- Department of Pathology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Nuclear protein in testis (NUT) carcinoma (NC) is a rare, aggressive tumor with a typical NUTM1 gene rearrangement.
Methods: Herein, we report a series of 2 cases of sinonasal NC: one in a 16-year-old woman and one in a 37-year-old man. Immunohistochemistry (IHC) staining for NUT (C52B1), fluorescence in situ hybridization (FISH), and next generation sequencing (NGS) sequencing were performed to investigate the morphological and genetic features of sinonasal NC.
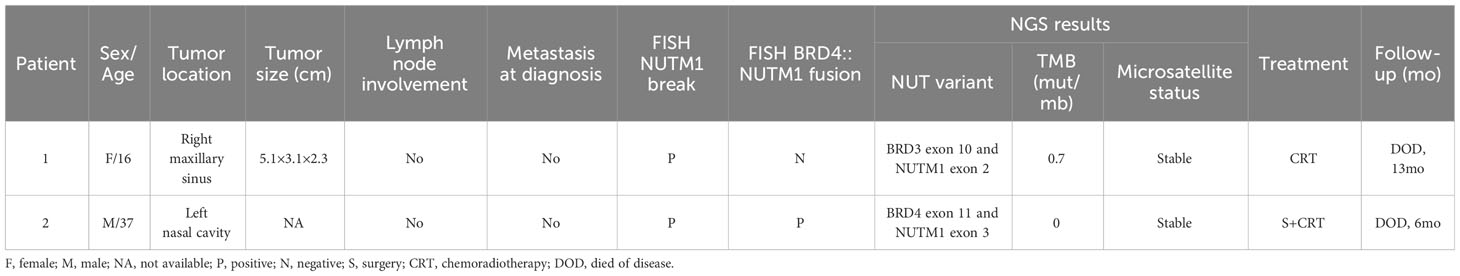
Results: The two cases presented similar pathological features and IHC markers, and typical morphological changes, including undifferentiated cells and abrupt keratinization, were observed, with numerous mitotic figures and widespread tumor necrosis. Diffuse expression of NUT, CK, p63, and p40 was noted, while the tumors were negative for synaptophysin, chromogranin A, S-100, EBV-ISH, and PD-L1. Both tumors harbored a NUTM1 rearrangement. Subsequent sequencing revealed a rare BRD3::NUTM1 fusion and a classic BRD4::NUTM1 fusion. In addition, MCL1 copy number gain (2.1), low tumor mutation burden and stable microsatellites, were also confirmed. Case 1 received surgery and chemoradiotherapy but died 13 months after local recurrence and subsequent lung and bone metastasis. Case 2 underwent chemoradiotherapy and unfortunately died from the disease 6 months later. A review of all previously reported cases of sinonasal NCs (n=55) revealed that these tumors occur more frequently in female pediatric patients (n=11, male: female =3:8), whereas this sex difference is not observed in adult patients (n=44, male: female =23:21). The median survival times of pediatric and adult patients were 17 and 13.8 months, respectively.
Conclusion: Sinonasal NC presents typical undifferentiated or poorly differentiated cells, abrupt keratinization features and heterogeneous genotypes, including BRD4::NUTM1 and BRD3::NUTM1 fusions, with low tumor mutation burden and stable microsatellites.
Introduction
Nuclear protein in testis carcinoma (NC) is a rare and aggressive genetically defined malignant neoplasm (1). NC was initially described in children and adolescents in 1991 (2, 3); however, the frequency of diagnoses in adults has increased in recent years. Although NC generally arises in the midline structures of the thorax or from the head and neck (HN), other sites, such as the kidney, bladder, and parotid gland, can also be involved (4). Furthermore, NC is considered the most clinically aggressive squamous carcinoma, with amedian overall survival (OS) duration of only 6.5 months; most patients succumb to rapid disease progression with early metastases to locoregional and distant sites, even with intensive treatment (4–6).
Genetically, the hallmark of NC is the t (7, 8) translocation, and this genetic aberration leads to the formation of the BRD4::NUTM1 fusion oncogene (9). The BRD-NUT oncoprotein has been demonstrated to block epithelial differentiation and maintain carcinoma cell growth. Most patients had the BRD4::NUTM1 fusion (78%); however, other fusion variants have also been described, including BRD3::NUTM1 (15%) and NSD3::NUTM1 (6%) (6). In addition, several novel fusion partner genes have identified in NUTM1-associated sarcoma (6).
NC is a poorly differentiated tumor that displays variable degrees of squamous differentiation and occasionally presents abrupt keratinization but lacks typical histological features. It is often difficult to diagnose, especially as the sinonasal tract gives rise to many tumors with undifferentiated morphologies, such as sinonasal undifferentiated carcinoma (SNUC) and poorly differentiated squamous cell carcinoma (PDSCC) (10, 11). Diagnosis is often assisted by the demonstration of NUT staining or NUTM1 rearrangement in reverse transcriptase–polymerase chain reaction (RT-PCR), fluorescence in situ hybridization (FISH), and next-generation sequencing (NGS) analyses (9).
