
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 23 January 2024
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1307716
 Xinzhen Xu1†
Xinzhen Xu1† Xiang Zhang1†
Xiang Zhang1† Xin Li2
Xin Li2 Ao Yu1
Ao Yu1 Xiqiang Zhang1
Xiqiang Zhang1 Shuohui Dong1
Shuohui Dong1 Zitian Liu1
Zitian Liu1 Zhiqiang Cheng1
Zhiqiang Cheng1 Kexin Wang1*
Kexin Wang1*Background: Placement of an indwelling transanal drainage tube (TDT) to prevent anastomotic leakage (AL) after anterior rectal cancer surgery has become a routine choice for surgeons in the recent years. However, the specific indwelling time of the TDT has not been explored. We performed this meta-analysis and considered the indwelling time a critical factor in re-analyzing the effectiveness of TDT placement in prevention of AL after anterior rectal cancer surgery.
Methods: Randomized controlled trials (RCTs) and cohort studies which evaluated the effectiveness of TDT in prevention of AL after rectal cancer surgery and considered the indwelling time of TDT were identified using a predesigned search strategy in databases up to November 2022. This meta-analysis was performed to estimate the pooled AL rates (Overall and different AL grades) and reoperation rates at different TDT indwelling times and stoma statuses.
Results: Three RCTs and 15 cohort studies including 2381 cases with TDT and 2494 cases without TDT were considered eligible for inclusion. Our meta-analysis showed that the indwelling time of TDT for ≥5-days was associated with a significant reduction (TDT vs. Non-TDT) in overall AL (OR=0.46,95% CI 0.34-0.60, p<0.01), grade A+B AL (OR=0.64, 95% CI 0.42-0.97, p=0.03), grade C AL (OR=0.35, 95% CI 0.24-0.53, p<0.01), overall reoperation rate (OR=0.36, 95%CI 0.24-0.53, p<0.01) and that in patients without a prophylactic diverting stoma (DS) (OR=0.24, 95%CI 0.14-0.41, p<0.01). There were no statistically significant differences in any of the abovementioned indicators (p>0.05) when the indwelling time of TDT was less than 5 days.
Conclusion: Extending the postoperative indwelling time of TDT to 5 days may reduce the overall AL and the need for reoperation in patients without a prophylactic DS.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023407451, identifier CRD42023407451.
Anastomotic leakage (AL) is a major complication of anterior rectal cancer surgery, and its incidence has been reported to range between 1- 30% (1, 2). The occurrence of AL could result in significantly more severe postoperative complications, higher rates of reoperations, increased hospital stay, and higher mortality. It can even affect the patients’ prognosis (3). The endoluminal pressure at the anastomotic site may be associated with AL (4). Placing a transanal drainage tube (TDT) can reduce the intraluminal pressure and help drain feces and gas at the anastomosis site (5, 6). This is likely to provide an ideal regional environment for anastomosis healing, thus reducing the incidence of AL. However, there is a controversy regarding the effectiveness of TDT placement for prevention of AL after anterior resection of rectal cancer (5, 7–13). Tamura et al. (10) analyzed 157 patients who underwent laparoscopic anterior resection with and without postoperative TDT, and reported that the AL rate was not statistically significant between the two groups (7.6% vs 10.3%, TDT vs. non-TDT). Zhao et al. (12) conducted a randomized clinical trial to assess the effect of TDTs in AL prevention after laparoscopic low anterior resection for rectal cancer in 576 patients and concluded that TDTs may not confer any benefit for AL prevention in patients who undergo laparoscopic low anterior resection for mid-low rectal cancer without preoperative radiotherapy. However, Kawada et al. (14) found that TDT significantly reduced the AL rate from 26.1% to 10.7% in laparoscopic low anterior resection in a retrospective study. The results of majority of the meta-analyses support the effectiveness of postoperative TDT placement in reducing the occurrence of AL (15–17).
Some studies (18, 19) have shown that the average time to confirm AL is approximately 7 postoperative days. This is because the anastomotic strength rapidly decreases in the early postoperative days until the fibroblasts and smooth muscle cells can synthesize large amounts of new collagen (20). However, we found that few studies contemplated the indwelling time of postoperative TDT placement, and reported that TDTs that were considered ineffective were often removed too early. For example, in the study by Zhao et al. (12) the indwelling time of TDT was only 4.2 days after surgery. In the studies of Chaline et al. (5) and Lee et al. (9), this time was only 4 and 3 postoperative days, respectively.
Hence, we performed a systematic review and meta-analysis of TDT and considered indwelling time as a critical factor to reanalyze the effectiveness of TDT placement in the prevention of AL after anterior rectal cancer surgery.
The meta-analysis was conducted in accordance with the PRISMA 2020 (21) and AMSTAR 2 (22) guidelines. A comprehensive literature search of PubMed, EMBASE, Cochrane Library, Clinicaltrials.gov, China Biology Medicine disc (CBMdisc), and China National Knowledge Infrastructure Whole Article Database (CNKI) databases were performed from inception to November 29, 2022 to determine the effect of all studies that compared the indwelling time of TDT on postoperative AL. All original research studies that compared the effectiveness of postoperative TDT placement and considered the indwelling time of TDT were included. The subject terms or keywords of the literature search were: “rectal cancer\tumor\neoplasm”, “anastomotic leaks\leakage” and “transanal drainage tube\catheter”. Original studies included in the relevant meta-analysis were also screened to identify other eligible studies.
The inclusion criteria were: (1) original research studies that included patients with rectal cancer who underwent laparoscopic anterior resection; (2) randomized controlled trials (RCTs) or cohort studies, regardless of their publication status and languages; (3) studies that included patients with postoperative indwelling TDT; (4) studies which assessed the associations of TDT with AL and reoperation. Patients could be of any age, sex, country, and race. Moreover, patients who received preoperative neoadjuvant therapy and diverting stoma (DS) were also included.
