- 1Department of Otolaryngology, Regional Specialist Hospital Wroclaw, Research & Development Centre, Wroclaw, Poland
- 2Faculty of Medicine, Wroclaw University of Science and Technology, Wroclaw, Poland
- 3Institute of Human Genetics, Polish Academy of Sciences, Poznan, Poland
- 4Department of Laryngology, Faculty of Medical Sciences in Katowice, Medical University of Silesia in Katowice, Katowice, Poland
- 5Department of Otolaryngology Head Neck Oncology, Medical University of Lodz, Lodz, Poland
- 6Department of Otolaryngology Phoniatrics and Audiology, Nicolaus Copernicus University in Toruń, Bydgoszcz, Poland
- 7Department of Otolaryngology, Faculty of Medicine, Medical University of Gdansk, Gdansk, Poland
- 8Department of Otolaryngology, Medical University of Bialystok, Bialystok, Poland
- 9Radiation and Clinical Oncology Department, Maria Skłodowska-Curie National Research Institute of Oncology, Gliwice Branch, Gliwice, Poland
- 10Department of Radiotherapy I, The Greater Poland Cancer Centre, Poznan, Poland
- 11Department of Radiation Therapy, Oncology Chair, Medical University of Lodz, Lodz, Poland
- 12Department of Otolaryngology and Laryngological Oncology with Clinical Department of Cranio-Maxillofacial Surgery, Military Institute of Medicine - National Research Institute, Warsaw, Poland
- 13Department of Otorhinolaryngology Head and Neck Surgery, Medical University of Warsaw, Warsaw, Poland
- 14Department of Oncology, Wroclaw Medical University, Wroclaw, Poland
Summary: The algorithm of follow-up in patients with head and neck cancer (HNC) has been prepared by a board of Polish Head Neck and Oncology Experts. The aim of this research is to focus on the specificity of HNC monitoring, to review the current trends in follow-up, and to adapt the evidence-based medicine international standards to the capabilities of the local healthcare service.
Materials and methods: The first methodological step was to categorize HNCs according to the estimated risk of failure after the adequate first-line treatment and according to the possibility of effective salvage treatment, resulting in improved overall survival. The final method used in this work was to prepare an authors’ original monitoring algorithm for HNC groups with a high, moderate, and low risk of recurrence in combination with a high or low probability of using an effective salvage.
Results: Four categories were established: Ia. low risk of recurrence + effective organ preservation feasible; Ib. low risk of recurrence + effective salvage feasible; II. moderate risk of recurrence + effective salvage feasible; III. high risk of recurrence + effective salvage feasible; and IV. high risk of recurrence + no effective salvage feasible. Follow-up visit consisting of 1. ENT examination + neck ultrasound, 2. imaging HN tests, 3. chest imaging, 4. blood tests, and 5. rehabilitation (speech and swallowing) was scheduled with a very different frequency, at the proposed monthly intervals, tailored to the needs of the group. The number of visits for individual groups varies from 1 to 8 in the first 2 years and from 1 to 17 in the entire 5-year monitoring period. Group IV has not been included in regular follow-up, visits on own initiative of the patient if symptomatic, or supportive care needs, having in mind that third-line therapy and immune checkpoint inhibitors are available.
Conclusion: Universal monitoring algorithm for HNC four groups with a high, moderate, and low risk of recurrence after the adequate treatment in combination with a high or low probability of using an effective salvage is an innovative approach to redeploying system resources and ensuring maximum benefit for patients with HNC.
Introduction
● The presented algorithms of follow-up in patients with head and neck cancer (HNC) are complementary to the basic document, which is the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines, in which, however, post-treatment monitoring is not an extensive topic.
● These algorithms have a practical focus and are a handy document for a wide range of otolaryngologists who are responsible for monitoring patients after treatment, often outside leading oncological centers.
● The novel approach is the categorization of all patients with HNC, regardless of primary location. All HNCs are classified into one of the four groups, based on the estimated risk of failure after first-line treatment and the possibility of applying an effective salvage associated with improved survival.
● On the basis of this assumption, four different monitoring algorithms were developed for HNCs, classified as low, medium, and high risk of recurrence and palliative group. Accordingly, there are four further follow-up paths to practical application.
Follow-up is a well-established method of care for oncological patients. It is a common practice for most types of cancer to monitor survivors after treatment (1–5). Up to date, there is no consensus on the optimal duration of post-treatment follow-up after HNC (6), although monitoring standards, outside the NCCN, have been provided by the following societies around the world: ASHNS (American Society for Head and Neck Society, 2016), SHNS (Society of Head and Neck Surgery, 1999), BAHNO (British Association of Head and Neck Oncologists, 2001), DAHANCA (Danish Head and Neck Cancer Group, 2013), and ELS (European Laryngological Society, 2014) - Head and Neck Cancer Committee Working Group (7).
This algorithm is prepared by a board of Polish Head Neck and Oncology Experts. The aim is to 1. focus on the specifics of HNC follow-up and review the current world trends; 2. generate input data for an innovative approach to create new, simplified guidelines by categorizing the risk of recurrence and the chance for salvage for all HNCs; and 3. adapt the created algorithm to international standards and the capabilities of the local health service.
Definition for post-treatment monitoring
Follow-up is a long-lasting and regular maintenance of contact with and re-examination of a patient, especially following oncological treatment, at specified intervals, in a medically documented manner and using specified technical means.
Rationale for post-treatment monitoring
Patient monitoring programs are based on the assumption that a possible asymptomatic recurrence will be characterized by a lower stage of advancement recurrent tumour, node (rTN) than in the case of a patient reporting due to the appearance of ailments. If a diagnosis is made during a routine, scheduled visit, then there is a greater chance of implementing effective salvage therapy.
Outcome measures for post-treatment monitoring
Success in monitoring patients with cancer is firstly measured by the percentage of detected recurrences that can be qualified for subsequent radical treatment. Other measures include the percentage of effective salvage treatment and higher disease-related survival (5, 6, 8). Extending the patient’s survival time on the scale of the entire observed population reduces the number of disease-related deaths.
The specificity of HNC follow-up
However, for HNC, long-term routine follow-up remains a matter of debate (9–13). The benefits of routine follow-up for patients with HNC measured by extending the patient’s survival time have not been proven (14–18). Nevertheless, there are some additional issues bound with follow-up ideas (14, 19). Monitoring also aims to 1. assess the effectiveness of treatment; 2. early diagnose and treat complications, therapy failures, and sequelae (20); 3. detect a second primary tumor (SPT) (19, 21–23); and 4. implement psychological care and provide the patient with constant contact with the center, where he was treated (24–28).
Premises for logistics, cost-effectiveness, and maximization of health benefit
Organization of efficient outpatient care for patients with HNC constitutes a huge organizational challenge. Post-treatment monitoring puts increasing pressure on healthcare resources due to rising morbidity and, at the same time, rising survival rates. Therefore, many centers are analyzing a new approach to the observation of patients with HNC, giving them the opportunity to choose their own monitoring program (5, 6, 29). Determining the optimal schedule of follow-up visits for assessment for patients with HNC depends on multiple factors.
The first group of variables depends on the characteristics of the tumor. It is stratified by primary location, baseline tumor, node, metastases (TNM) stage, associated risk of recurrence, histological features of the tumor (e.g., Ki67), Human Papilloma Virus (HPV) status, final histological result with assessment of risk factors (R0, R1, R2, and tumor cell emboli in the microscopic vessels, perineurium infiltration, and extracapsular spread), and concepts and methods of treatment (surgical/radiotherapy/radiochemotherapy (RT/RTCT), definitive, palliative, and supportive).
The second group of variables depends on the patient status. The patient’s willingness to cooperate with medical staff, compliance, and checkup visits’ attendance should be of note. It is often a derivative of the distance from the treatment center, communication possibilities, age, education, health awareness, general condition, and cooperation with the family. Well-established risk factors for recurrence include older age, site of primary, male sex, smoking habit, and negative HPV status (30). Some prognostic factors are also predictors. HPV positivity indicates a better response to chemioradiotherapy (CRT). An advanced age is bound with a worse response, and elderly are usually not fit for re-irradiation or salvage surgery (16, 18).
The third group of variables depends on the capabilities of the treatment and monitoring center. This includes human resources, premises, access to imaging tests, and organizational efficiency in coordinating patient monitoring. The IT (Information Technology) infrastructure, efficient communication, and media (phones, text messages, and e-mails) availability are crucial.
Because there is no clear answer on how efficient and cost-effective monitoring is and what the real chances for recurrence, metastasis, or second primary cancer cure are, innovative rules of follow-up have been introduced. Salvage with the intention of radical curation is possible in less than 50% of monitored patients and applies only to those initially treated in the early stages of cancer. That is, the greatest therapeutic benefit is achieved by selected patients, in whom reducing the intensity of monitoring, i.e., limiting the number of visits, could jeopardize the patient’s chances of re-treatment with the organ preservation intent. Patients with extensive cancer have the lowest profit; even after early detection of recurrence, it is difficult to suggest any further treatment option in this group (6). The monitoring algorithms for individual primary locations and cancer stages according to the Cohort Study with Parametric Modelling of Event-Free Survival by Lee et al. (5) was the quintessence of a new therapeutic approach and a starting point for the development of Polish HNC follow-up recommendations (31).
Method
The first methodological step was to categorize HNCs according to the estimated risk of failure after the adequate first-line treatment and according to the possibility of effective salvage treatment associated with improved survival. The use of a prognostic model for patients with HNC receiving care at medical centers in developed countries was recommended and followed in the methods section (online at http://www.oncologiq.nl).
The final method used in this work was to prepare an authors’ original monitoring algorithm for HNC groups with a high, moderate, and low risk of recurrence in combination with a high or low probability of using an effective salvage.
The main research assumption and the premise for creating recommendations is to answer a question on how to optimize the HNC follow-up and is shown in Figure 1.
