
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 15 January 2024
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1297870
This article is part of the Research TopicAcute Leukemias: Molecular Characterization, Leukemia-Initiating Cells, and Influence of the Microenvironment Vol. IIView all 9 articles
Introduction: The prognostic role of Wilms’ tumor 1 (WT1) gene expression at diagnosis in children with B cell precursor acute lymphoblastic leukemia (BCP-ALL) is still controversial.
Methods: We detected the WT1 transcript levels of 533 de novo pediatric BCP-ALL patients using TaqMan-based real-time quantitative PCR and analyzed their clinical features.
Results: The WT1 transcript levels differed among the distinct molecularly defined groups, with the highest levels in the KMT2A rearrangements (KMT2A-r) group. According to the results of the X-tile software, all patients were divided into two groups: WT1/ABL ≥ 0.24% (group A) and <0.24% (group B). The proportions of patients whose age was ≥10 years old, with immunophenotype of Pro-B, belonging in high-risk group, or with minimal residual disease (MRD) ≥ 0.01% at week 12 were significantly higher in group A than in group B. In the B-other group, WT1 overexpression was an independent risk factor of overall survival (OS) rate (P = 0.042), and higher MRD ≥ 0.01% at week 12 was associated with lower OS rate (P<0.001) and event-free survival rate (P<0.001). Moreover, the subgroup analysis revealed that, in patients with initial WBC<50 × 109/L or MRD<0.1% at day 33 or MRD<0.01% at week 12 or in the standard-risk group, WT1 overexpression led to a poorer outcome in comparison with those with WT1 downexpression (P<0.05).
Discussion: Therefore, pediatric BCP-ALL with WT1 overexpression had unique clinico-pathological characteristics and poor treatment response. In B-other patients, WT1 overexpression at diagnosis predicted an inferior prognosis. The WT1 gene may serve as a biomarker for monitoring residual disease in the B-other population, especially in children in the standard-risk group.
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy, originating from lymphatic stem cell progenitors, and B-cell precursor acute lymphoblastic leukemia (BCP-ALL) accounts for approximately 80% of childhood ALL (1). Recently, the 5-year overall survival (OS) rate of pediatric BCP-ALL in developed countries has approached 90% due to the development of a risk-guided treatment system and the progress of therapeutic techniques (2). Molecular genetic abnormalities at initial diagnosis, such as ETV6-RUNX1, TCF3-PBX1, BCR-ABL1, and KMT2A rearrangements (KMT2A-r), and MRD at various stages of treatment are the most crucial indicators to determine risk stratification and prognosis (2). However, for other BCP-ALL (B-other) populations with no predictive molecular genetic abnormalities, it is necessary to identify new genetic markers to further improve the risk stratification system and select the most appropriate clinical therapeutic schedules for these patients.
Located on human chromosome 11p13, the Wilms tumor 1 (WT1) gene is primarily expressed in normal tissues such as the kidney, reproductive system, and hematological system. It encodes a transcription factor and is involved in biological processes such as embryonic development, cell proliferation, differentiation, apoptosis, invasion, and metastasis (3). WT1 is typically overexpressed in individuals with acute leukemia and expressed at low levels in healthy hematopoietic stem/progenitor cells (4). Although many studies have explored the prognostic value of WT1 expression in acute myeloid leukemia (AML) (5, 6), there is still a lack of research on the prognostic significance of WT1 expression in ALL, particularly in pediatric BCP-ALL (7). Therefore, it is necessary to conduct comprehensive investigations in children with BCP-ALL.
In this study, we retrospectively analyzed the data of 533 children with BCP-ALL admitted to our center, with the aim to explore the prognostic significance of WT1 expression and its correlation with other clinical characteristics in these children.
From January 2012 to December 2018, 533 consecutive children (aged 0 to 18) newly diagnosed with de novo BCP-ALL were included.
The diagnosis of BCP-ALL was based on the morphological, immunophenotypic, cytogenetic, and molecular criteria as measured by standard techniques.
The risk stratification was assessed according to NCI risk criteria, cytogenetic subtypes, and treatment response (8).
Real-time quantitative PCR (RQ-PCR), based on TaqMan, was used to detect the quantities of WT1 transcripts at diagnosis as well as TCF3-PBX1, ETV6-RUNX1, BCR-ABL1, and KMT2A-r fusion transcripts, which had been described in our previous studies (3).
The WT1 transcript level was ultimately calculated as the ratio of WT1 transcript copies to ABL copies using the control gene Abelson (ABL) as a reference. MRD was detected by multi-parameter flow cytometry with a sensitivity of 0.01% (9).
All patients received a modified version of the Berlin–Frankfurt–Munster regimen. The entire treatment cycle consisted of induction therapy, consolidation therapy, reinduction therapy interspersed with consolidation therapy, and maintenance therapy. The details of the protocol are presented in our previous reports (8, 10).