The exclusion criteria were: (1) case-control studies, case reports, reviews, conference abstracts, and dissertations; (2) studies with duplicate data; (3) studies with insufficient data; (4) studies related to TDT used as the treatment for AL; and (5) studies related to anal balloon or anal stent.
The title, abstract, and full text of the studies retrieved from the literature search were screened independently by two researchers. Any discrepancies were resolved through discussion with a senior professor. Two authors performed data extraction and collation separately, and any differences were resolved through dialogues until a consensus was reached or a third author was consulted. All extracted data were collated in an Excel spreadsheet. Data collected from the retrieved studies included publication journal, impact factor, first author’s name, whether TDT was effective based on the conclusion reached in the included study, publication year, study type, country, sample size, patient characteristics (age, sex, body mass index), DS, neoadjuvant therapy (radiochemotherapy or chemotherapy), AL and reoperation sample size, AL and reoperation rates, tube type, tube diameter, tube placement, tube position, decision to remove the TDT, the indwelling time of TDT, procedure type, stapling technique, tumor location, anastomosis location, and AL grading sample size (grade A+B, grade C). AL severity was graded according to the International Rectal Cancer Study Group (23), however, the sample of A- and B-grade AL were combined since grade A AL was rarely reported.
All data preprocessing and analyses were conducted using R statistical software (R Foundation for Statistical Computing, Vienna, Austria). Publication bias was assessed by visual inspection of the funnel plot generated by the R software. Odds ratio (ORs) and 95% confidence intervals (CIs) were calculated for all dichotomous variables (Mantel–Haenszel statistical method). I2 was used to assess the heterogeneity of the resulting evidence. If there was no evidence of heterogeneity, a fixed-effects model was used. Otherwise, a random-effects model was used. Since the heterogeneity among cohort studies was expected to be high due to their diversity, the random-effects meta-analysis approach was the default choice. Sensitivity analysis was performed if there were studies that significantly impacted the study heterogeneity. p-value for overall effect was calculated, and significance was set at p < 0.05.
The overall AL rate was the key metric. On this basis, subgroup analyses were performed, considering the indwelling time of TDT (≥ five days or <5 days). Time reported in the studies was used for time statistics if the specific indwelling time was reported. If time periods were reported, the median value was used. Based on the above subgroups, we focused on two additional indicators of DS and AL grading (A+B and C grades) to investigate the relationship between the indwelling time of TDT and both the abovementioned parameters.
A total of 274 relevant studies were identified in the initial search. Five additional studies were identified from the reference list of TDT-related meta-analysis (7–9, 14, 24). A total of 36 studies were subjected to full-text review. Eighteen publications were excluded: two were abstracts only, one was accompanied by colon cancer, two were single-arm trials, and 13 had insufficient clinical trials data. Therefore, a total of 18 studies met the inclusion criteria (Figure 1).
Of all the included studies, three were RCTs (Figure 2) (10, 12, 25) and 15 were observational studies (prospective or retrospective studies) (4–9, 11, 13, 14, 24, 26–30). A total of 4805 patients were included in this meta-analysis, 2381 with TDT and 2494 without TDT. All studies reported the occurrence of AL; however, two RCTs (10, 12) did not report reoperation. The indwelling time of the TDT ranged between 3-7 days. A total of 14 studies (4, 6–8, 10, 11, 13, 14, 25–30) reported that the TDTs’ indwelling time was ≥5 days, suggesting that postoperative day 5 might be a critical time. Of the 18 included studies, only 6 reported on the time to confirm postoperative AL, and of these, 5 reported on the time to postoperative AL of 5 days or more - 5.8 d (27), 6.5 d (11), 6.8 d (24), and 10.1 d (13), respectively - and one of these reported on the division of AL into an early leakage group (POD ≤ 5, n = 9) and a late leakage group (POD ≥ 6, n = 16) (14). Therefore, we chose postoperative day 5 as the cutting point. As for the funded status of the included studies, three (11, 25, 27) of the included studies reported having scientific funding, the rest were non-profit, and none of the studies received funding from healthcare companies. The funded status of all included studies were collected in Table 1B. The essential characteristics of all the studies are summarized in Tables 1–3.
The effectiveness of TDT placement after anterior resection of rectal cancer is controversial (5, 7–13). However, most of the included studies (4, 6, 14, 24–30) confirmed that postoperative TDT placement was effective in reducing the occurrence of AL and reoperation (Table 4, Figure 3).
