
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol., 23 November 2023
Sec. Cancer Molecular Targets and Therapeutics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1320621
The limitations of current cancer therapies, including the increasing prevalence of multidrug resistance, underscore the urgency for more effective treatments. One promising avenue lies in the repurposing of existing drugs. This review explores the impact of phenothiazines, primarily used as antipsychotic agents, on key mechanisms driving tumor growth and metastasis. The cationic and amphiphilic nature of phenothiazines allows interaction with the lipid bilayer of cellular membranes, resulting in alterations in lipid composition, modulation of calcium channels, fluidity, thinning, and integrity of the plasma membrane. This is especially significant in the setting of increased metabolic activity, a higher proliferative rate, and the invasiveness of cancer cells, which often rely on plasma membrane repair. Therefore, properties of phenothiazines such as compromising plasma membrane integrity and repair, disturbing calcium regulation, inducing cytosolic K-RAS accumulation, and sphingomyelin accumulation in the plasma membrane might counteract multidrug resistance by sensitizing cancer cells to membrane damage and chemotherapy. This review outlines a comprehensive overview of the mechanisms driving the anticancer activities of phenothiazines derivates such as trifluoperazine, prochlorperazine, chlorpromazine, promethazine, thioridazine, and fluphenazine. The repurposing potential of phenothiazines paves the way for novel approaches to improve future cancer treatment.
Cancer remains a complex and heterogeneous disease that poses a significant global health challenge. Drug resistance and side effects restrict the effectiveness of existing therapies, emphasizing the need for new and effective treatments (1). In recent years, drug repurposing has emerged as a promising strategy for identifying new anticancer agents, given its potential to rapidly develop drugs with established safety profiles and known pharmacokinetic properties (2).
Phenothiazines belong to important antipsychotic drugs used for schizophrenia and bipolar disorder treatment (3, 4). They demonstrate a broad spectrum of biological activities in mammalian cancer cells, as well as pathogenic bacteria and fungi with antipsychotic, antiemetic, antihistaminic, and anti-inflammatory properties (5, 6). Beyond psychiatric use, phenothiazines may act as potential anticancer agents, targeting processes involved in tumor growth and metastasis (7, 8).
Cancer cells are exposed to membrane stress due to their enhanced metabolic activity (9), making them more reliant on an effective plasma membrane repair mechanism to restore membrane integrity and avoid cell death (10). Annexins, a group of essential plasma membrane repair proteins, are often overexpressed in cancer cells (11, 12). They are characterized by their calcium-dependent binding to anionic phospholipids and the ability to aggregate vesicles and fuse membranes (13, 14). Despite excessive research on annexin-mediated membrane repair and annexins’ ability to accumulate and fuse with membranes (15–17), pharmacological approaches to impair membrane repair in cancer cells need to be elucidated. Compromising plasma membrane repair makes cancer cells more susceptible to membrane damage and cell death (18, 19).
Phenothiazine derivatives interfere with plasma membrane junctions, induce lipid phase separation (20, 21), and, as amphiphilic drugs, modify cell membrane properties. They achieve this by altering lipid composition, disrupting lipid rafts (22), thinning the plasma membrane (23), and modulating calcium channels (24). These properties are important aspects in cancer therapy, as phenothiazines have been shown to counteract multidrug resistance in various types of cancer cells and sensitize them to chemotherapy (25).
This review aims to provide a comprehensive overview of the molecular mechanisms underlying the anticancer activity of phenothiazines by influencing the biophysical properties of the plasma membrane. We will summarize current advances in understanding the therapeutic potential of established phenothiazines and their effects on plasma membrane integrity, while discussing the prospects of repurposing these drugs for cancer therapy.
Phenothiazines represent a class of cationic and amphiphilic compounds characterized by the presence of two phenyl rings and thiazine ring containing sulfur and nitrogen atoms (Figure 1). An alkyl bridge is linked to the nitrogen atom within the thiazine ring (26). Phenothiazines are a group of heterocyclic neuroleptic agents known as dopamine receptor blockers that also affect GABA-mediated inhibitory synaptic transmission in cultured hippocampal neurons (27). Additionally, they demonstrated the capacity to inhibit voltage-gated Kv1.3 channels in T lymphocytes (28).
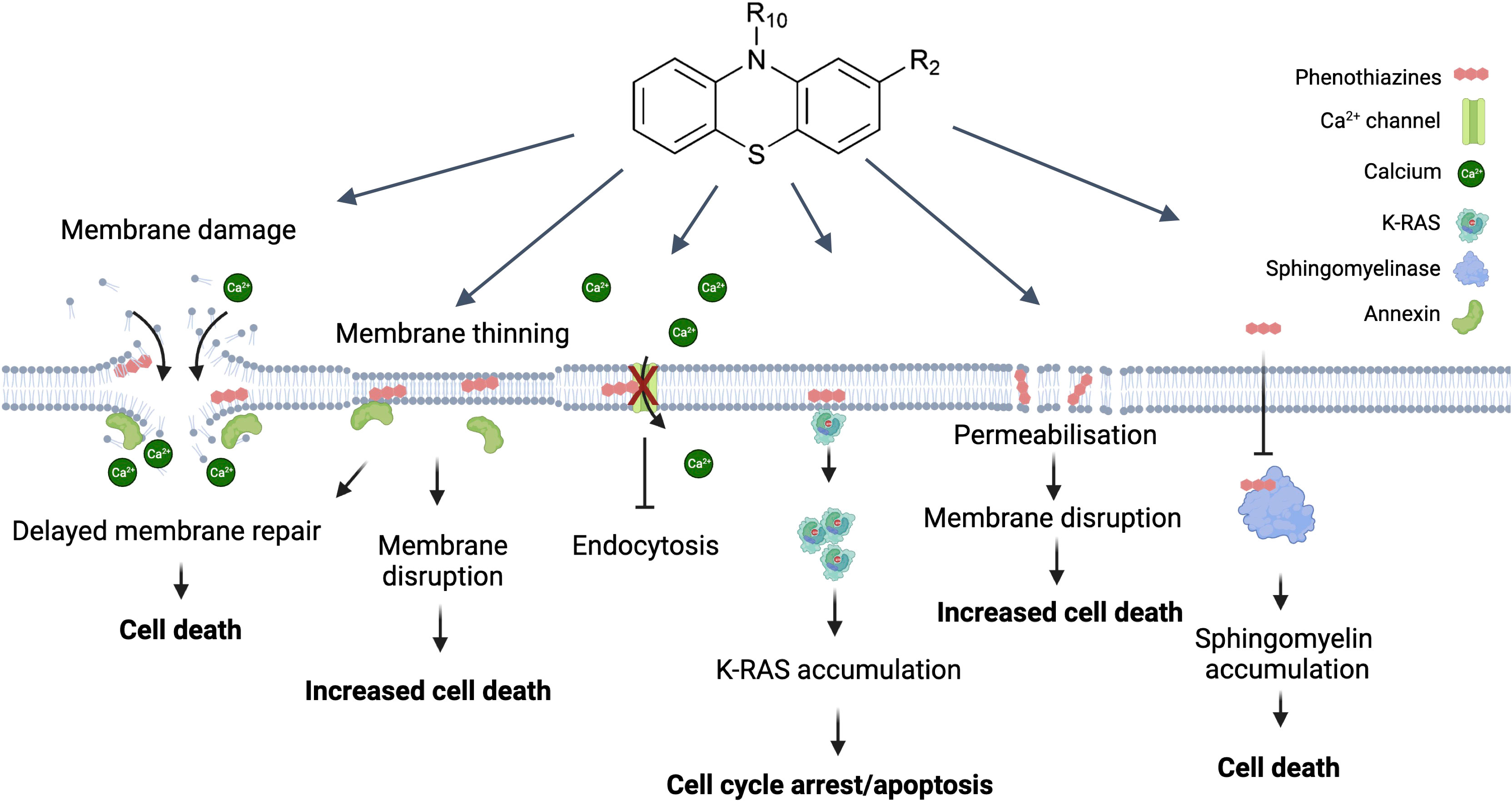
Figure 1 Direct effects of phenothiazine derivates on the plasma membrane and their potential as anti-cancer drugs. The most direct effect caused by phenothiazines is disruption of the plasma membrane. This causes a rapid influx of Ca2+ which causes depolarization of the actin filaments and activates the membrane repair machinery. The presence of phenothiazines can delay membrane resealing and thus lead to cell death. Similarly, phenothiazines can primarily induce membrane thinning and increased membrane permeabilization which can then also lead to membrane disruption. In contrast to the increased Ca2+ influx associated with membrane disruption phenothiazines can also inactivate the Ca2+ channels causing Ca2+ dysregulation affecting multiple cellular functions including growth. A more specific effect on growth is the interaction between phenothiazine and K-RAS, causing K-RAS to dissociate from the plasma membrane and accumulate in the cytosol, leading to cell cycle arrest and/or apoptosis. Finally, phenothiazines can inhibit the sphingomyelinase leading to sphingomyelin accumulation in the plasma membrane and subsequent cell death. Created with Biorender.