The B-other group was defined as BCP-ALL children with no TCF3-PBX1, ETV6-RUNX1, BCR-ABL1, or KMT2A-r. Complete remission (CR) was defined as the presence of normal hematopoiesis with less than 5% bone marrow blast cells and the absence of an extramedullary disease. OS and event-free survival (EFS) were measured from the date of diagnosis. The endpoint events of EFS were the first relapse or death while in remission, and the endpoint event of the OS was death regardless of the cause. The last follow-up date was June 30, 2022.
All data were analyzed by using SPSS 26.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were compared using Mann–Whitney U-test, and categorical variables were compared using the chi-square test or Fisher exact test. OS and EFS were estimated using the Kaplan–Meier method and compared using the log-rank test. Potential prognostic factors were considered in a Cox proportional hazards regression model in multivariate analyses. X-tile 3.6.1 software (Yale University, New Haven, CT, USA) was used to analyze the optimal cutoff value for the WT1 transcript level. According to the results of the X-tile software, the optimal cutoff value for the WT1 transcript level was 0.24% in children with BCP-ALL (Supplementary Figure S1). A two-sided P < 0.05 was considered statistically significant.
A total of 533 children with BCP-ALL were enrolled in the study, of which 302 were male (Table 1). The median age at diagnosis was 5.0 years (range, 0.3–16.0 years).
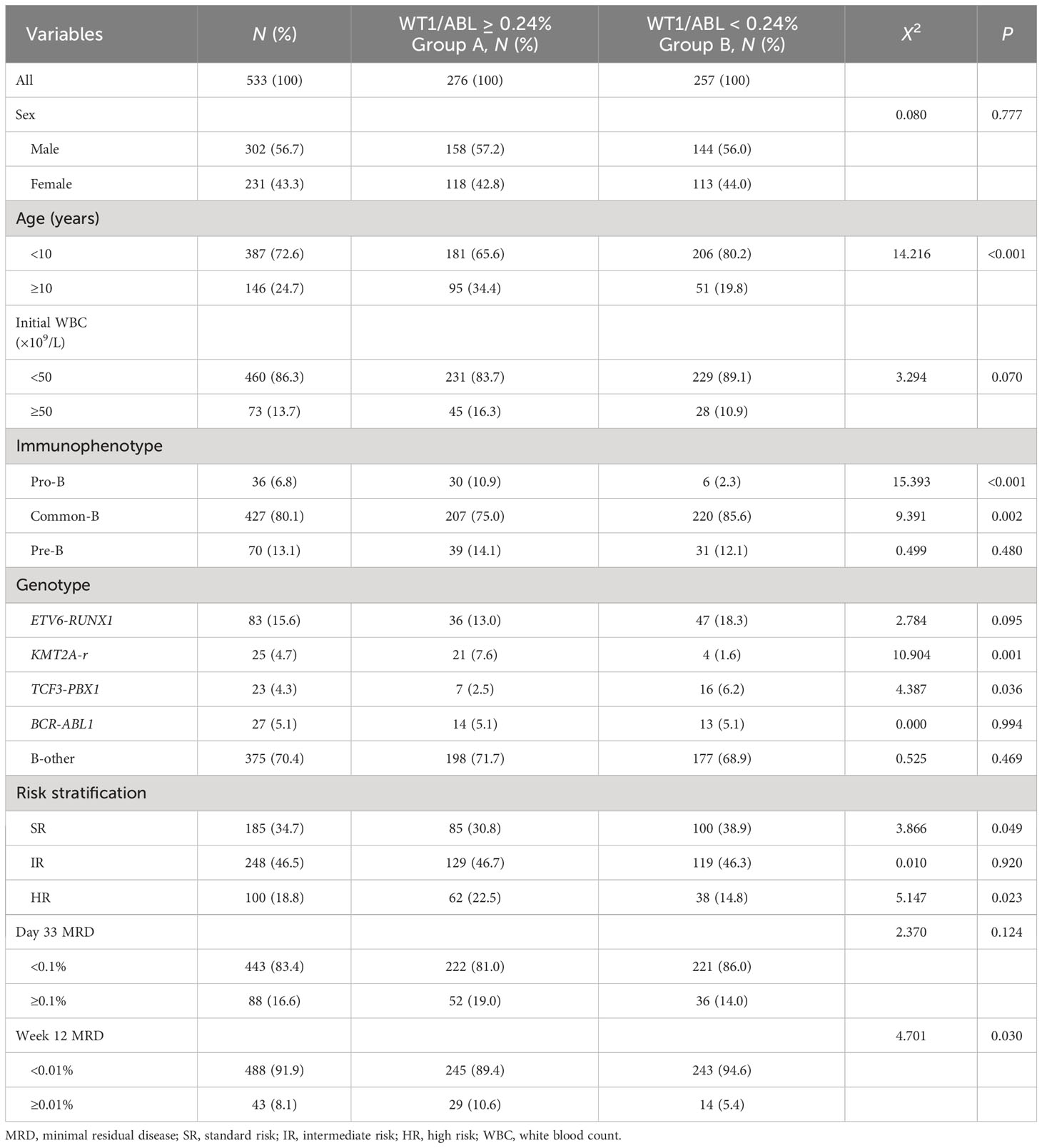
Table 1 Association of WT1 transcript levels with clinical features and treatment response of children with BCP-ALL.
