- Clinical Laboratory, Lishui People’s Hospital, The Sixth Affiliated Hospital of Wenzhou Medical University, Lishui, Zhejiang, China
Background: There are many studies regarding the use of systemic immune-inflammation index (SII) to help predict oral squamous cell carcinoma (OSCC) prognosis, but findings have been inconsistent. The present meta-analysis was conducted to determine whether SII could contribute to predicting OSCC prognosis.
Methods: PubMed, Embase, Cochrane Library and Web of Science databases were thoroughly searched from their inceptions through August 20, 2023. The role of SII in predicting OSCC prognosis was determined through combined hazard ratios (HRs) with relevant 95% confidence intervals (CIs). Correlations of SII with clinicopathological characteristics of OSCC patients were analyzed based on combined odds ratios (ORs) with 95% CIs.
Results: This meta-analysis utilized 11 articles in total, involving 3,464 patients. According to the results, an elevated SII was markedly associated with dismal overall survival (OS) (HR=1.85, 95%CI=1.48-2.29, p<0.001) and poor disease-free survival (DFS) (HR=1.77, 95%CI=1.20-2.61, p=0.004) of OSCC. Moreover, a higher SII was markedly correlated with stage T3-T4 (OR=2.47, 95%CI=1.40-4.37, p=0.002), TNM stage III-IV (OR=2.29, 95%CI=1.53-3.44, p<0.001), and low differentiation (OR=1.74, 95%CI=1.25-2.43, p=0.001).
Conclusion: According to the present meta-analysis, an increased SII is significantly associated with dismal OS and DFS, advanced tumor stage and poor differentiation in OSCC. SII could be a potential and important biomarker for clinical management and predicting the prognosis of patients with OSCC.
Systematic review registration: https://inplasy.com/inplasy-2023-9-0033/), identifier INPLASY202390033.
Introduction
Head and neck cancer (HNC) is the sixth most common cancer across the world, affecting nearly 650,000 patients and contributing to 350,000 deaths every year (1, 2). Oral squamous cell carcinoma (OSCC), has the highest morbidity in HNC and constitutes 48% of all HNC cases (3). Moreover, OSCC includes cancers that occur in the lips, gums, tongue, mouth, and palate (4). Although there have been improvements in multidisciplinary collaboration and comprehensive therapy, such as surgery, radiotherapy, and chemotherapy, OSCC has had a low 5-year survival rate (under 50%) over the past two decades (5). Nowadays, the tumor-node-metastasis (TNM) classification system is widely used to guide the selection of treatment strategies and predict survival outcomes; however, patients of an identical TNM stage can have diverse disease courses (6). Therefore, identifying reliable and cost-effective prognostic markers for OSCC patients is urgently needed to intervene treatment measures and improve overall prognosis.
Accumulating evidence has shown that cancer-related immune and inflammatory responses have pivotal effects on tumor occurrence, growth, invasion, and progression (7). Many blood-based indexes that reflect inflammatory statuses have been identified as prognostic biomarkers in different cancer types. These indexes include neutrophil-to-lymphocyte ratio (NLR) (8), platelet-to-lymphocyte ratio (PLR) (9), C-reactive protein/albumin ratio (CAR) (10), lymphocyte-monocyte ratio (LMR) (11) and lymphocyte-to-C-reactive protein ratio (LCR) (12). Systemic immune-inflammation index (SII), a hematological parameter, is calculated by the following formula: SII = (platelet number × neutrophil number)/lymphocyte number. Moreover, SII has been widely demonstrated to significantly predict diverse cancer prognostic outcomes, such as thyroid cancer (13), cholangiocarcinoma (14), hepatocellular carcinoma (HCC) (15), glioma (16), and pancreatic cancer (17). The ability of SII to predict OSCC prognosis has been explored previously, but no consistent findings have been reported (18–28). For example, a higher SII was reported as a distinct prognostic indicator of OSCC in certain articles (19, 26, 28). In contrast, some researchers indicated the absence of any obvious association of SII with survival outcomes in OSCC (23–25). Consequently, to identify the precise impact of SII on predicting OSCC prognosis, this work carried out comprehensive literature retrieval for meta-analysis. Furthermore, the relationship between SII and clinicopathological features of OSCC patients was also investigated.
Materials and methods
Study guideline and protocol registration
The present study was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (29), and registered in INPLASY (registration ID: INPLASY202390033, https://inplasy.com/inplasy-2023-9-0033/).
Literature retrieval
Literature was retrieved from the PubMed, Embase, Cochrane Library and Web of Science databases, starting with the earliest possible date through August 20, 2023. The following terms were used to search and select literature for the meta-analysis: (systemic immune-inflammatory index or SII or systemic immune-inflammation index or systemic-immune-inflammation index) and (oral squamous cell carcinoma or OSCC or oral cancer or tongue cancer or mouth cancer or oral carcinoma or oral cavity cancer or lip cancer or gingiva cancer). More details about these search strategies are provided in Supplementary File 1. Only English publications were considered. Besides, references in each publication were manually retrieved to identify the possible relevant articles.
Study eligibility criteria
Included studies had the following features (1): pathological diagnosis of primary OSCC (2); explored a relationship between pre-treatment SII and OSCC prognosis (3); hazard ratios (HRs) with 95% confidence intervals (CIs) can be determined according to the available data (4); the threshold SII is identified; and (5) articles written in the English language. Exclusion criteria were as follows (1): meeting abstracts, reviews, letters, comments, and case reports (2); does not have sufficient or available data (3); contains overlapped patients; and (4) animal studies.
Data collection and quality evaluation
Qualified publications were evaluated by two independent reviewers (JZ, SD), who also extracted data. Any discrepancy was settled through negotiation until a consensus was reached. Data collected included, first author, publication year, study country/region, sample size, age, gender, study center, study design, study period, tumor subsite, TNM stage, treatment, threshold, threshold determination approach, survival outcomes, survival analysis type, follow-up, HRs and 95% CIs. Our primary and secondary outcomes were overall survival (OS) and disease-free survival (DFS), separately. We employed the Newcastle–Ottawa Scale (NOS) for assessing study quality (30). The NOS contains three perspectives, selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points), with a total score of 0-9 points. NOS scores ≥ 6 indicate high-quality.
Statistical analysis
Significance of SII in predicting OSCC prognosis was estimated based on combined HRs with 95% CIs. Additionally, I2 statistics and Cochrane’s Q test were utilized to evaluate inter-study heterogeneity. The random-effects model was utilized in the case of obvious heterogeneity (I2>50%, P<0.10), otherwise, a fixed-effects model was applied. The source of heterogeneity was detected by different factors-stratified subgroup analyses. Correlations of SII with clinicopathological characteristics of OSCC were evaluated through combined odds ratios (ORs) as well as 95% CIs. Sensitivity analysis was used to compare pooled effects, by eliminating one individual study in the sequence and observing any potential changes to the result, repeating the process for each study. We performed Egger’s and Begg’s tests for assessing publication bias, and conducted statistical analyses using Stata version 12.0 (Stata Corporation, College Station, TX, USA). P-values < 0.05 were defined as statistically significant differences.