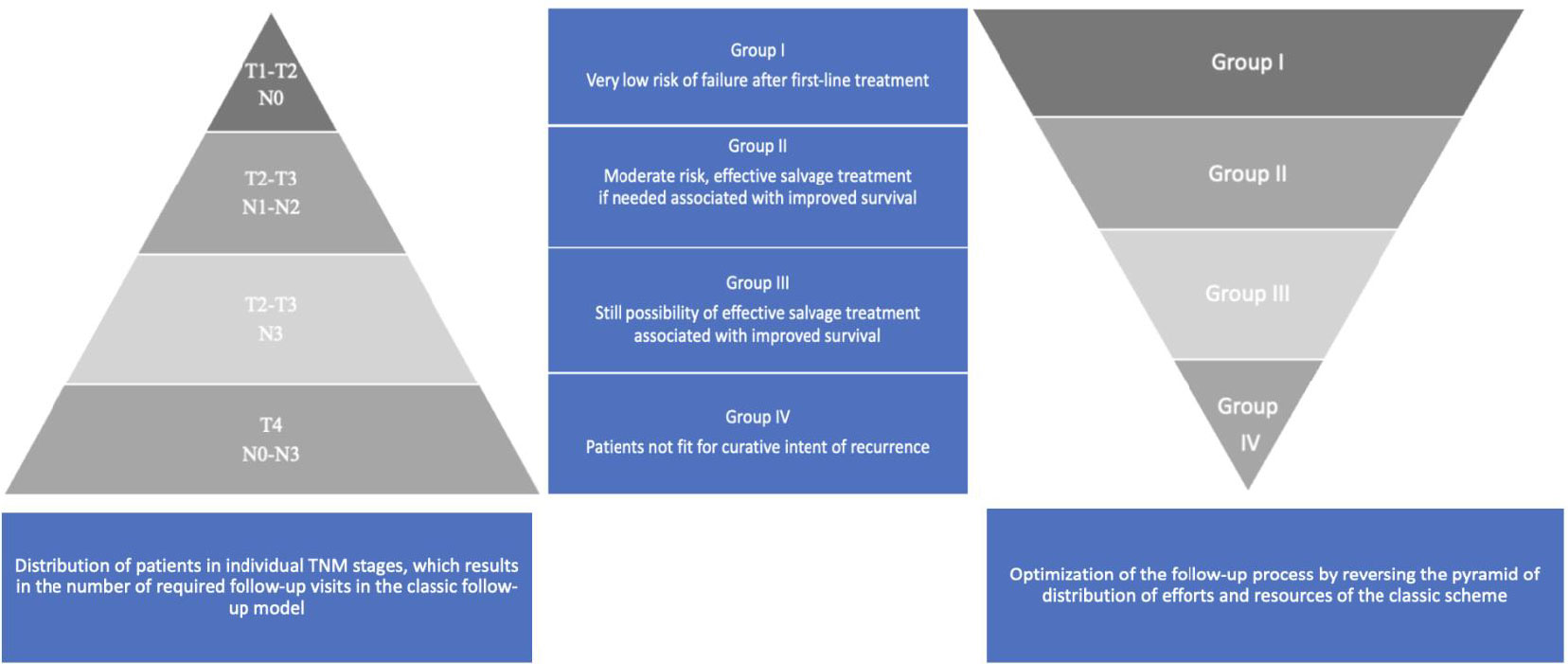
Figure 1 HNCs grouped according to the estimated risk of failure after first-line treatment and according to the possibility of effective salvage treatment associated with improved survival (online at http://www.oncologiq.nl). RT - Radiotherapy alone, RT/CT - Radio chemotherapy. ↑- upgrade to the higher group if there are adverse prognostic factors, increasing the risk. 1Larynx - Anterior Commissure, Posterior Paraglottic space involvement,. 2Larynx - prior tracheotomy, 3 Larynx - N advancement. 4 Larynx - N advancement, prior tracheotomy. 5.6 Patient treated as negative if the EBV, HPV status unknown.
Results
The monitoring algorithms for distinct primary locations—larynx, nasopharynx, oropharynx, hypopharynx, oral cavity, and particular cancer stages (TNM)—were created. The primary treatment modality was taken into consideration as well as second- and third-line treatment capabilities. Except the malignant sinonasal and salivary gland tumors, the common benign entities, such as inverted papilloma (IP) and pleomorphic adenoma (PA) follow-up schedules, were also enlisted.
Four categories of HNC were established: Ia. low risk of recurrence + effective organ preservation feasible; Ib. low risk of recurrence + effective salvage feasible; II. moderate risk of recurrence + effective salvage feasible; III. high risk of recurrence + effective salvage feasible; and IV. high risk of recurrence + no effective salvage feasible (Tables 1A–F).

Table 1a HNCs grouped according to the estimated risk of failure after first-line treatment and according to the possibility of effective salvage treatment associated with improved survival (online at http://www.oncologiq.nl) - LARYNX.
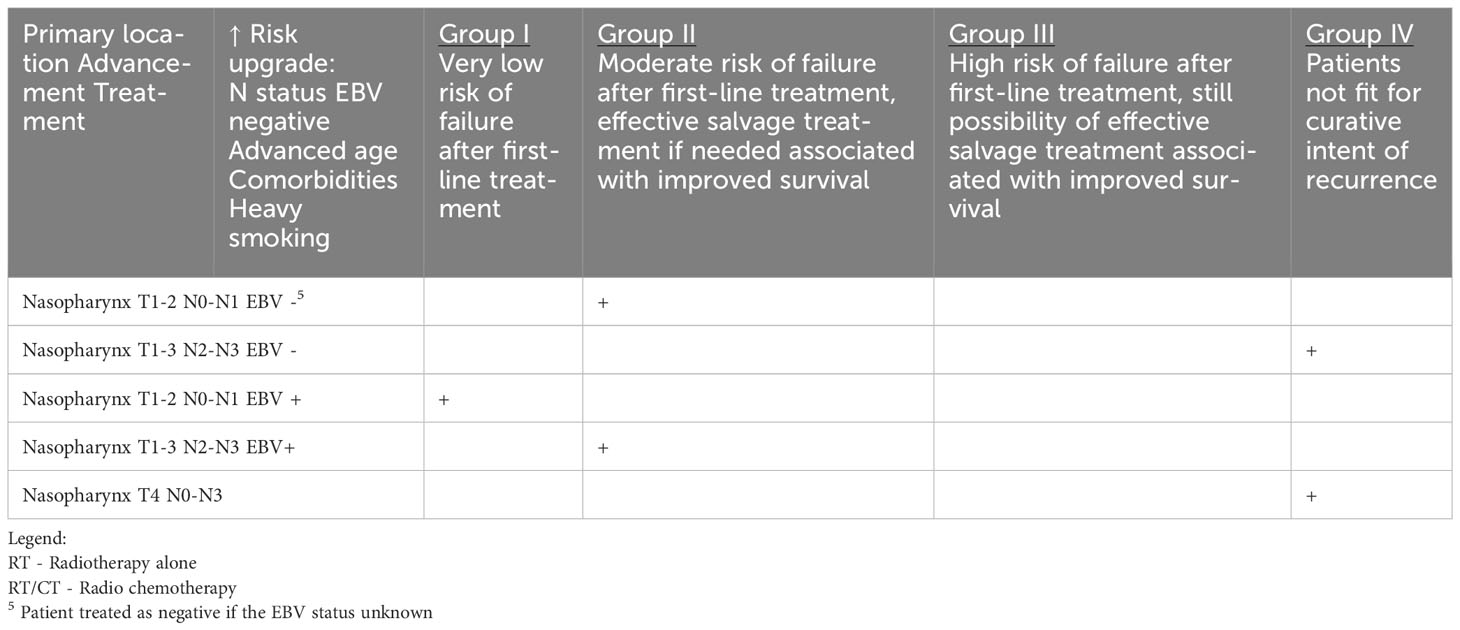
Table 1b HNCs grouped according to the estimated risk of failure after first-line treatment and according to the possibility of effective salvage treatment associated with improved survival (online at http://www.oncologiq.nl) - NASOPHARYNX.
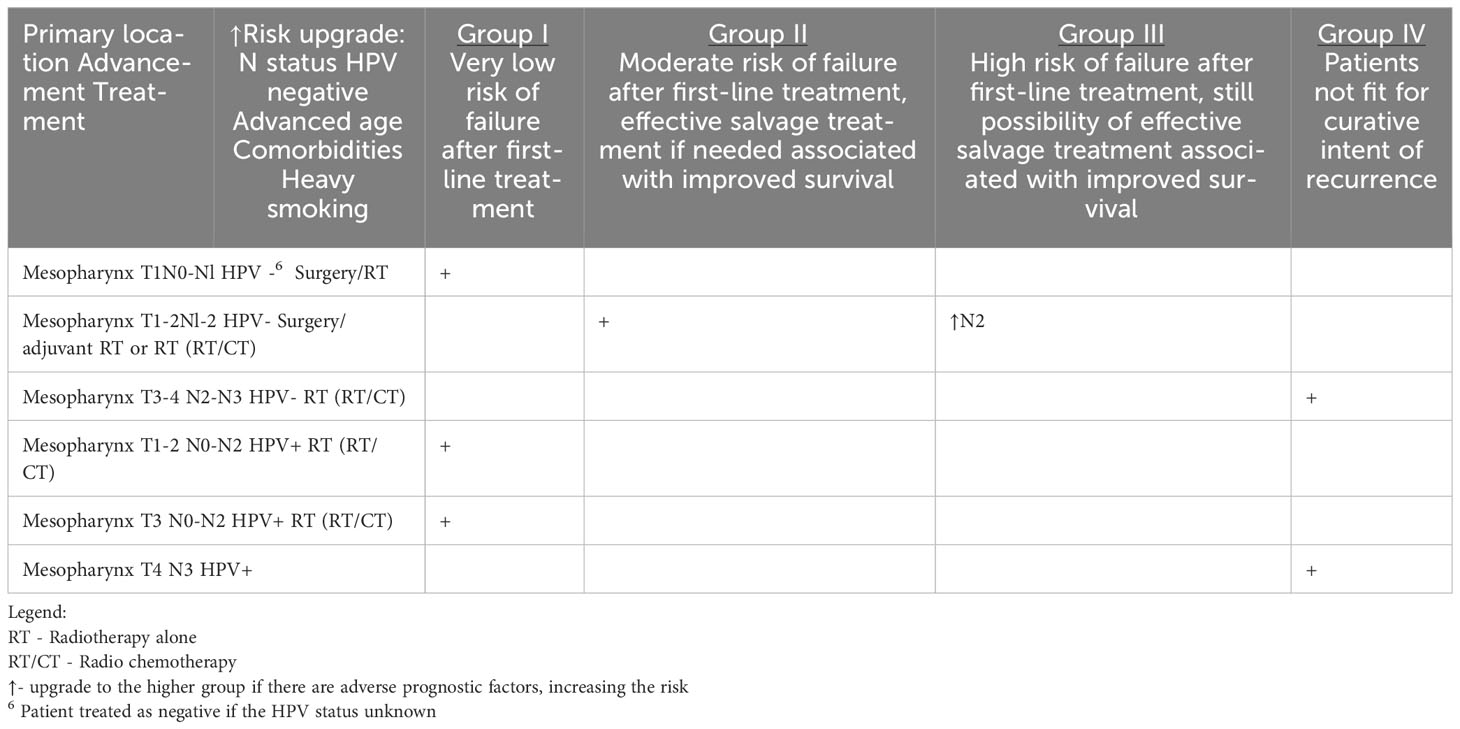
Table 1c HNCs grouped according to the estimated risk of failure after first-line treatment and according to the possibility of effective salvage treatment associated with improved survival (online at http://www.oncologiq.nl) – MESOPHARYNX.