After induction therapy, 527 (99.2%) patients achieved CR. In total, 82 children eventually relapsed, with a median relapse time of 19.2 months (range, 2.8–68.5 months), and 59 children died, including 49 deaths from relapse.
The 5-year OS rate and EFS rate of the entire cohort were 89.2 ± 1.4% and 83.2 ± 1.6%, respectively, with a median follow-up time of 64.6 months (range, 0.8–126.7 months).
The median WT1 transcript level at diagnosis in the whole population was 0.26% (range, 0–251.2%). The median WT1 transcript levels in ETV6-RUNX1, TCF3-PBX1, KMT2A-r, BCR-ABL1, and B-other groups were 0.19% (range, 0.01–8.0%), 0.15% (range, 0.01–2.62%), 14.5% (range, 0.01–251.19%), 0.27% (range, 0–3.1%), and 0.26% (range, 0–87.7%), respectively (P < 0.001). The WT1 transcript level in the KMT2A-r group was significantly higher than that in the other groups (Figure 1).
According to the results of the X-tile software, the patients were divided into two groups: WT1/ABL ≥ 0.24% (group A) and < 0.24% (group B). The rates of age ≥10 years old, immunophenotype of Pro-B, genotype of KMT2A-r and high-risk patients in group A were significantly higher than those in group B (P < 0.05). In group A, patients with MRD ≥ 0.01% at week 12 accounted for 10.6%, which was significantly higher than that in group B (P < 0.05). However, the level of WT1 transcript was not associated with sex, initial white blood count, or MRD levels on day 33. The clinical features and treatment response of patients with BCP-ALL with different WT1 transcript levels are summarized in Table 1.
