
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 09 January 2024
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1308432
Nuclear protein of the testis (NUT) midline carcinoma (NMC) is a rare tumor, with particularly low incidence in the lungs, and a correspondingly poor prognosis. To determine the clinicopathological characteristics, outcomes, and prognostic factors of primary pulmonary NMC, a case was reported and a systematic review was performed. Twenty-nine records, including ours, involving 62 cases, were finally included. The median age at diagnosis was 29.5 years. At presentation, the most common symptoms at presentation were cough (47.50%) and chest/back pain (37.50%). In terms of diagnosis, 32.14% of NMC cases were identified through immunohistochemistry (IHC); However, a greater number of cases may be misdiagnosed initially, and ultimately, the diagnosis of NMC was confirmed through a combination of IHC and fluorescence in situ hybridization (FISH). Despite the clinical application of various chemotherapy-based treatments, the actual effectiveness remains unsatisfactory. Furthermore, Cox regression analysis of multiple factors identified male gender and concurrent presence of pleural effusion as indicators of shorter survival time in patients. These results emphasize the importance of increased diagnostic awareness among clinical and pathology practitioners concerning NMC. While there is currently no established standard for treating NMC, a treatment approach combining multiple methods shows promise for future research. Concurrently, clinical and foundational investigations addressing variables such as gender and the presence of pleural effusion may yield valuable insights into the diagnosis and treatment of NMC.
Nuclear protein of the testis (NUT) midline carcinoma (NMC) is a rare, extremely aggressive and poorly differentiated squamous cell carcinoma with a dismal prognosis (1, 2). In the most of patients, however, the disease is already advanced at the time of detection, leading rapid progression to death. While various treatments have been reported to be used after diagnosis, NMC remains a challenging clinical problem with limited treatment options (3–6).
Primary pulmonary NMC has been reported less frequently, and its prognosis is even more unfavorable (7). Sholl et al. reported the mOS was only 2.2 months (1). Many questions regarding the epidemiology, histopathology, clinical presentation, and treatments of primary pulmonary NMC remain unanswered. Herein, to help clinicians better recognize and understand this disease, we reported a cases from our group to describe the clinical characteristics of primary pulmonary NMC. An additional 61 cases from published studies were pooled to summarize the clinical characteristics, prognosis, and prognostic factors for the outcomes of patients with the primary pulmonary NMC.
A 23-year-old female patient was admitted to the respiratory ward on June 8, 2020, with a complaint of “chest pain, cough for more than one month, aggravated with dyspnea for two weeks”. She had no significant medical history and denied a history of smoking. On admission, all other aspects of the physical examination, except for diminished breath sounds detected during auscultation of the right lung, revealed no abnormalities. A chest computed tomography (CT) scan showed a substantial amount of pleural effusion on the right side, with incomplete expansion of the right lung (Figure 1).
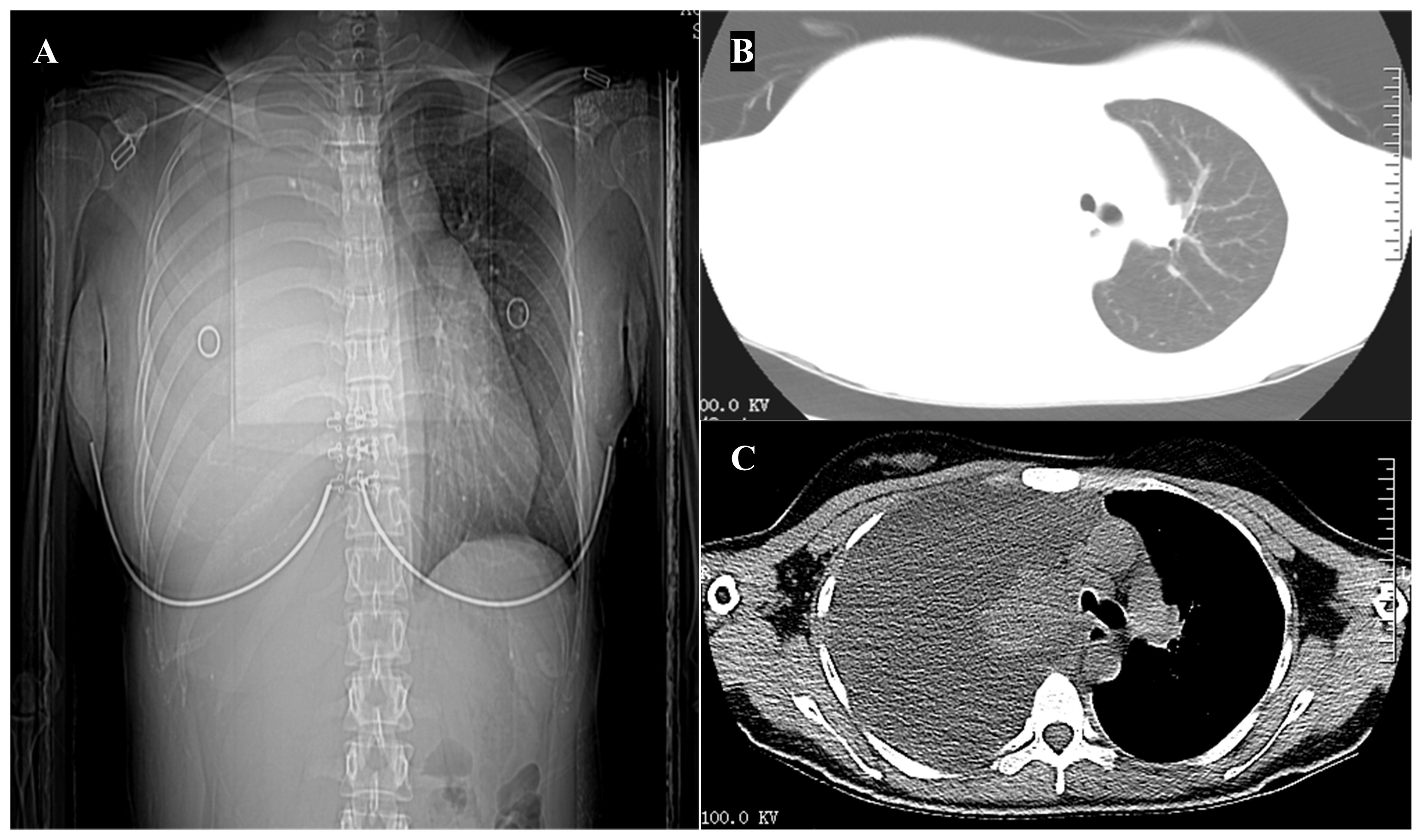
Figure 1 Imaging on hospitalization. (A) Chest x-ray demonstrated a right-sided pleural effusion. (B, C) Computed tomography scan of the chest showing a substantial amount of pleural effusion on the right side, with incomplete expansion of the right lung.
The patient underwent chest tube insertion for drainage. And the pleural fluid analysis revealed a red blood cell count of 0.009×1012/L, a total protein concentration of 37.1 g/L, an adenosine deaminase activity (ADA) of 7.87 U/L, a glucose concentration of 2.59 mmol/L, and a lactate dehydrogenase (LDH) activity of 390 U/L. The pleural fluid carcino-embryonic antigen (CEA) level was 204.68 ng/mL. The blood biochemistry results showed an albumin (ALB) level of 34.9 g/L, a globulin level of 15.7 g/L, a glucose level of 4.18 mmol/L, and a LDH level of 226 U/L. The serum CEA level was 16.72 ng/mL, the squamous cell carcinoma (SCC) antigen level was 1.32 ng/mL, and the neuron-specific enolase (NSE) level was 26.7 ng/mL.
Histopathological examination of the pleural effusion sample revealed the presence of cancer cells (Figure 2A). Immunohistochemical (IHC) analysis demonstrated positive expression of BerEP4, MOC31, p53, and CK7, CK5/6, EMA and P63, while negative expression of GATA-3, TTF-1, WT-1, ER, CDX-2, CK20, Napsin A, CD30, S-100, CD3, CD5, CD117, TdT and Villin. The preliminary pathological diagnosis, taking into account the results of the aforementioned immunohistochemistry, was a squamous cell carcinoma phenotype.