Results
Study screening
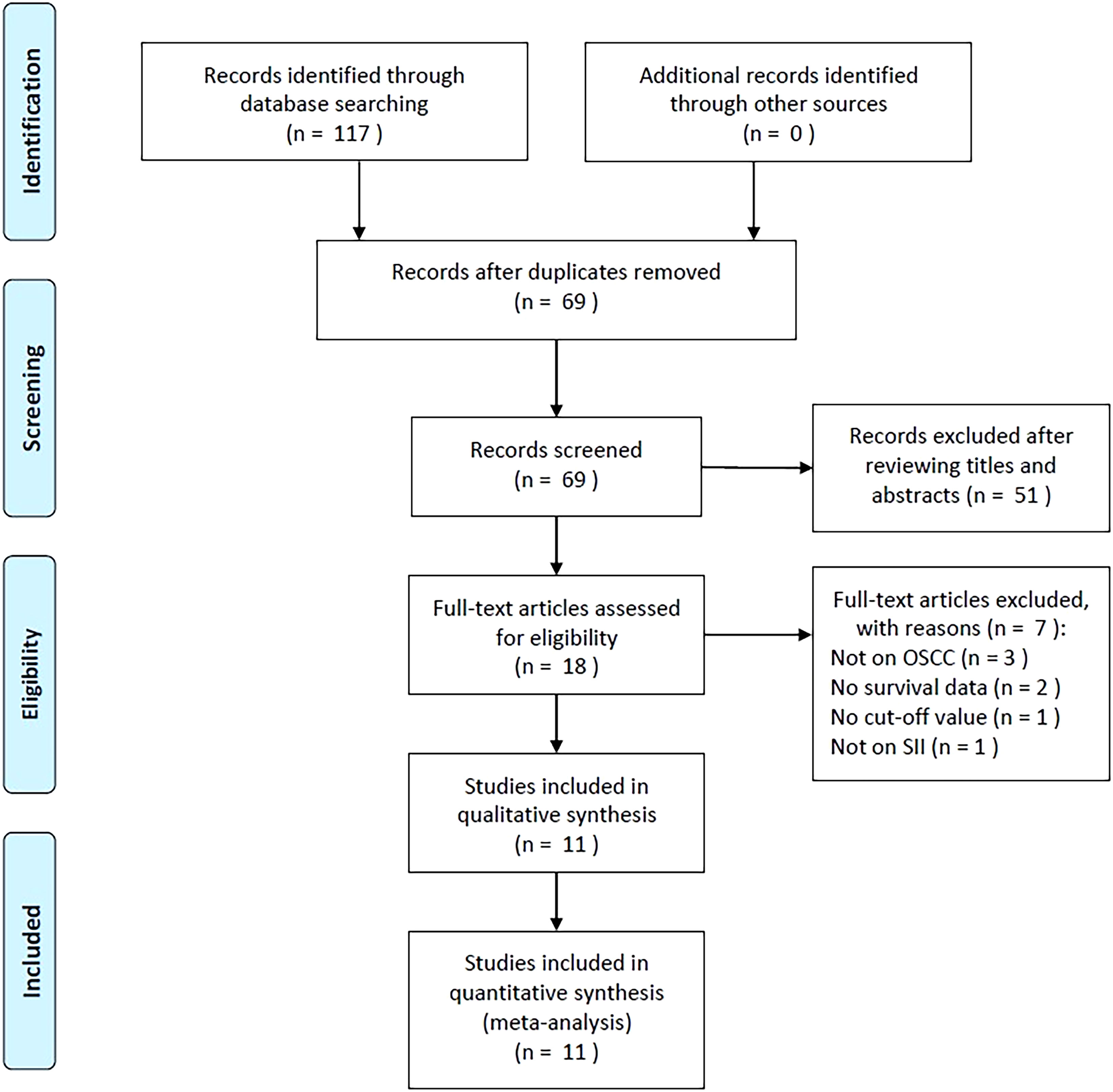
There were 117 articles obtained initially, among which 69 were retained following the removal of duplicates (Figure 1). Through title- and abstract-selection, 51 articles were then excluded due to irrelevance. Full-text review of the remaining 18 articles was conducted, among which, seven were eliminated for the following reasons, not focused on OSCC (n=3), no survival data provided (n=2), no cut-off value (n=1), and no report on SII (n=1). Ultimately, 11 articles were utilized for the remainder of the analysis, involving a total of 3,464 patients (18–28) (Figure 1, Table 1).
Enrolled study features
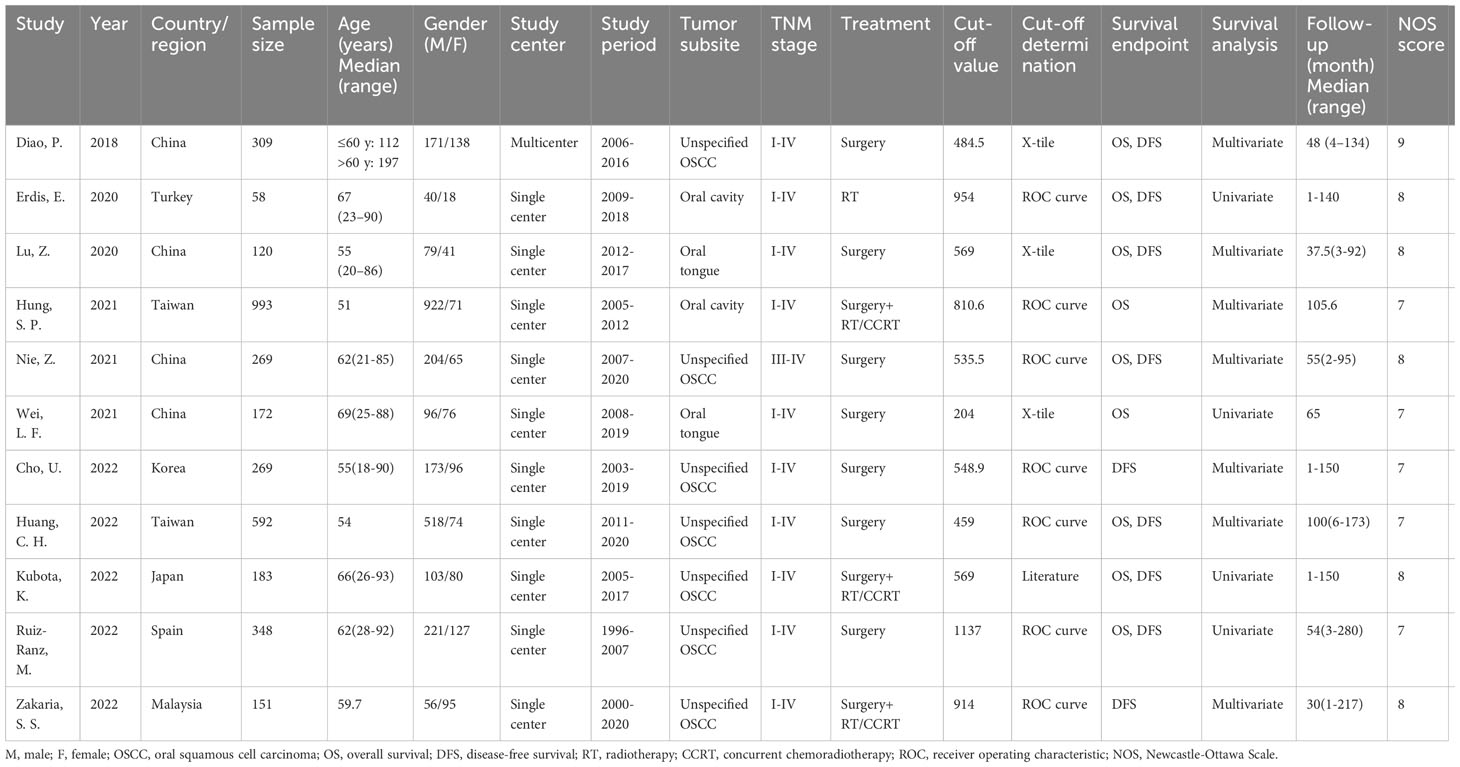
Table 1 provides baseline study features (18–28). All included studies were retrospective in nature, published in the English language and had publication years ranging from 2018 to 2022. Four studies were carried out in China (18, 20, 22, 23), two in Taiwan (21, 25), and one each in Turkey (19), Korea (24), Japan (26), Spain (27), and Malaysia (28). Sample sizes ranged from 58-993 (median, 269). Ten articles described single center studies (19–28) and one was a multicenter study (18). Seven studies recruited patients with OSCC (18, 22, 24–28), two recruited oral cavity cancer cases (19, 21), and two involved tongue cancer cases (20, 23). Ten articles described studies involving patients with TNM stage I-IV (18–21, 23–28), whereas one study only included stage III-IV patients (22). Seven studies treated patients with surgery (18, 20, 22–25, 27), three studies used surgery and concurrent chemoradiotherapy (CCRT) (21, 26, 28), and one study only applied radiotherapy (RT) (19). The threshold SII ranged from 204-1,137 (median, 569) in all 11 studies. Seven articles described the threshold through receiver operating characteristic curve (19, 21, 22, 24, 25, 27, 28), three studies applied the X-tile software (18, 20, 23), whereas another one was determined using previous literature (26). Nine articles reported a prognostic effect of SII for OS (18–23, 25–27) and nine mentioned a relationship between SII and DFS (18–20, 22, 24–28) in OSCC. Seven articles mentioned HRs with 95% CIs based on multivariate regression (18, 20–22, 25, 26, 28) and four studies used univariate analyses (19, 23, 24, 27). For all enrolled articles, NOS scores were from 7-9 (median, 8), demonstrating high quality (Table 1).