Phenothiazines demonstrate a broad spectrum of biological activities in mammalian cancer cells, as well as pathogenic microorganisms, which include bacteria (29), fungi, and protozoa. These compounds exhibit antipsychotic, antiemetic, antihistaminic, and anti-inflammatory properties and have been used in the treatment of a wide range of diseases (6).
Phenothiazines exert their anticancer effects through multiple mechanisms (30). They inhibit cell proliferation by targeting different stages of the cell cycle (8), including DNA repair (31) and microtubule dynamics (32). In addition, they also modulate signaling pathways such as, PDK1/Akt and MAPK/ERK1/2, which are involved in cancer progression and survival (7, 33). Other studies support the induction of apoptosis by phenothiazines through inhibiting the Akt/mTOR pathway, leading to decreased cell proliferation (33, 34).
Phenothiazines also inhibit angiogenesis, the formation of new blood vessels necessary for tumor growth, by inhibiting the production of VEGF (vascular endothelial growth factor) and VEGF-mediated signaling. Additionally, phenothiazines modulate other molecular pathways involved in angiogenesis, such as the MAPK signaling pathway (6, 35).
Furthermore, phenothiazines can induce oxidative stress by generating reactive oxygen species (ROS) or inhibiting antioxidant enzymes. This oxidative stress leads to DNA damage, mitochondrial dysfunction, and cell death. Cancer cells, which often have higher levels of oxidative stress, are particularly susceptible to this cytotoxicity (36).
Phenothiazines disrupt the integrity of cell membranes via their intercalation with the lipid bilayer (22). These compounds accumulate selectively within the lipid membrane and have profound effects on its biophysical properties (20, 37). By influencing membrane fluidity and organization, phenothiazines can impact crucial membrane-dependent processes such as signal transduction, ion channel activity, and membrane repair mechanisms (23). The study of the complex interaction between phenothiazines and membrane dynamics gives significant insight into their multiple pharmacological activities and highlights their potential as therapeutic agents in various contexts of disease.
The plasma membrane is a vital component of all living cells, serving as a selective barrier between the intracellular and extracellular environments. Maintaining membrane integrity is essential for cellular homeostasis, since membrane disturbances may impair function and result in cell death (38, 39). The plasma membrane is composed of a phospholipid bilayer containing various proteins and molecules. Integral membrane proteins, like receptors, transporters, and channels, are pivotal for specific cellular functions and external interactions. The plasma membrane is involved in cell signaling through surface receptors that sense external signals like hormones or neurotransmitters. These signals are conveyed into the cell, triggering specific responses crucial for communication, growth, differentiation, and survival (40).
Furthermore, the plasma membrane contributes to maintaining cellular homeostasis by regulating the balance of ions, nutrients, and waste products. This regulation ensures that the intracellular environment remains stable and suitable for cellular function. Additionally, the membrane facilitates cellular adhesion, allowing cells to interact with neighboring cells and form tissues and organs (41).
Beyond its structural and functional roles, the plasma membrane is dynamic and capable of remodeling and reorganizing in response to various stimuli (41). It can change its shape, form specialized structures such as microvilli or pseudopodia, and undergo processes such as endocytosis and exocytosis, allowing an internalization or release of substances (42).
Compromised membrane integrity is closely associated with the induction of cell death pathways (38). Understanding repair mechanisms is crucial for unraveling the complex relationship between membrane integrity and cellular homeostasis, offering therapeutic opportunities in conditions like cancer. A notable characteristic of metastatic cancer cell membranes is that lipid content may change over time. For example, cells undergoing metastasis reduce their cholesterol levels and increase their fluidity and plasticity to facilitate penetration into blood arteries (43). Additionally, reduced cholesterol levels disrupt lipid raft formation and can affect the localization and activity of membrane-associated proteins, influencing important cellular processes such as proliferation, apoptosis, and invasion (44, 45). Calcium ions (Ca2+) are crucial molecules involved in intracellular signaling, which is important for cell proliferation and survival (46). Repair of plasma membrane wounds is initiated by the influx of Ca2+ and the recruitment of Ca2+-regulated proteins, particularly annexins (13, 47). Annexin protein family members (ANXA) (in mammals: ANXA1-11 and ANXA13) play a crucial role in membrane fusion and wound healing (14). They are recruited to the damaged plasma membrane by binding to negatively charged phospholipids, facilitating membrane reshaping and fusion, thus promoting effective resealing. Annexins have diverse properties that contribute to membrane shaping and enable customized responses for efficient repair (15, 16, 48, 49).
Understanding membrane repair mechanisms opens novel avenues to target these processes and develop novel potential therapeutic strategies.