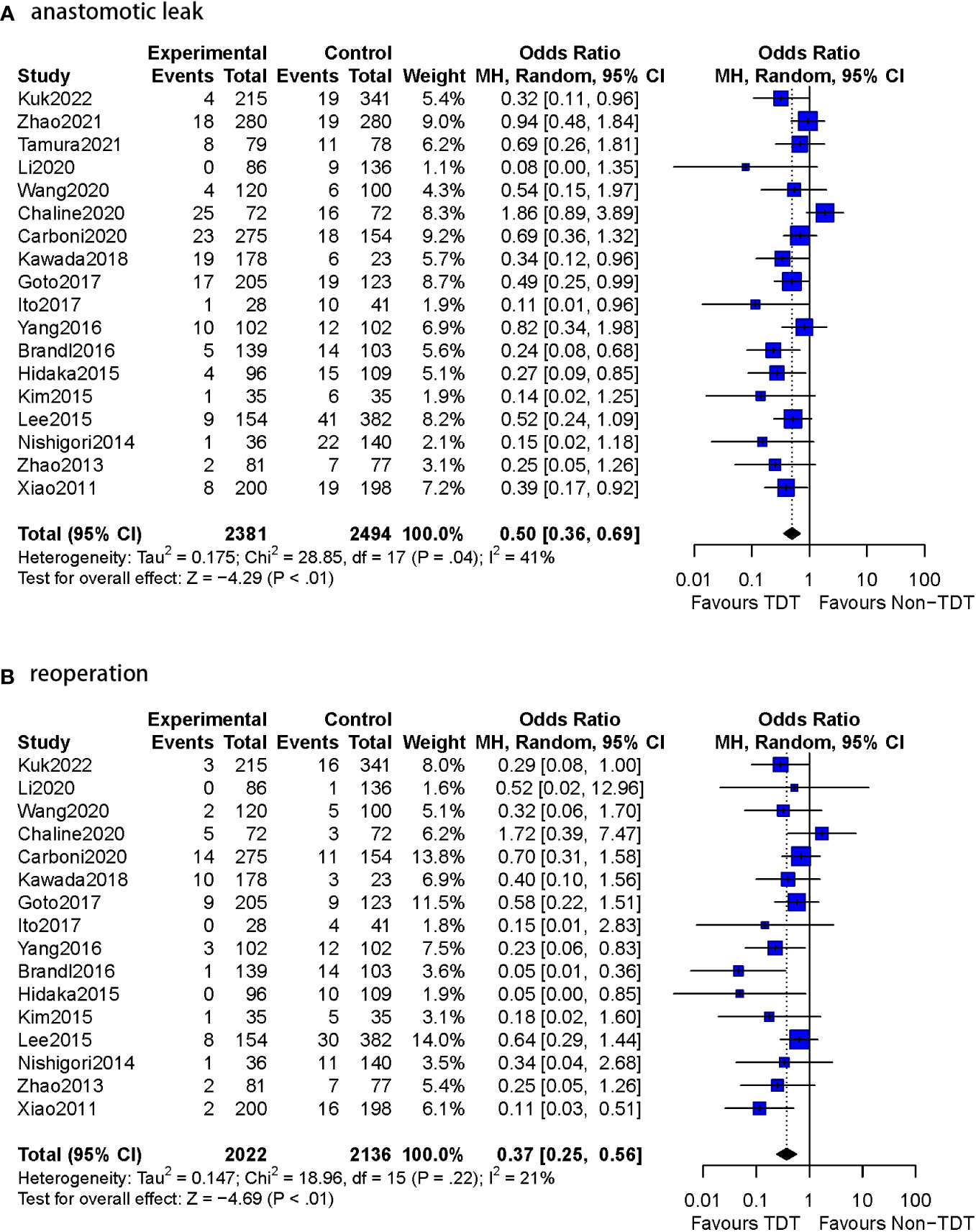
Figure 3 Meta-analysis results for whole studies of AL and reoperation. (A): anastomotic leak. (B): reoperation.
1. TDT indwelling time ≥ 5 days group (14 studies)
1.1 AL rate
The postoperative AL rate was used as an outcome indicator in all 14 studies (n=3393 patients). A total of 5.9% (102/1736) patients with TDT were reported to have AL compared to 10.8% (179/1657) in the non-TDT group (OR=0.46, 95%CI 0.34-0.60, p<0.01). No heterogeneity was observed in this analysis (I2 = 0%, p = 0.55) (Figure 4).
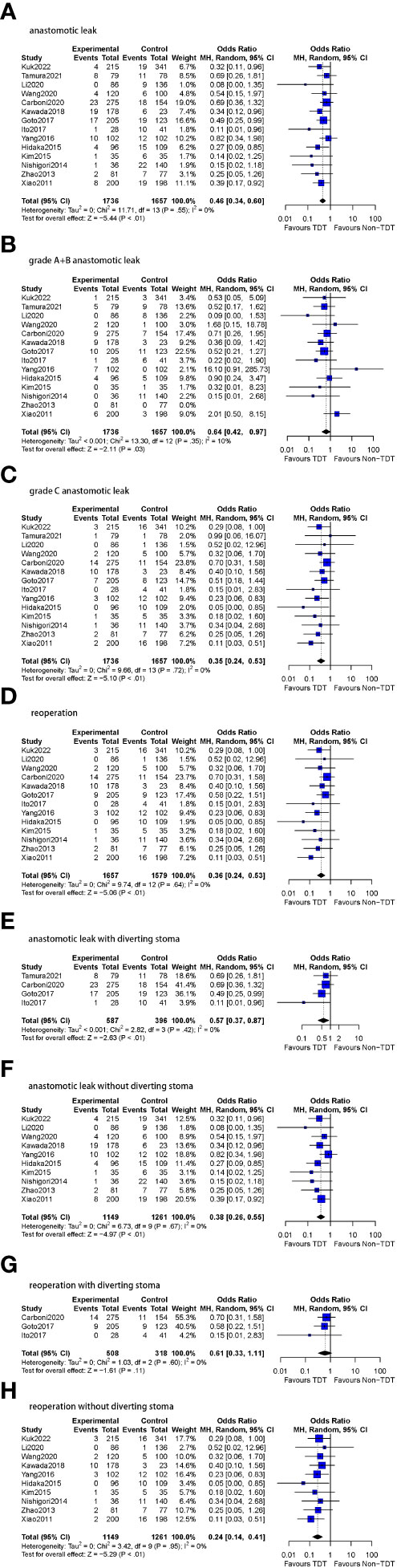
Figure 4 Meta-analysis results for TDT indwelling time ≥5 days subgroup. (A): anastomotic leak. (B): grade A+B anastomotic leak. (C): grade C anastomotic leak. (D): reoperation. (E): anastomotic leak with diverting stoma. (F): anastomotic leak without diverting stoma. (G): reoperation with diverting stoma. (H): reoperation without diverting stoma.