To date, few cases of sinonasal NC have been reported, and its clinicopathological and molecular features have not been sufficiently clarified. Herein, we report 2 cases of sinonasal NC and their clinical and pathological presentations. We also reviewed sinonasal NC and molecular information.
Materials and methods
Patients and clinical samples
In this study, two sinonasal NC cases, a 16-year-old woman and a 37-year-old man were identified by NUT immunohistochemistry among 118 primary head and neck poorly squamous cell carcinomas at the West China Hospital of Sichuan University between January 2016 and December 2020, as previously described (12). All sinonasal NC tumors, including surgical resection specimens for Case 1 and endoscopic biopsies for Case 2, were sampled, fixed in 10% formalin, embedded in paraffin, stained with hematoxylin and eosin (H&E) and reviewed by two experienced pathologists (LL. J and SL).
Immunohistochemistry and Epstein–Barr virus status analyses
A series of markers were assessed in addition to NUT (clone C52B1, CST, MA, USA), including Ki-67 (clone 9-40, Roche, AZ, USA), p40 (clone ZR8), CK5/6 (clone D5/16B4), anti-pankeratin (clone AE1/AE3), p63 (clone UMAB4), CD34 (clone EP88), synaptophysin (clone UMAB237), CD99 (clone 12E7), chromogranin A (clone LKZH10), p53 (clone D0-7), EGFR (clone EP22), and p16 (clone ICI). EBV status was assessed via EBER in situ hybridization (EBV-ISH) (Dako, no. Y520001). NUT protein positivity was defined as “strong” when there was speckled nuclear staining in more than 50% of the tumor nuclei (13). Positive PD-L1 expression was detected using the tumor proportion score (TPS) method (percentage of PD-L1-positive tumor cells over the total number of tumor cells on the whole entire slide) (14).
Fluorescence in situ hybridization
Two sinonasal NCs were subjected to further FISH analysis using a NUTM1 break-apart probe and a BRD4::NUTM1 fusion probe. All probes were commercially purchased from Anbiping (Guangzhou, China). FISH slides were observed under a 100× objective using a fluorescence microscope (Leica DM6000, Wetzlar, Germany). Scoring was performed by two independent pathologists (MC and SL) with expertise in FISH analysis. Samples were considered FISH-positive if over 15% of the 100 scored tumor cells harbored NUTM1 break-apart or BRD4::NUTM1 fusion signals (13).
Next generation sequencing
A targeted DNA-based NGS panel (YuanSu™ panel, Zhiben, Shanghai, China), including a 701-gene panel that included all coding exons of 638 key cancer-related genes and selected introns of 63 commonly rearranged genes in solid tumors, was performed [10]. The total DNA of the lesion and corresponding normal tissues was isolated from 5-μm-thick slices of FFPE samples using a DNA FFPE kit (Qiagen, Valencia, CA, USA). Fragments 200 to 400 base pairs in size were selected with beads and hybridized with the capture probe baits. Hybrid selection was subsequently performed with magnetic beads, and PCR amplification was carried out. The concentration of the DNA samples was determined using the Qubit 3.0 dsDNA assay (Thermo Fisher Scientific, Waltham, MA). Paired-end 2×150 bp sequencing was performed on the MiSeq DX platform (Illumina, San Diego, CA, USA) (15). The sequenced data were analyzed by STAR Fusion software (16).
Results
Clinical history of the two sinonasal NCs
Clinical and molecular information of the 2 sinonasal NCs is shown in Table 1. The patient in Case 1 was a 16-year-old woman with a 2-month history of right-sided nasal congestion, intractable epistaxis, tinnitus and hearing loss. When her symptoms progressed, a computed tomography (CT) head scan with contrast was performed, demonstrating a large enhancing sinonasal tumor measuring 5.1×3.1×2.3 cm that filled the right maxillary sinus area and extended to the adjacent structures (Figure 1B). Magnetic resonance imaging (MRI) of the brain with and without contrast demonstrated a sphenoid sinus mass (Figure 1C). However, no obvious abnormalities were observed in the brain. Laboratory evaluation demonstrated normal blood counts, normal renal function, uric acid and lactate dehydrogenase, mild hypercalcemia and aspartate aminotransferase elevation.