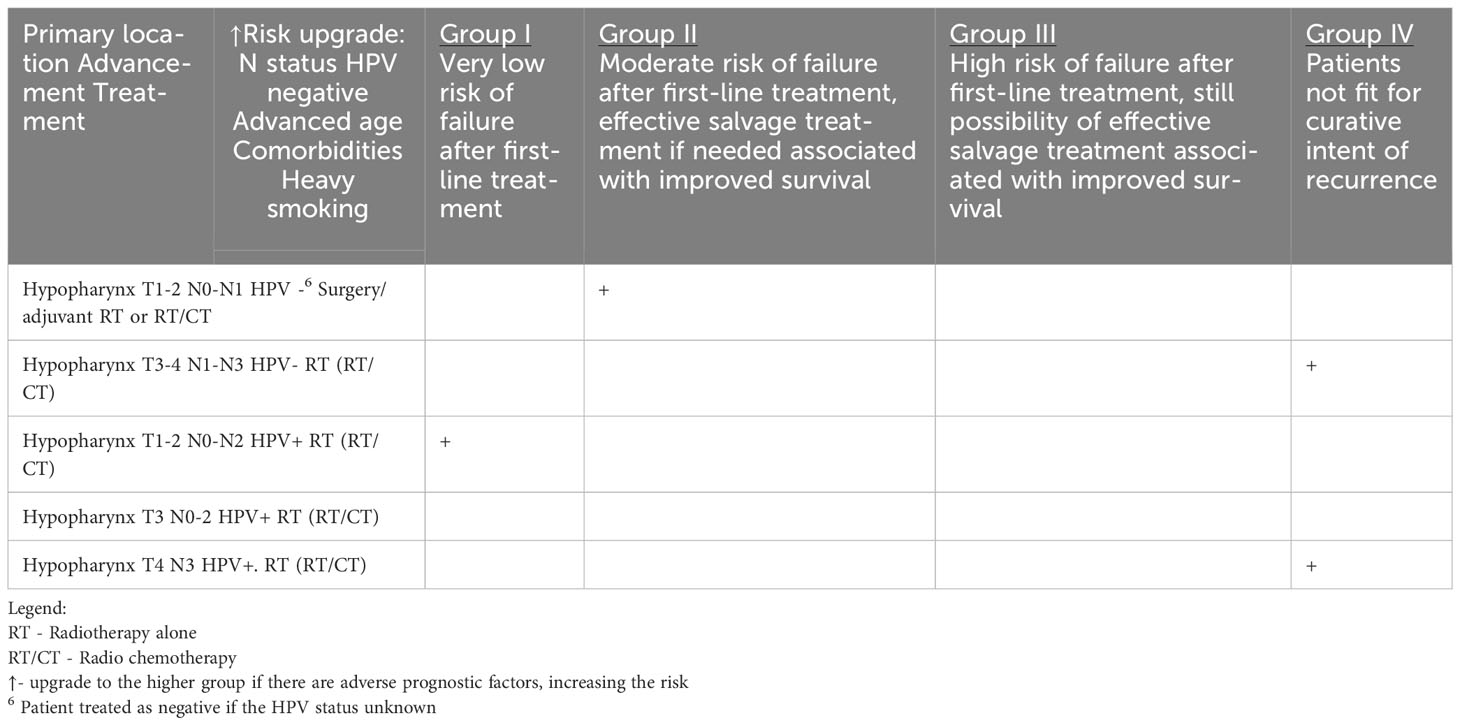
Table 1d HNCs grouped according to the estimated risk of failure after first-line treatment and according to the possibility of effective salvage treatment associated with improved survival (online at http://www.oncologiq.nl)- HYPOPHARYNX.
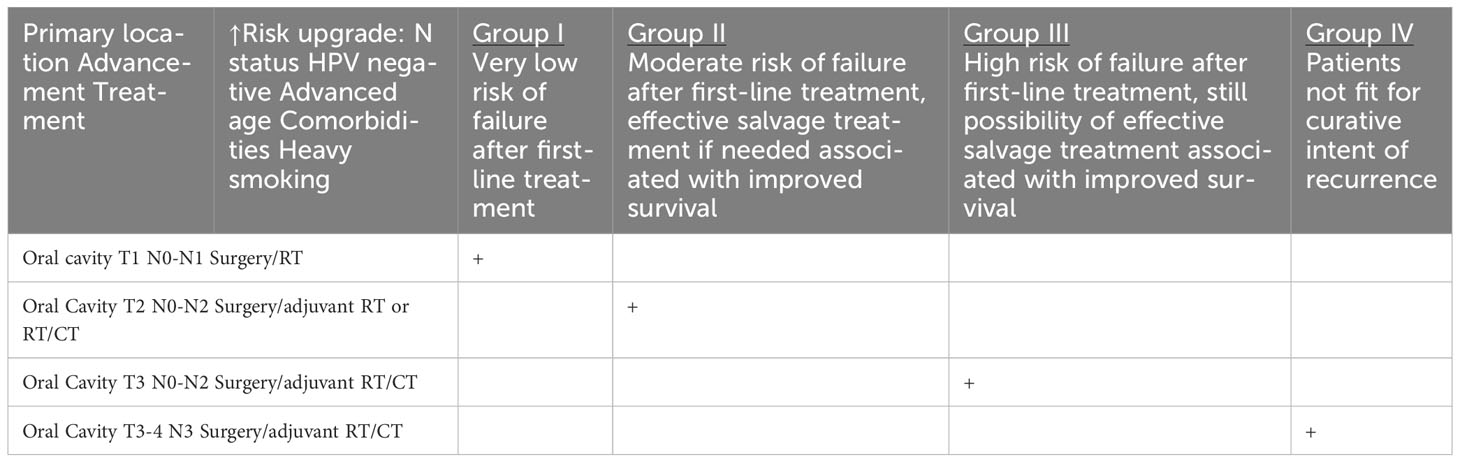
Table 1e HNCs grouped according to the estimated risk of failure after first-line treatment and according to the possibility of effective salvage treatment associated with improved survival (online at http://www.oncologiq.nl) ORAL CAVITY.
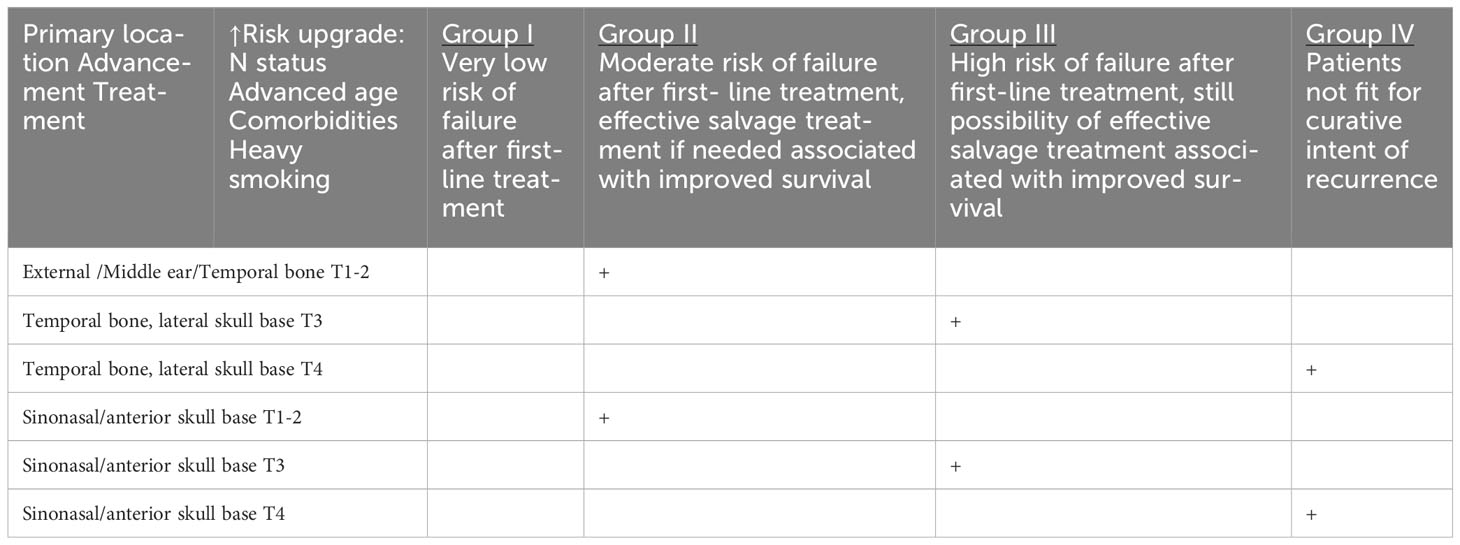
Table 1f HNCs grouped according to the estimated risk of failure after first-line treatment and according to the possibility of effective salvage treatment associated with improved survival (online at http://www.oncologiq.nl).
Follow-up visit consisting of 1. ENT examination + neck ultrasound, 2. imaging HN tests, 3. chest imaging, 4. blood tests, and 5. rehabilitation (speech and swallowing) was scheduled with a very different frequency, at the proposed monthly intervals, depending on the group. The number of visits for individual groups varies from 1 to 8 in the first 2 years and from 1 to 17 in the entire 5-year monitoring period. Group IV has not been included in regular follow-up, visits on own initiative of the patient if symptomatic, or supportive care needs, having in mind that third-line therapy and immune checkpoint inhibitors is available (Tables 1A–F).
Discussion
The author’s model assumes a far-reaching differentiation of follow-up schemes depending on the prognosis, risk of recurrence, and chances for potential effective salvage in patients with HNC. What is innovative in this study is the grouping of patients according to the risk of recurrence and the chances of providing effective treatment and not according to the primary location of the cancer. The justification for such construction of the monitoring system is the limited amount of money and human resources that can be allocated to the exponentially growing needs of HNC oncology patients. The proposed model is based on many years of the authors’ own experiences and literature reports with particular emphasis on the evolving global trends in this area.