1.1.1 AL rate (grade A+B)
All 14 studies included in this subgroup reported A and B-level AL (n=3393 patients). A total of 3.1% (54/1736) patients were assessed to have grade A or BAL compared to 4.1% (68/1657) patients following no TDT (OR=0.64, 95%CI 0.42-0.97, p=0.03). Low heterogeneity was found in this analysis (I2 = 10%, p = 0.35).
1.1.2 AL rate (grade C)
All 14 studies included in this subgroup reported C-level AL (n=3393 patients). In patients with a TDT placed for ≥5 days, 2.6% (46/1736) patients were diagnosed with grade CAL compared to 6.0% (100/1657) patients following no TDT (OR=0.35, 95%CI 0.24-0.53, p<0.01). No heterogeneity was found in this analysis (I2 = 0%, p = 0.72).
1.2 Reoperation rate
Thirteen of the 14 studies in this subgroup reported reoperation rates (n=3236 patients). The specific number of reoperation cases was not reported in two studies (10, 12). Reoperations were reported in 2.8% (47/1657) of the patients with TDT compared to 7.0% (110/1579) patients without TDT (OR=0.36, 95%CI 0.24-0.53, p<0.01). No heterogeneity was observed (I2 = 0%, p = 0.64).
1.3 DS
Of the 14 studies included in the subgroup analysis, four (n=983 patients) reported DS and 10 (n=2410 patients) did not.
1.3.1 AL
In the patients who with prophylactic DS, AL was reported in a total of 8.3% (49/587) patients with TDT in comparison to 14.6% (58/396) patients without TDT (OR=0.57, 95%CI 0.37-0.87, p<0.01), and no heterogeneity was found (I2 = 0%, p = 0.42). In the patients who without prophylactic DS, AL was reported in 9.6% (121/1261) patients without TDT compared to 4.6% (53/1149) patients with TDT (OR=0.38, 95%CI 0.26-0.55, p<0.01), and no heterogeneity was found as well (I2 = 0%, p = 0.67).
1.3.2 Reoperation rate
Three of the four studies reported the reoperation rate of patients with DS (n=826 patients). Reoperations were performed in 4.5% (23/508) patients with TDT and in 7.5% (24/318) patients without TDT (OR=0.61, 95%CI 0.33-1.11, p=0.11), and no heterogeneity was found (I2 = 0%, p = 0.60). All 10 studies reported reoperation rates of patients without DS (n=2410 patients). 2.1% (24/1149) patients with TDT underwent reoperations in comparison to 6.8% (86/1261) patients without TDT (OR=0.24, 95%CI 0.14-0.41, p<0.01). Similarly, no heterogeneity was observed (I2 = 0%, p = 0.95).
2. TDT indwelling time < 5 days group (4 studies)
2.1 AL rate
Postoperative AL rate was reported in all four studies in which the TDT indwelling time was <5 days (n=1482 patients). 8.8% (57/645) patients with TDT were reported to have AL compared to 10.8% (90/837) in the non-TDT group, with a pooled OR of 0.72 (95%CI 0.33-1.57, p=0.41). Substantial heterogeneity was observed in this analysis (I2 = 74%, p<0.01) (Figure 5).
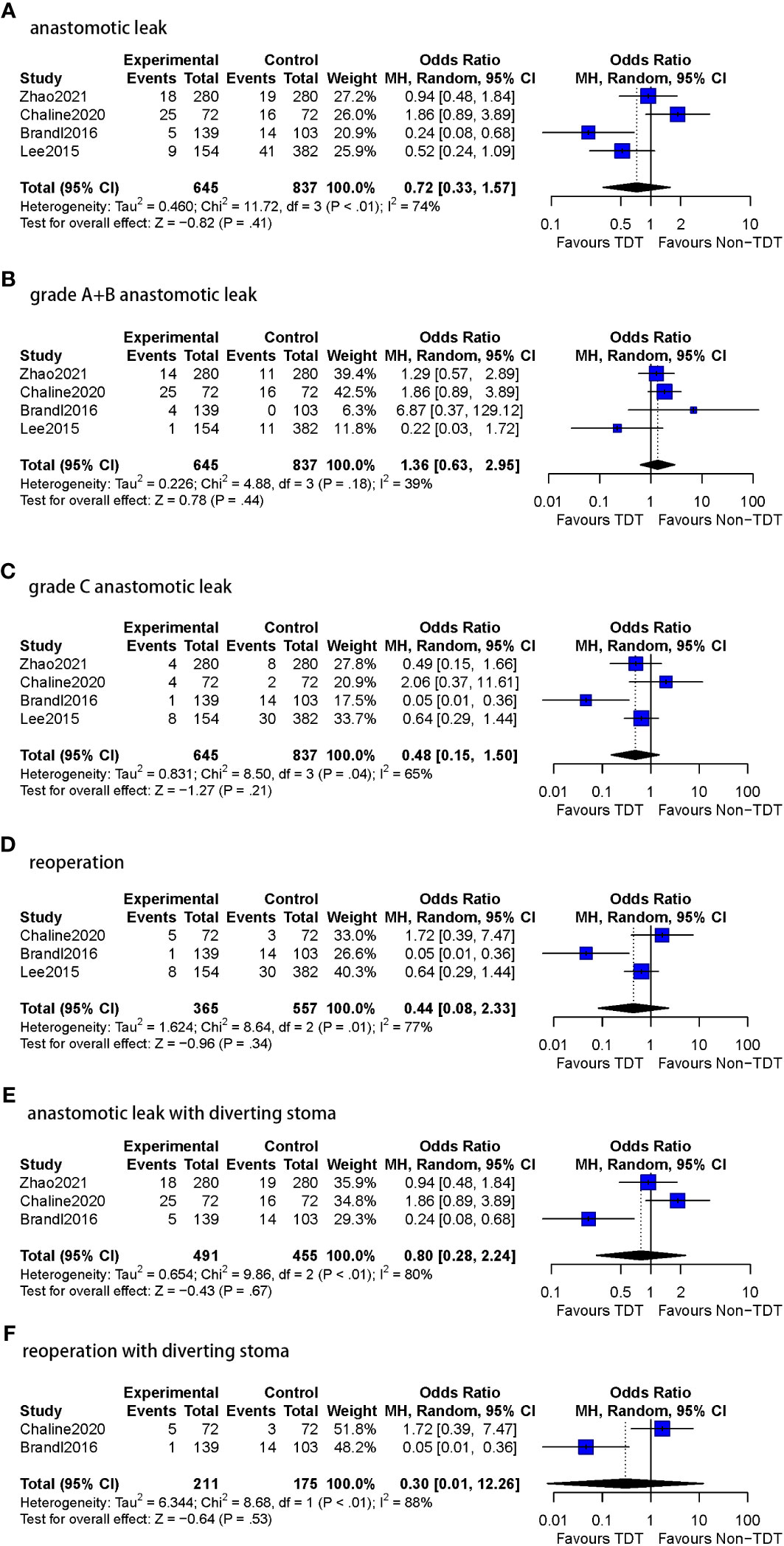
Figure 5 Meta-analysis results for TDT indwelling time less than 5 days subgroup. (A): anastomotic leak. (B): grade A+B anastomotic leak. (C): grade C anastomotic leak. (D): Reoperation. (E): anastomotic leak with diverting stoma. (F): reoperation with diverting stoma.
2.1.1 AL rate (grade A+B)
All four studies included in this subgroup reported A and B grades of AL (n=1482 patients). Among patients with a TDT placed for <5 days, 6.8% (44/645) were assessed to have grade A or BAL compared to 4.5% (38/837) following no TDT (OR=1.36, 95%CI 0.63-2.95, p=0.44). Moderate heterogeneity was observed (I2 = 39%, p = 0.18).
2.1.2 AL rate (grade C)