Multiple studies support the notion that phenothiazines exert therapeutic effects by modulating membrane function (72, 73). Derivatives of phenothiazine have demonstrated the ability to induce a range of alterations in the structure of cell membranes through molecular interactions with lipid bilayers in cancer cells (25). In this context, we have investigated the impact of various well-known phenothiazines on the plasma membrane of cancer cells and their ability to inhibit repair upon membrane damage (Table 1).
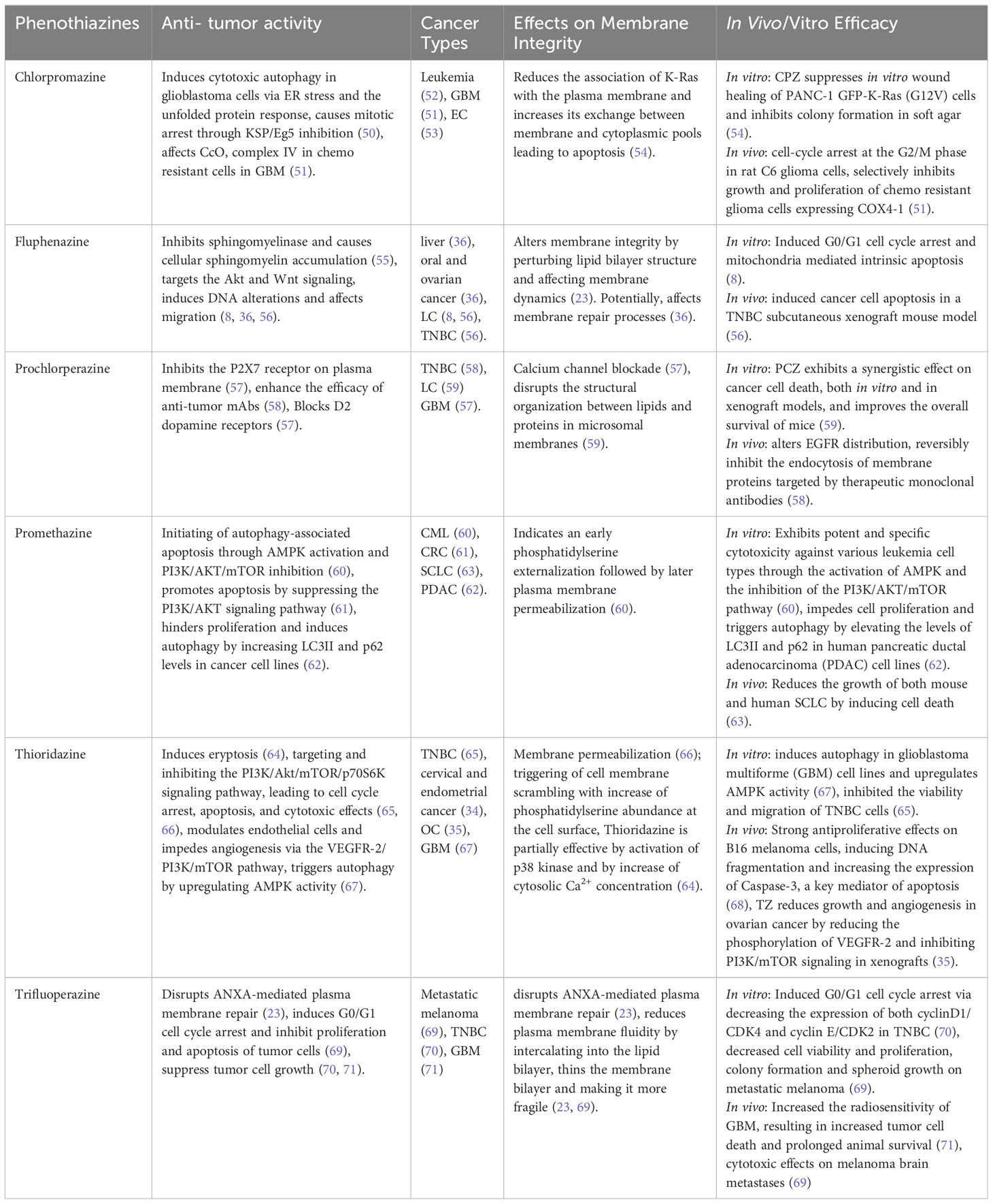
Table 1 Phenothiazines effecting cell membrane integrity and their respective anti-tumor activities.
TFP has been shown to induce lysosomal membrane permeabilization (69) and conformational alterations in membrane organization, caused by a reorganization of the surrounding lipids (74).
Moreover, TFP offers great potential as an inhibitor of plasma membrane repair that sensitizes cancer cells to plasma membrane damage (23). The findings of our study demonstrate that TFP intercalation in the plasma membrane induces membrane thinning and sensitizes cells to membrane injury and cell death. Moreover, the cationic properties of TFP compromise ANXA2 binding to the membrane, delaying the recruitment of ANXA proteins and weakening their attachment to the membrane. This further reduces their ability to induce ANXA4 and ANXA6-mediated membrane curvature around the damaged areas of the membrane (49, 75). This cascade of events initiated by TFP compromises the overall membrane repair response, leaving ruptures unrepaired and sensitizing cells to potential spontaneous injury and death (23).
Other in vitro experiments have shown that TFP induces cell cycle arrest and apoptosis in different cancer cell lines, including triple-negative breast cancer (TNBC) and brain metastases (70). Both in vitro and in vivo xenograft models demonstrated TFP binding to calmodulin (CaM), inhibiting glioblastoma proliferation and invasion by targeting Ca2+ signals (76). This interaction may have a significant impact on the inositol 1,4,5-triphosphate receptor (IP3R), a Ca2+ release channel located in intracellular Ca2+ stores, and IP3R-mediated Ca2+ release (24, 77, 78). Moreover, TFP has demonstrated the ability to enhance the radiosensitivity of glioblastoma multiforme (GBM), resulting in increased tumor cell mortality and extended survival (71). These findings highlight the potential of TFP as an anticancer agent with the ability to sensitize cancer cells to plasma membrane damage and target Ca2+ signals in glioblastoma, offering new possibilities for therapeutic interventions in cancer treatment.
PCZ, as primarly an antipsychotic and antiemetic medication, shows promise in cancer therapy by targeting specific cancer-related molecules, including KRAS mutants. PCZ binds to KRAS mutants’ GTP-binding sites, inhibiting their continuous activation. Additionally, the combination of PCZ and irradiation treatment synergistically increases the radiosensitivity of xenografted mice by downregulating the Ras/Raf/MEK/ERK signaling pathway and reducing the clonogenic survival of KRAS-mutant NSCLC. This combination treatment activates p-ATM, p53, and p21 proteins, leading to cell cycle arrest (59). PCZ also modulates plasma membrane P2X7 receptors, leading to the inhibition of P2X7-mediated Ca2+ entry, and potential impacts on cellular processes such as proliferation and apoptosis (57). PCZ disrupts the structural organization between lipids and proteins in microsomal membranes, thereby altering the activity and regulation of integral membrane proteins (79). Moreover, studies have shown that PCZ can reversibly inhibit the in vivo endocytosis of membrane proteins (58).