Current follow-up models
Numerous medical societies have issued recommendations for monitoring patients with HNC (13, 32, 33), but these have been based mainly on expert opinion rather than solid evidence. Therefore, monitoring schedules were and still are often set arbitrarily by physicians in everyday clinical practice. This approach is associated with excessive frequency of visits or, conversely, too infrequent planning of visits and, secondarily, insufficient sensitivity in detecting recurrences. Both deviations either impose unnecessary economic and financial burdens on healthcare systems or are sometimes ineffective (34). NCCN Clinical Practice Guidelines used to provide a framework for high-value survivorship care for patients with HNC. In general, published guidelines recommend inspection every 1 to 3 months in the first year, every 2 to 6 months in the second year, every 4 to 8 months from the third to the fifth year, and annually thereafter. The number of follow-up visits for 5 years after treatment ranges from 11 to 27 visits (5, 13, 33, 34).
To sum up, patients were usually seen about 15 times over the 5 years. Taking into account the stage of the tumor and overall mortality, this number and timing of follow-up visits according to some authors is adequate for the needs of patients with stage II–IV disease, whereas those with stage I disease may be considered for discharge after the third year if they are told about the risk factors, signs, and symptoms of recurrent disease, and surveillance can be conduct in primary care (35). However, the latest opinions underline that emphasis should be placed on monitoring those patients who can be offered effective salvage (17). The authors have illustrated this regularity and relationship in Table 2. Healthcare resources should, therefore, be reallocated in a way that reduces ineffective checkup visits while increasing expenditures and organizing multi-specialty care, including the care of speech therapists, swallowing specialists, dentists, and psychologists for patients with a better chance of recovery and professional activity (17, 36). Patients who are not fit for curative intent of recurrence may receive less intensive imaging, only when turned symptomatic (5, 17). On the contrary, taking into consideration resource allocation patterns and infrastructure density, the therapeutic landscape of locally advanced and recurrent and/or metastatic disease has been rapidly changing with the advent of immune checkpoint inhibitors and better utilization of local approaches (16).
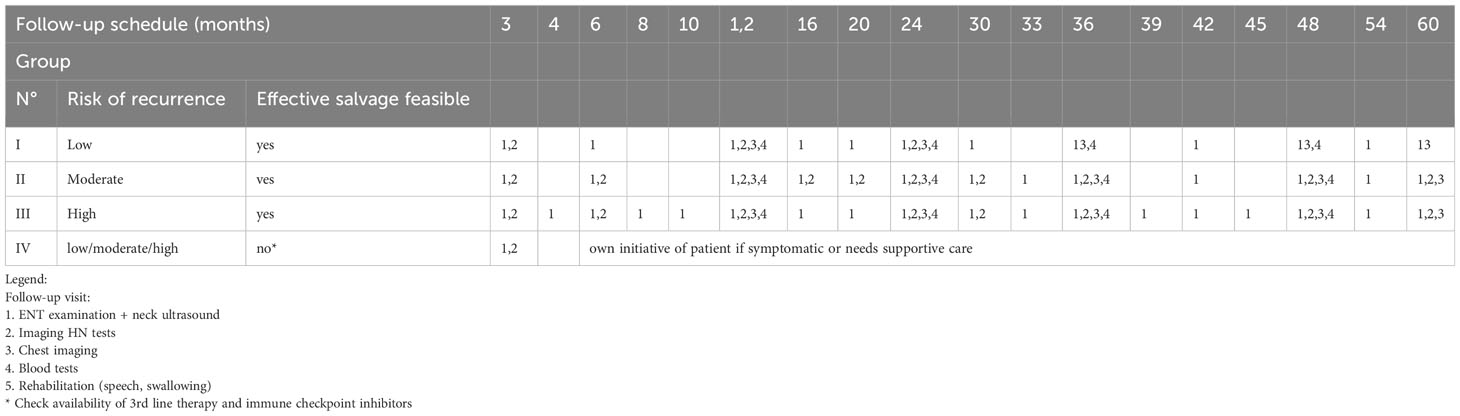
Table 2 Proposed universal monitoring algorithm for HNC groups with a high, moderate and low risk of recurrence after the adequate treatment in combination with a high or low probability of using an effective salvage.
Tools for conducting follow-up visits
Tools for conducting patient visits are widely standardized and do not raise controversy. Some centers supplement the classical instrumentation with narrow band imaging (NBI) or high-speed video laryngoscopy, but it is not obligatory.
Traditionally, the follow-up of patients with HNC is clinician-led with lack of standardization in approaches to the Multidisciplinary Team. The identified role of Allied Healthcare Professionals (AHPs) was not only to improve the quality of life (QoL) and symptom control rather than to detect recurrence but also to identify groups who will require more intensive AHP input (37).
Controversy still exists regarding the value of surveillance imaging past the first post-treatment baseline scan in patients who are asymptomatic (1, 38). A decision-analytic Markov model was developed to assess the cost utility of two alternative follow-up programs with a lifetime horizon: program of frequent radiological assessments (maximal approach) compared with a symptom-driven surveillance (minimal approach). In probabilistic sensitivity analysis, 72% of the results lie below the €40,000 threshold (55% below €25,000), and the conclusion has been drawn that an intensive post-treatment follow-up with scheduled radiological assessments over time might be cost-effective compared with symptom-driven surveillance in patients with HNC (39). Similarly, lifetime cost-effectiveness of PET-CT–guided management from a UK secondary care perspective has been proven (40).
Patients with a HNC index tumor have a high risk of second neoplasms located in the lung. In order to achieve an early diagnosis of these SPTs, it would be advisable to establish screening protocols based on the use of low-dose lung CT, which should be maintained indefinitely during the follow-up period (41).
Specificity of follow-up in different primary HNC locations
When planning the monitoring scheme, up to now, more importance has been attached to the location of the primary and loco-regional tumor advancement and, thus, to the prognosis of survival (35). The key feature of the algorithm presented in this paper is the elimination of divisions into individual primaries in favor of considering patients in terms of the common denominator of the possibility of organ preservation, effective salvage, or inability to undertake salvage treatment. Nevertheless, we discuss some strategic points in organ aspects.
Larynx
Despite the small size of the organ itself, the larynx is an extremely heterogeneous primary location, in which the prognosis for cure ranges from 99% for early glottic cancer to a few percent only with advanced processes in the supraglottis at the border of the hypopharynx. An important aspect is the possibility of preserving the organ in early stages of primary and recurrence advancement, in situations where salvage treatment can be carried out using sparing surgical techniques (42–45).
In advanced stages of laryngeal cancer qualified for amputation treatment, the prognosis, role of monitoring, and its effectiveness are completely different (46). The Markov chain model was developed and intended to evaluate the effectiveness of pre-symptomatic detection of breast cancer recurrence, but the model allowed us to make a comparison between the current protocol and various alternatives in larynx cancer survivors (47). Three different follow-up strategies were compared—the current schedule, no follow-up, and the perfect follow-up—in which all recurrences were detected asymptomatically. Compared with no follow-up, the current schedule showed a gain in life expectancy with a range from 0.3 years to 1.5 years that decreased with advancing age. Abolishing the current follow-up schedule raised the disease-specific mortality rate; the increase ranged from 2.8% to 5.9% (48). After total laryngectomy, the prognosis is poor after the development of recurrence. Curative therapy could only be offered to 27.5% of these patients, and only 5% of them were finally disease free (48).
The basis for the diagnosis of vocal fold recurrence is endoscopic examination with stroboscopy as the gold standard (49). However, there are some limitations associated with conventional white light imaging together with stroboscopy in terms of laryngeal cancer follow-up. Application of NBI, as one of biological endoscopic evaluation, has created a new direction due to filtering of microvessels, which indicate neoangiogenesis. This technique enabled the assessment of the pathological vessels in previously treated cancerous laryngeal mucosa and detected recurrence early during the follow-up, especially in a difficult-to-visualize site such as the hypopharynx. On the other hand, an additional tool to stroboscopy is high-speed videoendoscopy, which is an accurate method for an objective assessment of vocal fold oscillations (50).
Oral cavity
In oral cavity, salvage treatment saves only 22% of patients with recurrent disease. Over half of patients with recurrence were detected at pre-scheduled follow-up visits; minority were detected at extra visits at the patients’ own request. The necessity and cost-effectiveness of a routine follow-up schedule can thus be questioned, given that there is a very limited effect on survival. After 2 years, follow-up should be tailored to the individual needs of patients for supportive care and monitoring of late side effects of the treatment (19, 51).
Surveillance imaging is critical during follow-up period to detect recurrence in oral cavity cancer for effective salvage surgery. MR imaging has potentially high value to detect recurrent tumor especially after primary surgery without flap reconstruction up to developed new technical application as T2 weighted image (T2WI) in Diffusion-weighted magnetic resonance imaging (DWI or DW-MRI) technique that could be more sensitive with flaps surgery (52). Fluorodeoxyglucose (FDG)-PET seems to be the most reliable tool for loco-regional surveillance of patients after resections with flap reconstruction (53).
Pharynx
Regardless of the location of the malignancy in the nasopharynx, oropharynx, or hypopharyx and its histological type, the postoperative care is similar and consists of head and neck examination including videoendoscopy and dental assessment of the oral cavity and areas exposed to radiotherapy. Epstein-Barr Virus (EBV) DNA monitoring in the blood should be considered in nasopharyngeal carcinoma, but the clinical benefit of this is not defined. In addition to the above general guidelines for all areas of the pharynx, the location of a neoplasm in the oropharynx specifically requires consideration of HPV testing. Some centers may recommend HPV testing during follow-up visits to assess the persistence or clearance of HPV infection, which can help inform prognosis and guide further management decisions.
The recurrence of hypopharyngeal cancer is a high-risk of fatal disease that is associated with poor prognosis and high risk of complications due to salvage treatment. Complication rate is higher in salvage mainly because of high percentage of prior chemotherapy and generally poor outcome of hypopharynx cancer (11, 54, 55). A 5-year overall survival (OS) of 27% for salvage (pharyngo)laryngectomy after primary chemoradiation was observed (55). As was reported the mean time to recurrence before salvage surgery was 7.5 months (56).
Oropharyngeal squamous cell carcinomas 5-year OS oscillated between 21% and 44%, but, nowadays, it is mostly dependent on HPV-positive or HPV-negative disease (57). HPV-positive tumors generally have a more favorable outcome, but still about 10% of patients could experience (loco)regional failure. Nevertheless, HPV-positive oropharyngeal squamous cell carcinomas (OPSCCs) do have a better outcome in salvage surgery (58). The recurrence-free survival is longer in HPV-positive tumors and OS in HPV-positive patients with disease progression after locoregional failure and after salvage surgery is more satisfactory (58).
