- 1Department of Thoracic Surgery, The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University, Huaian, China
- 2Department of Respiratory and Critical Care Medicine, The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University, Huaian, China
Objectives: The objective of this study is to investigate whether the evaluation of postoperative outcomes or overall survival in patients who undergo surgery for esophageal cancer can be achieved by assessing sarcopenia using psoas muscle mass index and peak expiratory flow.
Methods: This retrospective study analyzed the clinical data of 356 elderly patients (≥ 65 years) who had undergone radical surgery for esophageal cancer. Muscle mass and muscle strength were assessed by psoas muscle mass index (bilateral psoas area/height2) and peak expiratory flow, using preoperative computed tomography and spirometry, respectively. Sarcopenia is defined as a condition where both the psoas muscle mass index and peak expiratory flow fall below their gender-specific cutoff values. Survival and postoperative complications were compared between patients with and without sarcopenia.
Results: Out of the 356 elderly individuals diagnosed with esophageal cancer, 84 patients (23.6%) were found to have sarcopenia. The group with sarcopenia showed a notably higher occurrence of postoperative pneumonia (29.8% vs 16.9%, P < 0.001) and anastomotic leak (9.5% vs 3.7%, P < 0.05) compared to those without sarcopenia. Additionally, a multivariate analysis concluded that sarcopenia independently acted as a risk factor for postoperative pneumonia, possessing an odds ratio of 1.90 (P < 0.05). The survival rate after 3 years for individuals with sarcopenia was considerably lower than those without sarcopenia (57.8% vs 70.2%, P < 0.05). Sarcopenia was identified as an unfavorable prognostic factor for overall survival, with a hazard ratio of 1.51 (P < 0.05).
Conclusions: Preoperative sarcopenia diagnosed by psoas muscle mass index and peak expiratory flow is associated with reduced overall survival and adverse postoperative outcomes among elderly individuals suffering from esophageal cancer.
1 Introduction
Esophageal cancer (EC) ranks as the sixth leading cause of cancer-related death on a global scale (1). A considerable proportion of individuals diagnosed with esophageal cancer encounter varying degrees of malnutrition, ranging from 60% to 85%, making it the most prevalent malnutrition among all types of malignant tumors (2). The presence of malnutrition plays a significant role in determining the treatment response and prognosis of patients grappling with esophageal cancer. Moreover, this category of patients typically experiences notable alterations in body composition, such as a decrease in lean muscle mass and adipose tissue, which stem from cancer-related malnutrition and the activation of inflammatory cytokines. The severity of these alterations is further intensified by dysphagia resulting from tumor stenosis (3–5).
Sarcopenia is a degenerative disorder of the skeletal muscle, distinguished by a decline in both muscle mass and strength (6, 7). It frequently presents in individuals diagnosed with esophageal cancer, with prevalence rates varying from 16% to 75% (8). Studies have provided evidence that sarcopenia significantly heightens the risk of postoperative complications like pneumonia and anastomotic leak in esophageal cancer surgical patients (9–11). Additionally, it has been noted that sarcopenia negatively impacts overall survival and disease-free survival rates among these patients (9, 12, 13).
As per the consensus of the Asian Working Group on Sarcopenia and the European Working Group on Sarcopenia, the diagnosis of sarcopenia necessitates an assessment from two perspectives - muscle mass and muscle strength. Various methods are available to evaluate muscle mass, such as dual-energy X-ray absorptiometry, bioelectrical impedance analysis, magnetic resonance imaging, and computed tomography (CT). In addition, diminished muscle strength has been acknowledged as a vital diagnostic feature for sarcopenia and a reliable predictor of unfavorable consequences.Tests to measure muscle strength encompass grip strength, peak expiratory flow (respiratory muscle strength), and the chair stand test (leg muscle strength) (6, 7, 14).
However, previous investigations into sarcopenia in esophageal cancer have primarily focused on the diagnosis of reduced muscle mass, disregarding the evaluation of muscular strength (11, 15–17). This disregard could be attributed to the laborious process of gauging muscle strength and the accompanying expenses. CT imaging has the capability to assess muscle mass through the utilization of the skeletal muscle index (SMI) and psoas muscle mass index (PMI) (6). In comparison to SMI, PMI offers advantages such as well-defined boundaries, a smaller area of measurement, and the ability to measure individual muscles, rendering it more suitable for the assessment of preoperative patients in clinical practice (18). Peak expiratory flow (PEF) denotes the maximum airflow produced during forced exhalation and exhibits an intrinsic correlation with respiratory muscle strength (19). The study conducted by Ridwan additionally identified that diminished PEF is independently linked to the occurrence of sarcopenia (20). Furthermore, researchers have identified respiratory sarcopenia, characterized by PEF (21, 22). Preoperative CT and spirometry represent standard procedures employed in the staging and evaluation of esophageal cancer patients before surgical intervention. In our study, we employ CT images to capture PMI alterations, allowing for the evaluation of changes in muscle mass, while simultaneously utilizing PEF measurements from spirometry to assess variations in muscle strength. This approach offers a more pragmatic and convenient means of diagnosis.
This retrospective study utilizes the parameters of PMI and PEF to diagnose preoperative sarcopenia in elderly patients diagnosed with esophageal cancer, and investigates the impact of sarcopenia on postoperative complications and overall survival.
2 Materials and methods
2.1 Study design and participants
This retrospective investigation was carried out at the Department of Thoracic Surgery, located in the Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University. The study enrolled individuals diagnosed with esophageal cancer who underwent surgical intervention from January to December 2020. The investigation focused on patients who fulfilled the following inclusion criteria: (1) age of 65 years or above, (2) received an abdominal CT scan within a fortnight prior to the procedure, (3) pathology reports confirmed the presence of squamous cell carcinoma or adenocarcinoma, and (4) underwent McKeown surgery. Subjects meeting any of the subsequent exclusion criteria were excluded from the study: (1) received neoadjuvant treatment before the operation, (2) lacked comprehensive clinical or imaging data, (3) had other malignancies, (4) necessitated conversion to open surgery during the procedure, or (5) exhibited distant metastasis. This investigation strictly adhered to the ethical principles outlined in the Declaration of Helsinki and received approval from the Medical Ethics Committee of our hospital under the reference number KY-2022-037-01.