CPZ is known for its evident interactions with biological membranes. It accumulates in membranes and modulates their permeability and fluidity, contributing to the biochemical and pharmacological effects of phenothiazines (73, 80). As an antipsychotic drug, CPZ antagonizes the CNS dopamine D2 receptor (DRD2) and reduces the postsynaptic effect of dopamine (81). CPZ has also demonstrated potential as an anticancer agent through interactions with key cancer-related proteins, including p53, YAP, Ras protein, ion channels, and MAPKs, influencing cell cycle regulation, cancer growth, metastasis, resistance to chemotherapy, and stemness (50, 82). CPZ has shown a suppression of cell growth in chemoresistant glioma cells and glioma stem cells. In terms of its mechanism of action, CPZ inhibited the activity of cytochrome c oxidase (CcO, complex IV) in chemoresistant cells while leaving chemosensitive cells unaffected, and it had no impact on other mitochondrial complexes (51). CPZ also disrupts Ca2+ signaling, raising intracellular Ca2+, altering Ca2+ homeostasis, and causing cytotoxicity in glioblastoma cells (83, 84). Furthermore, CPZ induces endoplasmic reticulum (ER) stress and unfolded protein response (UPR), influencing cell fate through autophagy (50). The interaction between CPZ and negatively charged phospholipids has demonstrated a reduction of the link between oncogenic K-Ras and the plasma membrane, hence causing an increase in the cytosolic pool of K-Ras, followed by cell cycle arrest and apoptosis in cancer cells (53, 54).
PMTZ, as an initial-generation antihistamine, antipsychotic, sedative, and antiemetic drug, has shown a wide range of effects on several cancer types. PMTZ induces cell death in leukemia by activating AMPK and inhibiting the PI3K/AKT/mTOR pathway, leading to autophagy-associated apoptosis (60, 61). In chronic myeloid leukemia (CML), increasing concentrations of PMTZ have been associated with early phosphatidylserine externalization, followed by subsequent plasma membrane permeabilization (60). In colorectal cancer (CRC), PMTZ not only suppresses the proliferation of cancer cells but also initiates mitochondrial apoptosis through the PI3K/AKT pathway (61). Additionally, research has illuminated PMTZ’s capacity to induce autophagy in pancreatic ductal adenocarcinoma (PDAC), where it functions as an antagonist of proliferation (62). Furthermore, PMTZ has demonstrated a potent inhibitory impact on the proliferation of both human and murine small cell lung cancer (SCLC). Its ability to inhibit the growth of human H82 SCLC xenografts demonstrates its potential as a diverse and effective anticancer treatment (63).
TZ shows promise as a multifaceted anticancer agent with the ability to induce apoptosis, inhibit tumor growth, modulate angiogenesis, and target key signaling pathways involved in cancer progression. Earlier studies have demonstrated that TZ triggers eryptosis, the programmed death of red blood cells. This process is marked by disruption of the cell membrane, resulting in heightened binding of Annexin V to red blood cells situated on the cell surface, along with an elevation in cytosolic Ca2+ concentration and the activating p38 kinase (64). TZ exhibited inhibitory effects on TNBC cells, both in vitro and in vivo, by targeting the PI3K/AKT signaling pathway, resulting in G0/G1 cell cycle arrest, apoptosis, and mitochondrial dysfunction. This led to tumor growth suppression and the prevention of lung metastasis in TNBC models (65). TZ possesses the capability to suppress the PI3K/Akt/mTOR/p70S6K signaling pathway and exhibits cytotoxic effects on cervical and endometrial cancer cells through the induction of cell cycle arrest and apoptosis (34, 66). Moreover, TZ was found to disrupt signaling pathways downstream of PI3K, including Akt, PDK1, and mTOR, in ovarian tumor progression via vascular endothelial growth factor receptor 2 (VEGFR-2). This suggests that TZ can modulate endothelial cell function and inhibit angiogenesis through the VEGFR-2/PI3K/mTOR pathway, making it a potential anti-angiogenic agent in ovarian cancer (OC) treatment (35). Furthermore, TZ induces autophagy in GBM cell lines and upregulates AMPK activity (67). TZ has shown a strong antiproliferative effect on melanoma by inducing DNA fragmentation and increasing the expression of caspase-3 (68). These findings highlight the potential of TZ as a therapeutic agent against cancer.
Fluphenazine shows promising potential as a repurposed drug for cancer treatment, effectively reducing the viability of various types of cancers such as lung, TNBC, colon, liver, brain, leukemia, oral, ovarian, and skin (36). Fluphenazine shows anticancer properties, and its antitumor activity is mainly mediated by an effect on the cell cycle, proliferation, or apoptosis. This effect is partly mediated by the inhibition of the lysosomal enzyme sphingomyelinase which leads to increased cellular levels of sphingomyelin (55). It should also be noted that this mechanism differs from other known lysosomal-disrupting agents (85, 86). Furthermore, fluphenazine’s interaction with dipalmitoyl phosphatidylcholine (DPPC) bilayers, the main component of pulmonary surfactants, leads to the disruption of the lipid bilayer and the formation of an isotropic phase at higher concentrations. These interactions contribute to its multidrug-resistant (MDR) activity, which offers a potential strategy for cancer chemoprevention (87). In the context of TNBC and brain metastases, fluphenazine hydrochloride (Flu) was investigated. Flu effectively inhibited the survival of metastatic TNBC cells, inducing arrest of the G0/G1 cell cycle and mitochondrial-mediated intrinsic apoptosis in vitro. Pharmacokinetic studies in mice demonstrated favorable brain bioavailability of Flu for at least 24 hours. In particular, Flu exhibited strong antimetastatic effects in a mouse model of brain metastasis, achieving an impressive 85% inhibition rate. Furthermore, Flu showed a significant inhibition of spontaneous lung metastasis without severe side effects (56). These promising findings urge further research to evaluate Flu’s potential as a treatment option for metastatic TNBC and address the urgent need for novel therapeutic approaches.
Repurposing drugs offers innovative solutions that can exceed standard cancer treatments in effectiveness and safety. Phenothiazines show promise against drug resistance and cancer due to their unique properties, including hydrophobicity and specific structure (2, 6, 26). They exhibit diverse effects on cancer cells, including inhibiting proliferation, disrupting cell cycles, preventing metastasis, inducing apoptosis, and enhancing chemotherapy sensitivity (61, 65, 70, 82).
