. Gastroenterol (2015) 148(4):S1196. doi: 10.1016/S0016-5085(15)34084-1
155. Shah MA, Starodub A, Sharma S, Berlin J, Patel M, Wainberg ZA, et al. Andecaliximab/GS-5745 alone and combined with mFOLFOX6 in advanced gastric and gastroesophageal junction adenocarcinoma: results from a phase I study. Clin Cancer Res (2018) 24(16):3829–37. doi: 10.1158/1078-0432.CCR-17-2469
156. Winer A, Adams S, Mignatti P. Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol Cancer Ther (2018) 17(6):1147–55. doi: 10.1158/1535-7163.MCT-17-0646
157. Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discovery (2014) 13(12):904–27. doi: 10.1038/nrd4390
158. Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, Ramirez-Acuña JM, Perez-Romero BA, Guerrero-Rodriguez JF, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci (2020) 21(24):9739. doi: 10.3390/ijms21249739
159. Syed S, Takimoto C, Hidalgo M, Rizzo J, Kuhn JG, Hammond LA, et al. A phase I and pharmacokinetic study of Col-3 (Metastat), an oral tetracycline derivative with potent matrix metalloproteinase and antitumor properties. Clin Cancer Res (2004) 10(19):6512–21. doi: 10.1158/1078-0432.CCR-04-0804
160. Sauder DN, Dekoven J, Champagne P, Croteau D, Dupont E. Neovastat (AE-941), an inhibitor of angiogenesis: Randomized phase I/II clinical trial results in patients with plaque psoriasis. J Am Acad Dermatol (2002) 47(4):535–41. doi: 10.1067/mjd.2002.124702
161. Sandborn WJ, Bhandari BR, Randall C, Younes ZH, Romanczyk T, Xin Y, et al. Andecaliximab [Anti-matrix metalloproteinase-9] induction therapy for ulcerative colitis: A randomised, double-blind, placebo-controlled, phase 2/3 study in patients with moderate to severe disease. J Crohns Colitis (2018) 12(9):1021–9. doi: 10.1093/ecco-jcc/jjy049
162. Gossage DL, Cieslarová B, Ap S, Zheng H, Xin Y, Lal P, et al. Phase 1b study of the safety, pharmacokinetics, and disease-related outcomes of the matrix metalloproteinase-9 inhibitor andecaliximab in patients with rheumatoid arthritis. Clin Ther (2018) 40(1):156–65.e5. doi: 10.1016/j.clinthera.2017.11.011
Keywords: matrix metalloproteinases, colitis-associated cancer, colorectal cancer, inflammatory bowel disease, tissue inhibitors of metalloproteinases, enzymes, cell invasion, biomarkers
Citation: Sampaio Moura N, Schledwitz A, Alizadeh M, Patil SA and Raufman J-P (2024) Matrix metalloproteinases as biomarkers and therapeutic targets in colitis-associated cancer. Front. Oncol. 13:1325095. doi: 10.3389/fonc.2023.1325095
Received: 20 October 2023; Accepted: 26 December 2023;
Published: 15 January 2024.
Edited by:
Shuyun Rao, Feinstein Institute for Medical Research, United StatesReviewed by:
Mansoor-Ali Vaali-Mohammed, King Saud University, Saudi ArabiaNicolas Etique, UMR7369 Matrice extracellulaire et dynamique cellulaire (MEDyC), France
Hong Gu, Genhawk (Wuhan) Biotech Co., Ltd., China
Copyright © 2024 Sampaio Moura, Schledwitz, Alizadeh, Patil and Raufman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean-Pierre Raufman, anJhdWZtYW5Ac29tLnVtYXJ5bGFuZC5lZHU=
†These authors have contributed equally to this work and share first authorship
 Natalia Sampaio Moura
Natalia Sampaio Moura Alyssa Schledwitz
Alyssa Schledwitz Madeline Alizadeh
Madeline Alizadeh Seema A. Patil1
Seema A. Patil1