SII and OS of OSCC
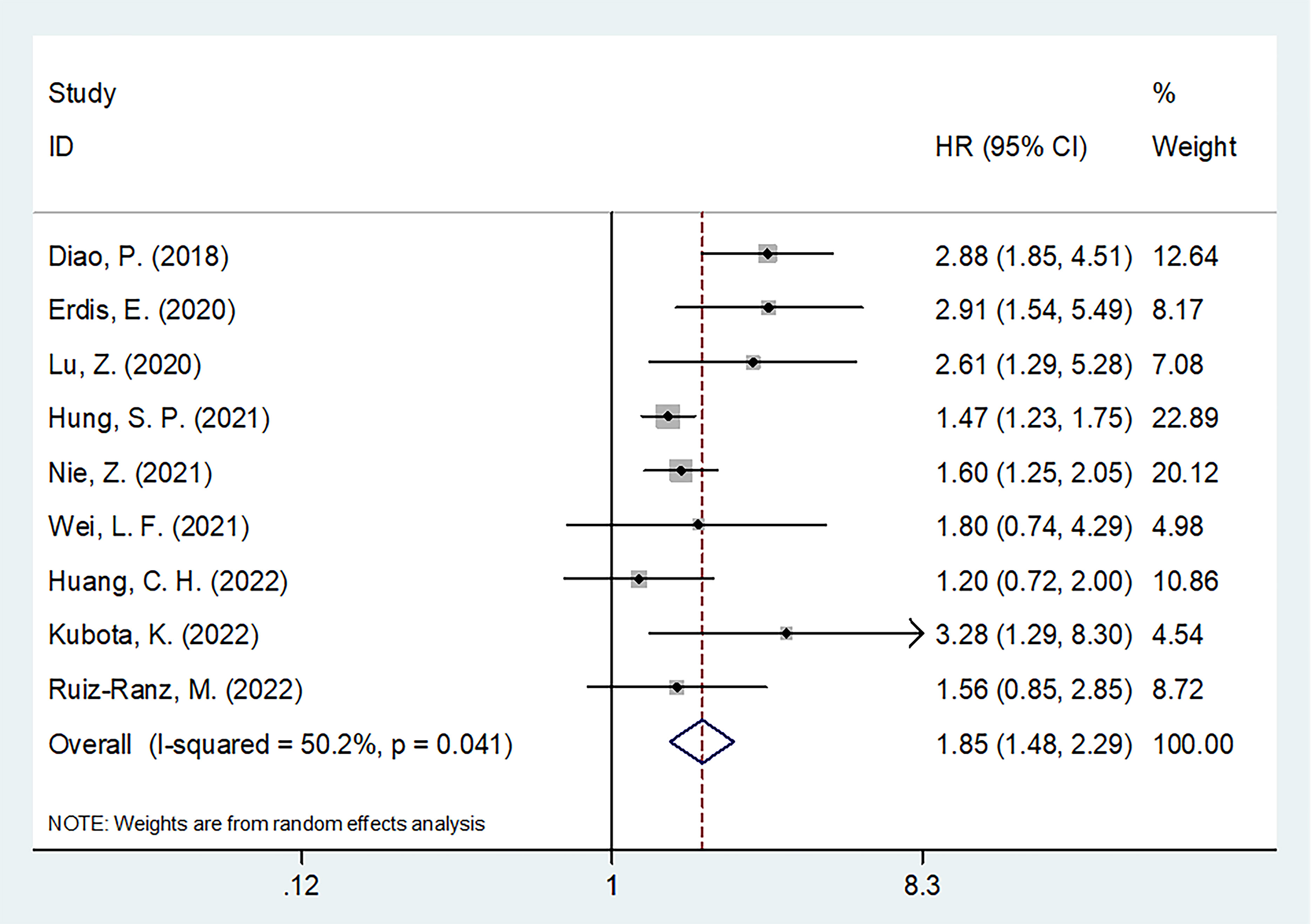
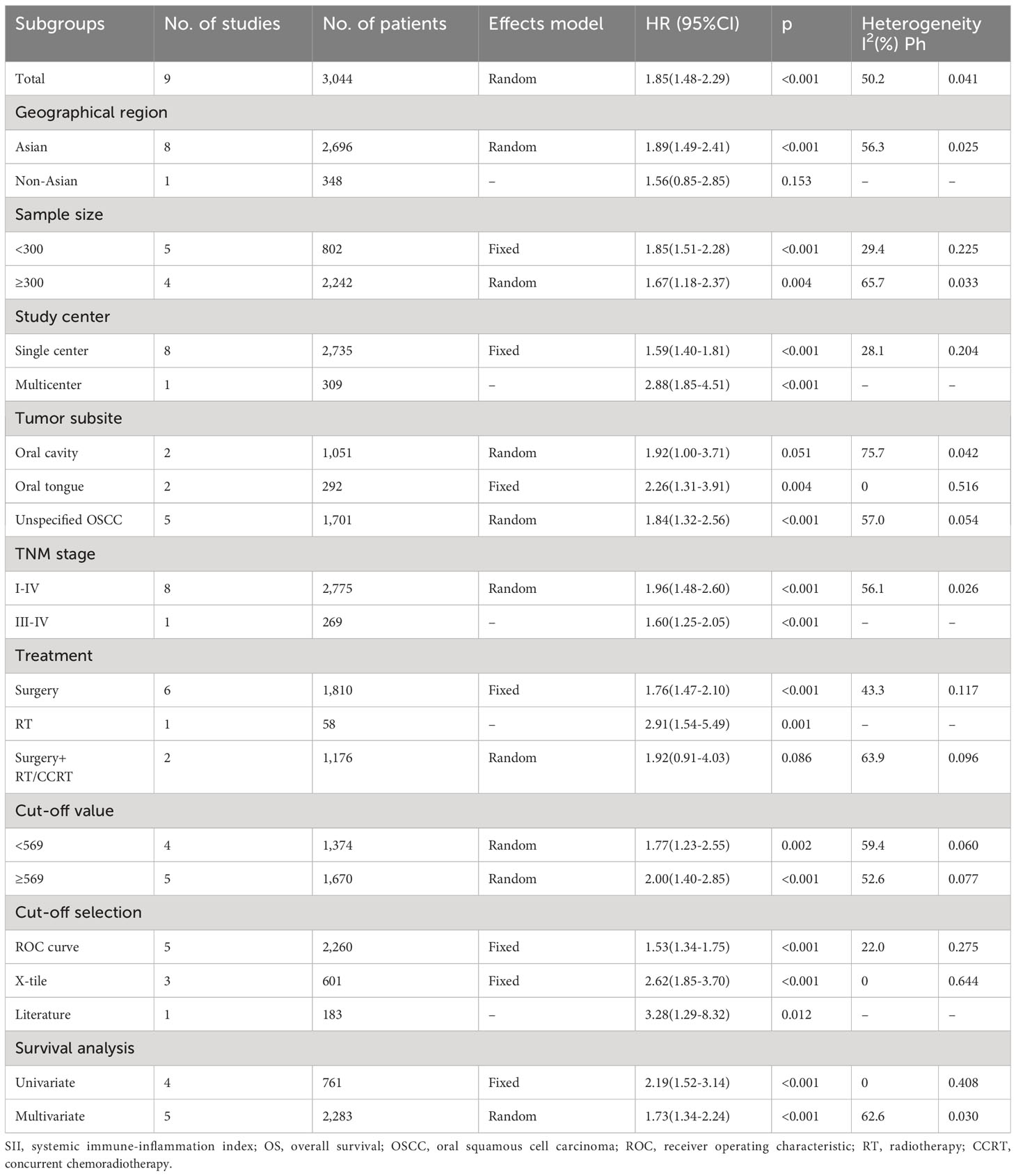
Nine articles, involving 3,044 patients (18–23, 25–27), mentioned a significance of SII to predict OS in OSCC. Due to significant heterogeneity (I2 = 50.2%, p=0.041), we selected the random-effects model. According to Figure 2 and Table 2, HR=1.85, 95%CI=1.48-2.29, and p<0.001, which indicates that a higher SII was markedly related to the dismal OS of OSCC patients. According to subgroup analyses, sample size, study center, TNM stage, threshold, threshold determination method, and survival analysis type did not affect the significant role of SII to predict OS (p<0.05; Table 2). Moreover, higher SII still significantly predicted poor OS in the following subgroups: in Asian regions (p<0.001), tongue tumor site (p=0.004) or OSCC (p<0.001), and patients who received surgery (p<0.001) or RT (p=0.001) (Table 2).