2.2 Information on clinical parameters
The extracted clinical data consisted of the subsequent details: age, gender, hypertension, diabetes mellitus, smoking history (smoking index ≥400), preoperative pulmonary function test (forced expiratory volume in 1 second [FEV1], peak expiratory flow [PEF]), preoperative laboratory parameters (albumin, pre-albumin), tumor stage (in accordance with the 8th edition of the UICC/AJCC staging criteria for esophageal cancer in 2017), body mass index (BMI), prognostic nutritional index (PNI), short-term outcomes (postoperative complications, hospital length of stay [LOS], 30-day mortality).
The deadline for follow-up was July 31, 2023, and patients were monitored through either outpatient appointments or telephone interviews. Overall survival was defined as the duration from surgery to the last follow-up appointment or death resulting from any cause.
2.3 Postoperative complications
Within a 30-day period following esophagectomy, we assessed the occurrence of postoperative complications demanding medical or surgical intervention for management. The diagnostic criteria for postoperative pneumonia detection within a 30-day period after surgery must adhere to the following three conditions concurrently: (1) the presence of at least two chest radiographs and, furthermore, a minimum of one pneumonia diagnosis; (2) fulfillment of at least one of the subsequent requirements: age ≥70 years, peripheral blood WBC count 12 × 109/L, and fever accompanied by altered consciousness (body temperature >38°C); (3) the manifestation of a minimum of two of the subsequent indications: occurrence of purulent sputum or alteration in sputum characteristics, heightened respiratory secretions, necessity to increase the frequency of sputum generation, or presence of dyspnea.
The identification of an anastomotic leak was achieved by means of esophagography or fistulography, in addition to the consequent discharge of saliva through the fistula. Pleural effusion and arrhythmia were diagnosed based on the criteria established by the Esophagectomy Complications Consensus Group (23). Overall complications are defined as the occurrence of one or more of the above.
2.4 Determination of sarcopenia
Retrospective collection and analysis of abdominal CT images from patients diagnosed with esophageal cancer took place prior to surgery, within a period of 14 days. Measurement of muscles was conducted within a HU range of −29 to +150 HU, and adjustments were made manually to address any issues pertaining to tissue boundaries. The calculation of PMI (bilateral psoas area/height2) involved adding the left and right psoas areas of the L3 segment and normalizing them in relation to height.
Hamaguchi et al. developed a new set of diagnostic criteria to evaluate low muscle mass on CT scans in the Asian population. Accordingly, the PMI thresholds for sarcopenia in men and women were 6.36 cm2/m2 and 3.92 cm2/m2, respectively (24). A Japanese cross-sectional study including 681 community-dwelling elderly individuals determined sex-specific thresholds for PEF to be 4.40 L/s for males and 3.21 L/s for females (21). Preoperative sarcopenia diagnosis relied on the presence of PMI and PEF measurements falling below the gender-specific cutoff values.
2.5 Statistical analysis
The normality of the collected data was evaluated by visually examining histograms and conducting the Shapiro-Wilk’s W-test. If the data followed a normal distribution, it was presented as the mean and standard deviation (SD); otherwise, it was presented as the median and interquartile range (IQR). Categorical data was presented as the number and percentage. In order to examine distinctions among groups regarding continuous data, we employed the Mann-Whitney U test, whereas the Pearson chi-squared test or Fisher’s exact probability method was utilized for categorical data. The determination of cutoff values for continuous variables was based on the median of the cohort (age [70 years]), defined class (BMI [18.5 kg/m2, 24 kg/m2]), predicted FEV1% [80%]), and thresholds reported in previous studies (pre-albumin [160 mg/L]) (25, 26).
Survival curves were generated using Kaplan-Meier analysis, and the log-rank test was utilized to compare differences. We developed a logistic regression model with the intent of identifying preoperative prognostic factors specifically related to postoperative pneumonia. The model incorporated all variables found to have a significance level of P < 0.1 in the initial univariate analysis. Univariate and multivariate analyses were conducted using Cox proportional hazards models, and variables with P values less than 0.1 at the univariate level were included in the multivariate model. Hazard ratios (HR) and corresponding 95% confidence intervals (CI) were calculated to determine the strength of associations. To conduct statistical analyses, we employed two software programs: SPSS version 26.0 developed by IBM Corp in Armonk, NY, USA and Prism 8 designed by GraphPad Software in La Jolla, CA, USA.
3 Results
During the period from January 2020 to December 2020, a total of 428 older individuals (aged ≥65 years) diagnosed with esophageal cancer underwent the McKeown surgical procedure at our medical facility. None of these patients were subjected to neoadjuvant therapy. Out of these individuals, 21 did not have CT scans performed within a 2-week window prior to the operation, 15 patients lacked complete clinical data, and 36 patients were lost to follow-up. Subsequently, we included a total of 356 patients who fulfilled the predetermined inclusion criteria for our study. Among the study population, there were 235 male patients and 121 female patients, with a median age of 70 years.