In the entire cohort of BCP-ALL, patients in group A (with WT1 overexpression) had inferior 5-year OS and EFS rates compared to those in group B (OS: 80.3 ± 4.7% vs. 90.5 ± 1.4%, P = 0.008; EFS: 79.8 ± 2.4% vs. 86.9 ± 2.1%, P = 0.021). However, multivariate analysis revealed that WT1 overexpression was not an independent risk factor of OS and EFS rate (Supplementary Table S1). We further analyzed the impact of WT1 overexpression in the B-other group. Patients with WT1 overexpression had significantly lower 5-year OS and EFS rates than patients with WT1 downexpression (OS: 73.8 ± 6.8% vs. 88.4 ± 1.8%, P = 0.007; EFS: 67.8 ± 7.5% vs. 82.1 ± 2.1%, P = 0.018). Multivariate analysis revealed that WT1 overexpression was an independent risk factor of OS rate, and a higher MRD level at week 12 was associated with lower OS and EFS rates (Table 2).
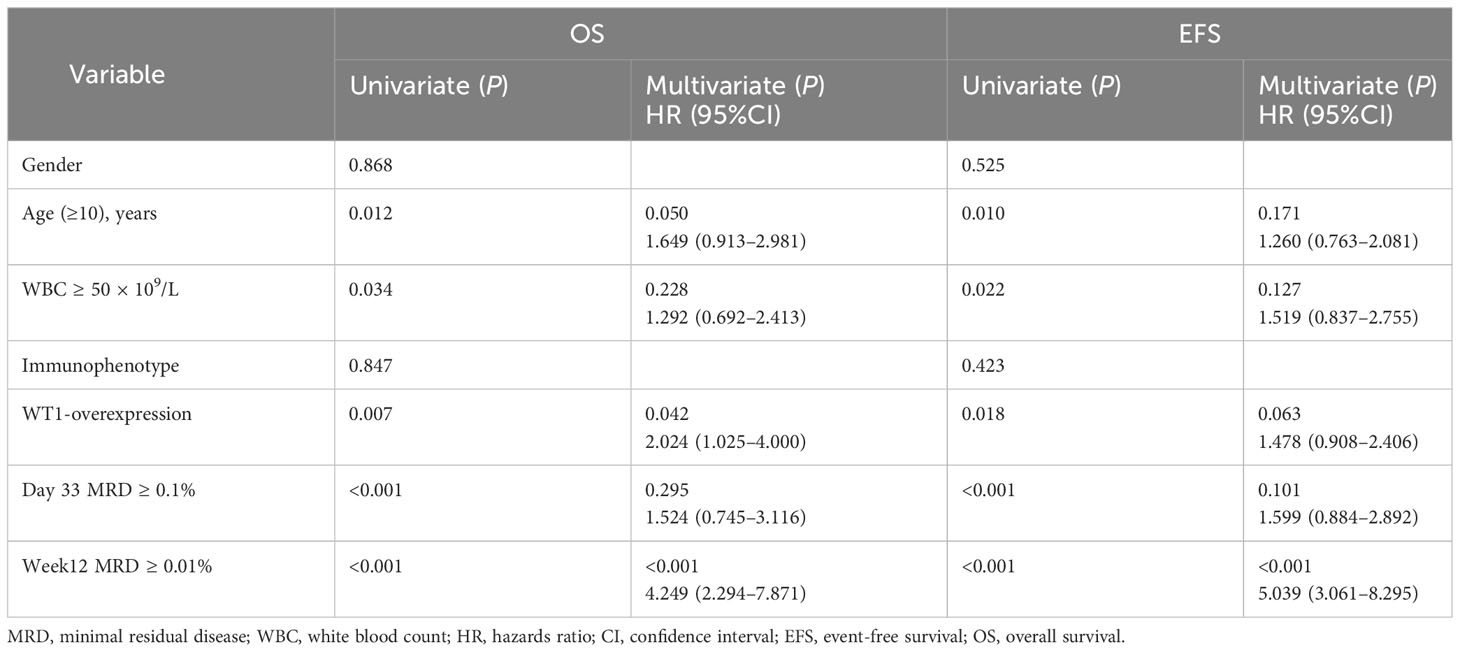
Table 2 Univariate and multivariate analysis of risk factors for overall survival and event-free survival in the B-other ALL patients.
We evaluated the prognostic significance of WT1 expression in different clinical and treatment response subgroups (age, WBC, genotype, risk classification, day 33 MRD, and week 12 MRD). In patients with initial WBC<50 × 109/L or in the standard-risk group or with MRD<0.1% at day 33, the OS and EFS rates tended to decline in the WT1 overexpression group compared with those in the WT1 downexpression group (P<0.05). Furthermore, patients with MRD<0.01% at week 12 had better EFS in the WT1 downexpression group than in the WT1 overexpression group (P = 0.034). As shown in Supplementary Table S2; Figures 2-4, the negative impact of WT1 overexpression was only observed in patients with initial WBC<50 × 109/L or good response to treatment.
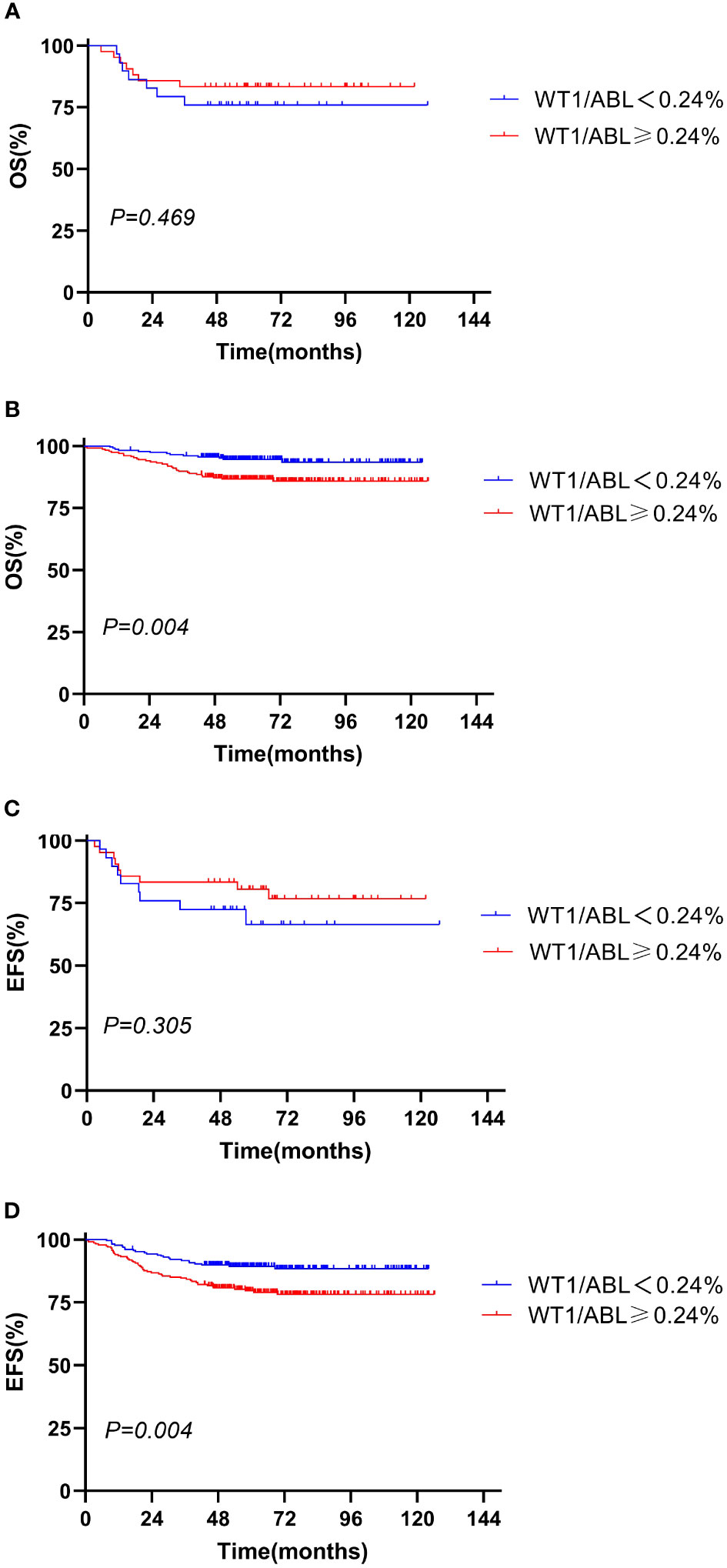
Figure 2 Overall survival and event-free survival according to the WT1 expression and initial WBC. (A, C) WBC ≥ 50 × 109/L; (B, D) WBC < 50 × 109/L).