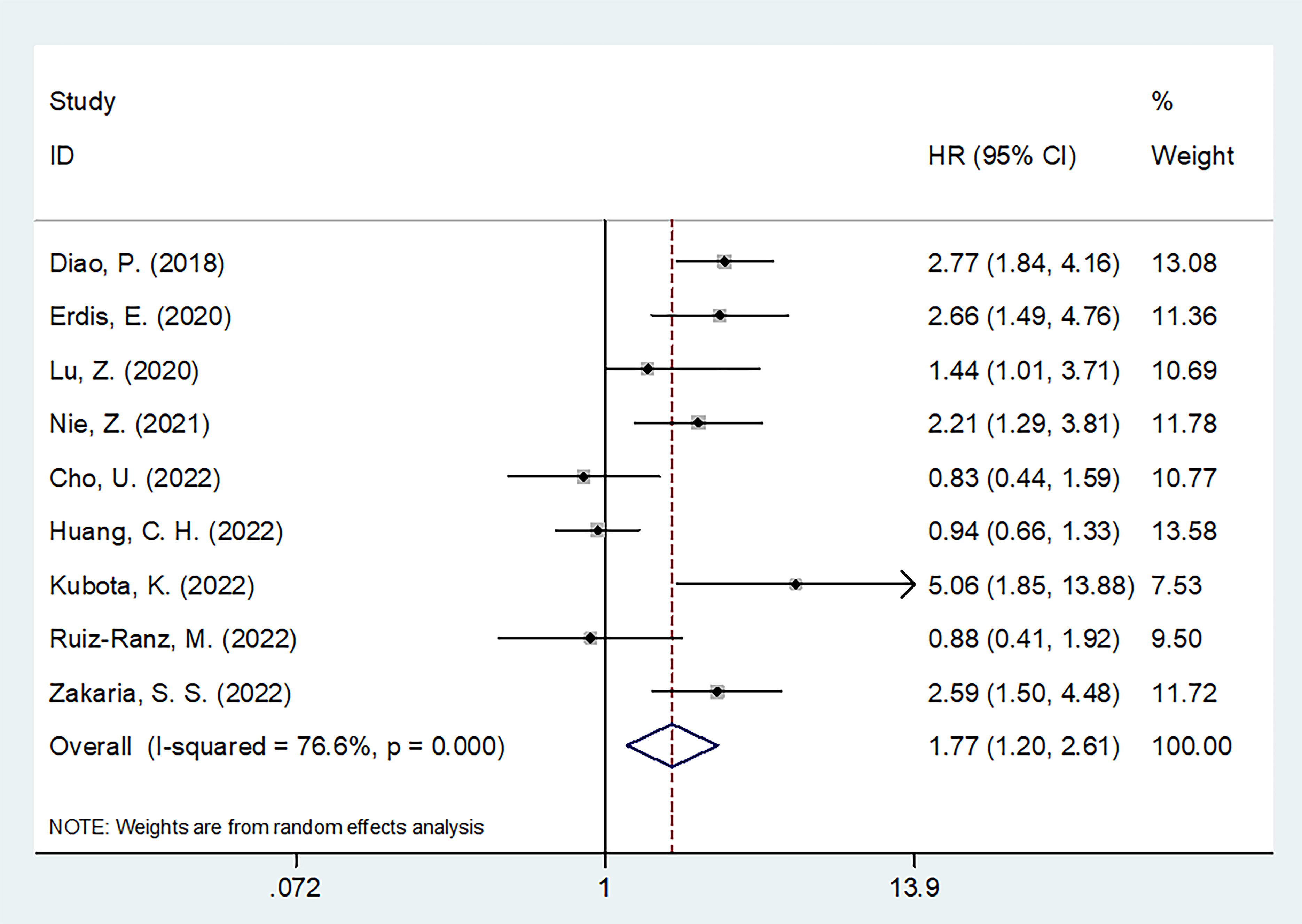
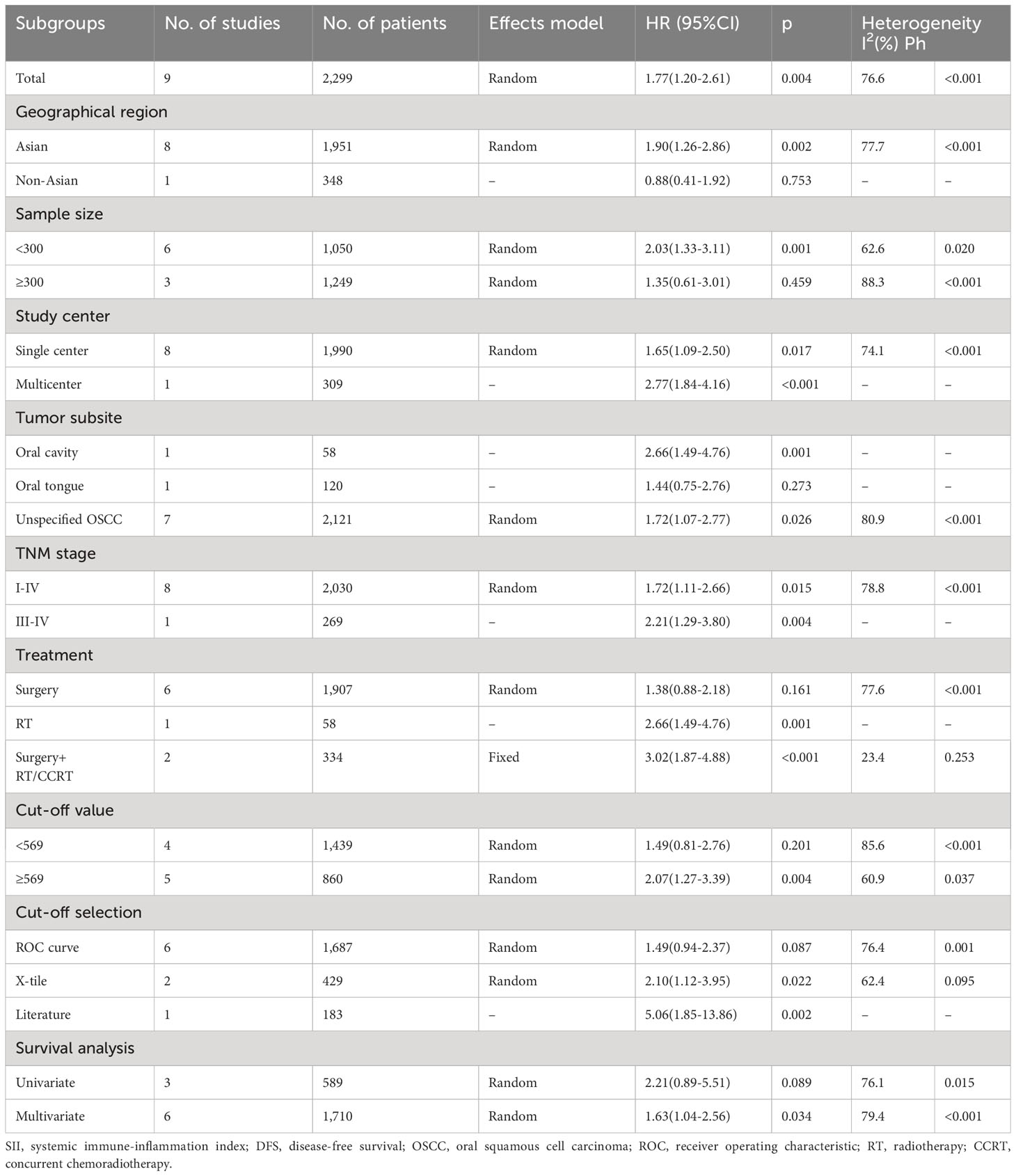
SII and DFS in OSCC
Altogether, nine articles, involving 2,299 patients (18–20, 22, 24–28), mentioned the prognostic effect of SII for DFS in OSCC. Based on our pooled results, higher SII was significantly related to inferior DFS in OSCC (HR=1.77, 95%CI=1.20-2.61, p=0.004) (Figure 3; Table 3). According to subgroup analyses, high SII significantly predicted DFS, and remained unaffected by the study center or TNM stage (p<0.05; Table 3). Additionally, elevated SII was markedly related to dismal DFS for the following subgroups: in Asian regions (p=0.002), sample size < 300 (p=0.001), multicenter studies (p<0.001), oral cavity tumor site (p=0.001) or OSCC (p=0.026), patients who received RT (p=0.001) or surgery + CCRT (p<0.001), SII threshold ≥ 569 (p=0.004), threshold determined by X-tile (p=0.022) or literature (p=0.002), and multivariate analysis (p=0.034) (Table 3).