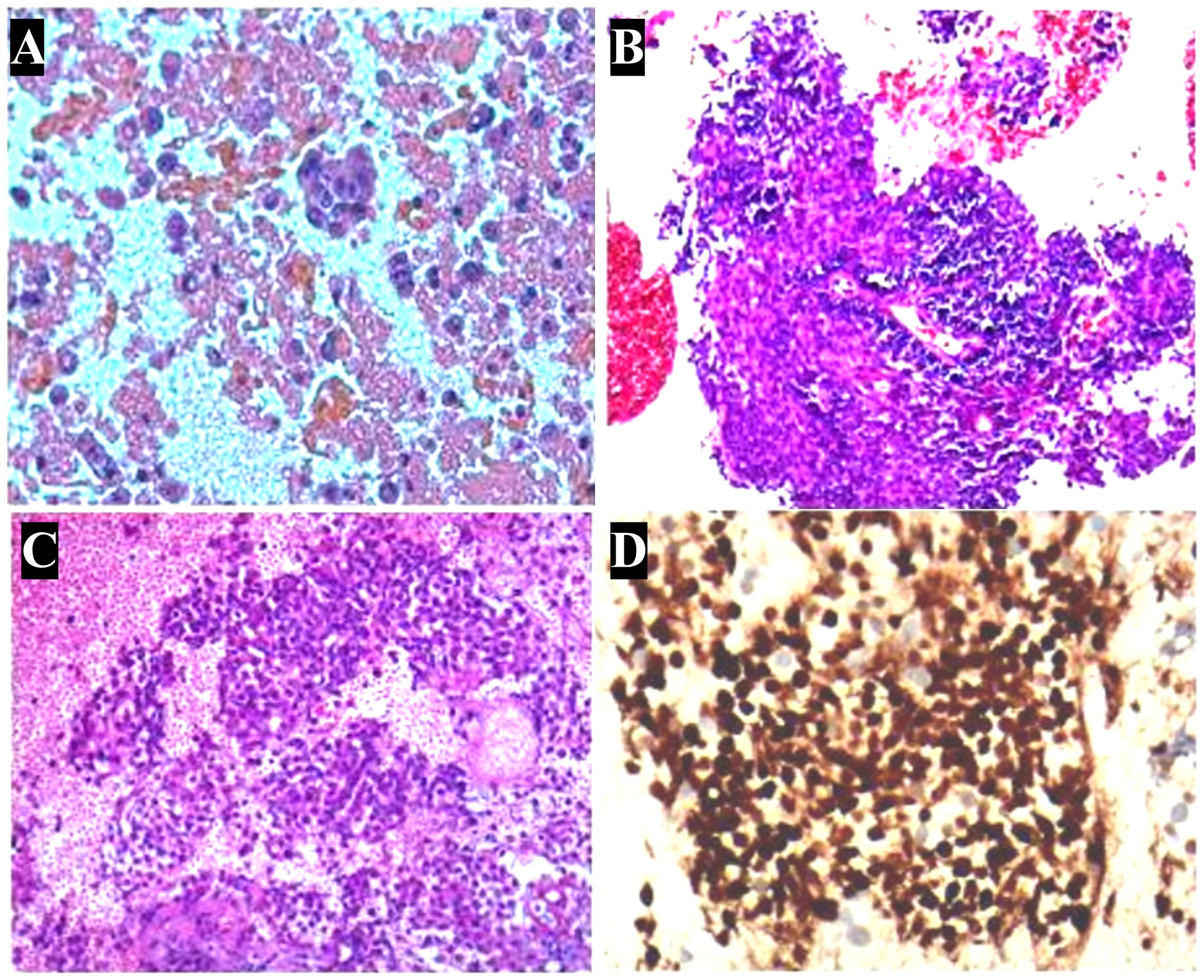
Figure 2 Pathologic characterization of tumor. (A) Hematoxylin and eosin stain of a cell block from pleural effusion sample shows a large number of cancer cells. (B) In aspiration cytology of a mediastinal lymph node, some tumor cells are medium-sized and poorly differentiated with scanty cytoplasm and hyperchromatic nuclei. (C) Biopsy of a mediastinal lymph node shows monotonous tumor cells and hyperchromatic nuclei. (D) Immunohistochemistry shows nuclear expression of NUT in the histology of a mediastinal lymph node. (A-D): ×400.
Given the suspicion of malignant pleural effusion, an electronic bronchoscopy and ultrasound bronchoscopy-guided transbronchial needle aspiration (EBUS-TBNA) procedure was performed on June 15, 2020. The results revealed the presence of metastatic malignant tumors (Figure 2B, C) with a positive staining for NUT in the IHC analysis (Figure 2D), while CD117, CD5, TdT were negative, and Ki67 was positive with a rate of 30%. Based on clinical and IHC findings, the pathologist considered the possibility of NUT carcinoma (NMC) in the histological tissue. Additional molecular testing with NUT FISH confirmed the diagnosis of NMC ultimately. On June 12th, 2020, a positron emission tomography (PET)-CT scan was conducted to evaluate the patient’s disease staging (Figure 3). The results revealed multiple high metabolic foci with localized lesions in the bones, indicating malignant lesions.
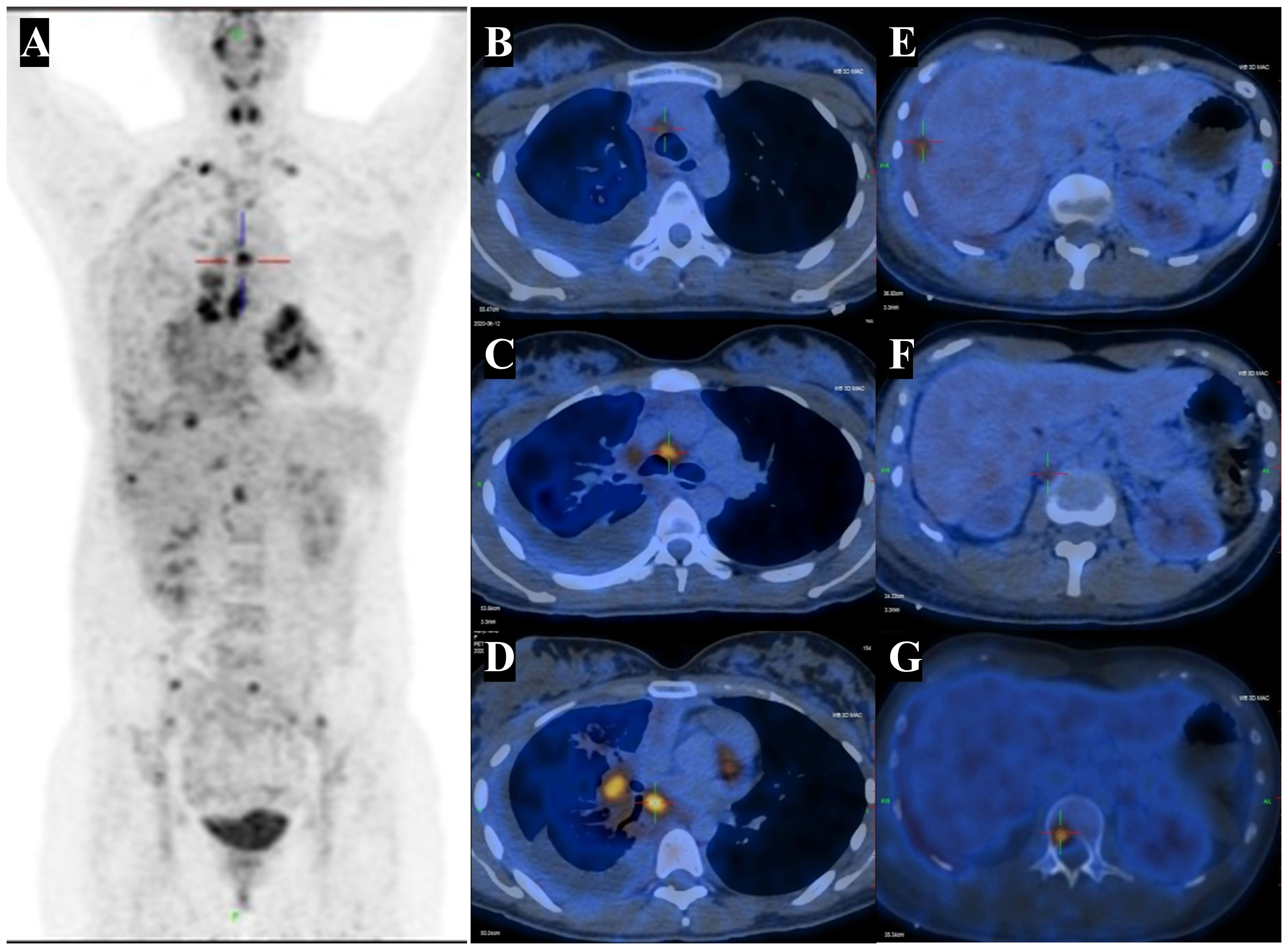
Figure 3 PET-CT highlights multiple hypermetabolic lesions. (A) Fused coronal PET-CT image shows an high metabolic foci in right lung cancer with lymph node, pleural and liver capsule, bone, as well as FDG-avid lesions in the right pleural effusion. (B-D) The right hilar and mediastinal lymph nodes with abnormal FDG uptake. SUVmax 4.4 - 7.8. (E) The abnormal FDG uptake in hepatic capsule with SUVmax 4.6 - 5.6. (F) The abnormal FDG uptake in retroperitoneal lymph nodes with SUVmax 3.4 - 3.6. (G) The abnormal FDG uptake in bone with SUVmax 3.9 - 5.8. CT, computed tomography; PET, positron emission tomography; FDG, fluorodeoxyglucose. SUVmax, maximum standardized uptake value.