Recurrent nasopharyngeal carcinoma is highly challenging option for treatment. Early detection of recurrence is mandatory. Salvage surgery is an option for carefully selected patients with a local recurrence (59, 60). The survival curves are distinctly separated within these stages [5-year OS of 72.0%, 55.1%, 21.1%, and 10.1% for surgical stages I, II, III, and IV, respectively (61)]. Three-year survival rates up to 60% after salvage surgery was observed mostly in T1 and T2 recurrent disease. Advanced stages with skull base, cranial nerve, and dural or brain involvement are associated with poor prognosis (62). It has been proven that surgical salvage may be superior to re-irradiation using intensity modulated radiation therapy (IMRT) (re-IMRT) in terms of laryngeal cancer (LC), OS, and QoL. Surgical salvage treatment is associated with significantly better OS compared with re-IMRT (5-year OS of 77.1% vs. 55.5%) (61, 62).
Salivary glands
The basic, cheap, readily available, and widely accepted tool for the assessment of the glands, and the bed after surgery of the benign salivary tumors is ultrasonography. Its only limitation is the depth of the tissues that can be assessed, so, in some cases, it should be replaced with MRI (63). In malignant tumors, a follow-up consisting of contrast enhanced MRI should be supplemented.
Salivary gland benign tumors
Majority of benign salivary gland tumors are located in the parotid gland with PA being the most frequent. Recurrence of PA after surgical treatment is reported in the literature at 2-8% (64, 65), and its malignant transformation is estimated at up to 6% (66). Risk factors for PA recurrence are capsule rupture, tumor spillage, margin status, satellite tumors, histological subtype, deep lobe location, younger age at diagnosis, and subsequent recurrence, and these cases should be included into group requiring more thorough follow-up (66, 67). PA recurrence in the parotid gland is often multinodular; thus, its early detection is essential to provide save for the facial nerve treatment (66). Secondary malignant rate (carcinoma ex PA in recurrent PA) is low, estimated in the published reports for 0% to 23%, and it may increase with number of recurrences, time, and microscopic severe dysplastic features (68).
Considering the above risk factors for recurrence and the potential for malignant transformation, patients after PA surgery should be divided into two groups: low and increased risk of recurrence. Patients with a low risk of local recurrence may self-examine (self-monitoring), and ultrasound examination of the operated area performed once a year. In the group with an increased risk of local recurrence, in addition to self-monitoring, in the first year after treatment, an ultrasound examination every 6 months, in subsequent years every 12 months, depending on the primary location, replacing it with an MRI examination should be considered.
Salivary gland malignant tumors
Salivary gland malignant tumors (SGMTs) are very heterogenous group (69), and they have different clinical course; more than 20 histological types have been distinguished, which may additionally be characterized by a low-grade, intermediate, high-grade, and variable grade of malignancy (Nishida). In addition to the histological structure, the prognosis is influenced by the primary location of the tumor (parotid, submandibular, sublingual gland, and minor salivary glands), sex, and patient’s age. In more than 50% of salivary malignancies, the perineural spread is observed, and this feature increases the risk of recurrence. Distant metastases are the most frequently located in lungs (80%) followed by bones (15%) (70). The best imaging technique for diagnosis of the primary SGMTs and for their follow-up is MRI (63). Recurrence of SGMT can occur many years after initial treatment; therefore, follow-up longer than 5 years is necessary.
Taking into consideration all described risk factors, we propose a follow-up consisting of contrast enhanced MRI every 6 to 12 months in the first 2 years after treatment, every 12 months in the subsequent years, and chest CT scan every 12 months. Initial for follow-up MRI should be performed 3 moths after the treatment is completed.
Adenoid cystic carcinoma is considered to be a high-grade cancer, and, with its tendency to perineural spread, it requires strict follow-up: MRI every 3 to 4 months in the first 2 years of follow-up, every 6 months during next 3 years, and every 12 months in subsequent years (69). Chest CT scan should be performed every 12 months as in other SGMTs. Because of its slow growth and high risk of recurrence and metastases many years after initial treatment, the lifelong follow-up is recommended.
Sinonasal, temporal bone, and skull base
Malignant neoplasms of the temporal bone constitute a small percentage of all HNC (only 0.2%), and the local advancement of the process is associated with a poor prognosis (71, 72). Local recurrences amount to 32.3% (73). The average rate of hidden metastases in the region II of the neck is 14%; in the local advancement, T3 is estimated at 21%. Considering the high rate of recurrences in the early postoperative period, follow-up should include a physical examination and MRI performed every 2 months in the first year, every 4 months in the second year, and every 6 months from the third to the fifth year (73, 74). Molecular markers are not yet significant in the diagnosis and follow-up of patients with temporal bone cancer (75). In follow-up, the need for auditory rehabilitation should be taken into account, with potential use of bone-integrated hearing implants (76).
Sinonasal malignancy surveillance strategies may warrant modifications of current protocols used for HNC. This is due to several factors, including a greater diversity of tumor histology and duration of post-treatment sinonasal inflammation (77, 78). Sinonasal cancer tends to exhibit a higher rate of local failure and occur in a delayed fashion compared with mucosal HNC. Moreover, the site of failure and time-varying risk of recurrence is histology-specific (79). Endoscopy has low sensitivity in recurrence detection, especially in the asymptomatic patient; CT, MRI (Khalili), and PET/CT (Ozturk) are useful although prolonged inflammation can lead to a high number of false positives (77). Following multimodality treatment of the skull base, patients may experience endocrine, visual, auditory, sinonasal, olfactory, and neurocognitive deficits, resulting in poor QoL. Thus, patients with sinonasal cancer would benefit from tailored survivorship programs to address not only the recurrence but also functional impairments resulting from disease and treatment toxicity (79). Regardless of the type of imaging, symptomatic presentation after treatment had a specificity of 91.0%, and the frequency of scans was not associated with the risk of recurrence. For the symptomatic presentation to be strongly associated with recurrence, patients should be investigated with appropriate imaging at presentation. This last research was in line with our conclusions. In the schemes proposed by the authors, patients with sinonasal and temporal bone cancer who underwent radical surgery with adjuvant RT are assigned to the fourth group.
The role of monitoring in side effects of therapy diagnostics and management
Follow-up has a significant impact on the timely management of the consequences and complications of cancer treatment. This extremely broad topic is not the subject of this study; however, we will list the most important issues.
Oncological treatment with chemotherapy or biological therapies causes a number of side effects and dysfunctions that affect the general health and QoL of patients. These include insufficiency of endocrine glands, central and peripheral nervous system, deafness, or atrophic mucositis.
Hypopituitarism after skull base radiotherapy has to be emphasized. Serum levels of cortisol, growth hormone, free T4, prolactin, insulin-like growth factor 2, luteinizing hormone, folliculotropic hormone, adrenocorticotropic hormone, and total and bioavailable testosterone should be annually tested. Thyrotropic hormone (TSH) alterations may indicate thyroid dysfunction or hypopituitarism in skull base irradiation patients (4). The need to monitor thyroid function is emphasized for elevated TSH levels that have been detected in 20%–25% of patients who have received neck irradiation; thus, TSH should be tested every 6 to 12 months in this group (13).
Oral cavity, oropharynx, and hypopharynx treatment influence the swallowing; thus, evaluations by a speech-language pathologist may be recommended to assess any swallowing and provide appropriate interventions if needed. Regular dental checkups and oral hygiene maintenance are important to manage any treatment-related dental and mucosal issues. Nasopharyngeal cancer treatment can affect hearing function; thus, regular otology evaluations are recommended to monitor conductive hearing loss and provide appropriate intervention if needed (13).
Laryngopharyngeal cancer treatment can impact voice quality and function; thus, speech-language pathologist evaluations are recommended to assess any voice changes and provide appropriate interventions if needed.
As the adjuvant radiotherapy is frequently applied in HNC treatment, the risk of radiation-induced malignancies in the head and neck region is increased. Therefore, like in each irradiated patient, the otorhinolaryngological examination, including endoscopy, should be performed every 1 to 3 months in the first year of follow-up, every 2 to 6 months in the second year, every 3 to 4 months in the years 3 to 5, and every 12 month in the next years (4).
Summarizing, the follow-up of patients with HNC should be multidisciplinary because of consequences of the primary tumor extent and treatment given. The importance of involving various specialists and coordinating care among them capture the complexity of optimal follow-up care in patients with HNC and include mainly radiotherapists, chemotherapists, and surgeons, and, in selected cases, endocrinologists, gastroenterologists, audiologists, gastroenterologists, dentists, peridontologists, and others.
Patients’ preferences regarding follow-up
Approximately 25% of patients disengaged from important follow-up care within 1 year. Lack of social support, depressive symptomatology, and single-treatment modality (80); unmarried status; a longer driving distance to the facility (although rurality was not associated with discontinuation); nor age, gender, or payer status (81) may be important correlates of discontinuation of care in patients with HNC. Other reported reasons of lost to follow-up are to enlist: 22% had difficulty scheduling an appointment, 30% had transportation barriers, 22% had personal or work obligations that prevented follow-up, 17% did not follow-up because they “felt better,” and 39% were following up with an otolaryngologist or oncologist closer to home (3). In addition, overall adherence to the American Cancer Society HNC Survivorship Care Guideline (82) in early post-treatment survivors occurred to be suboptimal (Salley JR). Understanding these barriers is critical to creating a patient-centered model that balances both clinical surveillance needs and reasonable expectations for patients. Improvements can be made to educate patients on the recommended length of follow-up and its importance (3).
Patients’ preferences regarding follow-up remain poorly investigated. The cross-sectional survey revealed that 89.1% preferred scheduled follow-up to self-referral, 57% favored fewer visits than the current standard, and 85.1% endorsed regular imaging. Moreover, patients’ preferences only partially correspond to current follow-up guidelines (83).
Patients’ preferences before and after treatment are closely connected with QoL. Improving it in patients with HNC is a very vast problem and exceeds the scope of this article. Although, it should be mentioned that existing literature for intervention studies focuses on educational, psychosocial, physical, and psychological symptom management; mindfulness; pharmacology; exercise; and telemedicine strategy. Appearance of HNC recurrence exacerbates the problem. However, using therapeutic coping mechanisms to control discomfort during and after treatment can affect mood and QoL well into the survivorship stage (84, 85).
HNC follow-up scheme optimization
Risk stratification–guided surveillance based on retrospectively collected local and distant recurrence of chemoradiation treated patients was presented in 2016 (86).
Personalized follow-up program (PFU) that provides a potential alternative to the standardized intense follow-up schedule was presented for all patients with HNC in 2022. PFU followed the NHS Long-Term Plan for Cancer, where each patient moved to a follow-up pathway that suits individual needs through personalized stratified follow-up programs (NHS England, 2019; https://doi.org/10.1016/j.annonc.2022.07.401).