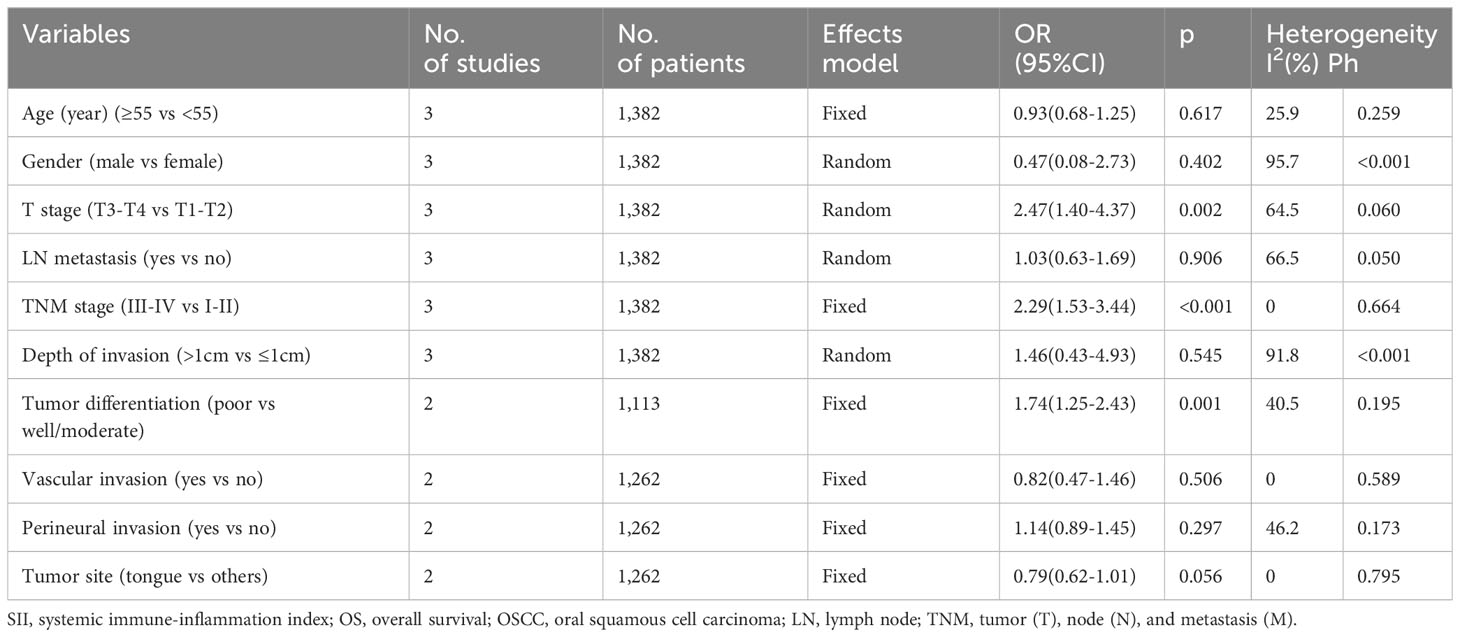
Association of SII with clinicopathological characteristics of OSCC
Three studies, encompassing 1,382 patients (20, 21, 24), presented data explaining a relationship of SII with OSCC clinicopathological features. According to the combined results, shown in Table 4, Figures 4 and 5, higher SII was remarkably related to stages T3-T4 (OR=2.47, 95%CI=1.40-4.37, p=0.002), TNM stages III-IV (OR=2.29, 95%CI=1.53-3.44, p<0.001), and low differentiation (OR=1.74, 95%CI=1.25-2.43, p=0.001). However, SII did not show any significant correlation with age (OR=0.93, 95%CI=0.68-1.25, p=0.617), gender (OR=0.47, 95%CI=0.08-2.73, p=0.402), tumor site (OR=0.79, 95%CI=0.62-1.01, p=0.056), lymph node metastasis (OR=1.03, 95%CI=0.63-1.69, p=0.906), invasion depth (OR=1.46, 95%CI=0.43-4.93, p=0.545), vascular invasion (OR=0.82, 95%CI=0.47-1.46, p=0.506), or perineural invasion (OR=1.14, 95%CI=0.89-1.45, p=0.297) (Table 4, Figures 4, 5).
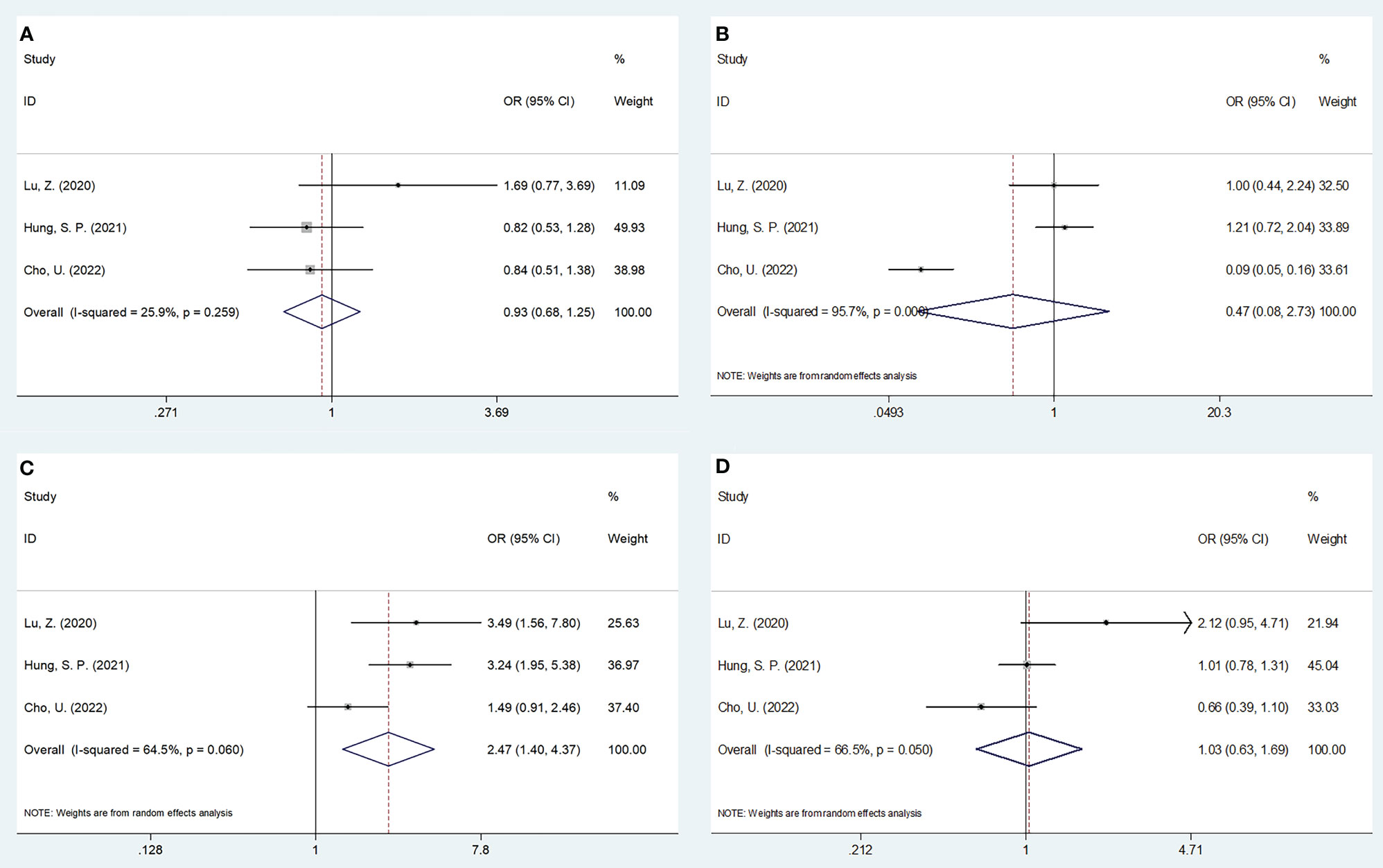
Figure 4 Forest plots on the association between SII and clinicopathological features in OSCC. (A) age (year) (≥55 vs <55); (B) gender (male vs female); (C) T stage (T3-T4 vs T1-T2); and (D) lymph node metastasis (yes vs no).
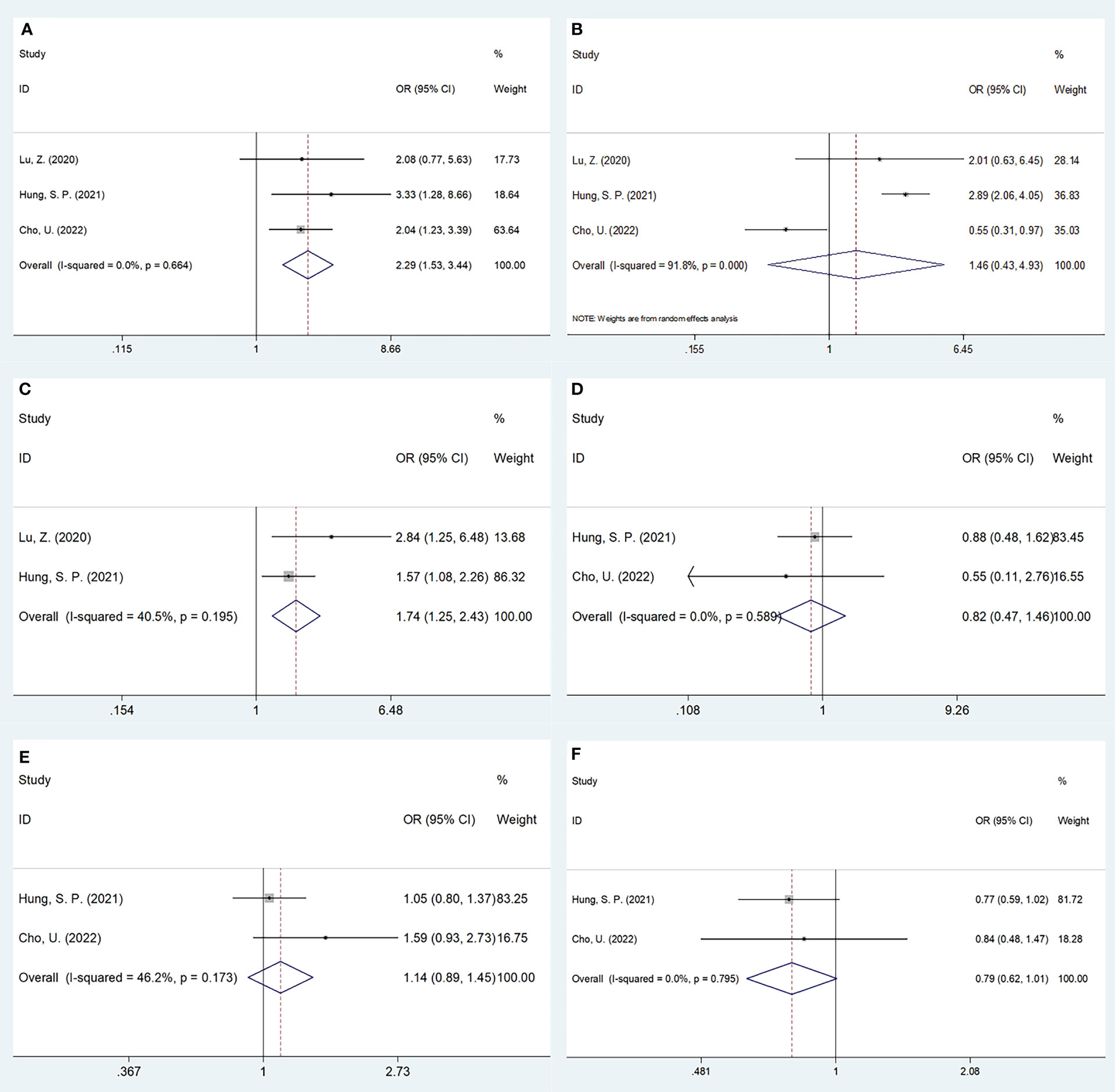
Figure 5 Forest plots on the association between SII and clinicopathological features in OSCC. (A) TNM stage (III-IV vs I-II); (B) depth of invasion (>1cm vs ≤1cm); (C) tumor differentiation (poor vs well/moderate); (D) vascular invasion (yes vs no); (E) perineural invasion (yes vs no); and (F) tumor site (tongue vs others).