3.1 Characteristics between sarcopenic and non-sarcopenic patients
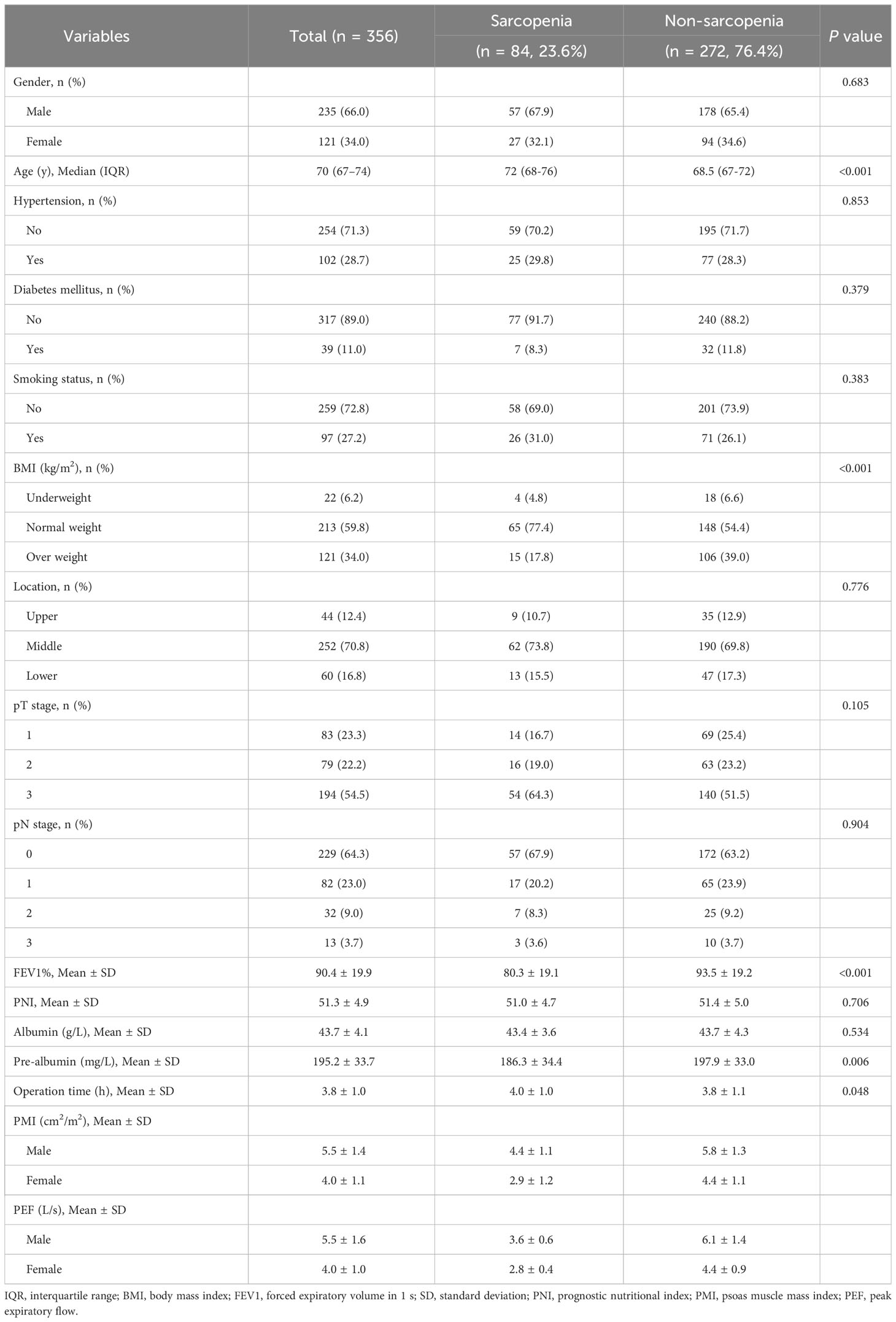
According to our criteria, a total of 84 patients (23.6%) were diagnosed with preoperative sarcopenia, while the remaining 272 patients did not exhibit signs of sarcopenia. Table 1 contains the clinical characteristics of both the sarcopenic and non-sarcopenic patients. It was observed that the sarcopenic patients displayed a significantly higher age compared to the non-sarcopenic patients. The occurrence rates of sarcopenia among the three age groups, specifically individuals aged 65-70, 71-75, and over 75, were found to be 15.7%, 30.7%, and 38.6% respectively. Additionally, when considering sarcopenia as a grouping factor, notable disparities were noted in terms of BMI (P < 0.001), FEV1% (P < 0.001), and pre-albumin levels (P = 0.006) within the various groups. Furthermore, it was observed that sarcopenic patients experienced a lengthier duration of surgical procedures compared to their non-sarcopenic counterparts (P = 0.048). Nevertheless, no significant distinctions were identified concerning other factors related to the host, laboratory indicators, or tumor-related factors between these two groups.
3.2 Sarcopenia and postoperative complications
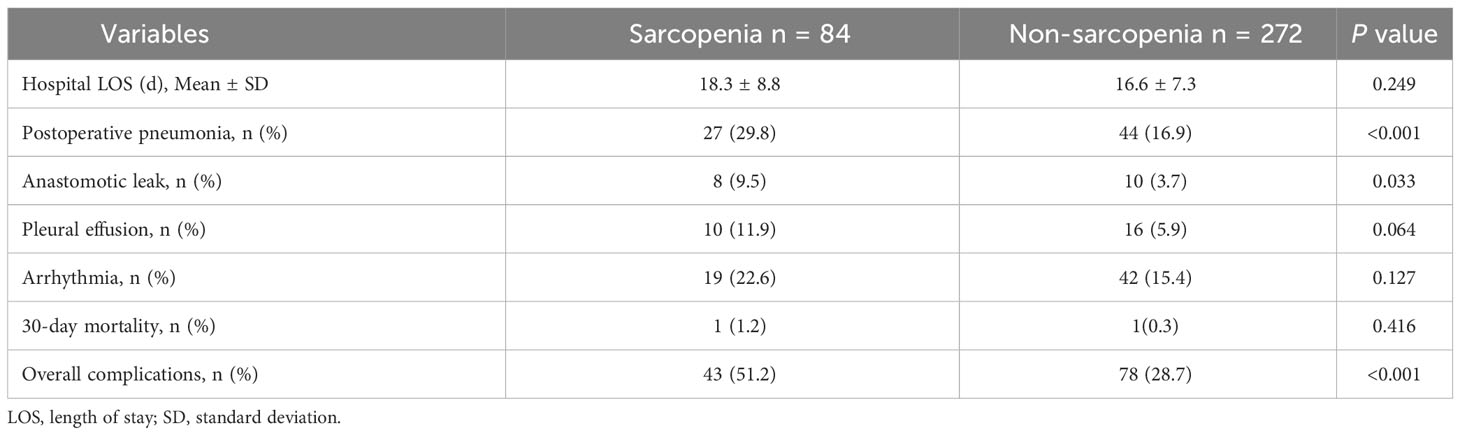
The occurrence of postoperative pneumonia exhibited a notably higher prevalence in the group with sarcopenia opposed to the non-sarcopenic group (29.8% vs 16.9%, P < 0.001). Additionally, patients with sarcopenia also manifested elevated rates of anastomotic leak (9.5% vs 3.7%, P = 0.033) and encountered a greater overall incidence of complications when compared to the non-sarcopenic group (Table 2). Table 3 lays out the risk factors for postoperative pneumonia. Through univariate analysis, it was disclosed that age (≥70y), history of smoking, FEV1% (<80%), operation time (≥4h), and sarcopenia were linked to the emergence of postoperative pneumonia. Following adjustment for age (≥70y) and smoking history in the multivariate analysis, sarcopenia was determined as an autonomous risk factor for pneumonia that arises as a consequence of esophageal cancer surgery (OR, 1.902; 95% CI, 1.040-3.479; P = 0.037).