Due to the diagnosis of NMC stage IV, the patient underwent two cycle of chemotherapy (paclitaxel albumin-bound and carboplatin) combined with immunotherapy (cetuximab) on June 20, 2020 and July 11, 2020. On August 27, 2020 (the third hospitalization), the patient was admitted for the third cycle of treatment, with a CEA level of 58.71 ng/mL upon admission, which was higher than before. Due to the patient’s upper abdominal and lumbar pain, palliative radiotherapy was administered to the cone metastasis site (GTV1 at L3, L4, and S1 with 40Gy/4Gy/10f; GTV2 at T11 and L1 with 32Gy/4Gy/8f) on the basis of aforementioned treatment schemes. On September 2, 2020, the patient was readmitted for treatment evaluation and had an elevated CEA level of 75.34 ng/mL. The patient experienced continuous disease progression, as evidenced by a right pneumothorax and left pleural effusion on chest CT on December 10, 2020 (Figure 4). The patient died of respiratory failure on January 9, 2021, after an overall disease course of approximately 7 months.
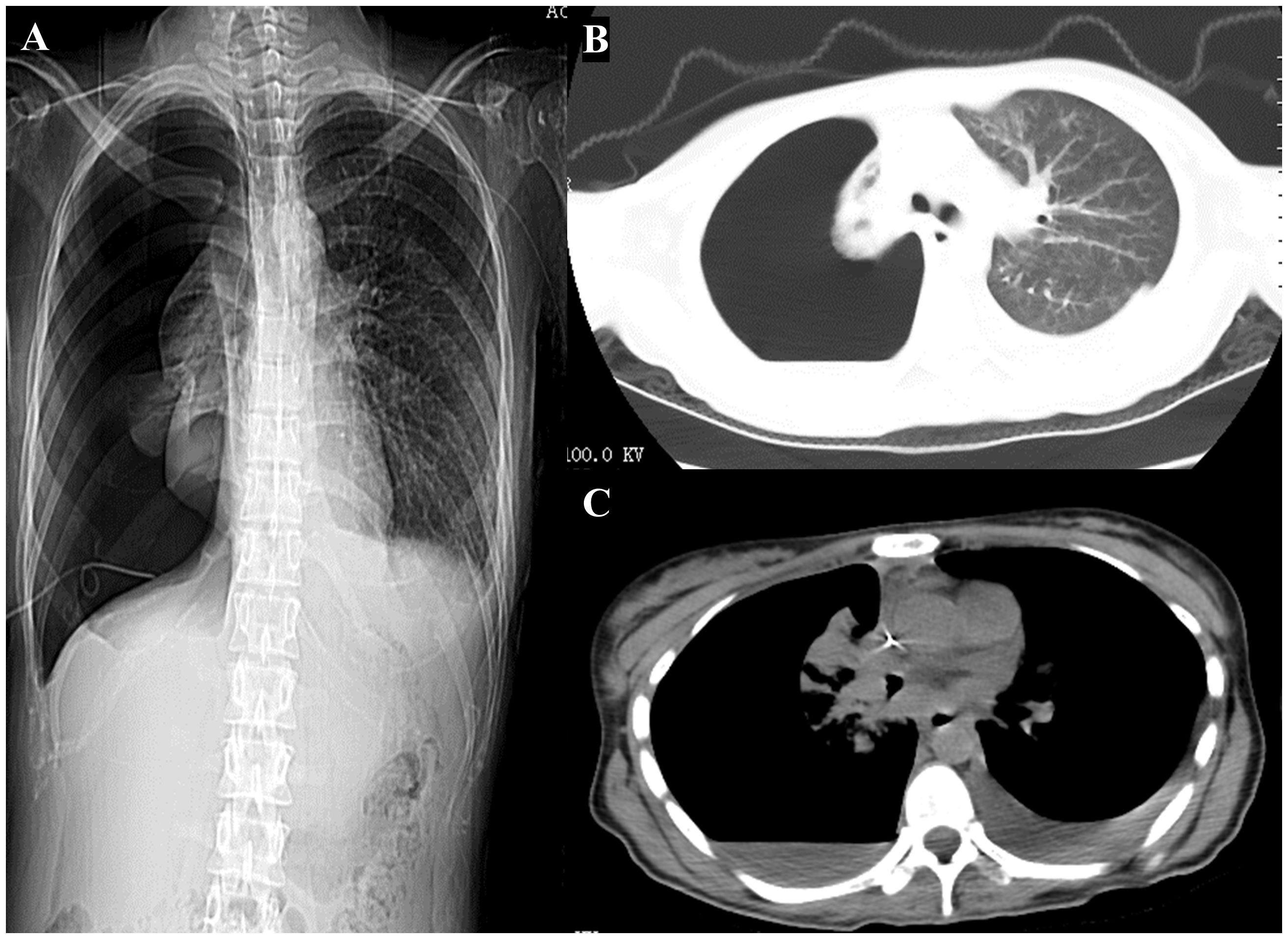
Figure 4 Computed tomography scan of the chest was performed during hers last hospitalization. (A) Chest x-ray imaging. (B, C) Computed tomography scan of the chest showing a right pneumothorax and left pleural effusion.
The study protocol was approved by the ethics committee of Renmin Hospital of Wuhan University (Ethical Application Ref:2023K-K173). A written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
At March 20, 2023, we conducted a literature search of the MEDLINE (PubMed) and Web of Science databases. The search terms included “nuclear protein of the testis”, “NUT” and “midline carcinoma” index words. Titles and abstracts were independently assessed by two reviewers (Wei Cao and Shiqian Zha). Any differences could be resolved through discussion or by hiring a third reviewer (Yixuan Wang) to reach consensus.
Inclusion criteria: (1) The literature should provide at least one of the survival data, other associated clinical characteristics or treatment. (2) Full text is available. (3) cases reported in the English literature. Exclusion criteria: (1) Literatures with repeated publications. (2) Reviews, meta-analysis, conference abstracts, or comment papers.
Three researchers (Wei Cao, Shiqian Zha and Yixuan Wang) extracted the data independently, and any disagreements were identified and resolved through discussions with another author (Zhou Pan). We extracted the following data from the included studies: study characteristics (first author, year of publication, study period, number of patients), patient age, gender, other metastasis site, clinical presentation, primary cancer location, presenting symptoms, histopathologic features, survival time and so on.
Analyses were conducted considering all included patients as one sample. Descriptive data were reported as median with interquartile range (IQR) or frequencies (%), respectively. Survival analysis was performed using Kaplan-Meier method. Univariate analysis was performed using Cox proportional hazards regression model, followed by a multivariate Cox regression analysis including only variables with a P value < 0.05 during univariate analysis. The analysis was performed using IBM SPSS statistical software package software (version 22.0, IBM Corp.) with a significance set at p < 0.05.
We conducted a comprehensive search of the literature and identified 254 articles for initial screening based on their titles and abstracts. Among these, 53 studies were selected for full-text review. After this process, we ultimately included 29 studies in our analysis, providing data on 61 cases of primary pulmonary NMC (Figure 5) (8–36). All of the studies included in our analysis provided information on survival times.