Maintaining cell membrane integrity is vital for survival. Cancer cells, much like normal cells, reprogram themselves to repair damaged membranes and avoid apoptosis (38). Phenothiazines are gaining scientific attention for their impact on membrane dynamics. They interact with the lipid bilayer and profoundly disturb the biophysical properties of cell membranes, such as fluidity and lipid organization, affecting downstream signal transduction and ion channel activity (20, 37). These compounds also inhibit annexin-mediated plasma membrane repair, which induces membrane thinning and reduces annexin-mediated membrane curvature (23). Disturbances in membrane repair machinery sensitize cells to membrane ruptures, ultimately triggering a cascade of cellular responses that culminate in cell death (15, 23, 39, 49). In addition, they may influence Ca2+ regulation by modifying the activation of Ca2+ receptors such as PMCA and IP3R, hence influencing downstream signaling cascades (24, 83, 84). Furthermore, phenothiazines suppress the PI3K/AKT (7, 34, 61, 65) pathway and interfere with critical cancer-related proteins like K-RAS (54), directing cellular outcomes toward cycle arrest, apoptosis, and reduced proliferation and survival. Their involvement in disturbing membrane permeability and sphingomyelin accumulation provides insights into the complex mechanisms driving cytotoxicity (21, 55, 86, 87).
The anticancer properties of phenothiazines may vary depending on their dosage, since it has been shown that clinically significant levels (~ 1-2 µM) might promote tumor growth (88, 89). However, the membrane-compromising actions of phenothiazines seem to need greater concentrations (~ 7-15 µM) (23). Consequently, the use of higher dosages may elevate the risk of potential side effects, particularly when taken in combination with chemotherapeutic agents. The inconsistent findings regarding these antipsychotic drugs in cancer cells underscore their concentration-dependent characteristics. The role of phenothiazines in cancer treatment may not only vary in relation to concentration but also in accordance with the cancer type. Hence, it is important to evaluate both aspects, when assessing the therapeutic potential of phenothiazines.
In summary, the multifaceted effects of phenothiazines on cellular membranes present significant potential for their repurposing in cancer therapy. Their ability to disrupt membrane integrity, inhibit repair processes, and modify critical cellular pathways positions them as intriguing options for the targeted therapy of cancer. A comprehensive understanding of their interaction with membrane dynamics introduces a fresh perspective for developing innovative therapeutic approaches to combat cancer and address various pathological conditions.
SM: Conceptualization, Writing – original draft, Writing – review & editing. SE: Writing – review & editing. JN: Conceptualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Work discussed here was supported by the Novo Nordisk Foundation (NNF18OC0034936), The Danish Cancer Society Scientific Committee (Knæk Cancer, R343-A19644), and NEYE-Fonden.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, et al. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med (2021) 9:20503121211034366. doi: 10.1177/20503121211034366
2. Singhal S, Maheshwari P, Krishnamurthy PT, Patil VM. Drug repurposing strategies for non-cancer to cancer therapeutics. Anticancer Agents Med Chem (2022) 22(15):2726–56. doi: 10.2174/1871520622666220317140557
3. Ohlow MJ, Moosmann B. Phenothiazine: the seven lives of pharmacology's first lead structure. Drug Discovery Today (2011) 16(3-4):119–31. doi: 10.1016/j.drudis.2011.01.001
4. Edinoff AN, Armistead G, Rosa CA, Anderson A, Patil R, Cornett EM, et al. Phenothiazines and their evolving roles in clinical practice: A narrative review. Health Psychol Res (2022) 10(4):38930. doi: 10.52965/001c.38930
5. Mosnaim AD, Ranade VV, Wolf ME, Puente J, Antonieta Valenzuela M. Phenothiazine molecule provides the basic chemical structure for various classes of pharmacotherapeutic agents. Am J Ther (2006) 13(3):261–73. doi: 10.1097/01.mjt.0000212897.20458.63
6. Varga B, Csonka A, Csonka A, Molnar J, Amaral L, Spengler G. Possible biological and clinical applications of phenothiazines. Anticancer Res (2017) 37(11):5983–93. doi: 10.21873/anticanres.12045
7. Choi JH, Yang YR, Lee SK, Kim SH, Kim YH, Cha JY, et al. Potential inhibition of PDK1/Akt signaling by phenothiazines suppresses cancer cell proliferation and survival. Ann New York Acad Sci (2008) 1138(1):393–403. doi: 10.1196/annals.1414.041
8. Xi H, Wu M, Ma H, Li S, Huang Q, Zhang Y, et al. Repurposing fluphenazine to suppress melanoma brain, lung and bone metastasis by inducing G0/G1 cell cycle arrest and apoptosis and disrupting autophagic flux. Clin Exp Metastasis (2023) 40(2):161–75. doi: 10.1007/s10585-023-10202-0
9. Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab (2016) 23(1):27–47. doi: 10.1016/j.cmet.2015.12.006
10. Jaiswal JK, Nylandsted J. S100 and annexin proteins identify cell membrane damage as the Achilles heel of metastatic cancer cells. Cell Cycle (2015) 14(4):502–9. doi: 10.1080/15384101.2014.995495
11. Liu X, Ma D, Jing X, Wang B, Yang W, Qiu W. Overexpression of ANXA2 predicts adverse outcomes of patients with Malignant tumors: a systematic review and meta-analysis. Med Oncol (2015) 32(1):392. doi: 10.1007/s12032-014-0392-y
12. Qi H, Liu S, Guo C, Wang J, Greenaway FT, Sun MZ. Role of annexin A6 in cancer. Oncol Lett (2015) 10(4):1947–52. doi: 10.3892/ol.2015.3498
13. Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol (2005) 6(6):449–61. doi: 10.1038/nrm1661
14. Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev (2002) 82(2):331–71. doi: 10.1152/physrev.00030.2001
15. Lauritzen SP, Boye TL, Nylandsted J. Annexins are instrumental for efficient plasma membrane repair in cancer cells. Semin Cell Dev Biol (2015) 45:32–8. doi: 10.1016/j.semcdb.2015.10.028