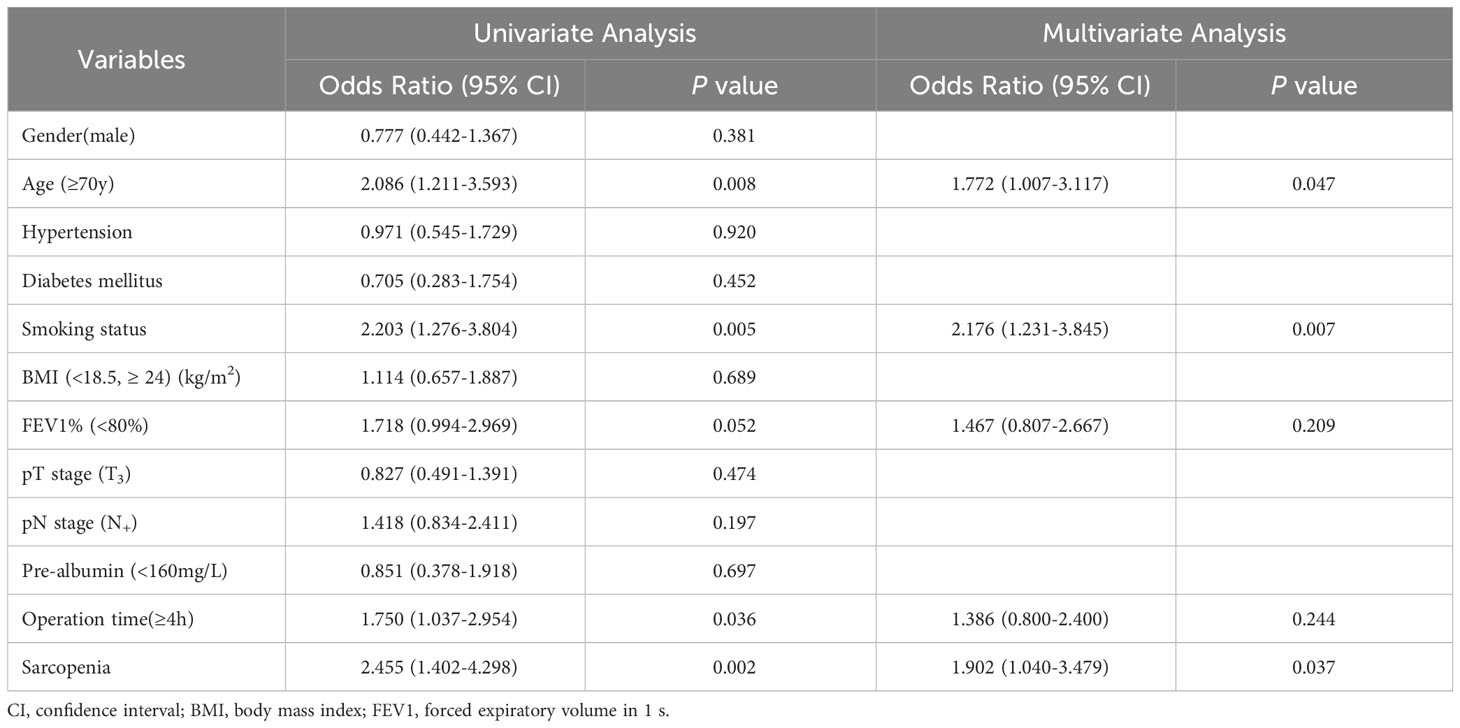
Table 3 Logistic regression model on postoperative pneumonia using univariate and multivariate analysis.
3.3 Prognostic significance of sarcopenia
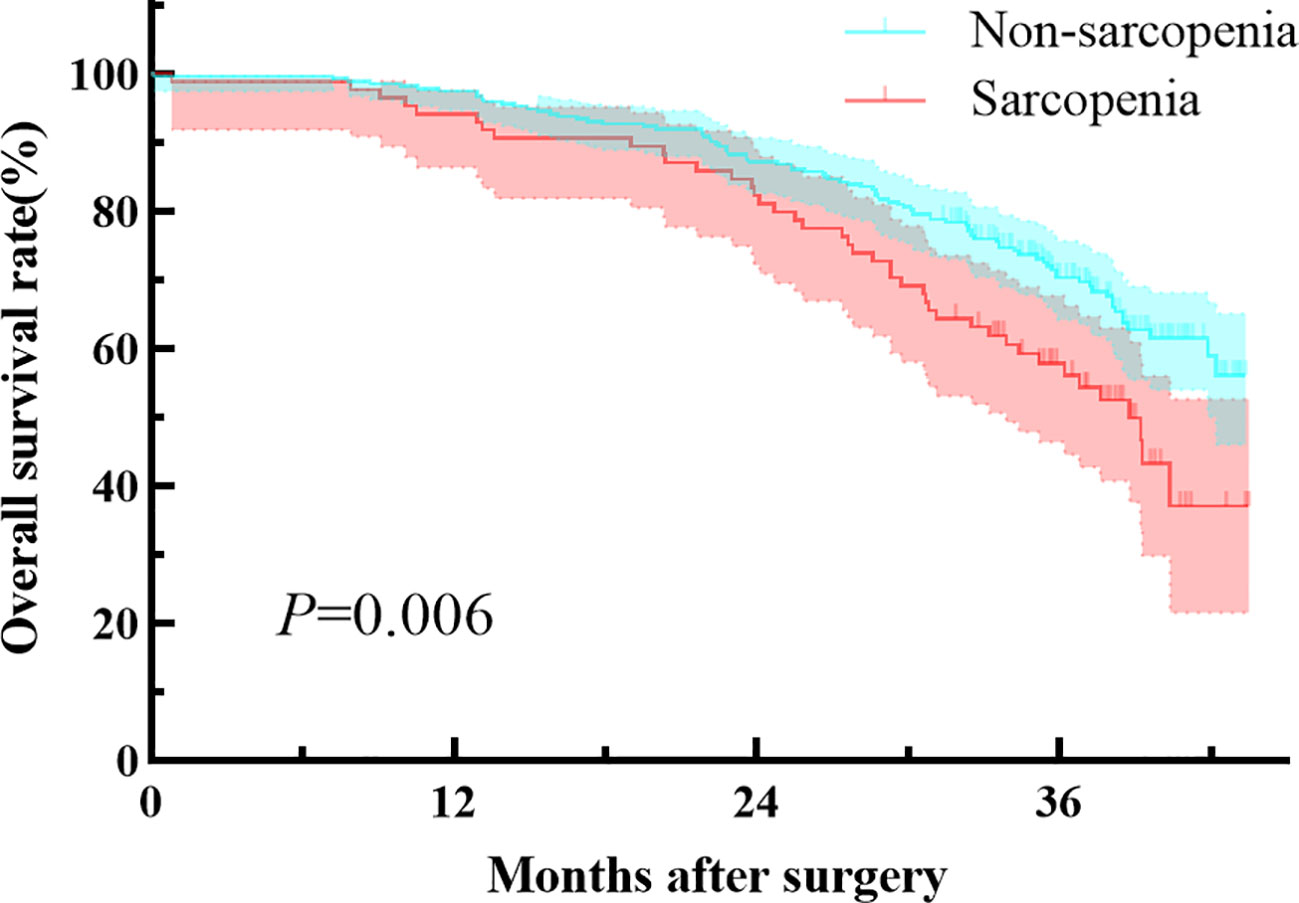
Patients with preoperative sarcopenia had worse overall survival compared to those without sarcopenia, with a median follow-up of 35.1 months (median 34.5 [IQR, 27.6-38.5] vs 35.2 [IQR, 31.9-38.9] months, P=0.006; Figure 1). The preoperative sarcopenia group and the non-sarcopenia group showed no statistical difference in the 2-year overall survival rate (82.1% vs 87.1%, P=0.377). However, patients with sarcopenia had significantly lower 3-year survival rate compared to those without sarcopenia (57.8% vs 70.2%, P=0.018). Table 4 presents the results of the Cox regression analysis of overall