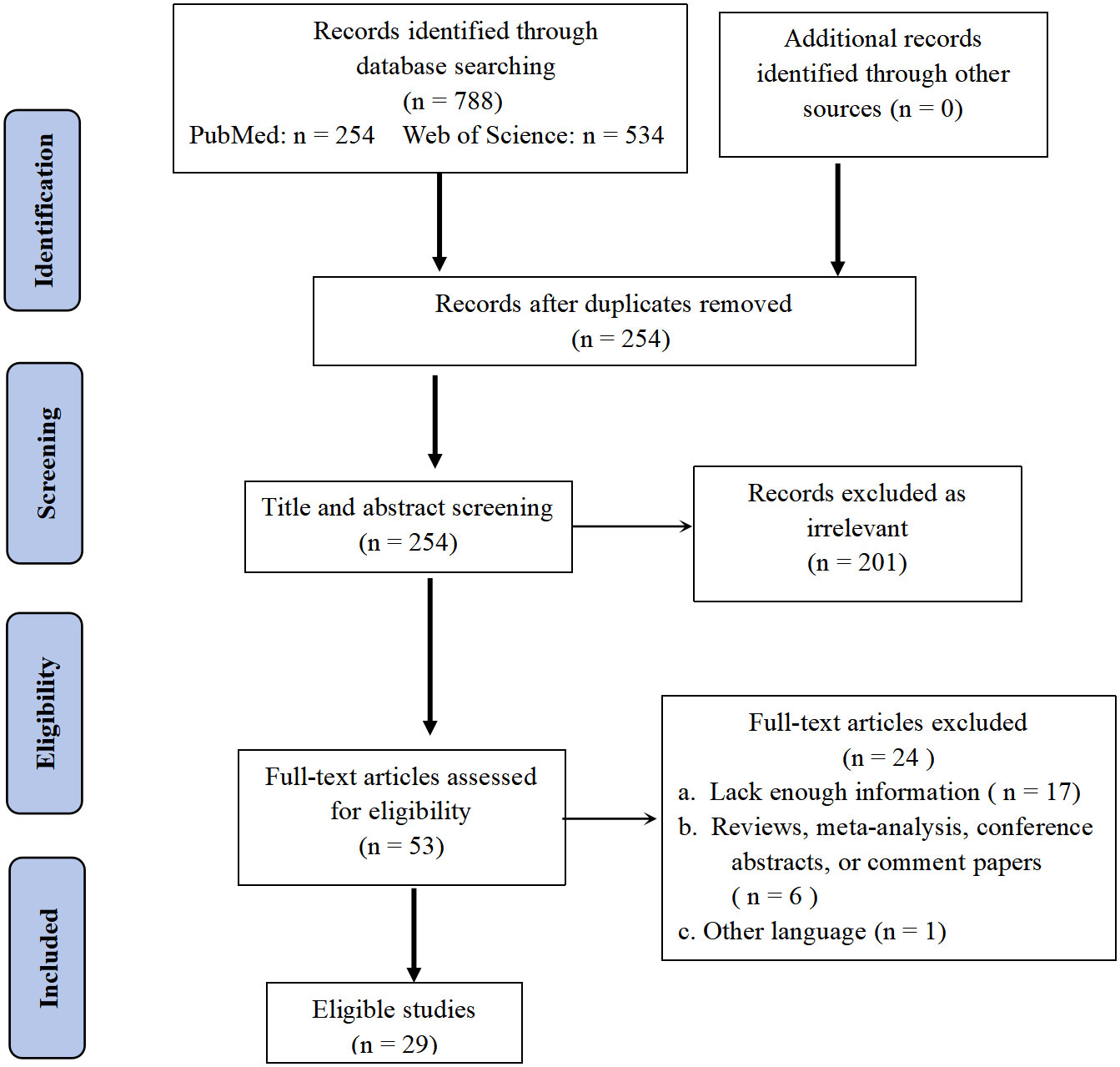
Figure 5 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) flow diagram of search strategy and study selection.
The main characteristics of 62 patients with Pulmonary NMC were summarized in the table below (Table 1). The median age at diagnosis was 29.5 years [Interquartile range (IQR): 18.5 - 36.8], with a female proportion of 46.77% (29/62). 24.19% of patients were diagnosed at less than 18 years old, while 70.97% were diagnosed between 18 - 60 years old. Among the patients with reported smoking status (n = 24), 54.17% were smokers. The primary locations of the tumor were distributed as follows: right upper lobe (16.36%), right lower lobe (16.36%), right middle lobe (3.64%), left upper lobe (7.27%), left lower lobe (14.55%), and hilar region (20.0%). The tumor size was < 2 cm in 0%, 2 - 5 cm in 20.0%, 5 - 10 cm in 45.45%, and > 10 cm in 18.18% of the patients. The most common symptoms at presentation were cough (47.50%) and chest/back pain (37.50%). Metastasis was detected in 42 reported patients, most commonly in the bone (73.81%). The 29 patients presented with pleural effusion in 45 reported cases (64.44%).
Several markers, including CKpan, P63, TTF-1, CK6/7, EMA, CD99, CK5/P40, CD56, Syn, CD30, Vimentin, CAM5.2, and AE1/AE3 cytokeratins were tested. The Ki-67 index, which indicates the level of cell proliferation, was also measured. The majority of patients (92.31%) had positive CKpan expression. And P63 was expressed in 95.65% of patients. The median Ki-67 index was 60%, with an IQR of 40% - 70%. NUT midline carcinoma was the most commonly diagnosed pathological type among the 28 reported cases, with a frequency of 32.14%. Nonetheless, several other pathological types were frequently misdiagnosed, including undifferentiated carcinoma (17.86%), squamous cell carcinoma (14.29%), small-cell carcinomas (10.72%), primary neuroectodermal tumor (7.14%), epithelial carcinoma (7.14%), non-small-cell carcinomas (7.14%), and Ewing’s sarcoma (3.57%).
Regarding therapy, the majority of patients received chemotherapy alone (45%), chemoradiotherapy (CRT) (18.33%) and Combined targeted therapy with chemoradiotherapy (CRT) (11.67%) (Figure 6). The mOS was 4.85 months (IQR: 3.0 - 10.0 months) (Table 1).
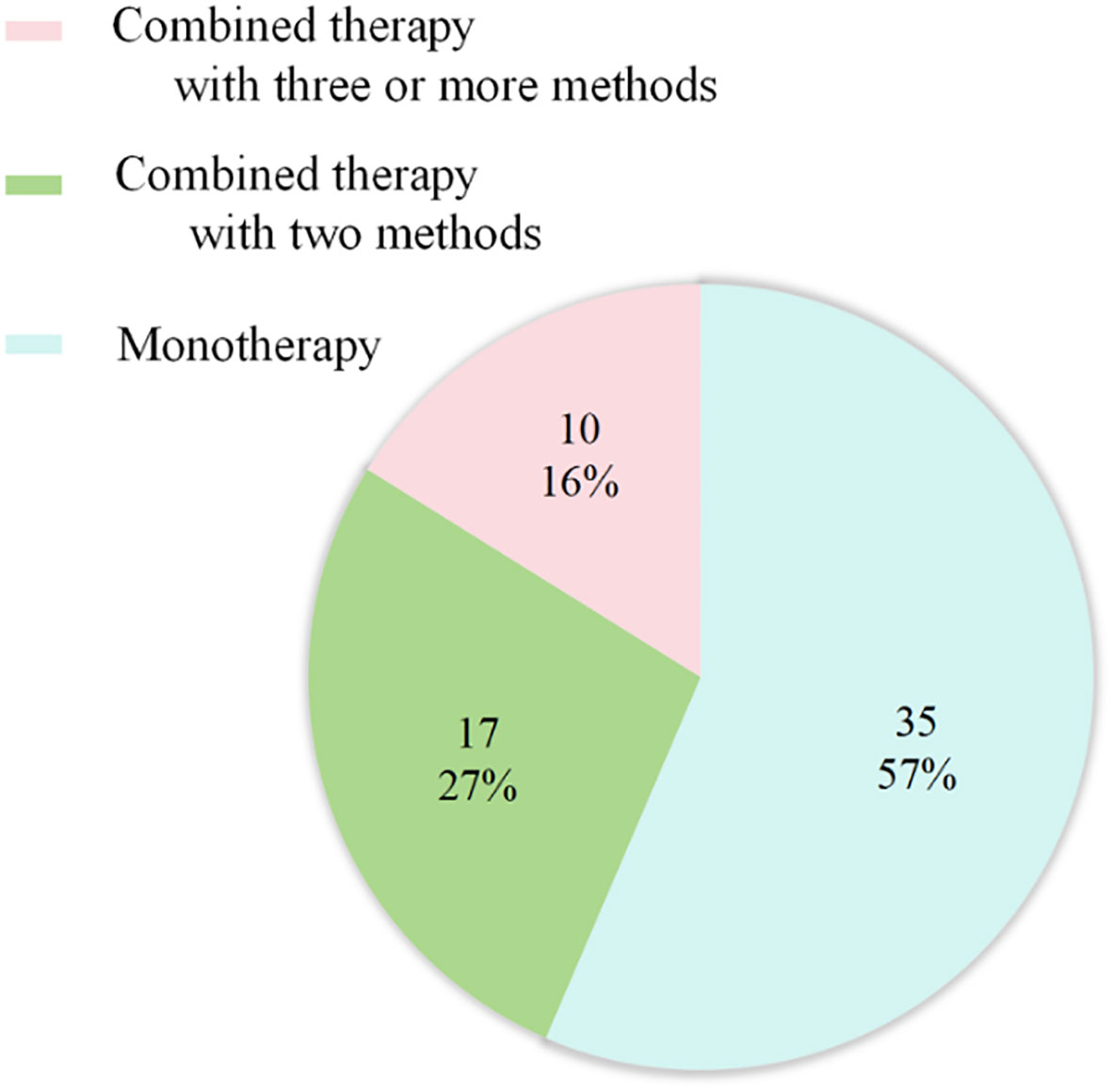
Figure 6 Treatment strategies for primary pulmonary NMC (n = 62). 35 cases received Monotherapy, of which 27 with chemotherapy; 17 cases treated with combined therapy with two methods, of which 11 with chemoradiotherapy. 10 cases treated with combined therapy with three or more methods, including 7 patients undergoing the combination of chemoradiotherapy with target therapy.