16. Boye TL, Nylandsted J. Annexins in plasma membrane repair. Biol Chem (2016) 397(10):961–9. doi: 10.1515/hsz-2016-0171
17. Berg Klenow M, Iversen C, Wendelboe Lund F, Mularski A, Busk Heitmann AS, Dias C, et al. Annexins A1 and A2 accumulate and are immobilized at cross-linked membrane-membrane interfaces. Biochemistry (2021) 60(16):1248–59. doi: 10.1021/acs.biochem.1c00126
18. Frandsen SK, McNeil AK, Novak I, McNeil PL, Gehl J. Difference in membrane repair capacity between cancer cell lines and a normal cell line. J Membr Biol (2016) 249(4):569–76. doi: 10.1007/s00232-016-9910-5
19. Jaiswal JK, Lauritzen SP, Scheffer L, Sakaguchi M, Bunkenborg J, Simon SM, et al. S100A11 is required for efficient plasma membrane repair and survival of invasive cancer cells. Nat Commun (2014) 5:3795. doi: 10.1038/ncomms4795
20. Hendrich AB, Wesołowska O, Michalak K. Trifluoperazine induces domain formation in zwitterionic phosphatidylcholine but not in charged phosphatidylglycerol bilayers. Biochim Biophys Acta (BBA) - Biomembranes (2001) 1510(1-2):414–25. doi: 10.1016/S0005-2736(00)00373-4
21. Hendrich AB, Michalak K, Wesolowska O. Phase separation is induced by phenothiazine derivatives in phospholipid/sphingomyelin/cholesterol mixtures containing low levels of cholesterol and sphingomyelin. Biophys Chem (2007) 130(1-2):32–40. doi: 10.1016/j.bpc.2007.07.001
22. Wesołowska O, Michalak K, Hendrich AB. Direct visualization of phase separation induced by phenothiazine-type antipsychotic drugs in model lipid membranes. Mol Membrane Biol (2011) 28(2):103–14. doi: 10.3109/09687688.2010.533706
23. Heitmann ASB, Zanjani AAH, Klenow MB, Mularski A, Sønder SL, Lund FW, et al. Phenothiazines alter plasma membrane properties and sensitize cancer cells to injury by inhibiting annexin-mediated repair. J Biol Chem (2021) 297(2):101012. doi: 10.1016/j.jbc.2021.101012
24. Kang S, Hong J, Lee JM, Moon HE, Jeon B, Choi J, et al. Trifluoperazine, a well-known antipsychotic, inhibits glioblastoma invasion by binding to calmodulin and disinhibiting calcium release channel IP3R. Mol Cancer Ther (2017) 16(1):217–27. doi: 10.1158/1535-7163.MCT-16-0169-T
25. Michalak K, Wesolowska O, Motohashi N, Molnar J, Hendrich A. Interactions of phenothiazines with lipid bilayer and their role in multidrug resistance reversal. Curr Drug Targets (2006) 7(9):1095–105. doi: 10.2174/138945006778226570
26. Jaszczyszyn A, Gasiorowski K, Swiatek P, Malinka W, Cieslik-Boczula K, Petrus J, et al. Chemical structure of phenothiazines and their biological activity. Pharmacol Rep (2012) 64(1):16–23. doi: 10.1016/S1734-1140(12)70726-0
27. Mozrzymas JW, Barberis A, Michalak K, Cherubini E. Chlorpromazine inhibits miniature GABAergic currents by reducing the binding and by increasing the unbinding rate of GABAA receptors. J Neurosci (1999) 19(7):2474–88. doi: 10.1523/JNEUROSCI.19-07-02474.1999
28. Teisseyre A, Michalak K. The voltage- and time-dependent blocking effect of trifluoperazine on T lymphocyte Kv1. 3 channels Biochem Pharmacol (2003) 65(4):551–61. doi: 10.1016/S0006-2952(02)01561-7
29. Dastidar SG, Kristiansen JE, Molnar J, Amaral L. Role of phenothiazines and structurally similar compounds of plant origin in the fight against infections by drug resistant bacteria. Antibiotics (Basel) (2013) 2(1):58–72. doi: 10.3390/antibiotics2010058
30. Sroda-Pomianek K, Michalak K, Palko-Labuz A, Uryga A, Swiatek P, Majkowski M, et al. The combined use of phenothiazines and statins strongly affects doxorubicin-resistance, apoptosis, and Cox-2 activity in colon cancer cells. Int J Mol Sci (2019) 20(4):955. doi: 10.3390/ijms20040955
31. Gangopadhyay S, Karmakar P, Dasgupta U, Chakraborty A. Trifluoperazine stimulates ionizing radiation induced cell killing through inhibition of DNA repair. Mutat Res (2007) 633(2):117–25. doi: 10.1016/j.mrgentox.2007.05.011
32. Ghinet A, Moise IM, Rigo B, Homerin G, Farce A, Dubois J, et al. Studies on phenothiazines: New microtubule-interacting compounds with phenothiazine A-ring as potent antineoplastic agents. Bioorg Med Chem (2016) 24(10):2307–17. doi: 10.1016/j.bmc.2016.04.001
33. Colturato-Kido C, Lopes RM, Medeiros HCD, Costa CA, Prado-Souza LFL, Ferraz LS, et al. Inhibition of autophagy enhances the antitumor effect of thioridazine in acute lymphoblastic leukemia cells. Life (2021) 11(4):365. doi: 10.3390/life11040365
34. Kang S, Dong SM, Kim B-R, Park MS, Trink B, Byun H-J, et al. Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells. Apoptosis (2012) 17(9):989–97. doi: 10.1007/s10495-012-0717-2
35. Park MS, Dong SM, Kim B-R, Seo SH, Kang S, Lee E-J, et al. Thioridazine inhibits angiogenesis and tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian cancer xenografts. Oncotarget (2014) 5(13):4929–34. doi: 10.18632/oncotarget.2063
36. Duarte D, Vale N. Antipsychotic drug fluphenazine against human cancer cells. Biomolecules (2022) 12(10):1360. doi: 10.3390/biom12101360
37. Hidalgo AA, Caetano W, Tabak M, Oliveira ON Jr. Interaction of two phenothiazine derivatives with phospholipid monolayers. Biophys Chem (2004) 109(1):85–104. doi: 10.1016/j.bpc.2003.10.020
38. Dias C, Nylandsted J. Plasma membrane integrity in health and disease: significance and therapeutic potential. Cell Discovery (2021) 7(1):4. doi: 10.1038/s41421-020-00233-2
39. Andrews NW, Corrotte M. Plasma membrane repair. Curr Biol (2018) 28(8):R392–R7. doi: 10.1016/j.cub.2017.12.034
40. Nicolson GL. Cell membrane fluid-mosaic structure and cancer metastasis. Cancer Res (2015) 75(7):1169–76. doi: 10.1158/0008-5472.CAN-14-3216
41. Blazek AD, Paleo BJ, Weisleder N. Plasma membrane repair: A central process for maintaining cellular homeostasis. Physiol (Bethesda) (2015) 30(6):438–48. doi: 10.1152/physiol.00019.2015
42. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P, et al. Transport into the cell from the plasma membrane: endocytosis. In: Molecular Biology of the Cell, 4th. New York: Garland Science (2002).