survival. The univariate analysis identified age (≥70y), pT stage (T3), pN stage (N+), and sarcopenia as factors associated with adverse overall survival. Additionally, the multivariate analysis confirmed that the presence of sarcopenia was a significant prognostic factor for overall survival (HR, 1.510; 95% CI, 1.033-2.206; P = 0.033).
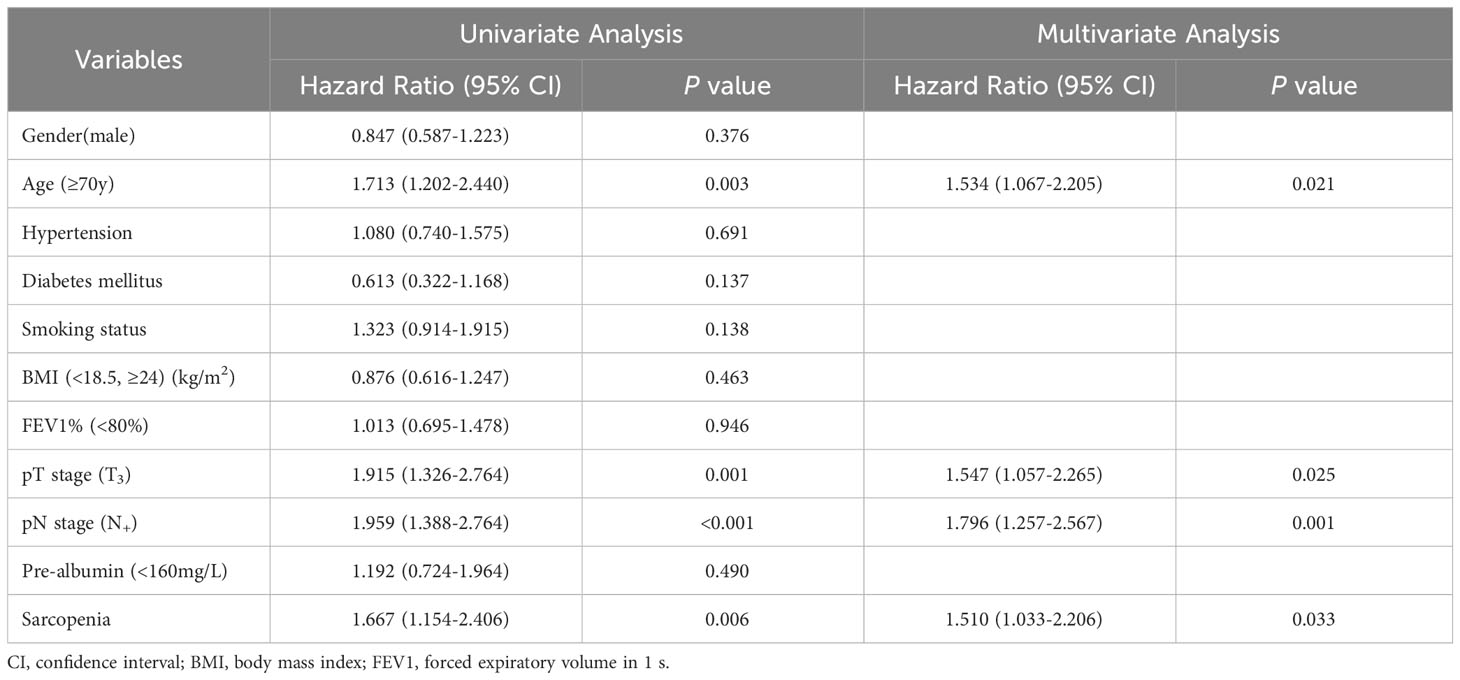
Table 4 Cox proportional hazard model of clinical characteristics on OS using univariate and multivariate analysis.
4 Discussion
This study demonstrated that sarcopenia, diagnosed by PMI and PEF, was identified as an independent risk factor for overall survival in elderly patients with resectable esophageal cancer. It was also found to be associated with adverse postoperative outcomes, and independently related to the occurrence of postoperative pneumonia.