As for survival after diagnosis of pulmonary NMC, univariate Cox analysis revealed that cases with males exhibited poorer prognosis than females [unadjusted hazard ratio (HR) 1.97, 95% CI: 1.16-3.33, P = 0.012], while cases with pleural effusion (unadjusted HR 2.68, 95% CI: 1.36–5.31, P = 0.005) or tumor size more than 10 cm (unadjusted HR 3.31, 95% CI: 1.30 - 8.44, P = 0.012) demonstrated worse outcomes than cases without pleural effusion or tumor size between 2 cm to 5 cm. However, age, smoking history, various biomarkers, site of metastasis, and different treatment methods were not significantly associated with survival (Table 2).
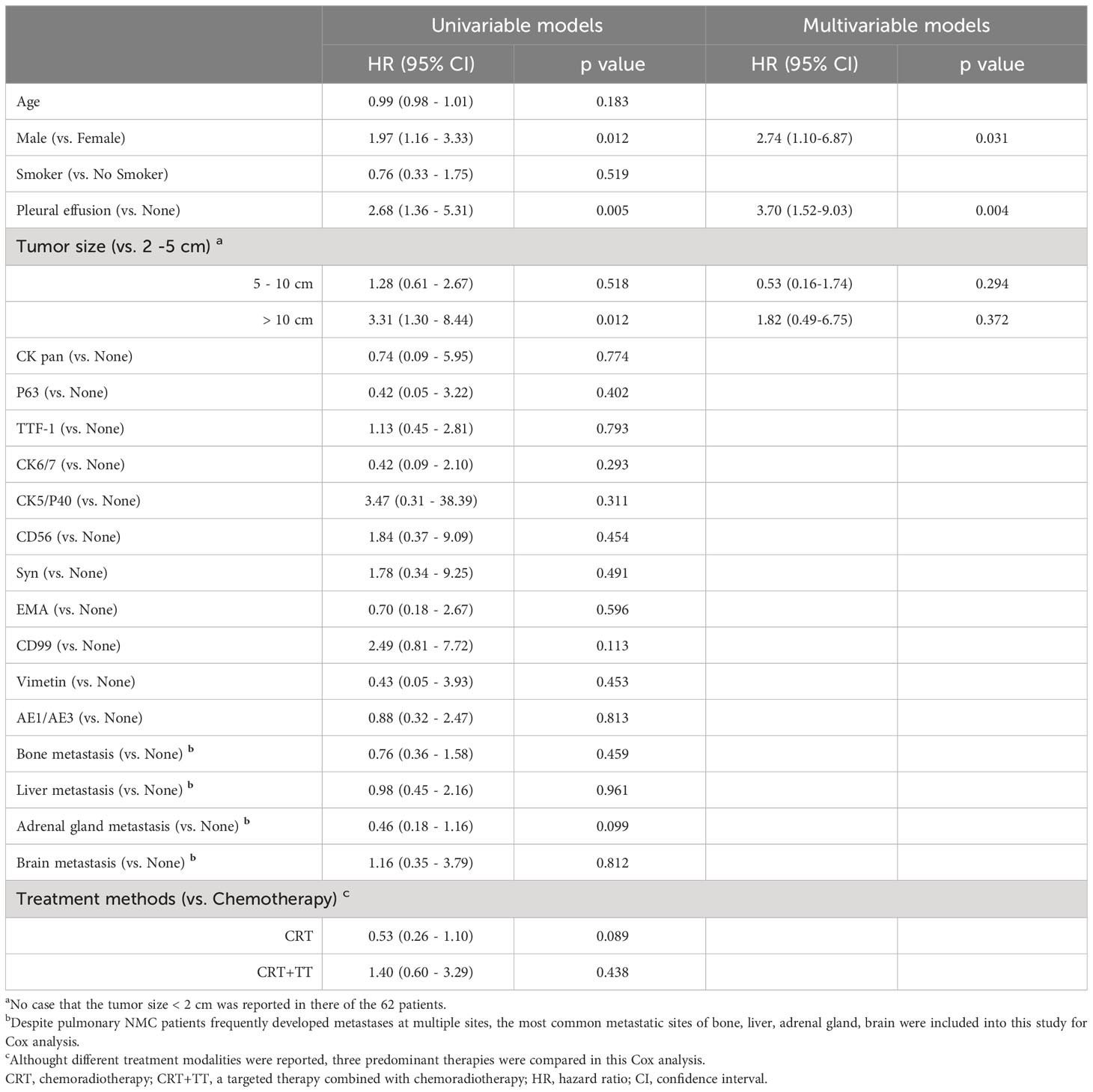
Table 2 Univariable and multivariable Cox models of characteristics that may affect survival to investigate risk factors for pulmonary NMC in 62 patients.
Kaplan-Meier analysis showed that gender (P = 0.007), tumor size (P = 0.011) and the presence of pleural effusion (P = 0.002) predicted a poor OS. While the different treatment methods (P = 0.078) exhibited not significantly associated with OS (Figure 7).
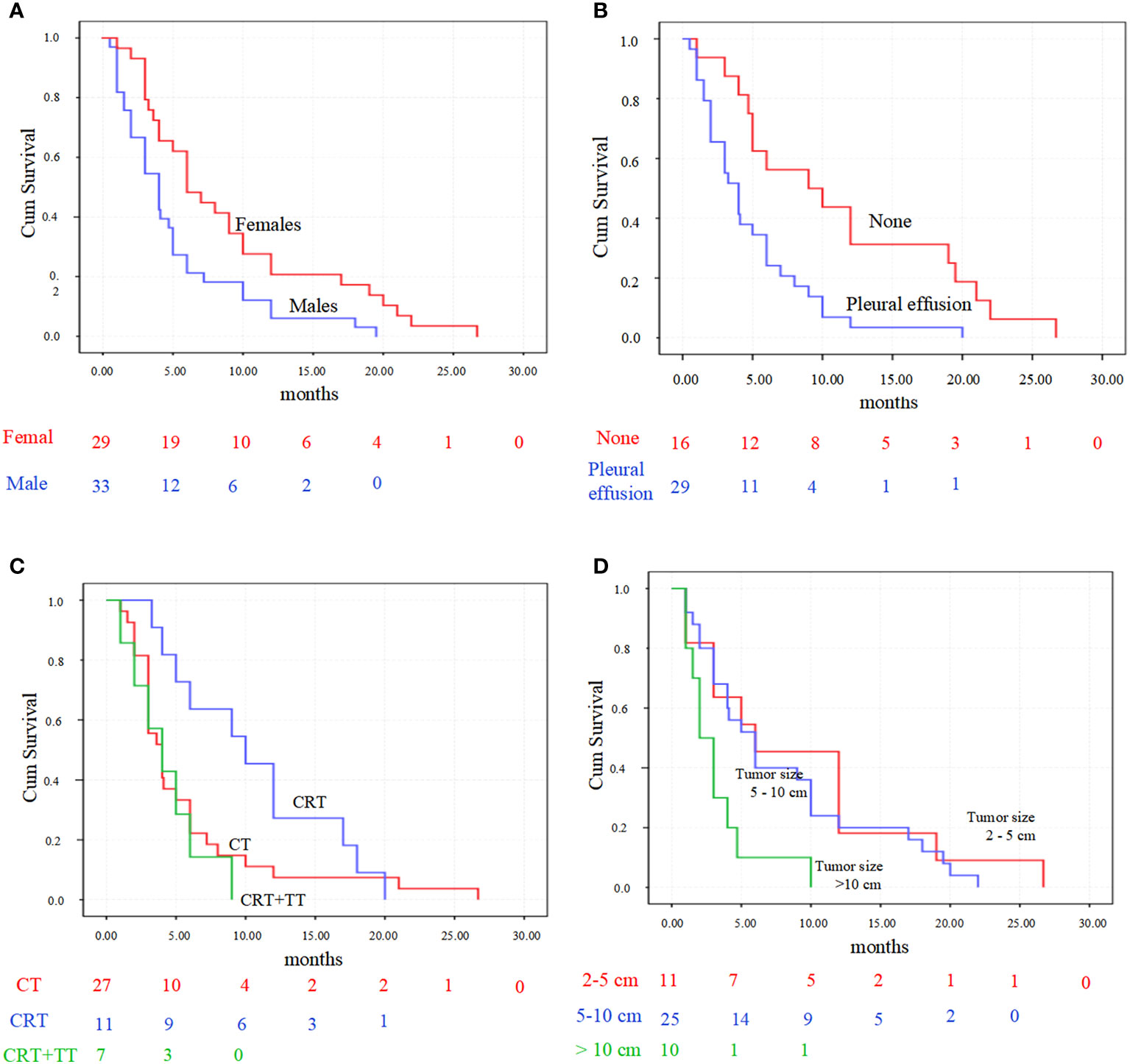
Figure 7 Kaplan-Meier survival analysis for primary pulmonary NMC patients. (A) patients’ overall survival accordingly with gender (P = 0.007). (B) survival was significantly longer in patient with pleural effusion than those without (P = 0.002). (C) overall survival comparison between the three predominant treatment strategies (P = 0.078). (D) overall survival comparison between the three tumor sizes (P = 0.011). CT, chemotherapy; CRT, chemoradiotherapy; CRT+TT, the combination of chemoradiotherapy with target therapy.