All four studies included in this subgroup reported grade C AL (n=1482 patients). In patients with a TDT placed for <5 days, 2.6% (17/645) were diagnosed with grade CAL compared to 6.5% (54/837) with no TDT (OR=0.48, 95%CI 0.15-1.50, p=0.21). Moderate heterogeneity was observed (I2 = 65%, p = 0.04).
2.2 Reoperation rate
Three of the four studies in this subgroup reported reoperation rates (n=922 patients). Reoperations were reported in 3.8% (14/365) patients with TDT in comparison to 8.4% (47/557) patients without TDT (OR=0.44, 95%CI 0.08-2.33, p=0.34). Substantial heterogeneity was observed in this analysis (I2 = 77%, p = 0.01).
2.3 DS
In this subgroup, three studies (n=946 patients) reported DS and only one study (n=536 patients) did not.
2.3.1 AL rate
Three of the four studies reported DS in the less than 5 days TDT indwelling time subgroup. AL was reported in 9.8% (48/491) patients with TDT in comparison to 10.8% (49/455) patients without TDT (OR=0.80, 95%CI 0.28-2.24, p=0.67), and substantial heterogeneity was observed (I2 = 80%, p<0.01).
Only one study in this subgroup did not report DS. AL was reported in 5.8% (9/154) patients with TDT compared to 10.7% (41/382) patients without TDT (OR=0.52, 95%CI 0.24-1.09, p=0.08).
2.3.2 Reoperation rate
In the two of three studies reporting DS in the less than 5 days TDT indwelling time subgroup (n=386 patients), reoperations were performed in 2.8% (6/211) patients with TDT and in 9.7% (17/175) patients without TDT (OR=0.30, 95%CI 0.01-12.26, p=0.53), substantial heterogeneity was found as well (I2 = 88%, p<0.01).
Only one study reported the reoperation rate in patients without DS (n=536). 5.2% (8/154) patients with TDT underwent reoperations compared to 7.9% (30/382) without TDT (OR=0.64, 95%CI 0.29-1.44, p=0.28).
Substantial heterogeneity was found in the subgroup of TDT indwelling time of < 5 days. In the AL indicator, heterogeneity was slightly reduced after individual study (5) was excluded; However, substantial heterogeneity remained (I2 = 59%, p=0.09). Heterogeneity was reduced to 24% after individual study (24) was excluded from the meta-analysis of reoperations in the subgroup (p=0.73). Guo et al. (15) concluded that in the study by Challine et al. (5), temporary diverting stoma were systemically constructed for all patients after anterior resection for rectal cancer, which may have contributed to the differences from other studies in the pooled analysis.
According to the Egger test, there was evidence of publication bias in the meta-analysis of AL (p=0.0005), reoperation (p=0.0114), and AL without DS (p=0.0032) in the subgroup with TDT indwelling time ≥5 days (Figure 6).
We conducted a systematic review and meta-analysis of the indwelling time of TDT after anterior rectal cancer surgery. After conducting a systematic analysis of the preliminary data statistics, we finalized the subgroups as ≥5-day and <5-day subgroups depending on the indwelling time of the TDT postoperatively. The vast majority of the 18 included studies (14/18) had TDT indwelling time of 5 days or more, which is also consistent with our clinical experience. The results showed that the indwelling time of TDT for ≥5-days was associated with a significant reduction (TDT vs. Non-TDT) in overall AL, grade A+B AL, grade C AL, overall reoperation rate and that in patients without a prophylactic DS. There were no statistically significant differences in any of the abovementioned indicators when the indwelling time of TDT was less than 5 days.