Sarcopenia, characterized by progressive loss of muscle mass and strength, is a skeletal muscle disorder. Muscle function can be reliably assessed using muscle strength, which has been demonstrated in recent studies (6). The occurrence of sarcopenia can be indicated by a decrease in muscle strength, which is accompanied by the loss of muscle mass. The diagnosis of sarcopenia can be further supported by confirming the loss of muscle mass (7). The strength of respiratory muscles can be determined by measuring PEF, and low PEF is independently associated with the development of sarcopenia. This association is supported by a recent cross-sectional study conducted in the community (14, 20, 21). Our research findings indicate that patients with low PMI and low PEF have a significantly lower 3-year survival rate. These patients are also more susceptible to complications such as postoperative pneumonia and anastomotic leak. Therefore, the combined assessment of muscle mass and muscle strength provides a comprehensive evaluation of the patient’s skeletal muscle status.
Previous investigations have demonstrated that sarcopenia is linked to inferior overall survival in individuals diagnosed with esophageal cancer post-surgery (27–29). A comprehensive analysis revealed that patients affected by sarcopenia exhibited significantly lower 3-year (51.6% vs. 65.4%, P < 0.001) and 5-year overall survival rates (41.2% vs 52.2%, P = 0.018) in contrast to those without sarcopenia (12). Another systematic review, comprising six studies on long-term outcomes after esophagectomy, arrived at similar findings, underscoring sarcopenia as an autonomous risk factor contributing to unsatisfactory overall survival subsequent to surgery (8). Our research aligns with these outcomes. In patients undergoing neoadjuvant treatment, sarcopenia was also identified as a predictor of poorer overall survival (30–32). Consequently, our study excluded individuals receiving neoadjuvant therapy. Sarcopenia signifies a state of severe malnourishment. Our investigation uncovered that the sarcopenic cohort presented lower pre-albumin levels in comparison to the non-sarcopenic group, potentially leading to detrimental effects on long-term prognosis (33). Moreover, the inferior long-term prognosis can be attributed to the systemic inflammatory response incited by sarcopenia. Skeletal musculature not only functions in exercising and locomotion but also actively engages in intricate immunological and inflammatory processes by secreting diverse cytokines (34–36). Additionally, sarcopenia may heighten the occurrence of adverse reactions to radiotherapy and chemotherapy for esophageal cancer. Individuals affected by sarcopenia demonstrate an augmented susceptibility towards chemotherapy-induced toxic reactions when subjected to platinum-based chemotherapy regimens, which may ultimately result in dosage reduction or even discontinuation of chemotherapy, thereby profoundly influencing treatment outcomes (37–39). Our findings substantially support the clinical value of sarcopenia in nutritional risk assessment and prognostic evaluation.
Various studies have consistently shown that sarcopenia is linked to adverse perioperative results in individuals diagnosed with esophageal cancer, even though the diagnostic approaches employed may differ (40–43). Xu’s study identified sarcopenia, as diagnosed by low PMI, as an independent risk factor for postoperative pneumonia in these patients (44). Fehrenbach’s study also concluded that sarcopenia patients had an increased risk of major complications such as anastomotic leak and postoperative pneumonia, which aligns with our findings (40). However, a meta-analysis indicated that sarcopenia only correlated with postoperative pulmonary complications, not anastomotic leak, cardiac complications, or surgical site infection (8). It’s important to note that differences in diagnostic methods and the age distribution may have influenced these conclusions. Nakashima et al. reported that sarcopenia was an independent risk factor for anastomotic leak in patients aged 65 years and older, but not in those younger than 65 (11). Our study specifically focused on patients aged 65 years and older and found a correlation between sarcopenia and both postoperative pneumonia and anastomotic leak. Age plays a critical role in the development of sarcopenia, as elderly patients often experience more significant malnutrition and have impaired healing abilities, increasing their vulnerability to anastomotic leak (45, 46). Therefore, subgrouping by age is essential in studying sarcopenia. Additionally, elderly patients with sarcopenia are more susceptible to postoperative pneumonia, likely due to a decline in respiratory muscle strength (47). The PEF value reflects patients’ coughing ability, and effective coughing is crucial in preventing postoperative pneumonia (48). Consequently, preoperative detection of sarcopenia can significantly contribute to the perioperative assessment of patients.
A notable strength of this research is its employment of a convenient diagnostic technique that can be derived from CT scans and spirometry conducted before surgery. These procedures do not require extra radiation exposure or intricate measurements in a clinical environment. Furthermore, the study included a substantial number of participants who had undergone surgical removal of esophageal cancer, ensuring a homogeneous cohort. Moreover, there were adequate occurrences of events for each variable in all statistical analyses. The data collection regarding preoperative information and complications was carried out retrospectively in a logical manner, and the outcomes were adjusted for numerous common baseline factors.
There are some limitations to this investigation. Initially, the research was carried out retrospectively in a solitary establishment. Consequently, these discoveries necessitate validation through expansive, population-oriented prospective investigations. Furthermore, the inquiry depended on information concerning muscle mass and muscle strength acquired from spirometry and CT imaging prior to surgery, all at a solitary instance. In order to delve deeper into the prognostic importance of muscle mass and muscle strength in aged patients with esophageal cancer, forthcoming research should encompass longitudinal evaluations of alterations occurring both before and after surgery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