In multivariate Cox analysis, gender (HR 2.74, 95% CI 1.10 - 6.87, p = 0.031) and the presence of pleural effusion (HR 3.70, 95% CI 1.52 - 9.03, p = 0.004) remained significant predictors of survival after adjusting for other variables. Other factors were not significant.
Primary pulmonary NMC is an exceedingly rare disease, and its pathogenesis remains unclear. Current evidence suggests that rearrangement of the NUT carcinoma family member 1 (NUTM1) gene located on chromosome 15 q l4 may be the predominant causative factor in the development of NMC (37). Consequently, positive results for NUT IHC or molecular testing techniques (such as FISH, PCR, and second-generation sequencing) are considered diagnostic criteria for NMC (30, 35, 38, 39). In this study, we conducted a comprehensive collection and analysis of cases of pulmonary NMC, with the objective of enhancing our understanding of the epidemiological, pathological, and immunohistochemical features of this disease.
In terms of epidemiology, primary pulmonary NMC mainly affected children and young adults, with a median age of 29.5 years (range: 6-74 years), younger than in other reports (38, 40). Both genders are equally affected. The tumors could be located in any segment of the lung, with about 50% of nodules measuring between 5-10 cm during diagnosis, indicating the onset of the disease was occult. And symptoms of pulmonary NMC were often non-specific, with cough being the most common symptom. Other symptoms might include chest pain, dyspnea, chest or back pain and so on.
Given the non-specific histological characteristics of NMC (2, 8, 28, 40, 41), most cases expressed p63, p40, CK6/7, EMA, CD99, Vimentin, and AE1/AE3 cytokeratins. In addition to these markers, NMC might also express TTF-1, CD56, Syn, CD30, and CAM5.2, which often leaded to misdiagnosis. For squamous cell carcinoma of the lung, which is typically associated with older male patients and a history of smoking. Conversely, NMC should be considered in the case of a non-smoking or younger patient (38). Becase midline structures was the common site for the occurrence of NMC, all thymic carcinomas should be evaluated for the possibility of NMC, and IHC could be utilized to identify NUT-positive cases. In this research, we reported a 23-year-old female with pleural effusion, which is positive for the squamous differentiation marker P63. However, given the absence of non-keratinizing thymic carcinoma metastasis, the possibility of NMC was considered. When tumor cells in NMC express Syn, misdiagnosis as small cell lung carcinoma might occur (42). However, small cell carcinoma is common in elderly patients with elevated serum NSE levels and is positive for CD56 and TTF-1, but negative for NUT, P63, P40 (43). But additionally, there are also some specific situations that need to be pointed out. The spectrum of small round blue cell tumours (including leukaemias) can enter the differential diagnosis in a subset of NMC expressing CD34 and CD99 and/or lacking expression of p40 and keratin (2, 44–46). NMC may express CD99, leading to potential confusion with extraskeletal Ewing’s sarcoma (46, 47). However, this malignancy is extremely rare in the lungs and mediastinum, warranting further molecular testing to confirm diagnosis. FISH testing can distinguish NMC from extraskeletal myxoid chondrosarcoma by the absence of EWSR1 breakage in NUTM1 (28, 48–50). The coexpression of TTF-1 with focal or multifocal p63 is not uncommon in certain lung tumors that simultaneously express neuroendocrine markers (1, 51), warranting consideration for the possibility of NMC. NMC also needed consideration in the differential diagnosis of poorly differentiated squamous cell carcinoma, small cell carcinoma, and combined small cell and squamous cell carcinoma, particularly when there is abrupt transition from small cell tumour areas to foci of squamous differentiation (11, 52). Therefore, when encountering the aforementioned specific situations again, the possibility of NMC should be considered. Concurrently, employ IHC for NUT protein immunohistochemical staining, and, contingent upon the specific context, undertake FISH molecular testing to facilitate discrimination (17, 38, 52).
NMC has yet to establish a standardized treatment protocol. Surgical resection, chemotherapy, and radiation therapy are proposed as potential treatments for primary pulmonary NMC (10, 16). Surgical intervention may be the preferred method for solid tumors and could significantly improve survival outcomes for patients in the early stage (10, 29). However, in this study, distant metastases were present in over 73.81% of patients, and only three cases were identified without any metastases. Thus, most NMC patients are diagnosed after the optimal surgical window has passed, making chemotherapy the primary treatment option. Nonetheless, there was controversy regarding the efficacy of various treatment modalities in prolonging survival and required larger sample size data for practical efficacy (10). Presently, various treatments based on tumor characteristics have been reported, including immunotherapy, targeted therapy, and anti-angiogenic therapy, as well as the combination of chemotherapy, immunotherapy (44, 53), targeted therapy (18, 54), and anti-angiogenic therapy (2). Despite this, the therapeutic effectiveness of these approaches has mainly been based on case reports or small sample studies. Targeted therapies, exemplified by bromodomain and extra-terminal Motif (BET) inhibitors, have attracted considerable attention and are perceived to hold promising prospects for clinical treatment (17). As BET proteins are crucial for the functioning of every cell in the body, and BET inhibitors are generally unable to selectively target cell types, targeting not only BRD4 but also BRD2 or BRD3, becomes imperative to minimize potential side effects (55, 56). Achieving selective targeting of cells by BET inhibitors would significantly alleviate these side effects.
In terms of survival, primary pulmonary NMC had a shorter survival time than overall NMC survival. Further analysis of factors affecting patient survival revealed that male gender and the presence of pleural effusion were high-risk factors for shorter OS. Although there was no significant difference in gender incidence, the reason for shorter survival in males needs further investigation. At present, there exists a paucity of research elucidating the specific mechanisms contributing to the diminished survival prognosis of male patients afflicted with NMC (9, 52, 56, 57). Delving into the following aspects could provide insights into the diminished survival rates observed in male NMC patients: disparate responses post-treatment, variations in hormonal levels, immune system reactions, and tumor staging at the time of diagnosis. Over half of the patients experienced pleural effusion in this study. The presence of pleural effusion generally indicated that tumor metastasis to the pleura and suggested disease progression to stage IV, with a worse prognosis. However, considering that distant metastases to other sites had little impact on survival time, studying the possible mechanisms and interventions for pleural effusion metastasis might provide possible directions for treatment. The patient reported in this paper was treated with multiple methods, including anti-angiogenic drugs, which might have played a role in delaying disease progression. It should be noted that the treatment combined with multiple methods would be a promising direction for future research.
Some of the limitations of this study were the small sample size and the retrospective design. Given the rarity of pulmonary NMC, the 62 cases reported in this study were sourced from 29 articles spanning a 13-year period. Despite our meticulous efforts to extract information comprehensively and reflect patient baseline data exhaustively, it was imperative to acknowledge the inherent potential for heterogeneity, particularly in the realms of treatment modalities and diagnostic criteria. Therefore, strengthening animal experiments and clinical trials might provide direction for future treatments.