43. Szlasa W, Zendran I, Zalesinska A, Tarek M, Kulbacka J. Lipid composition of the cancer cell membrane. J Bioenerg Biomembr (2020) 52(5):321–42. doi: 10.1007/s10863-020-09846-4
44. Buschiazzo J, Ialy-Radio C, Auer J, Wolf JP, Serres C, Lefevre B, et al. Cholesterol depletion disorganizes oocyte membrane rafts altering mouse fertilization. PLoS One (2013) 8(4):e62919. doi: 10.1371/journal.pone.0062919
45. Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest (2005) 115(4):959–68. doi: 10.1172/JCI200519935
46. Kang SS, Han KS, Ku BM, Lee YK, Hong J, Shin HY, et al. Caffeine-mediated inhibition of calcium release channel inositol 1,4,5-trisphosphate receptor subtype 3 blocks glioblastoma invasion and extends survival. Cancer Res (2010) 70(3):1173–83. doi: 10.1158/0008-5472.CAN-09-2886
47. Bendix PM, Simonsen AC, Florentsen CD, Hager SC, Mularski A, Zanjani AAH, et al. Interdisciplinary synergy to reveal mechanisms of annexin-mediated plasma membrane shaping and repair. Cells (2020) 9(4):1029. doi: 10.3390/cells9041029
48. Koerdt SN, Ashraf APK, Gerke V. Annexins and plasma membrane repair. Curr Top Membr (2019) 84:43–65. doi: 10.1016/bs.ctm.2019.07.006
49. Mularski A, Sonder SL, Heitmann ASB, Pandey MP, Khandelia H, Nylandsted J, et al. Interplay of membrane crosslinking and curvature induction by annexins. Sci Rep (2022) 12(1):22568. doi: 10.1038/s41598-022-26633-w
50. Matteoni S, Matarrese P, Ascione B, Ricci-Vitiani L, Pallini R, Villani V, et al. Chlorpromazine induces cytotoxic autophagy in glioblastoma cells via endoplasmic reticulum stress and unfolded protein response. J Exp Clin Cancer Res (2021) 40(1):347. doi: 10.1186/s13046-021-02144-w
51. Oliva CR, Zhang W, Langford C, Suto MJ, Griguer CE. Repositioning chlorpromazine for treating chemoresistant glioma through the inhibition of cytochrome c oxidase bearing the COX4-1 regulatory subunit. Oncotarget (2017) 8(23):37568–83. doi: 10.18632/oncotarget.17247
52. Zhelev Z, Ohba H, Bakalova R, Hadjimitova V, Ishikawa M, Shinohara Y, et al. Phenothiazines suppress proliferation and induce apoptosis in cultured leukemic cells without any influence on the viability of normal lymphocytes. Cancer Chemother Pharmacol (2004) 53(3):267–75. doi: 10.1007/s00280-003-0738-1
53. Cui Y, Wu H, Yang L, Huang T, Li J, Gong X, et al. Chlorpromazine sensitizes progestin-resistant endometrial cancer cells to MPA by upregulating PRB. Front Oncol (2021) 11:665832. doi: 10.3389/fonc.2021.665832
54. Eisenberg S, Giehl K, Henis YI, Ehrlich M. Differential interference of chlorpromazine with the membrane interactions of oncogenic K-Ras and its effects on cell growth. J Biol Chem (2008) 283(40):27279–88. doi: 10.1074/jbc.M804589200
55. Klutzny S, Lesche R, Keck M, Kaulfuss S, Schlicker A, Christian S, et al. Functional inhibition of acid sphingomyelinase by Fluphenazine triggers hypoxia-specific tumor cell death. Cell Death Disease (2017) 8(3):e2709–e. doi: 10.1038/cddis.2017.130
56. Xu F, Xia Y, Feng Z, Lin W, Xue Q, Jiang J, et al. Repositioning antipsychotic fluphenazine hydrochloride for treating triple negative breast cancer with brain metastases and lung metastases. Am J Cancer Res (2019) 9(3):459–78.
57. Hempel C, Norenberg W, Sobottka H, Urban N, Nicke A, Fischer W, et al. The phenothiazine-class antipsychotic drugs prochlorperazine and trifluoperazine are potent allosteric modulators of the human P2X7 receptor. Neuropharmacology (2013) 75:365–79. doi: 10.1016/j.neuropharm.2013.07.027
58. Chew HY, De Lima PO, Gonzalez Cruz JL, Banushi B, Echejoh G, Hu L, et al. Endocytosis inhibition in humans to improve responses to ADCC-mediating antibodies. Cell (2020) 180(5):895–914.e27. doi: 10.1016/j.cell.2020.02.019
59. Sad K, Parashar P, Tripathi P, Hungyo H, Sistla R, Soni R, et al. Prochlorperazine enhances radiosensitivity of non-small cell lung carcinoma by stabilizing GDP-bound mutant KRAS conformation. Free Radic Biol Med (2021) 177:299–312. doi: 10.1016/j.freeradbiomed.2021.11.001
60. Medeiros HCD, Colturato-Kido C, Ferraz LS, Costa CA, Moraes VWR, Paredes-Gamero EJ, et al. AMPK activation induced by promethazine increases NOXA expression and Beclin-1 phosphorylation and drives autophagy-associated apoptosis in chronic myeloid leukemia. Chemico-Biological Interactions (2020) 315:108888. doi: 10.1016/j.cbi.2019.108888
61. Tan X, Gong L, Li X, Zhang X, Sun J, Luo X, et al. Promethazine inhibits proliferation and promotes apoptosis in colorectal cancer cells by suppressing the PI3K/AKT pathway. Biomed Pharmacother (2021) 143:112174. doi: 10.1016/j.biopha.2021.112174
62. Avendano-Felix M, Aguilar-Medina M, Bermudez M, Lizarraga-Verdugo E, Lopez-Camarillo C, Ramos-Payan R. Refocusing the use of psychiatric drugs for treatment of gastrointestinal cancers. Front Oncol (2020) 10:1452. doi: 10.3389/fonc.2020.01452
63. Jahchan NS, Dudley JT, Mazur PK, Flores N, Yang D, Palmerton A, et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discovery (2013) 3(12):1364–77. doi: 10.1158/2159-8290.CD-13-0183
64. Lang E, Modicano P, Arnold M, Bissinger R, Faggio C, Abed M, et al. Effect of thioridazine on erythrocytes. Toxins (2013) 5(10):1918–31. doi: 10.3390/toxins5101918
65. Song Y, Li L, Chen J, Chen H, Cui B, Feng Y, et al. Thioridazine hydrochloride: an antipsychotic agent that inhibits tumor growth and lung metastasis in triple-negative breast cancer via inducing G0/G1 arrest and apoptosis. Cell Cycle (2020) 19(24):3521–33. doi: 10.1080/15384101.2020.1850969
66. Spengler G, Csonka A, Molnar J, Amaral L. The anticancer activity of the old neuroleptic phenothiazine-type drug thioridazine. Anticancer Res (2016) 36(11):5701–6. doi: 10.21873/anticanres.11153
67. Cheng H-W, Liang Y-H, Kuo Y-L, Chuu C-P, Lin C-Y, Lee M-H, et al. Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Disease (2015) 6(5):e1753–e. doi: 10.1038/cddis.2015.77
68. Gil-Ad I, Shtaif B, Levkovitz Y, Nordenberg J, Taler M, Korov I, et al. Phenothiazines induce apoptosis in a B16 mouse melanoma cell line and attenuate in vivo melanoma tumor growth. Oncol Rep (2006) 15(1):107–12. doi: 10.3892/or.15.1.107
69. Zhang X, Ding K, Ji J, Parajuli H, Aasen SN, Espedal H, et al. Trifluoperazine prolongs the survival of experimental brain metastases by STAT3-dependent lysosomal membrane permeabilization. Am J Cancer Res (2020) 10(2):545–63.