PETNECK2 is a program of research (NIHR200861) with an embedded randomized controlled trial designed to determine whether patient-initiated follow-up (PIFU) is more effective than regular follow-up for HNC. PETNECK2 builds on the successful results of the first PETNECK study, where a 3-month PET-CT scan reliably detected patients requiring neck dissection, avoiding unnecessary surgery for those at low risk of recurrence, and reducing harm and costs (87). Current PETNECK2 trial will compare a new model of PIFU with routine scheduled follow-up. UK clinicians were enthusiastic about the PETNECK2 trial but had concerns that PIFU may not suit disengaged patients and may aggravate patient anxiety/fear of recurrence and delay detection of recurrence (88).
The next Individualized Follow-Up for Head and Neck Cancer (INFLUENCE) study offered a decision-aided choice between standardized or individualized follow-up after 1.5 years of uncomplicated guideline-prescribed follow-up. Standardized follow-up entails continuing the 5-year guideline-prescribed schedule. Individualized follow-up means the patient only attends the outpatient clinic on their own initiative in case of physical symptoms or supportive care needs (15). Patients are educated on self-examination and when a control visit is necessary (6). Moreover, the same authors point out that 1 year of follow-up for oral cavity squamous cell carcinoma (SCC) and 1.5 years for oropharynx-, larynx-, and hypopharynx SCC suffice for the goal of detecting disease manifestations after treatment (6).
A pilot-tested clinical informatics intervention, HN-STAR, was developed to elicit concerns online from HNC survivors prior to a routine oncology clinic visit. HN-STAR then presents tailored evidence-based clinical recommendations as a clinical decision support tool to be used during the visit where the oncology clinician and survivor select symptom management strategies and other actions. This generates a survivorship care plan (SCP). Online elicitation of health concerns occurs 3, 6, and 9 months after the clinic visit, generating an updated SCP each time. HN-STAR encompasses important methods of improving survivorship care (89).
HPV and EBV as a game changers in HNC surveillance strategies
HPV or EBV status is rarely considered when determining surveillance plans. In particular, HPV+ and HPV− OPC are two distinct entities that require 4 and 12 follow-up visits, respectively (17). The presented current findings reveal an urgent need for individualized surveillance strategies in patients with HNC cancers’ HPV- and EBV-based etiology. Thus, a patient-tailored assessment plan should integrate prognostic factors based on HPV or EBV positivity (90). However, overlapping high-risk features may necessitate a modification of the recommended follow-up visits, for example, in favor of more frequent visits for patients with HPC+ OPC with a smoking history of more than 10 pack years (17).
Conclusions
The presented algorithm summarizes HNC follow-up problems. This paper is also a call to action for implementing the recommended strategies. We wanted to highlight areas where further research is needed to refine follow-up care in HNC.
Multidisciplinary cooperation and the development and refinement of follow-up recommendations by the experts of the individual scientific societies are essential. The need for more specific recommendations stems from the specificity of the primary tumor site, considering the histological specificity of the tumor.
Novel approaches and technologies in follow-up care should be explored in more depths, i.e., established visualization technique such as biological endoscopy (NBI), and liquid biopsy should be integrated into the standard practice with all potential benefits and challenges.
Universal monitoring algorithm for HNC four groups with a high, moderate, and low risk of recurrence after the adequate treatment in combination with a high or low probability of applying an effective salvage is an absolutely innovative approach to redeploying system resources and ensuring maximum benefit for patients with HNC.
The division of all HNCs into four different monitoring paths ensures standardization of procedures, repeatability of the scheme, and redistribution of forces and resources to groups that can get the greatest therapeutic benefit from dense meshes of the visits network.
The presented algorithm practically eliminates from follow-up visits a very large group of the most advanced patients, with no chance for further curative treatment. Nevertheless, intensive palliative support should be planned for this group in dedicated places, which should be the subject of another study.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
MW: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. JM: Conceptualization, Writing – original draft, Writing – review & editing. WP: Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. PB: Writing – original draft, Writing – review & editing. BM: Writing – original draft, Writing – review & editing. MR: Writing – original draft, Writing – review & editing. KS: Writing – review & editing. PM: Writing – original draft, Writing – review & editing. JF: Writing – review & editing. DJ: Writing – review & editing. KN: Writing – review & editing. AM: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research received an internal research grant from Medical University of Lodz, Poland (503/1-036-03/503-11-002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Digonnet A, Hamoir M, Andry G, Haigentz M, Takes RP, Silver CE, et al. Post-therapeutic surveillance strategies in head and neck squamous cell carcinoma. Eur Arch Oto-Rhino-Laryngol (2013) 270:1569–80. doi: 10.1007/s00405-012-2172-7
2. Manikantan K, Khode S, Dwivedi RC, Palav R, Nutting CM, Rhys-Evans P, et al. Making sense of post-treatment surveillance in head and neck cancer: when and what of follow-up. Cancer Treat Rev (2009) 35:744–53. doi: 10.1016/j.ctrv.2009.08.007
3. Dimon EL, Simmons JK, Ziegler A, Bollman M, Bur A, Nallani R, et al. Assessment of conditions leading to lost-to-follow-up of head and neck cancer patients. Am J Otolaryngol - Head Neck Med Surg (2022) 43:5–9. doi: 10.1016/j.amjoto.2022.103443
4. Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, et al. Head and neck cancers, version 2.2020. JNCCN J Natl Compr Cancer Network (2020) 18:873–98. doi: 10.6004/jnccn.2020.0031
5. Bl H, Pe B, Rv K, As F, Albieri V, So D, et al. treatment in adult cancer survivors (Review). Cochrane Library (2019) 2019:1–77. doi: 10.1002/14651858.CD012425.pub2.www.cochranelibrary.com
6. van de Weerd C, Ebbers T, Smilde DEM, van Tol-Geerdink JJ, Takes RP, van den Broek GB, et al. Evaluation of a remote monitoring app in head and neck cancer follow-up care. Cancer Med (2023) 12:15552–66. doi: 10.1002/cam4.6202
7. Simo R, Bradley P, Chevalier D, Dikkers F, Eckel H, Matar N, et al. European Laryngological Society: ELS recommendations for the follow-up of patients treated for laryngeal cancer. Eur Arch Oto-Rhino-Laryngol (2014) 271:2469–79. doi: 10.1007/s00405-014-2966-x
8. Wierzbicka M, Milecki P, Katulska K MA. Nowotwory głowy i szyi. Via Medica (2018). pp. 202–212 p.
9. Pagh A, Vedtofte T, Lynggaard CD, Rubek N, Lonka M, Johansen J, et al. The value of routine follow-up after treatment for head and neck cancer. A National Survey from DAHANCA. Acta Oncol (Madr) (2013) 52:277–84. doi: 10.3109/0284186X.2012.741324
10. Pagh A, Grau C, Overgaard J. A longitudinal study of follow-up activities after curative treatment for head and neck cancer. Acta Oncol (Madr) (2015) 54:813–9. doi: 10.3109/0284186X.2015.1028591
11. Chung EJ, Jeong WJ, Jung YH, Kwon SK, Kwon TK, Ahn SH, et al. Long-term oncological and functional outcomes of induction chemotherapy followed by (chemo)radiotherapy vs definitive chemoradiotherapy vs surgery-based therapy in locally advanced stage III/IV hypopharyngeal cancer: Multicenter review of 266 cases. Oral Oncol (2019) 89:84–94. doi: 10.1016/j.oraloncology.2018.12.015
12. Jackowska J, Abelak Y, Piersiala K, Wierzbicka M. The effectiveness of the follow-up of patients after surgery due to cancer of the head and neck. J Comp Eff Res (2018) 7:765–73. doi: 10.2217/cer-2017-0096
13. National Comprehensive Cancer Network. NCCN guidelines: head and neck cancer . Available at: https://www.nccn.org/guidelines/guidelines-detailcategory (Accessed January 4, 2022).