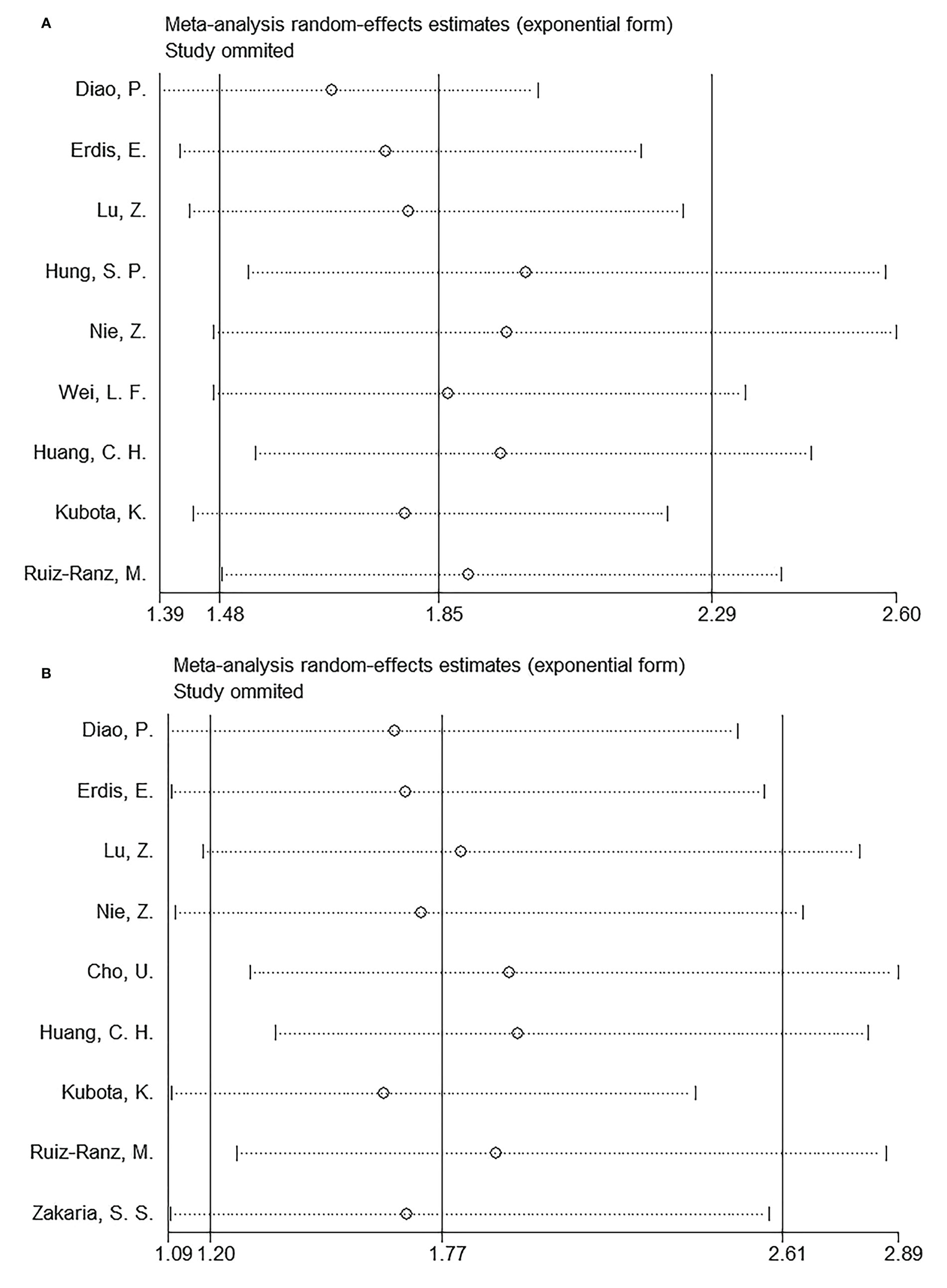
Sensitivity analysis
Every article was removed individually during each sensitivity analysis. Results were recalculated each time, based on the remaining studies’ OS and DFS. According to Figure 6, in the overall analysis of OS and DFS, there was no significant difference after eliminating each work, suggesting the reliability of our combined results.
Publication bias
Begg’s funnel plots and the Egger’s test were conducted to assess possible publication bias. The funnel plots observed in Figure 7 show symmetry, suggesting no significant publication bias for OS (p=0.175 and p=0.082 upon Begg’s and Egger’s tests, separately) or DFS (p=1 and p=0.542 upon Begg’s and Egger’s tests, separately).
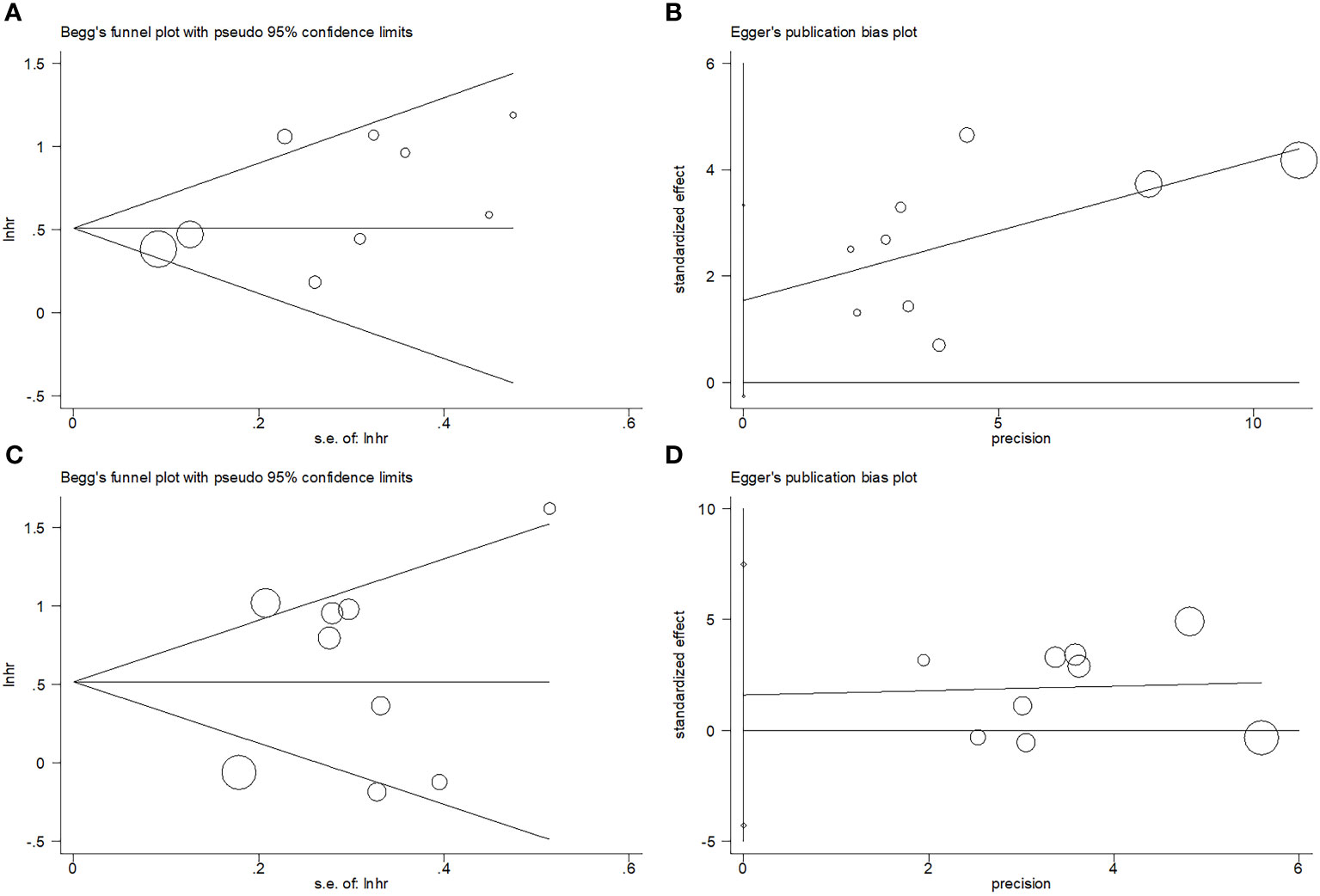
Figure 7 Publication bias test. (A) Begg’s test for OS, p=0.175; (B) Egger’s test for OS, p=0.082; (C) Begg’s test for DFS, p=1; and (D) Egger’s test for DFS, p=0.542.