While NMC could be diagnosed through positive NUT immunohistochemical staining or FISH testing for NUT gene mutations, the disease was typically diagnosed at an advanced stage, leading to reduced survival. Therefore, increasing awareness of this disease might help clinicians and pathologists to make an early diagnosis, potentially extending patients’ survival. As genetic mutations play a key role in the pathophysiology of the disease, intensifying efforts in the foundational and clinical evaluation of targeted therapies -exemplified by BET inhibitors- and a range of combinatorial treatments, would offer substantial potential in furnishing a robust theoretical foundation. This, in turn, could significantly contribute to the development of standardized therapeutic protocols. The current study highlights the negative impact of male gender and pleural effusion on the prognosis of pulmonary NMC. Additional investigation of these factors might provide new insights for disease management and treatment.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
DZ: Data curation, Formal analysis, Funding acquisition, Methodology, Software, Writing – original draft. WC: Conceptualization, Formal analysis, Investigation, Methodology, Writing – review & editing. SZ: Conceptualization, Investigation, Supervision, Writing – review & editing. YW: Data curation, Investigation, Methodology, Software, Writing – review & editing. ZP: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. JZ: Data curation, Formal analysis, Investigation, Supervision, Visualization, Writing – review & editing. KH: Formal analysis, Funding acquisition, Methodology, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Hubei Province, China (Grant No. 2023AFB055), the Fundamental Research Funds for the Central Universities (2042022kf1116), the Open Project of Hubei Key Laboratory (2023KFZZ030) and National Natural Science Foundation of China (82270101).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1308432/full#supplementary-material
1. Sholl LM, Nishino M, Pokharel S, Mino-Kenudson M, French CA, Janne PA, et al. Primary pulmonary NUT midline carcinoma: clinical, radiographic, and pathologic characterizations. J Thorac Oncol (2015) 10(6):951–9. doi: 10.1097/JTO.0000000000000545
2. Li X, Shi H, Zhang W, Bai C, He M, Ta N, et al. Immunotherapy and targeting the tumor microenvironment: current place and new insights in primary pulmonary NUT carcinoma. Front Oncol (2021) 30:690115. doi: 10.3389/fonc.2021.690115
3. Salati M, Baldessari C, Bonetti LR, Messina C, Merz V, Cerbelli B, et al. NUT midline carcinoma: Current concepts and future perspectives of a novel tumour entity. Crit Rev Oncol Hematol (2019) 144:102826. doi: 10.1016/j.critrevonc.2019.102826
4. Lauer UM, Hinterleitner M, Horger M, Ohnesorge PV, Zender L. NUT carcinoma-an underdiagnosed malignancy. Front Oncol (2022) 26:914031. doi: 10.3389/fonc.2022.914031
5. Patil VM, Toshniwal A, Noronha V, Joshi A, Menon N, Banavali S, et al. NUT midline carcinoma. Clin Oncol (R Coll Radiol) (2019) 31(10):732. doi: 10.1016/j.clon.2019.06.012
6. Fekkar A, Emprou C, Lefebvre C, Ferretti G, Stephanov O, Pissaloux D, et al. Thoracic NUT carcinoma: Common pathological features despite diversity of clinical presentations. Lung Cancer (2021) 158:55–9. doi: 10.1016/j.lungcan.2021.06.008
7. Benito Bernáldez C, Romero Muñoz C, Almadana Pacheco V. NUT midline carcinoma of the lung, a rare form of lung cancer. Arch Bronconeumol (2016) 52(12):619–21. doi: 10.1016/j.arbres.2016.04.011
8. Zhang Y, Han K, Dong X, Hou Q, Li T, Li L, et al. Case report and literature review: primary pulmonary NUT-midline carcinoma. Front Oncol (2021) 30:700781. doi: 10.3389/fonc.2021.700781
9. Taniyama TK, Nokihara H, Tsuta K, Horinouchi H, Kanda S, Fujiwara Y, et al. Clinicopathological features in young patients treated for small-cell lung cancer: significance of immunohistological and molecular analyses. Clin Lung Cancer (2014) 15(3):244–7. doi: 10.1016/j.cllc.2013.06.012
10. Lemelle L, Pierron G, Fréneaux P, Huybrechts S, Spiegel A, Plantaz D, et al. NUT carcinoma in children and adults: A multicenter retrospective study. Pediatr Blood Cancer (2017) 64(12). doi: 10.1002/pbc.26693
11. Karakuş E, Poyraz A, Oğuz Erdogan AS, Emir S, Özyörük D. NUT midline carcinoma of the lung in a six-year-old child. Fetal Pediatr Pathol (2017) 36(6):472–4. doi: 10.1080/15513815.2017.1392662
12. Al Diffalha S, Al Aukla N, Hasan S, Dickinson S, Khalil F. NUT midline carcinoma: A rare Malignancy. Cancer Control (2017) 24(2):202–6. doi: 10.1177/107327481702400214
13. Tanaka M, Kato K, Gomi K, Yoshida M, Niwa T, Aida N, et al. NUT midline carcinoma: report of 2 cases suggestive of pulmonary origin. Am J Surg Pathol (2012) 36(3):381–8. doi: 10.1097/PAS.0b013e31824230a8
14. Ueki H, Maeda N, Sekimizu M, Yamashita Y, Moritani S, Horibe K. A case of NUT midline carcinoma with complete response to gemcitabine following cisplatin and docetaxel. J Pediatr Hematol Oncol (2014) 36(8):e476–80. doi: 10.1097/MPH.0000000000000082
15. Nelson BA, Lee EY, French CA, Bauer DE, Vargas SO. BRD4-NUT carcinoma of the mediastinum in a pediatric patient: multidetector computed tomography imaging findings. J Thorac Imaging (2010) 25(3):W93–6. doi: 10.1097/RTI.0b013e3181b5d84d
16. Xie XH, Wang LQ, Qin YY, Lin XQ, Xie ZH, Liu M, et al. Clinical features, treatment, and survival outcome of primary pulmonary NUT midline carcinoma. Orphanet J Rare Dis (2020) 15(1):183. doi: 10.1186/s13023-020-01449-x
17. Stathis A, Zucca E, Bekradda M, Gomez-Roca C, Delord JP, de la Motte Rouge T, et al. Clinical response of carcinomas harboring the BRD4-NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discovery (2016) 6(5):492–500. doi: 10.1158/2159-8290.CD-15-1335
18. Virarkar M, Mallery M, Saleh M, Ramani NS, Morani AC, Bhosale P. Clinical, radiographic, pathologic characterization and survival outcomes of nuclear protein of the testis carcinoma. J Comput Assist Tomogr (2021) 45(3):431–41. doi: 10.1097/RCT.0000000000001163
19. Zhou L, Yong X, Zhou J, Xu J, Wang C. Clinicopathological analysis of five cases of NUT midline carcinoma, including one with the gingiva. BioMed Res Int (2020) 13:9791208. doi: 10.1155/2020/9791208
20. Lee JK, Louzada S, An Y, Kim SY, Kim S, Youk J, et al. Complex chromosomal rearrangements by single catastrophic pathogenesis in NUT midline carcinoma. Ann Oncol (2017) 28(4):890–7. doi: 10.1093/annonc/mdw686
21. Policarpio-Nicolas ML, de Leon EM, Jagirdar J. Cytologic findings of NUT midline carcinoma in the hilum of the lung. Diagn Cytopathol (2015) 43(9):739–42. doi: 10.1002/dc.23291
22. Kuroda S, Suzuki S, Kurita A, Muraki M, Aoshima Y, Tanioka F, et al. Cytological features of a variant NUT midline carcinoma of the lung harboring the NSD3-NUT fusion gene: A case report and literature review. Case Rep Pathol (2015) 2015:572951. doi: 10.1155/2015/572951
23. Shenoy KD, Stanzione N, Caron JE, Fishbein GA, Abtin F, Lluri G, et al. Midline carcinoma expressing NUT in Malignant effusion cytology. Diagn Cytopathol (2019) 47(6):594–8. doi: 10.1002/dc.24150
24. Engleson J, Soller M, Panagopoulos I, Dahlén A, Dictor M, Jerkeman M. Midline carcinoma with t(15;19) and BRD4-NUT fusion oncogene in a 30-year-old female with response to docetaxel and radiotherapy. BMC Cancer (2006) 16:6:69. doi: 10.1186/1471-2407-6-69
25. Suzuki S, Kurabe N, Ohnishi I, Yasuda K, Aoshima Y, Naito M, et al. NSD3-NUT-expressing midline carcinoma of the lung: first characterization of primary cancer tissue. Pathol Res Pract (2015) 211(5):404–8. doi: 10.1016/j.prp.2014.10.013
26. Shatavi S, Fawole A, Haberichter K, Jaiyesimi I, French C. Nuclear protein in testis (NUT) midline carcinoma with a novel three-way translocation (4;15;19)(q13;q14;p13.1). Pathology (2016) 48(6):620–3. doi: 10.1016/j.pathol.2016.06.010