14. Imbimbo M, Alfieri S, Botta L, Bergamini C, Gloghini A, Calareso G, et al. Surveillance of patients with head and neck cancer with an intensive clinical and radiologic follow-up. Otolaryngol - Head Neck Surg (United States) (2019) 161:635–42. doi: 10.1177/0194599819860808
15. Newton C, Beaver K, Clegg A. Patient initiated follow-up in cancer patients: A systematic review. Front Oncol (2022) 12:954854. doi: 10.3389/fonc.2022.954854
16. Szturz P, Van Laer C, Simon C, Van Gestel D, Bourhis J, Vermorken JB. Follow-up of head and neck cancer survivors: tipping the balance of intensity. Front Oncol (2020) 10:688. doi: 10.3389/fonc.2020.00688
17. Lee HI, Lee J, Lee JH, Wu HG, Kim JH, Kim Y EK. Evaluation of optimal assessment schedules for surveillance after definitive locoregional treatment of locally advanced head and neck cancer: A retrospective cohort study with parametric modeling of event-free survival. JAMA Otolaryngol Head Neck Surg (2022) 148:1059–67. doi: 10.1001/jamaoto.2022.2561
18. De Felice F, Musio D, Tombolini V. Follow-up in head and neck cancer: A management dilemma. Adv Otolaryngol (2015) 2015:1–4. doi: 10.1155/2015/703450
19. Brands MT, Brennan PA, Verbeek ALM, Merkx MAW, Geurts SME. Follow-up after curative treatment for oral squamous cell carcinoma. A critical appraisal of the guidelines and a review of the literature. Eur J Surg Oncol (2018) 44:559–65. doi: 10.1016/j.ejso.2018.01.004
20. Milas ZL, Neelands B, Trufan SJ, Benbow J, Carrizosa D, Brickman DS, et al. Survivorship care plans improve the identification of post-radiation hypothyroidism after head and neck cancer treatment. Anticancer Res (2022) 42:429–4437. doi: 10.21873/anticanres.15943
21. Mroueh R, Nevala A, Haapaniemi A, Pitkäniemi J, Salo T, Mäkitie AA. Risk of second primary cancer in oral squamous cell carcinoma. Head Neck (2020) 42:1848–58. doi: 10.1002/hed.26107
22. Badwelan M, Muaddi H, Ahmed A, Lee KT, Tran SD. Oral squamous cell carcinoma and concomitant primary tumors, what do we know? A review of the literature. Curr Oncol (2023) 30:3721–34. doi: 10.3390/curroncol30040283
23. Brands MT, Campschroer G, Merkx MAW, Verbeek ALM, van Dijk BAC, Geurts SME. Second primary tumours after squamous cell carcinoma of the oral cavity. Eur J Surg Oncol (2021) 47:1934–9. doi: 10.1016/j.ejso.2021.03.242
24. Crowder SL, Najam N, Sarma KP, Fiese BH, Arthur AE. Head and neck cancer survivors: a qualitative study. Support Care Cancer (2022) 29:4349–56. doi: 10.1007/s00520-020-05981-1.Quality
25. Brennan KE, Hall SF, Yoo J, Rohland SL, Theurer J, Peng Y, et al. Routine follow-up care after curative treatment of head and neck cancer: A survey of patients’ needs and preferences for healthcare services. Eur J Cancer Care (Engl) (2019) 28:1–15. doi: 10.1111/ecc.12993
26. Deuning-Smit E, Custers JAE, Miroševič Š, Takes RP, Jansen F, Langendijk JA, et al. Prospective longitudinal study on fear of cancer recurrence in patients newly diagnosed with head and neck cancer: Course, trajectories, and associated factors. Head Neck (2022) 44:914–25. doi: 10.1002/hed.26985
27. Bl H, Pe B, Rv K, As F, Albieri V, So D, et al. treatment in adult cancer survivors (Review). Eur J Surg Oncol (2018) 2019:559–65. doi: 10.1002/14651858.CD012425.pub2.www.cochranelibrary.com
28. van Overveld LFJ, Takes RP, Turan AS, Braspenning JCC, Smeele LE, Merkx MAW HR, et al. Needs and preferences of patients with head and neck cancer in integrated care. Clin Otolaryngol (2018) 43:553–61. doi: 10.1111/coa.13021
29. Wierzbicka M, Waśniewska E, Jackowska J, Leszczyńska M, Szyfter W. 2012 Mar-Apr;66(2):138-47. P SW. Problematyka monitorowania chorych leczonych z powodu nowotworów głowy i szyi [Routine follow-up in patients treated of head and neck cancer]. Otolaryngol Pol (2012) 66:137–47. doi: 10.1016/S0030-6657(12)70762-X
30. Joshi A, Calman F, O’Connell M, Jeannon JP, Pracy P, Simo R. Current trends in the follow-up of head and neck cancer patients in the UK. Clin Oncol (2010) 22:114–8. doi: 10.1016/j.clon.2009.11.004
31. Wierzbicka M, Waśniewska E, Jackowska J, Leszczyńska M SW. Problematyka monitorowania chorych leczonych z powodu nowotworów głowy i szyi [Routine follow-up in patients treated of head and neck cancer]. Otolaryngol Pol (2012) 66:138–47. doi: 10.1016/S0030-6657(12)70762-X
32. Machiels JP, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol (2020) 31:1462–75. doi: 10.1016/j.annonc.2020.07.011
33. Simo R, Homer J, Clarke P, Mackenzie K, Paleri V, Pracy P, et al. Follow-up after treatment for head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol (2016) 130:S208–11. doi: 10.1017/s0022215116000645
34. Nocon CC, Kennedy A, Jaffe J, Pruitt J, Kuchta K BM. Costs associated with imaging surveillance after treatment for head and neck cancer. JAMA Otolaryngol - Head Neck SurgeryAMA Otolaryngol Head Neck Surg (2021) 147:632–7. doi: 10.1001/jamaoto.2021.0835
35. Kanatas A, Bala N, Lowe D, Rogers SN. Outpatient follow-up appointments for patients having curative treatment for cancer of the head and neck: Are the current arrangements in need of change? Br J Oral Maxillofac Surg (2014) 52:681–7. doi: 10.1016/j.bjoms.2014.06.017
36. Schwartz DL, Barker J, Chansky K, Yueh B, Raminfar L, Drago P, et al. Postradiotherapy surveillance practice for head and neck squamous cell carcinoma - Too much for too little? Head Neck (2003) 25:990–9. doi: 10.1002/hed.10314
37. Rocke J, Mclaren O, Hardman J, Garas G, Smith ME, Ishii H, et al. The role of allied healthcare professionals in head and neck cancer surveillance: A systematic review. Clin Otolaryngol (2020) 45:83–98. doi: 10.1111/coa.13471
38. Suenaga Y, Kitajima K, Ishihara T, Sasaki R, Otsuki N, Nibu K, et al. FDG-PET/contrast-enhanced CT as a post-treatment tool in head and neck squamous cell carcinoma: comparison with FDG-PET/non-contrast-enhanced CT and contrast-enhanced CT. Eur Radiol (2016) 26:1018–30. doi: 10.1007/s00330-015-3902-1
39. Meregaglia M, Cairns J, Licitra L, Bossi P. The use of intensive radiological assessments in routine surveillance after treatment for head and neck cancer: An economic evaluation. Eur J Cancer (2018) 93:89–98. doi: 10.1016/j.ejca.2018.01.082
40. Smith AF, Hall PS, Hulme CT, Dunn JA, McConkey CC, Rahman JK, et al. Cost-effectiveness analysis of PET-CT-guided management for locally advanced head and neck cancer. Eur J Cancer (2017) 85:6–14. doi: 10.1016/j.ejca.2017.07.054
41. León X, García J, Pujol A, de Juan J, Vásquez R, Quer M, et al. Relationship between the transcriptional expression of PIM1 and local control in patients with head and neck squamous cell carcinomas treated with radiotherapy. Eur Arch Oto-Rhino-Laryngol (2022) 279:3679–84. doi: 10.1007/s00405-021-07223-4
42. Roedel RMW, Matthias C, Wolff HA, Schindler P, Aydin T, Christiansen H. Transoral laser microsurgery for recurrence after primary radiotherapy of early glottic cancer. Auris Nasus Larynx (2010) 37:474–81. doi: 10.1016/j.anl.2009.11.004
43. Russo E, Costantino A, Veneroni MV, Festa BM, Pellini R, Campo F, et al. Transoral laser microsurgery in recurrent laryngeal cancer: A systematic review and meta-analysis. Laryngoscope (2023) 133:1425–33. doi: 10.1002/lary.30332
44. Cai Z, Yue H, Chen L, Xv Y, Li Y, Tang B, et al. Salvage transoral laser microsurgery for early local recurrence of glottic squamous cell cancer. J Otolaryngol - Head Neck Surg (2023) 52:1–10. doi: 10.1186/s40463-023-00628-7
45. Piazza C, Paderno A, Sjogren EV, Bradley PJ, Eckel HE, Mäkitie A, et al. Salvage carbon dioxide transoral laser microsurgery for laryngeal cancer after (chemo)radiotherapy: a European Laryngological Society consensus statement. Eur Arch Oto-Rhino-Laryngol (2021) 278:4373–81. doi: 10.1007/s00405-021-06957-5
46. Wulff NB, Dalton SO, Wessel I, Arenaz Búa B, Löfhede H, Hammerlid E, et al. Health-related quality of life, dysphagia, voice problems, depression, and anxiety after total laryngectomy. Laryngoscope (2022) 132:980–8. doi: 10.1002/lary.29857
47. Somda SMA, Leconte E, Boher JM, Asselain B, Kramar A, Filleron T. Optimal scheduling of post-therapeutic follow-up of patients treated for cancer for early detection of relapses. Stat Methods Med Res (2016) 25:2457–71. doi: 10.1177/0962280214524178
48. Ritoe SC, Bergman H, Krabbe PFM, Kaanders JHAM, van den Hoogen FJA, Verbeek ALM, et al. Cancer recurrence after total laryngectomy: treatment options, survival, and complications. Head Neck (2006) 28:383–8. doi: 10.1002/hed.20350
49. Boscolo Nata F, Gardenal N, Giudici F, Tirelli G. The role of NBI with flexible video-endoscope in the follow-up of head and neck cancer patients: a prospective study. Eur Arch Otorhinolaryngol (2022) 279(4):2133–41. doi: 10.1007/s00405-021-07016-9
50. Malinowski J, Pietruszewska W, Stawiski K, Kowalczyk M, Barańska M, Rycerz A, et al. High-speed videoendoscopy enhances the objective assessment of glottic organic lesions: a case-control study with multivariable data-mining model development. Cancers (Basel) (2023) 15(14):3716. doi: 10.3390/cancers15143716
51. Kissun D, Magennis P, Lowe D, Brown JS, Vaughan ED, Rogers SN. Timing and presentation of recurrent oral and oropharyngeal squamous cell carcinoma and awareness in the outpatient clinic. Br J Oral Maxillofac Surg (2006) 44:371–6. doi: 10.1016/j.bjoms.2005.08.010
52. Jajodia A, Mandal G, Yadav V, Khoda J, Goyal J, Pasricha S, et al. Adding MR diffusion imaging and t2 signal intensity to neck imaging reporting and data system categories 2 and 3 in primary sites of postsurgical oral cavity carcinoma provides incremental diagnostic value. AJNR Am J Neuroradiol (2022) 43(7):1018–23. doi: 10.3174/ajnr.A7553
53. Müller J, Hüllner M, Strobel K, Huber GF, Burger IA, Haerle SK. The value of 18F-FDG-PET/CT imaging in oral cavity cancer patients following surgical reconstruction. Laryngoscope (2015) 125:1861–8. doi: 10.1002/lary.25326
54. Elbers JBW, Al-Mamgani A, van den Brekel MWM, Jóźwiak K, de Boer JP, Lohuis PJFM, et al. Salvage surgery for recurrence after radiotherapy for squamous cell carcinoma of the head and neck. Otolaryngol - Head Neck Surg (United States) (2019) 160:1023–33. doi: 10.1177/0194599818818443
55. Putten L, Bree R, Doornaert PA, Buter J, Eerenstein SEJ, Rietveld DHF, et al. Salvage surgery in post-chemoradiation laryngeal and hypopharyngeal carcinoma: outcome and review. Acta Otorhinolaryngol Ital (2015) 35:162–72.