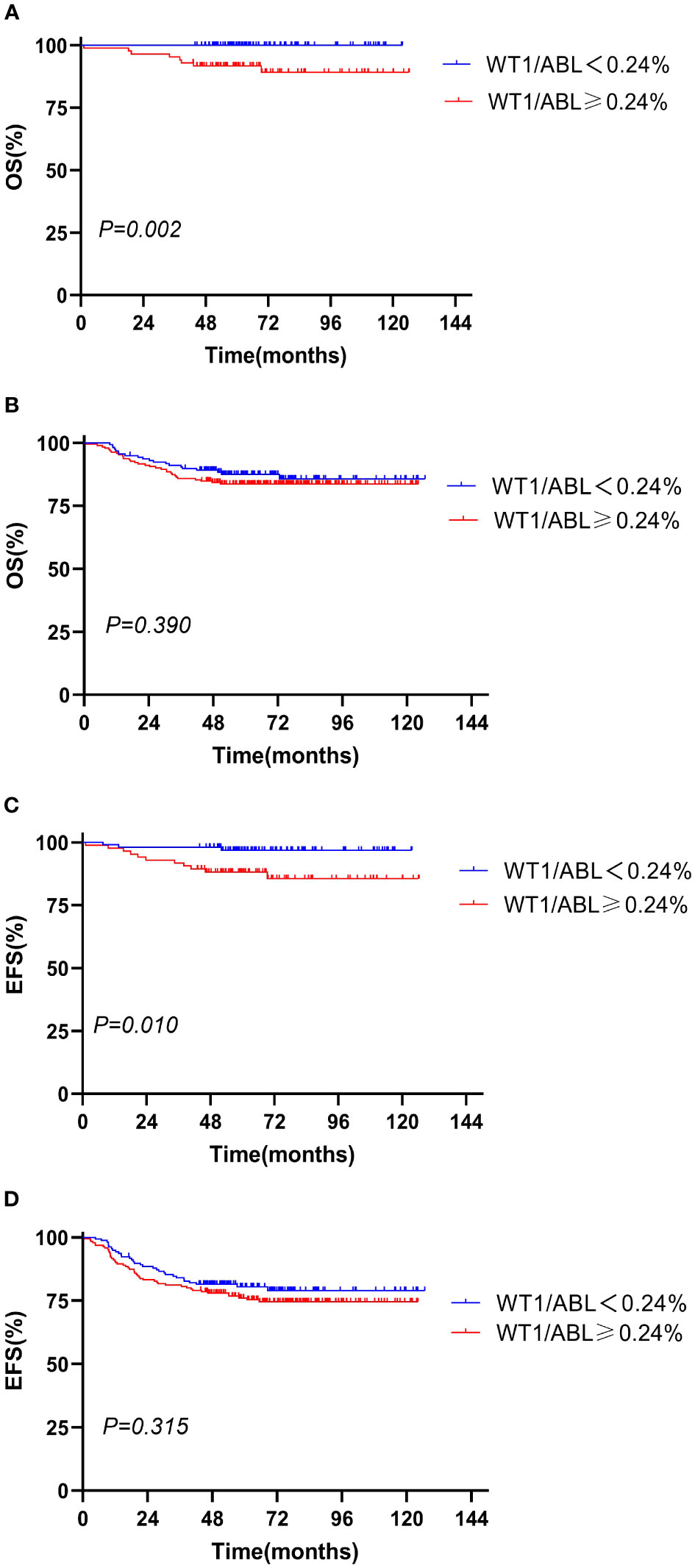
Figure 3 Overall survival and event-free survival according to the WT1 expression and risk stratification. (A, C) Standard risk; (B, D) intermediate risk and high risk.