In most of the included studies, TDT was maintained until at least postoperative day (POD) 5. This may differ from what most surgeons believe, that postoperative AL does not occur only in the early postoperative period. Several studies have reported that the mean time to postoperative AL was ≥5 days, and that most occurred on POD 7 (18, 19, 31). Li et al. (19) defined AL occurring ≤ 5 days postoperatively as very early AL (vE-AL). AL occurring during this period is considered fatal; thus, POD 5 was considered the optimal cutoff time to distinguish truly life-threatening early AL requiring urgent reoperation. Several factors may have contributed to these findings. First, during the early postoperative period, the anal sphincter is usually tight due to factors such as pain, tension, or inflammation, which may lead to increased pressure in the rectal lumen as stool or gas passes through the anastomosis, thus interfering with anastomosis healing (4). Kwada et al. reported that the daily fecal volume increased until POD 3 or 4 and significantly decreased on POD 5 (14), which means that the pressure in the rectal lumen increases until POD 5, leading to an increased risk of AL. Second, the strength of the anastomosis is mainly dependent on the collagen fibrils within the submucosal layer. During the first few days after rectal surgery, collagen at the anastomosis degrades, and the strength of the anastomosis depends on the suture- or staple-holding capacity of the existing collagen. AL is more likely to occur at this stage until a large amount of collagen can be resynthesized within one or two postoperative days. AL occurs when the radial force at the anastomosis exceeds the resistance generated by sutures, staples, and early scars. Bursting pressure approaches 100% on POD 7, after which the intestine generally bursts outside the anastomotic site (20). In colon, the strength at the anastomosis site has been reported to reach only 30% of the initial strength after 48 h of surgery (32) and reach 50% after one week (20). These findings suggest that premature TDT removal may be detrimental to postoperative anastomotic healing.
We analyzed the number and rates of occurrence of grade A+B and grade C of AL in the included studies. Some of the included studies did not report accurate data on AL classification, so patients with grade C AL were considered as those requiring reoperation for statistical purposes. The results may not be accurate because patients with AL, not only grade C AL, can be treated surgically.
In clinical practice, surgeons often use a prophylactic DS to reduce the occurrence of AL in patients at high risk for postoperative AL. Preventive ileostomy can reduce the passage of stool and gas through the anastomosis and reduce the pressure in the colorectal lumen to prevent AL (4). In particular, prophylactic ileostomy can reduce the incidence of clinically significant AL, resulting in lower reoperation rates in rectal cancer (33). However, preventive ileostomy has disadvantages such as inconvenience, high incidence of stoma complications, poor patient subjective perception, and the need for a secondary surgery to close the stoma (34), which increases the hospital and surgical costs and produces a secondary trauma to the patient. TDT can also reduce postoperative pressure in the rectal lumen and be an alternative to preventive ileostomy in patients with a clear low risk of postoperative AL (35). The effect of DS on AL is not negligible. We also created statistics for this purpose. And our findings suggest that different stoma status does not impact the effect of TDT at a specific indwelling time (Table 5).
Tumor location has now been identified as an independent important risk factor for postoperative AL (36). Tumors less than 5 cm from the anal verge are 6.5 times more likely to develop AL after surgery compared to those situated greater than 5 cm from the anal verge. We aimed to investigate the relationship between anastomotic position and AL after rectal cancer surgery at specific TDT indwelling times. This may be a very important factor affecting AL after rectal cancer surgery. Since anastomotic positions were not reported in some of the included studies, tumor positions were counted as the replacement of the anastomotic positions. However, the data on tumor locations reported in the included studies were inaccurate and in different ways, which did not allow valid grouping; therefore, no further analysis was performed. The relevant data reported in the included studies were organized in the Tables 2, 1.
For unresectable rectal cancer, Guadagni et al. (37) mention that the integration of hypoxic pelvic perfusion (HPP)/targeted therapies may be effective in terms of locally controlled symptom as well as long term outcomes in patients with unresectable rectal cancer. This may provide an important adjunct to surgery for rectal tumors that are difficult to resect, and pelvic perfusion therapy may allow for more complete local tumor clearance, which in turn reduces anastomotic tumor recurrence and AL.
The concept of enhanced recovery after surgery (ERAS) has been generally accepted in rectal cancer surgery, with the advantage of reduced length of hospital stay (LOS), lower costs, and decreased non-surgical complications (38). Regrettably, none of the studies included in the present meta-analysis referred to ERAS protocols, which may be a limitation of this study. However, we assumed that the presence of TDT may not be in conflict with early postoperative feeding and ERAS protocols. Early postoperative feeding usually begins with liquid diet which produces minimal amount of loose stool and is unlikely to obstruct to the TDT. An indwelling TDT can drain stools and gas out of the rectal lumen, decreasing the intraluminal-pressure, providing an ideal environment for anastomotic healing, and eventually reducing the risk of AL. To some extent, postoperative indwelling TDT is beneficial for patients and may represent a new part of ERAS protocols.
This is the first meta-analysis to analyze the effect of indwelling time of TDT on the effectiveness of TDT after rectal cancer anterior resection. However, this study also had some limitations. The inclusion of less number of RCTs may have affected the statistical power and reduced the persuasiveness of the results. In terms of subgroups, there were fewer studies in the <5-day subgroup, which may have led to selection bias, and substantial heterogeneity was found in this subgroup. In addition, the included studies differed in the type and diameter of TDT, which may have introduced heterogeneity into the results. We assume that in addition to indwelling time, factors such as placement of the TDT catheter tip (proximal to the anastomosis or at the anastomosis), caliber of the TDT, material, and number of drainage holes may also affect AL. Further studies are needed to investigate this aspect.