Discussion
Previously, the effect of SII to predict OSCC prognosis has been explored, but no consistent findings have been reported (18–28). This work combined results from 11 articles involving 3,464 patients. According to our results, an elevated SII was remarkably related to dismal OS and inferior DFS of OSCC. Moreover, SII had a stable role when predicting prognosis, as examined by sensitivity, subgroup, and publication basis analyses. Higher SII was also evidently related to T3-T4, TNM III-IV, and poor tumor differentiation. Taken together, a higher SII significantly predicted the short- and long-term survival of OSCC, which was also dramatically related to tumor metastasis and poor differentiation. To our knowledge, this is the first meta-analysis investigating whether SII could be used to predict OSCC prognosis.
To understand the biological mechanism behind SII’s prognostic value, it is necessary to understand the function of neutrophils, platelets, and lymphocytes. First, neutrophils release inflammatory mediators such as neutrophil elastase, interleukin-8 (IL-8) and matrix metalloproteinase-9 (MMP-9) which enhance tumor cell growth, migration and invasion (31). Increased neutrophil counts can also produce reactive oxygen species, nitric oxide, and arginase, resulting in disordered T cell activation (32). Consequently, the body loses its ability to target tumor cells, indirectly contributing to tumor progression (33). Second, platelets can protect cancer cells from natural killer cells and tumor necrosis factor-α (TNF-α) by using glycoprotein (GP) receptors and tumor cell integrin α vβ-dependent pathway (34). Platelets also induce epithelial-mesenchymal transition and support transendothelial migration in circulating tumor cells, ultimately protecting tumor cells from immune destruction and promoting distant metastasis (35, 36). Third, lymphocytes are responsible for the adaptive immune response and participate in cancer immunosurveillance and immunoediting. Tumor-infiltrating lymphocytes promote tumor cell apoptosis and remove dead cells by way of humoral and cellular immunity, and these processes are necessary for the host’s immune defense and surveillance (37). Therefore, SII has a biological rationale for its role in predicting OSCC prognosis. Notably, a recent single study by Yoshimura et al. investigated the prognostic effect of multiple inflammation-nutrition parameters including NLR, PLR, LMR, CRP-albumin ratio (CAR), Glasgow prognostic score (GPS), modified GPS (mGPS), prognostic nutritional index (PNI), controlling nutrition status (CONUT), and modified CONUT (mCONUT) in patients with OSCC receiving surgery (38). They found that a low PNI was associated with shorter OS and DFS in patients with OSCC through multivariate analysis (38). Although that study did not include SII for analysis in OSCC, their results were important to investigate mechanisms (38). In peripheral blood analyses, inflammation-related markers were mainly composed of upregulated factors (neutrophils, platelets, monocytes, and CRP) and downregulated factors (lymphocytes, albumin, total cholesterol, and hemoglobin). Different combinations of these factors became prognostic indicators and the prognostic parameters were more stable than using a single element.
Many recent studies have also reported that SII could be used to predict the prognosis of different cancer types by conducting meta-analyses (39–43). A meta-analysis on 2,101 patients conducted by Zeng et al. found that elevated pretreatment SII was markedly associated with poor OS and progression-free survival (PFS) in esophageal squamous cell carcinoma (39). According to Wang et al., SII could independently predict OS and PFS of nasopharyngeal carcinoma patients through a meta-analysis that included nine studies (40). In the meta-analysis, which included 833 patients conducted by Salazar-Valdivia et al., indicated that high SII values are related to poor OS and PFS of testicular cancer (41). Moreover, a recent meta-analysis, including 1,426 patients, indicated that higher SII was significantly related to dismal OS and PFS in glioma patients (42). According to Zhang et al., a higher SII is linked dramatically to dismal OS and worse PFS/biochemical recurrence-free survival (bRFS) of prostate cancer in their meta-analysis enrolling 8,133 patients (43). The results of this SII focused meta-analysis mostly conforms to those obtained in additional cancer types.
There were some limitations to be noted. First, every enrolled article had a retrospective design, which could introduce selection bias. Second, many enrolled articles were conducted in Asia (10 out of 11). Although the study region was not restricted, all included studies were published in English. Therefore, the findings of this work may be more applicable in Asian OSCC populations. Third, threshold SII was not uniform across the included studies, so there could be differences to each conclusion. Due to these limitations, more multi-regional prospective trials are still necessary to further validate the utility of SII when predicting the prognosis of OSCC patients.
Conclusions
In conclusion, this meta-analysis demonstrates that higher SIIs are significantly related to dismal OS and DFS in OSCC. Additionally, high SIIs are markedly related to advanced tumor stages and poor differentiation in OSCC. SII could be a potential and important biomarker for clinical management and prognosis prediction of OSCC patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. SD: Conceptualization, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1303132/full#supplementary-material
Abbreviations
SII, systemic immune-inflammation index; OSCC, oral squamous cell carcinoma; HR,hazard ratio; CI,confidence interval; OR,odds ratio; OS, overall survival; DFS, disease-free survival; HNC, head and neck cancer; TNM, Tumor-Node-Metastasis; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; CAR, C-reactive protein/albumin ratio; LMR, lymphocyte-monocyte ratio; LCR, lymphocyte-to-C-reactive protein ratio; HCC, hepatocellular carcinoma; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NOS, Newcastle–Ottawa Scale; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; ROC, receiver operating characteristic; MMP-9, matrix metalloproteinase-9; IL-8, interleukin-8; TNF-α, tumor necrosis factor α; CTCs, circulating tumor cells; TILs, tumor-infiltrating lymphocytes; PFS, progression-free survival; bRFS, biochemical recurrence-free survival.
References
1. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol (2019) 5(12):1749–68. doi: 10.1001/jamaoncol.2019.2996
2. Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet (2008) 371(9625):1695–709. doi: 10.1016/s0140-6736(08)60728-x
3. Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma–an update. CA: Cancer J Clin (2015) 65(5):401–21. doi: 10.3322/caac.21293
4. Chen SH, Hsiao SY, Chang KY, Chang JY. New insights into oral squamous cell carcinoma: from clinical aspects to molecular tumorigenesis. Int J Mol Sci (2021) 22(5):2252. doi: 10.3390/ijms22052252
5. van Harten AM, Brakenhoff RH. Targeted treatment of head and neck (Pre)Cancer: preclinical target identification and development of novel therapeutic applications. Cancers (Basel) (2021) 13(11):2774. doi: 10.3390/cancers13112774
6. Rahman N, MacNeill M, Wallace W, Conn B. Reframing histological risk assessment of oral squamous cell carcinoma in the era of UICC 8th edition TNM staging. Head Neck Pathol (2021) 15(1):202–11. doi: 10.1007/s12105-020-01201-8
7. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature (2017) 541(7637):321–30. doi: 10.1038/nature21349
8. Yasumatsu R, Wakasaki T, Hashimoto K, Nakashima K, Manako T, Taura M, et al. Monitoring the neutrophil-to-lymphocyte ratio may be useful for predicting the anticancer effect of nivolumab in recurrent or metastatic head and neck cancer. Head Neck (2019) 41(8):2610–8. doi: 10.1002/hed.25737
9. Qi Y, Zhang Y, Fu X, Wang A, Yang Y, Shang Y, et al. Platelet-to-lymphocyte ratio in peripheral blood: A novel independent prognostic factor in patients with melanoma. Int Immunopharmacol (2018) 56:143–7. doi: 10.1016/j.intimp.2018.01.019
10. Jiang Y, Gu H, Zheng X, Pan B, Liu P, Zheng M. Pretreatment C-reactive protein/albumin ratio is associated with poor survival in patients with 2018 FIGO stage IB-IIA HPV-positive cervical cancer. Pathol Oncol research: POR (2021) 27:1609946. doi: 10.3389/pore.2021.1609946
11. Shen H, Dang W, Su R, Zhang Z, Wu S, Li M, et al. Pretreatment lymphocyte-monocyte ratio (LMR) as a superior predictor of short-term progression outcomes in patients with gastric cancer receiving second- and later-line apatinib regimens. J Cancer Res Clin Oncol (2023) 149(12):10715–26. doi: 10.1007/s00432-023-04976-9
12. Okugawa Y, Toiyama Y, Fujikawa H, Kawamura M, Yasuda H, Yokoe T, et al. Cumulative perioperative lymphocyte/C-reactive protein ratio as a predictor of the long-term outcomes of patients with colorectal cancer. Surg Today (2021) 51(12):1906–17. doi: 10.1007/s00595-021-02291-9
13. Modica R, Minotta R, Liccardi A, Cannavale G, Benevento E, Colao A. Evaluation of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and systemic immune-inflammation index (SII) as potential biomarkers in patients with sporadic medullary thyroid cancer (MTC). J Pers Med (2023) 13(6):953. doi: 10.3390/jpm13060953
14. Yang J, Shu C, Shang X, Xu H, Wei N. Prognostic value of systemic immune-inflammation index-based nomogram in patients with extrahepatic cholangiocarcinoma treated by percutaneous transhepatic biliary stenting combined with (125)I seed intracavitary irradiation. Int J Gen Med (2023) 16:2081–94. doi: 10.2147/ijgm.S411577
15. Teng W, Cheng TA, Lin PT, Lin CC, Lin CY, Lin SM. Combination of systemic immune-inflammation index and albumin-bilirubin grade predict prognosis of regorafenib in unresectable hepatocellular carcinoma. Am J Cancer Res (2023) 13(6):2702–13.