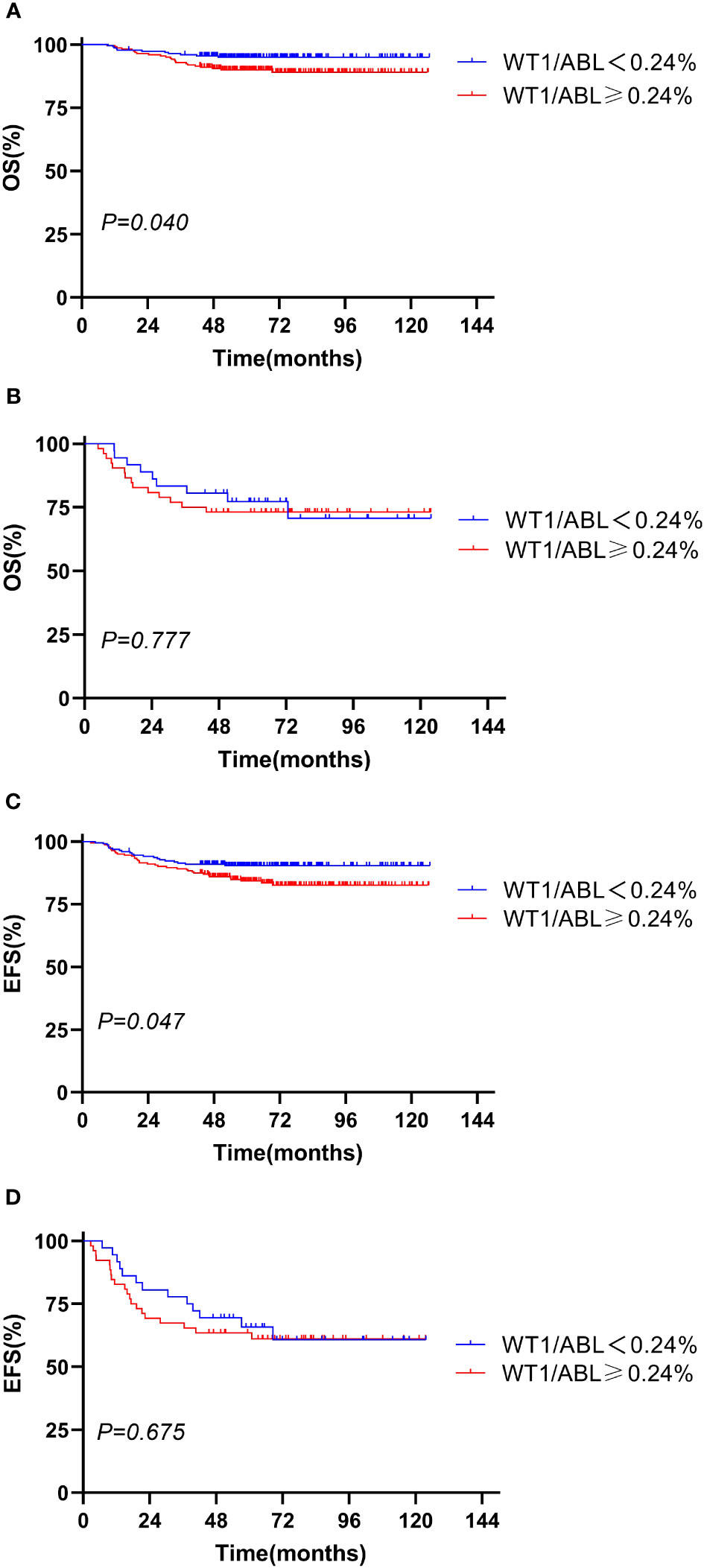
Figure 4 Overall survival and event-free survival according to the WT1 expression and day 33 minimal residual disease (MRD). (A, C) Day 33 MRD < 0.1%; (B, D) day 33 MRD ≥ 0.1%.
Currently, there are few studies on WT1 gene in pediatric BCP-ALL. We assessed the clinical specificity and prognostic value of WT1 expression in children with BCP-ALL in a comparatively large clinical data set and found a clear subgroup heterogeneity in the prognostic significance of WT1 gene.
In this study, children with WT1 overexpression had distinctive clinico-biological features, with WT1 overexpression taking place more frequently in the KMT2A-r+ subgroup or the Pro-B subgroup or elder children. These findings are in consonance with the outcomes of previous studies (3, 4, 11). According to the study of Boublikova L et al. (11), WT1 overexpression tended to occur in pediatric ALL with KMT2A-AFF1+ or initial age ≥10 years. Qin YZ et al. (3) and Wang S et al. (4) also reported that, in adult BCP-ALL, the WT1 transcript levels in the KMT2A rearrangement group were uniformly higher. However, a previous study with a small sample (n = 40) of pediatric ALL by Hagag A et al. (7) showed a remarkably older age in the negative WT1 gene expression group in comparison with the positive group, but with no essential differences between the two groups regarding other clinical presentations.