56. Dubsky PC, Stift A, Rath T, Kornfehl J. Salvage surgery for recurrent carcinoma of the hypopharynx and reconstruction using jejunal free tissue transfer and pectoralis major muscle pedicled flap. Arch Otolaryngol - Head Neck Surg (2007) 133:551–5. doi: 10.1001/archotol.133.6.551
57. van Weert S, Leemans CR. Salvage surgery in head and neck cancer. Oral Dis (2021) 27:117–24. doi: 10.1111/odi.13582
58. Fakhry C, Zhang Q, Nguyen-Tan PF, Rosenthal D, El-Naggar A, Garden AS, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol (2014) 32:3365–73. doi: 10.1200/JCO.2014.55.1937
59. Chan JYW. Surgical salvage of recurrent nasopharyngeal carcinoma. Curr Oncol Rep (2015) 17:7–12. doi: 10.1007/s11912-014-0433-x
60. Na’ara S, Amit M, Billan S, Cohen JT, Gil Z. Outcome of patients undergoing salvage surgery for recurrent nasopharyngeal carcinoma: A meta-analysis. Ann Surg Oncol (2014) 21:3056–62. doi: 10.1245/s10434-014-3683-9
61. You R, Zou X, Wang SL, Jiang R, Tang LQ, Zhang WD, et al. New surgical staging system for patients with recurrent nasopharyngeal carcinoma based on the AJCC/UICC rTNM classification system. Eur J Cancer (2015) 51:1771–9. doi: 10.1016/j.ejca.2015.05.014
62. Svajdova M, Sicak M, Dubinsky P, Slavik M, Slampa P, Kazda T. Recurrent nasopharyngeal cancer: Critical review of local treatment options including recommendations during the COVID-19 pandemic. Cancers (Basel) (2020) 12:1–19. doi: 10.3390/cancers12123510
63. Freling N, Crippa F, Maroldi R. Staging and follow-up of high-grade Malignant salivary gland tumours: The role of traditional versus functional imaging approaches – A review. Oral Oncol (2016) 60:157–66. doi: 10.1016/j.oraloncology.2016.04.016
64. Zbären P, Vander Poorten V, Witt RL, Woolgar JA, Shaha AR, Triantafyllou A, et al. Pleomorphic adenoma of the parotid: Formal parotidectomy or limited surgery? Am J Surg (2013) 205:109–18. doi: 10.1016/j.amjsurg.2012.05.026
65. Redaelli De Zinis LO, Piccioni M, Antonelli AR, Nicolai P, Lovin BD, Gidley PW, et al. Second primary tumours after squamous cell carcinoma of the oral cavity. Eur J Surg Oncol (2019) 125:134–48. doi: 10.1002/14651858.CD012425.pub2.www.cochranelibrary.com
66. Valstar MH, de Ridder M, van den Broek EC, Stuiver MM, van Dijk BAC, van Velthuysen MLF, et al. Salivary gland pleomorphic adenoma in the Netherlands: A nationwide observational study of primary tumor incidence, Malignant transformation, recurrence, and risk factors for recurrence. Oral Oncol (2017) 66:93–9. doi: 10.1016/j.oraloncology.2017.01.004
67. Kuriyama T, Kawata R, Higashino M, Nishikawa S, Inui T, Terada T, et al. Recurrent benign pleomorphic adenoma of the parotid gland: Facial nerve identification and risk factors for facial nerve paralysis at re-operation. Auris Nasus Larynx (2019) 46:779–84. doi: 10.1016/j.anl.2019.02.010
68. Witt RL, Nicolai P. Recurrent benign salivary gland neoplasms. Adv Otorhinolaryngol (2016) 78:63–70. doi: 10.1159/000442126
69. van Herpen C, Vander Poorten V, Skalova A, Terhaard C, Maroldi R, van Engen A, et al. Salivary gland cancer: ESMO–European Reference Network on Rare Adult Solid Cancers (EURACAN) Clinical Practice Guideline for diagnosis, treatment and follow-up †. ESMO Open (2022) 7:100602. doi: 10.1016/j.esmoop.2022.100602
70. Guzzo M, Locati LD, Prott FJ, Gatta G, McGurk M, Licitra L. Major and minor salivary gland tumors. Crit Rev Oncol Hematol (2010) 74:134–48. doi: 10.1016/j.critrevonc.2009.10.004
71. Wierzbicka M, Niemczyk K, Bruzgielewicz A, Durko M, Klatka J, Kopeć T, et al. Multicenter experiences in temporal bone cancer surgery based on 89 cases. PloS One (2017) 12:1–12. doi: 10.1371/journal.pone.0169399
72. McCracken M, Pai K, Cabrera CI, Johnson BR, Tamaki A, Gidley PW MNF. Temporal bone resection for squamous cell carcinoma of the lateral skull base: systematic review and meta-analysis. Otolaryngol Head Neck Surg (2023) 168:154–64. doi: 10.1177/01945998221084912
73. Cazzador D, Franz L, Tealdo G, Carobbio ALC, Ferraro M, Mazzoni A, et al. Survival outcomes in squamous cell carcinoma of the external auditory canal: A systematic review and meta-analysis. J Clin Med (2023) 12:2490–561. doi: 10.3390/jcm12072490
74. Seligman KL, Sun DQ, Ten Eyck PP, Schularick NM, Hansen MR. Temporal bone carcinoma: Treatment patterns and survival. Laryngoscope (2020) 130:E11–20. doi: 10.1002/lary.27877
75. Alessandrini L, Astolfi L, Franz L, Gentilin E, Mazzoni A, Zanoletti E, et al. Temporal bone squamous cell carcinoma: molecular markers involved in carcinogenesis, behavior, and prognosis: A systematic review. Int J Mol Sci (2022) 23:1–19. doi: 10.3390/ijms23094536
76. Benjamin RS, Cushing SL, Blakeman AW, Campos JL, Papsin BC, Gordon KA. Evaluating the use of a balance prosthesis during balance perturbations in children and young adults with cochleovestibular dysfunction. Sci Rep (2023) 13:1–14. doi: 10.1038/s41598-023-36613-3
77. Workman AD, Palmer JN, Adappa ND. Posttreatment surveillance for sinonasal Malignancy. Curr Opin Otolaryngol Head Neck Surg (2017) 25:86–92. doi: 10.1097/MOO.0000000000000330
78. Khalili S, Worrall DM, Brooks S, Morris SM, Farquhar D, Newman JG, et al. Endoscopy versus imaging: Analysis of surveillance methods in sinonasal Malignancy. Head Neck (2016) 38:1229–33. doi: 10.1002/hed.24413
79. Han AY, Nader ME, Lam K, Su SY. Current status of sinonasal cancer survivorship care. Head Neck (2023) 45(9):2458–68. doi: 10.1002/hed.27457
80. Howren MB, Christensen AJ, Pagedar NA, Leoncini E, Ricciardi W, Cadoni G, et al. Examination of risk factors for discontinuation of follow-up care in patients with head and neck cancer. Head Neck (2023) 36:631–9. doi: 10.1016/j.ejca.2021.09.046.Clinical
81. Seaman AT, Seligman KL, Nguyen KK, Al-Qurayshi Z, Kendell ND, Pagedar NA. Characterizing head and neck cancer survivors’ discontinuation of survivorship care. Cancer (2022) 128:192–202. doi: 10.1002/cncr.33888
82. Nekhlyudov L, Lacchetti C, Davis NB, Garvey TQ, Goldstein DP, Nunnink JC, et al. Head and neck cancer survivorship care guideline: American society of clinical oncology clinical practice guideline endorsement of the American cancer society guideline. J Clin Oncol (2017) 35:1606–21. doi: 10.1200/JCO.2016.71.8478
83. Mueller SA, Riggauer J, Elicin O, Blaser D, Trelle S, Giger R. Patients’ preferences concerning follow-up after curative head and neck cancer treatment: A cross-sectional pilot study. Head Neck (2019) 41:2174–81. doi: 10.1002/hed.25686
84. Senchak JJ, Fang CY, Bauman JR. Interventions to improve quality of life (QOL) and/or mood in patients with head and neck cancer (HNC): a review of the evidence. Cancers Head Neck (2019) 11:4:2. doi: 10.1186/s41199-019-0041-4.eCollection 2019
85. Thilges S, Mumby P, Sinacore J, Clark J, Czerlanis C. Implementing a cognitive behavioral intervention for patients with head and neck cancer. Support Care Cancer (2023) 31(8):476. doi: 10.1007/s00520-023-07948-4
86. Denaro N, Merlano MC, Russi EG. Follow-up in head and neck cancer: Do more does it mean do better? A systematic review and our proposal based on our experience. Clin Exp Otorhinolaryngol (2016) 9:287–97. doi: 10.21053/ceo.2015.00976
87. Mehanna H, McConkey CC, Rahman JK, Wong WL, Smith AF, Nutting C, et al. PET-NECK: A multicentre randomised Phase III non-inferiority trial comparing a positron emission tomography-computerised tomography-guided watch-and-wait policy with planned neck dissection in the management of locally advanced (N2/N3) nodal metastases in. Health Technol Assess (Rockv) (2017) 21:1–122. doi: 10.3310/hta21170
88. Lorenc A, Wells M, Fulton-Lieuw T, Nankivell P, Mehanna H, Jepson M, et al. Clinicians’ Views of patient-initiated follow-up in head and neck cancer: a qualitative study to inform the PETNECK2 trial. Clin Oncol (2022) 34:230–40. doi: 10.1016/j.clon.2021.11.010
89. Salz T, Ostroff JS, Nightingale CL, Atkinson TM, Davidson EC, Jinna SR, et al. The Head and Neck Survivorship Tool (HN-STAR) Trial (WF-1805CD): A protocol for a cluster-randomized, hybrid effectiveness-implementation, pragmatic trial to improve the follow-up care of head and neck cancer survivors. Contemp Clin Trials (2021) 107:1–30. doi: 10.1016/j.cct.2021.106448
90. Tsai CJ, McBride SM, Riaz N, Kang JJ, Spielsinger DJ, Waldenberg T, et al. Evaluation of substantial reduction in elective radiotherapy dose and field in patients with human papillomavirus-associated oropharyngeal carcinoma treated with definitive chemoradiotherapy. JAMA Oncol (2022) 8:364–72. doi: 10.1001/jamaoncol.2021.6416
Keywords: algorithm, head and neck cancer, follow-up, salvage, risk of failure, primary location, advancement
Citation: Wierzbicka M, Markowski J, Pietruszewska W, Burduk P, Mikaszewski B, Rogowski M, Składowski K, Milecki P, Fijuth J, Jurkiewicz D, Niemczyk K and Maciejczyk A (2023) Algorithms of follow-up in patients with head and neck cancer in relation to primary location and advancement. Consensus of Polish ENT Society Board and Head Neck Experts. Front. Oncol. 13:1298541. doi: 10.3389/fonc.2023.1298541
Received: 21 September 2023; Accepted: 31 October 2023;
Published: 12 December 2023.
Edited by:
Jeroen Meulemans, University Hospitals Leuven, BelgiumReviewed by:
Remo Accorona, ASST Grande Ospedale Metropolitano Niguarda, ItalyAlberto Paderno, University of Brescia, Italy
Copyright © 2023 Wierzbicka, Markowski, Pietruszewska, Burduk, Mikaszewski, Rogowski, Składowski, Milecki, Fijuth, Jurkiewicz, Niemczyk and Maciejczyk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wioletta Pietruszewska, d2lvbGV0dGEucGlldHJ1c3pld3NrYUB1bWVkLmxvZHoucGw=
 Małgorzata Wierzbicka
Małgorzata Wierzbicka Jarosław Markowski4
Jarosław Markowski4 Wioletta Pietruszewska
Wioletta Pietruszewska Paweł Burduk
Paweł Burduk Krzysztof Składowski
Krzysztof Składowski Kazimierz Niemczyk
Kazimierz Niemczyk