MZ: Writing – original draft, Formal analysis. YX: Writing – original draft, Formal analysis. MC: Writing – review & editing, Data curation. DX: Writing – review & editing, Data curation. KX: Writing – review & editing. WT: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Huaian Natural Science Research Program (HAB202201) and Jiangsu Provincial Medical Key Discipline Cultivation Unit (JWDS202233). These funding agencies were not involved in the study design, the collection, analysis and interpretation of data, writing the report, and the decision to submit the article for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1303877/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Bozzetti F, Mariani L, Lo Vullo S, SCRINIO Working Group, Amerio ML, Biffi R, et al. The nutritional risk in oncology: a study of 1,453 cancer outpatients. Support Care Cancer (2012) 20(8):1919–28. doi: 10.1007/s00520-012-1387-x
3. Anandavadivelan P, Lagergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol (2016) 13(3):185–98. doi: 10.1038/nrclinonc.2015.200
4. Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc (2016) 75(2):188–98. doi: 10.1017/S0029665115004279
5. Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla Ē, Prado CM. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc (2016) 75(2):199–211. doi: 10.1017/S002966511500419X
6. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing (2019) 48(1):16–31. doi: 10.1093/ageing/afy169
7. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc (2020) 21(3):300–7.e2. doi: 10.1016/j.jamda.2019.12.012
8. Boshier PR, Heneghan R, Markar SR, Baracos VE, Low DE. Assessment of body composition and sarcopenia in patients with esophageal cancer: a systematic review and meta-analysis. Dis Esophagus (2018) 31(8). doi: 10.1093/dote/doy047
9. Wang PY, Xu LD, Chen XK, Xu L, Yu YK, Zhang RX, et al. Sarcopenia and short-term outcomes after esophagectomy: A meta-analysis. Ann Surg Oncol (2020) 27(8):3041–51. doi: 10.1245/s10434-020-08236-9
10. Elliott JA, Doyle SL, Murphy CF, King S, Guinan EM, Beddy P, et al. Sarcopenia: prevalence, and impact on operative and oncologic outcomes in the multimodal management of locally advanced esophageal cancer. Ann Surg (2017) 266(5):822–30. doi: 10.1097/SLA.0000000000002398
11. Nakashima Y, Saeki H, Nakanishi R, Sugiyama M, Kurashige J, Oki E, et al. Assessment of sarcopenia as a predictor of poor outcomes after esophagectomy in elderly patients with esophageal cancer. Ann Surg (2018) 267(6):1100–4. doi: 10.1097/SLA.0000000000002252
12. Deng HY, Zha P, Peng L, Hou L, Huang KL, Li XY. Preoperative sarcopenia is a predictor of poor prognosis of esophageal cancer after esophagectomy: a comprehensive systematic review and meta-analysis. Dis Esophagus (2019) 32(3):doy115. doi: 10.1093/dote/doy115
13. Boshier PR, Klevebro F, Jenq W, Puccetti F, Muthuswamy K, Hanna GB, et al. Long-term variation in skeletal muscle and adiposity in patients undergoing esophagectomy. Dis Esophagus (2021) 34(11):doab016. doi: 10.1093/dote/doab016
14. Kera T, Kawai H, Hirano H, Kojima M, Fujiwara Y, Ihara K, et al. Relationships among peak expiratory flow rate, body composition, physical function, and sarcopenia in community-dwelling older adults. Aging Clin Exp Res (2018) 30(4):331–40. doi: 10.1007/s40520-017-0777-9
15. Nishi S, Miki Y, Imai T, Nambara M, Miyamoto H, Tamura T. et al. The evaluation of sarcopenia before neoadjuvant chemotherapy is important for predicting postoperative pneumonia in patients with esophageal cancer. Dig Surg (2023) 40(5):153–60. doi: 10.1159/000533185
16. Jogiat UM, Baracos V, Turner SR, Eurich D, Filafilo H, Rouhi A, et al. Changes in sarcopenia status predict survival among patients with resectable esophageal cancer. Ann Surg Oncol (2023) 30(12):7412–21. doi: 10.1245/s10434-023-13840-6
17. Harada T, Tsuji T, Ueno J, Hijikata N, Ishikawa A, Kotani D, et al. Association of sarcopenia with relative dose intensity of neoadjuvant chemotherapy in older patients with locally advanced esophageal cancer: A retrospective cohort study. J Geriatr Oncol (2023) 14(7):101580. doi: 10.1016/j.jgo.2023.101580
18. Zhang JX, Yan HT, Ding Y, Liu J, Liu S, Zu QQ, et al. Low Psoas-Muscle index is associated with decreased survival in hepatocellular carcinoma treated with transarterial chemoembolization. Ann Med (2022) 54(1):1562–9. doi: 10.1080/07853890.2022.2081872
19. Kera T, Kawai H, Ejiri M, Ito K, Hirano H, Fujiwara Y, et al. Respiratory sarcopenia is a predictor of all-cause mortality in community-dwelling older adults-The Otassha Study. J Cachexia Sarcopenia Muscle (2023) 14(4):1894–9. doi: 10.1002/jcsm.13266
20. Ridwan ES, Wiratama BS, Lin MY, Hou WH, Liu MF, Chen CM, et al. Peak expiratory flow rate and sarcopenia risk in older Indonesian people: A nationwide survey. PloS One (2021) 16(2):e0246179. doi: 10.1371/journal.pone.0246179
21. Kera T, Kawai H, Hirano H, Kojima M, Watanabe Y, Motokawa K, et al. Definition of respiratory sarcopenia with peak expiratory flow rate. J Am Med Dir Assoc (2019) 20(8):1021–5. doi: 10.1016/j.jamda.2018.12.013
22. Fujishima I, Fujiu-Kurachi M, Arai H, Hyodo M, Kagaya H, Maeda K, et al. Sarcopenia and dysphagia: Position paper by four professional organizations. Geriatr Gerontol Int (2019) 19(2):91–7. doi: 10.1111/ggi.13591
23. Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D’Journo XB, et al. International consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications consensus group (ECCG). Ann Surg (2015) 262(2):286–94. doi: 10.1097/SLA.0000000000001098
24. Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition (2016) 32(11-12):1200–5. doi: 10.1016/j.nut.2016.04.003
25. Caballero B. Humans against obesity: who will win? Adv Nutr (2019) 10(suppl_1):S4–9. doi: 10.1093/advances/nmy055