27. Puliyel MM, Mascarenhas L, Zhou S, Sapra A, Dal Cin P, French CA, et al. Nuclear protein in testis midline carcinoma misdiagnosed as adamantinoma. J Clin Oncol (2014) 32(15):e57–60. doi: 10.1200/JCO.2012.48.5300
28. Raza A, Cao H, Conrad R, Cobb C, Castelino-Prabhu S, Mirshahidi S, et al. Nuclear protein in testis midline carcinoma with unusual elevation of α-fetoprotein and synaptophysin positivity: a case report and review of the literature. Expert Rev Anticancer Ther (2015) 15(10):1199–213. doi: 10.1586/14737140.2015.1082909
29. Zhao R, Hua Z, Hu X, Zhang Q, Zhang J, Wang J. NUT carcinoma of the lung:A case report and literature analysis. Front Oncol (2022) 12:890338. doi: 10.3389/fonc.2022.890338
30. Jiang J, Ren Y, Xu C, Lin X. NUT midline carcinoma as a primary lung tumor treated with anlotinib combined with palliative radiotherapy: a case report. Diagn Pathol (2022) 17(1):4. doi: 10.1186/s13000-021-01188-y
31. Cao J, Chen D, Yang F, Yao J, Zhu W, Zhao C. NUT midline carcinoma as a primary lung tumor: a case report. J Thorac Dis (2017) 9(12):E1045–9. doi: 10.21037/jtd.2017.11.50
32. Teo M, Crotty P, O'Sullivan M, French CA, Walshe JM. NUT midline carcinoma in a young woman. J Clin Oncol (2011) 29(12):e336–9. doi: 10.1200/JCO.2010.32.7486
33. Ma Y, Feng J, Xiao X, Chen L. NUT midline carcinoma in the mediastinum in a ten-year-old boy. Arch Bronconeumol (Engl Ed) (2018) 54(10):539–41. doi: 10.1016/j.arbres.2017.09.010
34. Chang AI, Kim TS, Han J, Kim TJ, Choi JY. NUT midline carcinoma of the lung: computed tomography findings in 10 patients. J Comput Assist Tomogr (2021) 45(2):330–6. doi: 10.1097/RCT.0000000000001133
35. Zhang H, Kong W, Liang W. NUT midline carcinoma: A rare solid tumour characterized by chromosome rearrangement. Evid Based Complement Alternat Med (2022) 2022:3369895. doi: 10.1155/2022/3369895
36. Parikh SA, French CA, Costello BA, Marks RS, Dronca RS, Nerby CL, et al. NUT midline carcinoma: an aggressive intrathoracic neoplasm. J Thorac Oncol (2013) 8(10):1335–8. doi: 10.1097/JTO.0b013e3182a00f41
37. Chen J, Li M, Lu H. Nuclear protein in testis carcinoma of the lung. Transl Oncol (2023) 30:101640. doi: 10.1016/j.tranon.2023.101640
38. Xie M, Fu X, Wang W. Clinicopathological and molecular characterizations of pulmonary NUT midline carcinoma. Cancer Med (2021) 10(17):5757–64. doi: 10.1002/cam4.4096
39. Xu B, Chen JF, Sarungbam J, Tickoo S, Dickson BC, Reuter VE, et al. NUTM1-fusion positive Malignant neoplasms of the genitourinary tract: A report of six cases highlighting involvement of unusual anatomic locations and histologic heterogeneity. Genes Chromosomes Cancer (2022) 61(9):542–50. doi: 10.1002/gcc.23046
40. Yuan J, Xu Z, Guo Y. Diagnosis, treatment and prognosis of primary pulmonary NUT carcinoma: A literature review. Curr Oncol (2022) 29(10):6807–15. doi: 10.3390/curroncol29100536
41. Agaimy A, Fonseca I, Martins C, Thway K, Barrette R, Harrington KJ, et al. NUT carcinoma of the salivary glands: clinicopathologic and molecular analysis of 3 cases and a survey of NUT expression in salivary gland carcinomas. Am J Surg Pathol (2018) 42(7):877–84. doi: 10.1097/PAS.0000000000001046
42. Wu Z, Ma JY, Yang JJ, Zhao YF, Zhang SF. Primary small cell carcinoma of esophagus: report of 9 cases and review of literature. World J Gastroenterol (2004) 10(24):3680–2. doi: 10.3748/wjg.v10.i24.3680
43. Xinli W, Lixiao W, Baoqi D, Hu H, Qiang Z. Expression and clinicopathological significance of SOX11 in small-cell lung cancer. BioMed Res Int (2022) 2022:1707914. doi: 10.1155/2022/1707914
44. Giridhar P, Mallick S, Kashyap L, Rath GK. Patterns of care and impact of prognostic factors in the outcome of NUT midline carcinoma: a systematic review and individual patient data analysis of 119 cases. Eur Arch Otorhinolaryngol (2018) 275(3):815–21. doi: 10.1007/s00405-018-4882-y
45. Li W, Chastain K. NUT midline carcinoma with leukemic presentation mimicking CD34-positive acute leukemia. Blood (2018) 132(4):456. doi: 10.1182/blood-2017-07-796268
46. Wu Y, Mao A, Wang J. Primary Ewing's sarcoma (ES)/peripheral primitive neuroectodermal tumor (pPNET) of the tongue in a child: A case report. Oral Oncol (2022) 128:105818. doi: 10.1016/j.oraloncology.2022.105818
47. Wang GY, Thomas DG, Davis JL, Ng T, Patel RM, Harms PW, et al. EWSR1-NFATC2 translocation-associated sarcoma clinicopathologic findings in a rare aggressive primary bone or soft tissue tumor. Am J Surg Pathol (2019) 43(8):1112–22. doi: 10.1097/PAS.0000000000001260
48. Clanchy FIL. Rationale for early detection of EWSR1 translocation-associated sarcoma biomarkers in liquid biopsy. Cancers (Basel) (2021) 13(4):824. doi: 10.3390/cancers13040824
49. Todorovic E, Truong T, Eskander A, Lin V, Swanson D, Dickson BC, et al. Middle ear and temporal bone nonkeratinizing squamous cell carcinomas with DEK-AFF2 fusion: an emerging entity. Am J Surg Pathol (2020) 44(9):1244–50. doi: 10.1097/PAS.0000000000001498
50. Maffini F, French CA, Cameron MJ, Stufano V, Barberis M, Pisa E, et al. A case of NUT midline carcinoma with no HPV infection, slight EWSR1 rearrangement and strong expression of EGFR. Tumori (2013) 99(4):e152–5. doi: 10.1177/030089161309900423
51. Bishop JA, French CA, Ali SZ. Cytopathologic features of NUT midline carcinoma: A series of 26 specimens from 13 patients. Cancer Cytopathol (2016) 124(12):901–8. doi: 10.1002/cncy.21761
52. Farooq A, Kerper AL, Boland JM, Lo YC. Nuclear protein in testis (NUT) carcinoma: A comprehensive immunohistochemical analysis of 57 cases with consideration of interpretation and pitfall recognition. Arch Pathol Lab Med (2023). doi: 10.5858/arpa.2023-0178-OA
53. He M, Chernock R, Zhou S, Gondim M, Dehner LP, Pfeifer JD. Tumor mutation burden and checkpoint immunotherapy markers in NUT midline carcinoma. Appl Immunohistochem Mol Morphol (2020) 28(7):495–500. doi: 10.1097/PAI.0000000000000781
54. Liu Y, Li YY, Ke XX, Lu Y. The primary pulmonary NUT carcinomas and some uncommon somatic mutations identified by next-generation sequencing: a case report. AME Case Rep (2020) 30:4:24. doi: 10.21037/acr-19-168
55. Stathis A, Bertoni F. BET proteins as targets for anticancer treatment. Cancer Discovery (2018) 8(1):24–36. doi: 10.1158/2159-8290.CD-17-0605
56. Chau NG, Ma C, Danga K, Al-Sayegh H, Nardi V, Barrette R, et al. An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr (2019) 4(2):pkz094. doi: 10.1093/jncics/pkz094
57. Cousin S, Blay JY, Garcia IB, de Bono JS, Le Tourneau C, Moreno V, et al. Safety, pharmacokinetic, pharmacodynamic and clinical activity of molibresib for the treatment of nuclear protein in testis carcinoma and other cancers: Results of a Phase I/II open-label, dose escalation study. Int J Cancer (2022) 150(6):993–1006. doi: 10.1002/ijc.33861
Keywords: pulmonary, NUT midline carcinoma (NMC), survival, pleural effusion, nuclear protein of the testis
Citation: Zhao D, Cao W, Zha S, Wang Y, Pan Z, Zhang J and Hu K (2024) Primary pulmonary nuclear protein of the testis midline carcinoma: case report and systematic review with pooled analysis. Front. Oncol. 13:1308432. doi: 10.3389/fonc.2023.1308432
Received: 06 October 2023; Accepted: 21 December 2023;
Published: 09 January 2024.
Edited by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesReviewed by:
Hazem I. Assi, American University of Beirut, LebanonCopyright © 2024 Zhao, Cao, Zha, Wang, Pan, Zhang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke Hu, aHVrZS1ybWhvc3BpdGFsQDE2My5jb20=
†These authors have contributed equally to this work
‡ORCID: Ke Hu, orcid.org/0000-0001-9862-7239
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.