16. Jiang YT, Wang TC, Zhang W. Preoperative systemic immune-inflammation index is a potential biomarker in adult patients with high-grade gliomas undergoing radical resection. J Inflammation Res (2023) 16:3479–90. doi: 10.2147/jir.S423488
17. Van ‘t Land FR, Aziz MH, Michiels N, Mieog JSD, Bonsing BA, Luelmo SAC, et al. Increasing systemic immune-inflammation index during treatment in patients with advanced pancreatic cancer is associated with poor survival - A retrospective, multicenter, cohort study. Ann Surg (2023) 278(6):1018–23. doi: 10.1097/sla.0000000000005865
18. Diao P, Wu Y, Li J, Zhang W, Huang R, Zhou C, et al. Preoperative systemic immune-inflammation index predicts prognosis of patients with oral squamous cell carcinoma after curative resection. J Transl Med (2018) 16(1):365. doi: 10.1186/s12967-018-1742-x
19. Erdis E, Yucel B. Prognostic significance of inflammatory markers in patients with oral cavity cancers. Ent Updates (2020) 10(1):271–7. doi: 10.32448/entupdates.696940
20. Lu Z, Yan W, Liang J, Yu M, Liu J, Hao J, et al. Nomogram based on systemic immune-inflammation index to predict survival of tongue cancer patients who underwent cervical dissection. Front Oncol (2020) 10:341. doi: 10.3389/fonc.2020.00341
21. Hung SP, Chen PR, Ho TY, Chang KP, Chou WC, Lee CH, et al. Prognostic significance of the preoperative systemic immune-inflammation index in patients with oral cavity squamous cell carcinoma treated with curative surgery and adjuvant therapy. Cancer Med (2021) 10(2):649–58. doi: 10.1002/cam4.3650
22. Nie Z, Zhao P, Shang Y, Sun B. Nomograms to predict the prognosis in locally advanced oral squamous cell carcinoma after curative resection. BMC Cancer (2021) 21(1):372. doi: 10.1186/s12885-021-08106-x
23. Wei LF, Huang XC, Lin YW, Luo Y, Ding TY, Liu CT, et al. A prognostic model based on clinicopathological features and inflammation- and nutrition-related indicators predicts overall survival in surgical patients with tongue squamous cell carcinoma. Technol Cancer Res Treat (2021) 20:15330338211043048. doi: 10.1177/15330338211043048
24. Cho U, Sung YE, Kim MS, Lee YS. Prognostic role of systemic inflammatory markers in patients undergoing surgical resection for oral squamous cell carcinoma. Biomedicines (2022) 10(6):1268. doi: 10.3390/biomedicines10061268
25. Huang CH, Lue KH, Chen PR, Hsieh TC, Chou YF. Association between sarcopenia and immediate complications and mortality in patients with oral cavity squamous cell carcinoma undergoing surgery. Cancers (Basel) (2022) 14(3):785. doi: 10.3390/cancers14030785
26. Kubota K, Ito R, Narita N, Tanaka Y, Furudate K, Akiyama N, et al. Utility of prognostic nutritional index and systemic immune-inflammation index in oral cancer treatment. BMC Cancer (2022) 22(1):368. doi: 10.1186/s12885-022-09439-x
27. Ruiz-Ranz M, Lequerica-Fernández P, Rodríguez-Santamarta T, Suárez-Sánchez FJ, López-Pintor RM, García-Pedrero JM, et al. Prognostic implications of preoperative systemic inflammatory markers in oral squamous cell carcinoma, and correlations with the local immune tumor microenvironment. Front Immunol (2022) 13:941351. doi: 10.3389/fimmu.2022.941351
28. Zakaria SS, Ramanathan A, Mat Ripen Z, Ghani WMN, Yang YH, Vincent-Chong VK, et al. Prognostic abilities of pre- and post-treatment inflammatory markers in oral squamous cell carcinoma: stepwise modelling. Medicina (Kaunas Lithuania) (2022) 58(10):1426. doi: 10.3390/medicina58101426
29. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Internal Med (2009) 151(4):264–W64. doi: 10.7326/0003-4819-151-4-200908180-00135
30. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
31. Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol (2016) 28(2):187–96. doi: 10.1016/j.smim.2016.03.018
32. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
33. Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Internal Med (2000) 248(3):171–83. doi: 10.1046/j.1365-2796.2000.00742.x
34. Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell (2018) 33(6):965–83. doi: 10.1016/j.ccell.2018.03.002
35. Stanger BZ, Kahn ML. Platelets and tumor cells: a new form of border control. Cancer Cell (2013) 24(1):9–11. doi: 10.1016/j.ccr.2013.06.009
36. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb haemostasis: JTH (2011) 9(2):237–49. doi: 10.1111/j.1538-7836.2010.04131.x
37. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer (2011) 105(1):93–103. doi: 10.1038/bjc.2011.189
38. Yoshimura T, Suzuki H, Takayama H, Higashi S, Hirano Y, Tezuka M, et al. Prognostic value of inflammatory biomarkers in aged patients with oral squamous cell carcinoma. Front Pharmacol (2022) 13:996757. doi: 10.3389/fphar.2022.996757
39. Zeng X, Ye L, Luo M, Zeng D, Chen Y. Prognostic value of pretreatment systemic immune-inflammation index in Chinese esophageal squamous cell carcinoma patients receiving radical radiotherapy: A meta-analysis. Med (Baltimore) (2023) 102(25):e34117. doi: 10.1097/md.0000000000034117
40. Wang L, Qin X, Zhang Y, Xue S, Song X. The prognostic predictive value of systemic immune index and systemic inflammatory response index in nasopharyngeal carcinoma: A systematic review and meta-analysis. Front Oncol (2023) 13:1006233. doi: 10.3389/fonc.2023.1006233
41. Salazar-Valdivia FE, Valdez-Cornejo VA, Ulloque-Badaracco JR, Hernandez-Bustamante EA, Alarcón-Braga EA, Mosquera-Rojas MD, et al. Systemic immune-inflammation index and mortality in testicular cancer: A systematic review and meta-analysis. Diagnostics (Basel Switzerland) (2023) 13(5):843. doi: 10.3390/diagnostics13050843
42. Zhang S, Ni Q. Prognostic role of the pretreatment systemic immune-inflammation index in patients with glioma: A meta-analysis. Front Neurol (2023) 14:1094364. doi: 10.3389/fneur.2023.1094364
Keywords: SII, oral squamous cell carcinoma, meta-analysis, evidence-based medicine, prognostic markers
Citation: Zhang J and Dai S (2024) Prognostic and clinicopathological role of pretreatment systemic immune-inflammation index in patients with oral squamous cell carcinoma: a meta-analysis. Front. Oncol. 13:1303132. doi: 10.3389/fonc.2023.1303132
Received: 27 September 2023; Accepted: 31 December 2023;
Published: 16 January 2024.
Edited by:
Jason Chia-Hsun Hsieh, New Taipei Municipal TuCheng Hospital, TaiwanReviewed by:
Xiaobin Shang, Tianjin Medical University Cancer Institute and Hospital, ChinaXinmao Song, Fudan University, China
Hajime Suzuki, Kagoshima University, Japan
Copyright © 2024 Zhang and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Dai, ZGFpc2h1NTA5QDE2My5jb20=
 Jiliang Zhang
Jiliang Zhang Shu Dai
Shu Dai