26. Zhou YQ, Zhang XM, Chen ZQ, Wang JL, Qian YB, Xu RY. The prevalence of hypophosphatemia and refeeding-related hypophosphatemia in hospitalized patients requiring parental nutrition: a retrospective study. Support Care Cancer (2022) 30(8):6995–7003. doi: 10.1007/s00520-022-07141-z
27. Srpcic M, Jordan T, Popuri K, Sok M. Sarcopenia and myosteatosis at presentation adversely affect survival after esophagectomy for esophageal cancer. Radiol Oncol (2020) 54(2):237–46. doi: 10.2478/raon-2020-0016
28. Park A, Orlandini MF, Szor DJ, Junior UR, Tustumi F. The impact of sarcopenia on esophagectomy for cancer: a systematic review and meta-analysis. BMC Surg (2023) 23(1):240. doi: 10.1186/s12893-023-02149-6
29. Chen L, Yu G, Zhao W, Ye B, Shu Y. A possible combined appraisal pattern: predicting the prognosis of patients after esophagectomy. World J Surg Oncol (2023) 21(1):155. doi: 10.1186/s12957-023-03020-x
30. Wakefield CJ, Hamati F, Karush JM, Arndt AT, Geissen N, Liptay MJ, et al. Sarcopenia after induction therapy is associated with reduced survival in patients undergoing esophagectomy for locally-advanced esophageal cancer. J Thorac Dis (2021) 13(2):861–9. doi: 10.21037/jtd-20-2608
31. Järvinen T, Ilonen I, Kauppi J, Salo J, Räsänen J. Loss of skeletal muscle mass during neoadjuvant treatments correlates with worse prognosis in esophageal cancer: a retrospective cohort study. World J Surg Oncol (2018) 16(1):27. doi: 10.1186/s12957-018-1327-4
32. Yoon HG, Oh D, Ahn YC, Noh JM, Pyo H, Cho WK, et al. Prognostic impact of sarcopenia and skeletal muscle loss during neoadjuvant chemoradiotherapy in esophageal cancer. Cancers (Basel) (2020) 12(4):925. doi: 10.3390/cancers12040925
33. Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr (2010) 29(2):154–9. doi: 10.1016/j.clnu.2009.12.004
34. Tan X, Peng H, Gu P, Chen M, Wang Y. Prognostic significance of the L3 skeletal muscle index and advanced lung cancer inflammation index in elderly patients with esophageal cancer. Cancer Manag Res (2021) 13:3133–43. doi: 10.2147/CMAR.S304996
35. Lin JX, Lin JP, Xie JW, Wang JB, Lu J, Chen QY, et al. Prognostic value and association of sarcopenia and systemic inflammation for patients with gastric cancer following radical gastrectomy. Oncologist (2019) 24(11):e1091–101. doi: 10.1634/theoncologist.2018-0651
36. Matsunaga T, Miyata H, Sugimura K, Motoori M, Asukai K, Yanagimoto Y, et al. Prognostic significance of sarcopenia and systemic inflammatory response in patients with esophageal cancer. Anticancer Res (2019) 39(1):449–58. doi: 10.21873/anticanres.13133
37. Panje CM, Höng L, Hayoz S, Baracos VE, Herrmann E, Garcia Schüler H, et al. Skeletal muscle mass correlates with increased toxicity during neoadjuvant radiochemotherapy in locally advanced esophageal cancer: A SAKK 75/08 substudy. Radiat Oncol (2019) 14(1):166. doi: 10.1186/s13014-019-1372-3
38. Vega MC, Laviano A, Pimentel GD. Sarcopenia and chemotherapy-mediated toxicity. Einstein (Sao Paulo) (2016) 14(4):580–4. doi: 10.1590/S1679-45082016MD3740
39. Xu YY, Zhou XL, Yu CH, Wang WW, Ji FZ, He DC, et al. Association of sarcopenia with toxicity and survival in postoperative recurrent esophageal squamous cell carcinoma patients receiving chemoradiotherapy. Front Oncol (2021) 11:655071. doi: 10.3389/fonc.2021.655071
40. Fehrenbach U, Wuensch T, Gabriel P, Segger L, Yamaguchi T, Auer TA, et al. CT body composition of sarcopenia and sarcopenic obesity: predictors of postoperative complications and survival in patients with locally advanced esophageal adenocarcinoma. Cancers (Basel) (2021) 13(12):2921. doi: 10.3390/cancers13122921
41. Nagata K, Tsujimoto H, Nagata H, Harada M, Ito N, Kanematsu K, et al. Impact of reduced skeletal muscle volume on clinical outcome after esophagectomy for esophageal cancer: A retrospective study. Medicine (Baltimore) (2018) 97(30):e11450. doi: 10.1097/MD.0000000000011450
42. Soma D, Kawamura YI, Yamashita S, Wake H, Nohara K, Yamada K, et al. Sarcopenia, the depletion of muscle mass, an independent predictor of respiratory complications after oncological esophagectomy. Dis Esophagus (2019) 32(3):doy092. doi: 10.1093/dote/doy092
43. Poisson J, Martinez-Tapia C, Heitz D, Geiss R, Albrand G, Falandry C, et al. Prevalence and prognostic impact of cachexia among older patients with cancer: a nationwide cross-sectional survey (NutriAgeCancer). J Cachexia Sarcopenia Muscle (2021) 12(6):1477–88. doi: 10.1002/jcsm.12776
44. Xu Z, Wang Q, Zhang Z, Zhu Y, Chen Y, Tang D, et al. Association between preoperative diagnosis of sarcopenia and postoperative pneumonia in resectable esophageal squamous cell carcinoma patients: a retrospective cohort study. Front Oncol (2023) 13:1144516. doi: 10.3389/fonc.2023.1144516
45. Sousa AS, Guerra RS, Fonseca I, Pichel F, Amaral TF. Sarcopenia among hospitalized patients - A cross-sectional study. Clin Nutr (2015) 34(6):1239–44. doi: 10.1016/j.clnu.2014.12.015
46. Baranov NS, Slootmans C, van Workum F, Klarenbeek BR, Schoon Y, Rosman C. Outcomes of curative esophageal cancer surgery in elderly: A meta-analysis. World J Gastrointest Oncol (2021) 13(2):131–46. doi: 10.4251/wjgo.v13.i2.131
47. Okazaki T, Ebihara S, Mori T, Izumi S, Ebihara T. Association between sarcopenia and pneumonia in older people. Geriatr Gerontol Int (2020) 20(1):7–13. doi: 10.1111/ggi.13839
Keywords: esophageal cancer, sarcopenia, psoas muscle mass index, peak expiratory flow, complications, survival
Citation: Zhang M, Xiong Y, Chen M, Xu D, Xu K and Tian W (2023) Psoas muscle mass index and peak expiratory flow as measures of sarcopenia: relation to outcomes of elderly patients with resectable esophageal cancer. Front. Oncol. 13:1303877. doi: 10.3389/fonc.2023.1303877
Received: 03 October 2023; Accepted: 09 November 2023;
Published: 28 November 2023.
Edited by:
Xi Yang, Fudan University, ChinaReviewed by:
Yunkui Zhang, Shanxi Provincial Cancer Hospital, ChinaJiaping Li, First Affiliated Hospital of Wannan Medical College, China
Copyright © 2023 Zhang, Xiong, Chen, Xu, Xu and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keping Xu, ZG9jdG9yeHVrZXBpbmdAMTI2LmNvbQ==; Wenze Tian, aGF5eXR3ekBuam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Mingzhi Zhang
Mingzhi Zhang Yaqiong Xiong2†
Yaqiong Xiong2† Wenze Tian
Wenze Tian