Extending the postoperative indwelling time of TDT to 5 days may reduce the overall AL and the need for reoperation in patients without a prophylactic DS.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
XX: Data curation, Formal analysis, Writing – original draft. XZ: Conceptualization, Writing – review & editing. XL: Writing – original draft. AY: Data curation, Writing – original draft. XQZ: Data curation, Writing – original draft. SD: Writing – review & editing. ZL: Writing – review & editing, Formal analysis. ZC:Writing – original draft. KW: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Spinelli A, Anania G, Arezzo A, Berti S, Bianco F, Bianchi PP, et al. Italian multi-society modified Delphi consensus on the definition and management of anastomotic leakage in colorectal surgery. Updates Surg (2020) 72(3):781–92. doi: 10.1007/s13304-020-00837-z
2. van Helsdingen CP, Jongen AC, de Jonge WJ, Bouvy ND, Derikx JP. Consensus on the definition of colorectal anastomotic leakage: A modified Delphi study. World J Gastroenterol (2020) 26(23):3293–303. doi: 10.3748/wjg.v26.i23.3293
3. Chadi SA, Fingerhut A, Berho M, DeMeester SR, Fleshman JW, Hyman NH, et al. Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J Gastrointest Surg (2016) 20(12):2035–51. doi: 10.1007/s11605-016-3255-3
4. Wang Z, Liang J, Chen J, Mei S, Liu Q. Effectiveness of a transanal drainage tube for the prevention of anastomotic leakage after laparoscopic low anterior resection for rectal cancer. Asian Pacific J Cancer Prev (2020) 21(5):1441–4. doi: 10.31557/apjcp.2020.21.5.1441
5. Challine A, Lefèvre JH, Creavin B, Benoit O, Chafai N, Debove C, et al. Can a local drainage salvage a failed colorectal or coloanal anastomosis? A prospective cohort of 54 patients. Dis Colon Rectum (2020) 63(1):93–100. doi: 10.1097/dcr.0000000000001516
6. Hidaka E, Ishida F, Mukai S, Nakahara K, Takayanagi D, Maeda C, et al. Efficacy of transanal tube for prevention of anastomotic leakage following laparoscopic low anterior resection for rectal cancers: a retrospective cohort study in a single institution. Surg Endosc (2015) 29(4):863–7. doi: 10.1007/s00464-014-3740-2
7. Carboni F, Valle M, Levi Sandri GB, Giofrè M, Federici O, Zazza S, et al. Transanal drainage tube: alternative option to defunctioning stoma in rectal cancer surgery? Transl Gastroenterol Hepatol (2020) 5:6. doi: 10.21037/tgh.2019.10.16
8. Kim M-K, Won D-Y, Lee J-K, Kang W-K, Kim J-G, Oh ST. Comparative study between transanal tube and loop ileostomy in low anterior resection for mid rectal cancer: a retrospective single center trial. Ann Surg Treat Res (2015) 88(5):260–8. doi: 10.4174/astr.2015.88.5.260
9. Lee SY, Kim CH, Kim YJ, Kim HR. Impact of anal decompression on anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis. Langenbecks Arch Surg (2015) 400(7):791–6. doi: 10.1007/s00423-015-1336-5
10. Tamura K, Matsuda K, Horiuchi T, Noguchi K, Hotta T, Takifuji K, et al. Laparoscopic anterior resection with or without transanal tube for rectal cancer patients – A multicenter randomized controlled trial. Am J Surg (2021) 222(3):606–12. doi: 10.1016/j.amjsurg.2020.12.054
11. Yang CS, Choi GS, Park JS, Park SY, Kim HJ, Choi JI, et al. Rectal tube drainage reduces major anastomotic leakage after minimally invasive rectal cancer surgery. Colorectal Dis (2016) 18(12):O445–o452. doi: 10.1111/codi.13506
12. Zhao S, Zhang L, Gao F, Wu M, Zheng J, Bai L, et al. Transanal drainage tube use for preventing anastomotic leakage after laparoscopic low anterior resection in patients with rectal cancer: A randomized clinical trial. JAMA Surg (2021) 156(12):1151–8. doi: 10.1001/jamasurg.2021.4568
13. Zhao WT, Hu FL, Li YY, Li HJ, Luo WM, Sun F. Use of a transanal drainage tube for prevention of anastomotic leakage and bleeding after anterior resection for rectal cancer. World J Surg (2013) 37(1):227–32. doi: 10.1007/s00268-012-1812-9
14. Kawada K, Takahashi R, Hida K, Sakai Y. Impact of transanal drainage tube on anastomotic leakage after laparoscopic low anterior resection. Int J Colorectal Dis (2018) 33(3):337–40. doi: 10.1007/s00384-017-2952-z
15. Guo C, Fu Z, Qing X, Deng M. Prophylactic transanal drainage tube placement for preventing anastomotic leakage after anterior resection for rectal cancer: A meta-analysis. Colorectal Disease: Off J Assoc Coloproctol Great Britain Ireland (2022) 24(11):1273–84. doi: 10.1111/codi.16231
16. Rondelli F, Avenia S, De Rosa M, Rozzi A, Rozzi S, Chillitupa CIZ, et al. Efficacy of a transanal drainage tube versus diverting stoma in protecting colorectal anastomosis: a systematic review and meta-analysis. Surg Today (2023) 53(2):163–73. doi: 10.1007/s00595-021-02423-1
17. Chen H, Cai H-K, Tang Y-H. An updated meta-analysis of transanal drainage tube for prevention of anastomotic leak in anterior resection for rectal cancer. Surg Oncol (2018) 27(3):333–40. doi: 10.1016/j.suronc.2018.05.018