70. Feng Z, Xia Y, Gao T, Xu F, Lei Q, Peng C, et al. The antipsychotic agent trifluoperazine hydrochloride suppresses triple-negative breast cancer tumor growth and brain metastasis by inducing G0/G1 arrest and apoptosis. Cell Death Disease (2018) 9(10):1006. doi: 10.1038/s41419-018-1046-3
71. Zhang X, Xu R, Zhang C, Xu Y, Han M, Huang B, et al. Trifluoperazine, a novel autophagy inhibitor, increases radiosensitivity in glioblastoma by impairing homologous recombination. J Exp Clin Cancer Res (2017) 36(1):118. doi: 10.1186/s13046-017-0588-z
72. Samuels AM, Carey MC. Effects of chlorpromazine hydrochloride and its metabolites on Mg2+- and Na+,K+-ATPase activities of canalicular-enriched rat liver plasma membranes. Gastroenterology (1978) 74(6):1183–90. doi: 10.1016/0016-5085(78)90690-X
73. Bhise SB, Marwadi PR, Mathur SS, Srivastava RC. Liquid membrane phenomena in chlorpromazine action. Biophys Chem (1983) 17(3):187–92. doi: 10.1016/0301-4622(83)87003-3
74. Ruggiero AC, Meirelles NC. Effects of trifluoperazine on the conformation and dynamics of membrane proteins in human erythrocytes. Mol Genet Metab (1998) 64(2):148–51. doi: 10.1006/mgme.1998.2689
75. Florentsen CD, Kamp-Sonne A, Moreno-Pescador G, Pezeshkian W, Hakami Zanjani AA, Khandelia H, et al. Annexin A4 trimers are recruited by high membrane curvatures in giant plasma membrane vesicles. Soft Matter (2021) 17(2):308–18. doi: 10.1039/D0SM00241K
76. Vandonselaar M, Hickie RA, Quail JW, Delbaere LT. Trifluoperazine-induced conformational change in Ca(2+)-calmodulin. Nat Struct Biol (1994) 1(11):795–801. doi: 10.1038/nsb1194-795
77. Yeh CT, Wu AT, Chang PM, Chen KY, Yang CN, Yang SC, et al. Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer. Am J Respir Crit Care Med (2012) 186(11):1180–8. doi: 10.1164/rccm.201207-1180OC
78. Michikawa T, Hirota J, Kawano S, Hiraoka M, Yamada M, Furuichi T, et al. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,4,5-trisphosphate receptor. Neuron (1999) 23(4):799–808. doi: 10.1016/S0896-6273(01)80037-4
79. Dannenberg A, Zakim D. Effects of prochlorperazine on the function of integral membrane proteins. Biochem Pharmacol (1988) 37(7):1259–62. doi: 10.1016/0006-2952(88)90779-4
80. Maruoka N, Murata T, Omata N, Takashima Y, Tanii H, Yonekura Y, et al. Effects of chlorpromazine on plasma membrane permeability and fluidity in the rat brain: a dynamic positron autoradiography and fluorescence polarization study. Prog Neuropsychopharmacol Biol Psychiatry (2007) 31(1):178–86. doi: 10.1016/j.pnpbp.2006.08.019
81. Horn AS, Snyder SH. Chlorpromazine and dopamine: conformational similarities that correlate with the antischizophrenic activity of phenothiazine drugs. Proc Natl Acad Sci U S A (1971) 68(10):2325–8. doi: 10.1073/pnas.68.10.2325
82. Yde CW, Clausen MP, Bennetzen MV, Lykkesfeldt AE, Mouritsen OG, Guerra B. The antipsychotic drug chlorpromazine enhances the cytotoxic effect of tamoxifen in tamoxifen-sensitive and tamoxifen-resistant human breast cancer cells. Anticancer Drugs (2009) 20(8):723–35. doi: 10.1097/CAD.0b013e32832ec041
83. Chu C-S, Lin Y-S, Liang W-Z. The impact of the antipsychotic medication chlorpromazine on cytotoxicity through Ca2+ Signaling pathway in glial cell models. Neurotoxicity Res (2022) 40(3):791–802. doi: 10.1007/s12640-022-00507-5
84. Plenge-Tellechea F, Dominguez-Solis CA, Diaz-Sanchez AG, Melendez-Martinez D, Vargas-Medrano J, Sierra-Fonseca JA. Chlorpromazine and dimethyl sulfoxide modulate the catalytic activity of the plasma membrane Ca(2+)-ATPase from human erythrocyte. J Bioenerg Biomembr (2018) 50(1):59–69. doi: 10.1007/s10863-017-9741-9
85. Ellegaard A-M, Groth-Pedersen L, Oorschot V, Klumperman J, Kirkegaard T, Nylandsted J, et al. Sunitinib and SU11652 inhibit acid sphingomyelinase, destabilize lysosomes, and inhibit multidrug resistance. Mol Cancer Ther (2013) 12(10):2018–30. doi: 10.1158/1535-7163.MCT-13-0084
86. Petersen Nikolaj HT, Olsen Ole D, Groth-Pedersen L, Ellegaard A-M, Bilgin M, Redmer S, et al. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell (2013) 24(3):379–93. doi: 10.1016/j.ccr.2013.08.003
87. Perez-Isidoro R, Costas M. The effect of neuroleptic drugs on DPPC/sphingomyelin/cholesterol membranes. Chem Phys Lipids (2020) 229:104913. doi: 10.1016/j.chemphyslip.2020.104913
88. Wen Y, Zhang Y, Li J, Luo F, Huang Z, Liu K. Low concentration trifluoperazine promotes proliferation and reduces calcium-dependent apoptosis in glioma cells. Sci Rep (2018) 8(1):1147. doi: 10.1038/s41598-018-19413-y
Keywords: phenothiazines, repurposing, annexins, membrane biophysical properties, membrane integrity, cancer treatment, membrane repair, plasma membrane
Citation: Mehrabi SF, Elmi S and Nylandsted J (2023) Repurposing phenothiazines for cancer therapy: compromising membrane integrity in cancer cells. Front. Oncol. 13:1320621. doi: 10.3389/fonc.2023.1320621
Received: 12 October 2023; Accepted: 30 October 2023;
Published: 23 November 2023.
Edited by:
Tiago Rodrigues, Federal University of ABC, BrazilReviewed by:
Olga Wesolowska, Wroclaw Medical University, PolandCopyright © 2023 Mehrabi, Elmi and Nylandsted. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jesper Nylandsted, am5sQGNhbmNlci5kaw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.