This discrepancy may be associated with influences—for example, the number of participants and the detection procedure of WT1 gene. In comparison with previous studies, we had the largest sample size that makes our outcomes more convincing. Moreover, previous research (3, 4) have displayed that RQ-PCR utilizing ABL as a control gene is a sensitive and reliable procedure for the quantitative detection of the WT1 gene.
In pediatric ALL, data on WT1 as an indicator of MRD monitoring are scarce and conflicting, and the prognostic role of WT1 also remains unclear. In our study, there was an escalated proportion of children obtaining a negative MRD at week 12 in the WT1 downexpression group that parallels with a significantly higher OS rate. These findings do not entirely coincide with previous studies. Zhang R et al. (12) detected a WT1-RNA transcript level during the induction and consolidation therapy in 107 ALL children to assess its reliability as an MRD monitoring index. However, the outcomes showed that the WT1 gene was not an excellent marker for MRD assessment in childhood ALL. Boublikova L et al. (11) assessed the WT1 expression in 125 childhood ALL and found that either increased or decreased WT1 expression suggested an escalated likelihood of recurrence. The justifications for the differences in outcomes may be associated with the composition of patients and the cutoff value of WT1 gene. The cases enrolled in our survey were uniform, and all children were BCP-ALL. Furthermore, the X-tile software has been reported to calculate the optimal cutoff point by utilizing the minimum probability value of the log-rank chi-square test (13). We found the optimal cutoff value of the WT1 gene for predicting prognosis as calculated by X-tile: 0.24%, which was more concrete and has not been reported in previous studies.
Moreover, the subgroup analysis showed that the poor prognostic significance of WT1 overexpression was more obvious in the standard-risk (SR) group with initial WBC<50 × 109/L or good treatment response. This finding proposes for patients in the SR group, mainly for those with no particular risk-stratifying genes, that WT1 might be utilized as a continuously supervised biomarker to measure residual disease dynamics, just like MRD, consequently facilitating beforetime the identification of a high-risk subgroup. Unfortunately, several children in our cohort did not dynamically monitor the expression level of WT1 gene, so more subtle subgroup comparisons cannot be customized to further verify this conclusion.
WT1 is closely related to the occurrence, development, and prognosis of leukemia. However, its role in leukemogenesis has not been fully defined. The transcription factor encoded by WT1 gene is bidirectional, with the dual function of inhibiting tumor development and activating the transcription of oncogenes (14, 15). In leukemia, the overexpression of the WT1 gene has been reported to cause resistance of leukemic cells to chemotherapy (16–18), which can clarify the poor prognostic significance of WT1 overexpression in pediatric BCP-ALL in this survey.
In conclusion, the cytogenetically defined pediatric BCP-ALL subgroups have unique WT1 expression patterns at initial diagnosis. WT1 overexpression predicted a poor outcome in B-other patients. The WT1 gene may serve as a biomarker for monitoring residual disease in the B-other population, especially in children in the SR group.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of Peking University People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Y-jX: Writing – original draft. YW: Writing – original draft. L-pZ: Resources, Conceptualization, Writing – review & editing. A-dL: Data curation, Resources, Writing – review & editing. Y-pJ: Resources, Project administration, Supervision, Writing – review & editing. Y-xZ: Data curation, Resources, Writing – review & editing. H-mZ: Writing – review & editing, Project administration, Resources, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Foundation of 2018 Beijing Key Clinical Specialty Construction Project-Pediatrics (2199000726) and the State Natural Sciences Foundation project (82000151).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1297870/full#supplementary-material
1. Brown P, Inaba H, Annesley C, Beck J, Colace S, Dallas M, et al. Pediatric acute lymphoblastic leukemia, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2020) 18(1):81–112. doi: 10.6004/jnccn.2020.0001
2. Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica (2020) 105(11):2524–39. doi: 10.3324/haematol.2020.247031
3. Qin YZ, Jiang Q, Xu LP, Jiang H, Wang Y, Zhao XS, et al. The prognostic significance of Wilms' tumor gene 1 (WT1) expression at diagnosis in adults with Ph-negative B cell precursor acute lymphoblastic leukemia. Ann Hematol (2019) 98(11):2551–9. doi: 10.1007/s00277-019-03789-6
4. Wang S, Wang C, Li T, Wang W, Hao Q, Xie X, et al. WT1 overexpression predicted good outcomes in adult B-cell acute lymphoblastic leukemia patients receiving chemotherapy. Hematology (2020) 25(1):118–24. doi: 10.1080/16078454.2020.1735670
5. Ujj Z, Buglyo G, Udvardy M, Beyer D, Vargha G, Biro S, et al. WT1 expression in adult acute myeloid leukemia: assessing its presence, magnitude and temporal changes as prognostic factors. Pathol Oncol Res (2016) 22(1):217–21. doi: 10.1007/s12253-015-0002-0
6. Wang Y, Weng WJ, Zhou DH, Fang JP, Mishra S, Chai L, et al. Wilms tumor 1 mutations are independent poor prognostic factors in pediatric acute myeloid leukemia. Front Oncol (2021) 11:632094. doi: 10.3389/fonc.2021.632094
7. Hagag AA, Badraia IM, Hassan SM, EI-Lateef AEA. Prognostic impact of WT-1 gene expression in Egyptian children with acute lymphoblastic leukemia. Mediterr J Hematol Infect Dis (2016) 8(1):e2016008. doi: 10.4084/mjhid.2016.008
8. Xue YJ, Wang Y, Jia YP, Zuo YX, Wu J, Lu AD, et al. The role of minimal residual disease in specific subtypes of pediatric acute lymphoblastic leukemia. Int J Hematol (2021) 113(4):547–55. doi: 10.1007/s12185-020-03063-w
9. Zhao XS, Yan CH, Liu DH, Xu LP, Liu YR, Liu KY, et al. Combined use of WT1 and flow cytometry monitoring can promote sensitivity of predicting relapse after allogeneic HSCT without affecting specificity. Ann Hematol (2013) 92(8):1111–9. doi: 10.1007/s00277-013-1733-1
10. Wang Y, Xue YJ, Jia YP, Zuo YX, Lu AD, Zhang LP. Re-emergence of minimal residual disease detected by flow cytometry predicts an adverse outcome in pediatric acute lymphoblastic leukemia. Front Oncol (2020) 10:596677. doi: 10.3389/fonc.2020.596677
11. Boublikova L, Kalinova M, Ryan J, Quinn F, Marcaigh AO, Smith O, et al. Wilms' tumor gene 1 (WT1) expression in childhood acute lymphoblastic leukemia: a wide range of WT1 expression levels, its impact on prognosis and minimal residual disease monitoring. Leukemia (2006) 20(2):254–63. doi: 10.1038/sj.leu.2404047
12. Zhang R, Yang JY, Sun HQ, Jia H, Liao J, Shi YJ, et al. Comparison of minimal residual disease (MRD) monitoring by WT1 quantification between childhood acute myeloid leukemia and acute lymphoblastic leukemia. Eur Rev Med Pharmacol Sci (2015) 19(14):2679–88.
13. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res (2004) 10(21):7252–9. doi: 10.1158/1078-0432.CCR-04-0713
14. Yang L, Han Y, Suarez SF, Minden MD. A tumor suppressor and oncogene: the WT1 story. Leukemia (2007) 21(5):868–76. doi: 10.1038/sj.leu.2404624
15. Marlton P. The many facets of WT1 in acute myeloid leukemia: clarity remains elusive. Leuk Lymphoma (2014) 55(2):235–7. doi: 10.3109/10428194.2013.806804
16. Svensson E, Vidovic K, Lassen C, Richter J, Olofsson T, Fioretos T, et al. Deregulation of the Wilms' tumour gene 1 protein (WT1) by BCR/ABL1 mediates resistance to imatinib in human leukaemia cells. Leukemia (2007) 21(12):2485–94. doi: 10.1038/sj.leu.2404924
17. Zapata-Benavides P, Manilla-Munoz E, Zamora-Avila DE, Saavedra-Alonso S, Franco-Molina MA, Trejo-Avila LM, et al. WT1 silencing by RNAi synergizes with chemotherapeutic agents and induces chemosensitization to doxorubicin and cisplatin in B16F10 murine melanoma cells. Oncol Lett (2012) 3(4):751–5. doi: 10.3892/ol.2012.578
Keywords: B cell precursor acute lymphoblastic leukemia, pediatric, WT1 transcript levels, clinical characteristics, prognosis
Citation: Xue Y-j, Wang Y, Zhang L-p, Lu A-d, Jia Y-p, Zuo Y-x and Zeng H-m (2024) Prognostic significance of Wilms’ tumor gene 1 expression in children with B-cell precursor acute lymphoblastic leukemia. Front. Oncol. 13:1297870. doi: 10.3389/fonc.2023.1297870
Received: 20 September 2023; Accepted: 23 October 2023;
Published: 15 January 2024.
Edited by:
Spiros Vlahopoulos, University of Athens, GreeceReviewed by:
Michael James Burke, Medical College of Wisconsin, United StatesCopyright © 2024 Xue, Wang, Zhang, Lu, Jia, Zuo and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-min Zeng, cm15eWVrNjAwNEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.