18. Kanellos D, Pramateftakis MG, Vrakas G, Demetriades H, Kanellos I, Mantzoros I, et al. Anastomotic leakage following low anterior resection for rectal cancer. Techniques Coloproctol (2010) 14 Suppl 1:S35–7. doi: 10.1007/s10151-010-0620-1
19. Li Y-W, Lian P, Huang B, Zheng HT, Wang MH, Gu WL, et al. Very early colorectal anastomotic leakage within 5 post-operative days: a more severe subtype needs relaparatomy. Sci Rep (2017) 7:39936. doi: 10.1038/srep39936
20. Thompson SK, Chang EY, Jobe BA. Clinical review: Healing in gastrointestinal anastomoses, part I. Microsurgery (2006) 26(3):131–6. doi: 10.1002/micr.20197
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg (London England) (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
22. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (2017) 358:j4008. doi: 10.1136/bmj.j4008
23. Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery (2010) 147(3):339–51. doi: 10.1016/j.surg.2009.10.012
24. Brandl A, Czipin S, Mittermair R, Weiss S, Pratschke J, Kafka-Ritsch R. Transanal drainage tube reduces rate and severity of anastomotic leakage in patients with colorectal anastomosis: A case controlled study. Ann Med Surg (Lond) (2016) 6:12–6. doi: 10.1016/j.amsu.2016.01.003
25. Xiao L, Zhang WB, Jiang PC, Bu XF, Yan Q, Li H, et al. Can transanal tube placement after anterior resection for rectal carcinoma reduce anastomotic leakage rate? A single-institution prospective randomized study. World J Surg (2011) 35(6):1367–77. doi: 10.1007/s00268-011-1053-3
26. Goto S, Hida K, Kawada K, Okamura R, Hasegawa S, Kyogoku T, et al. Multicenter analysis of transanal tube placement for prevention of anastomotic leak after low anterior resection. J Surg Oncol (2017) 116(8):989–95. doi: 10.1002/jso.24760
27. Kuk JC, Lim DR, Shin EJ. Effect of transanal drainage tube on anastomotic leakage following low anterior resection for rectal cancer without a defunctioning stoma. Asian J Surg (2022) 45(12):2639–44. doi: 10.1016/j.asjsur.2021.12.026
28. Nishigori H, Ito M, Nishizawa Y, Nishizawa Y, Kobayashi A, Sugito M, et al. Effectiveness of a transanal tube for the prevention of anastomotic leakage after rectal cancer surgery. World J Surg (2014) 38(7):1843–51. doi: 10.1007/s00268-013-2428-4
29. Ito T, Obama K, Sato T, Matsuo K, Inoue H, Kubota K, et al. Usefulness of transanal tube placement for prevention of anastomotic leakage following laparoscopic low anterior resection. Asian J Endosc Surg (2017) 10(1):17–22. doi: 10.1111/ases.12310
30. Li Y, Gu F. Effectiveness of a large-calibre transanal drainage tube on the prevention of anastomotic leakage after anterior resection for rectal cancer. J buon (2020) 25(2):933–8.
31. Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it's later than you think. Ann Surg (2007) 245(2):254–8. doi: 10.1097/01.sla.0000225083.27182.85
32. Guyton KL, Hyman NH, Alverdy JC. Prevention of perioperative anastomotic healing complications: anastomotic stricture and anastomotic leak. Adv Surg (2016) 50(1):129–41. doi: 10.1016/j.yasu.2016.03.011
33. Mu Y, Zhao L, He H, Zhao H, Li J. The efficacy of ileostomy after laparoscopic rectal cancer surgery: a meta-analysis. World J Surg Oncol (2021) 19(1):318. doi: 10.1186/s12957-021-02432-x
34. Tan WS, Tang CL, Shi L, Eu KW. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg (2009) 96(5):462–72. doi: 10.1002/bjs.6594
35. Cho SH, Lee IK, Lee YS, Kim MK. The usefulness of transanal tube for reducing anastomotic leak in mid rectal cancer: compared to diverting stoma. Ann Surg Treat Res (2021) 100(2):100–8. doi: 10.4174/astr.2021.100.2.100
36. Zarnescu EC, Zarnescu NO, Costea R. Updates of risk factors for anastomotic leakage after colorectal surgery. Diagnostics (Basel) (2021) 11(12):2382. doi: 10.3390/diagnostics11122382
37. Guadagni S, Fiorentini G, Mambrini A, Masedu F, Valenti M, Mackay AR, et al. Multidisciplinary palliation for unresectable recurrent rectal cancer: hypoxic pelvic perfusion with mitomycin C and oxaliplatin in patients progressing after systemic chemotherapy and radiotherapy, a retrospective cohort study. Oncotarget (2019) 10(39):1–13. doi: 10.18632/oncotarget.26972
Keywords: anastomotic leakage, indwelling time, transanal drainage tube, anterior resection, rectal cancer
Citation: Xu X, Zhang X, Li X, Yu A, Zhang X, Dong S, Liu Z, Cheng Z and Wang K (2024) Effect of transanal drainage tube on prevention of anastomotic leakage after anterior rectal cancer surgery taking indwelling time into consideration: a systematic review and meta-analysis. Front. Oncol. 13:1307716. doi: 10.3389/fonc.2023.1307716
Received: 11 October 2023; Accepted: 31 December 2023;
Published: 23 January 2024.
Edited by:
Ugo Grossi, University of Padua, ItalyReviewed by:
Beatriz Martin-Perez, University Hospital of Badajoz, SpainCopyright © 2024 Xu, Zhang, Li, Yu, Zhang, Dong, Liu, Cheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kexin Wang, d2t4MzcyNkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.