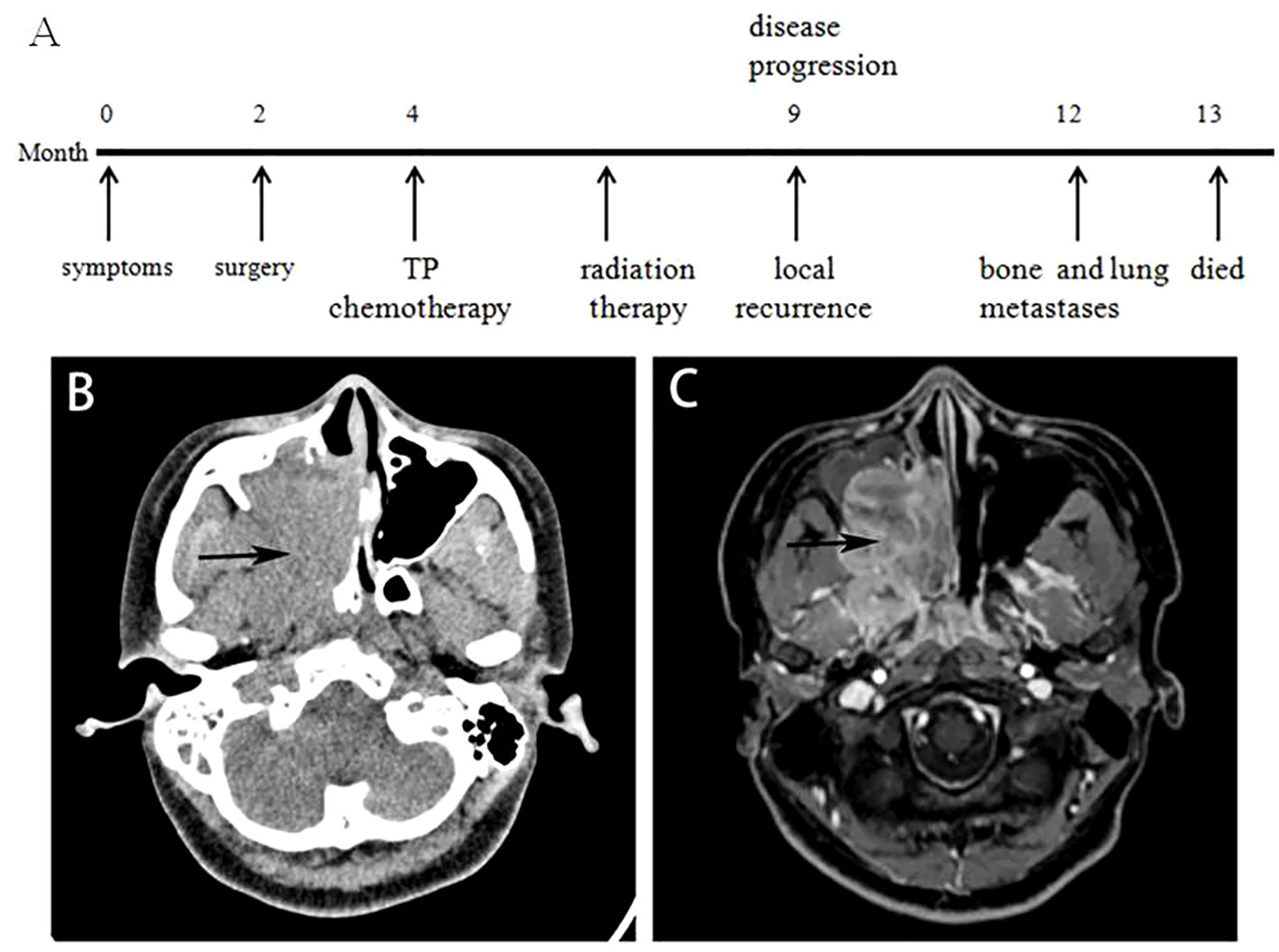
Figure 1 Imaging characteristics of Case 1. (A) Timeline of the diagnosis and treatment course of Case 1. (B) Contrast-enhanced CT scan of the head demonstrated a 5.1×3.1×2.3 cm sinonasal tumor filling the right maxillary sinus area and extending to the adjacent structures (black arrow). (C) Brain MRI with and without contrast demonstrating a sphenoid sinus mass.
The patient in Case 2 was a 37-year-old man with a 1-month history of left-sided nasal obstruction, headache, purulent and bloody nasal mucus, and a decreasing sense of smell. When the patient’s symptoms worsened, he was referred to otolaryngology, where a large left nasal cavity mass was noted. Further workup, including maxillofacial and chest examination, computed tomography, and facial magnetic resonance imaging, demonstrated a large heterogeneously enhancing infiltrative mass in the left nasal cavity and sinuses. Laboratory evaluation demonstrated normal blood counts, normal hepatic and renal function, uric acid, and lactate dehydrogenase. Under nasal endoscopy, the bilateral nasal mucosa showed chronic hyperemia, and some purulent secretions were attached. The left nasal cavity was narrowed with a gray soft tumor. A small incisional biopsy revealed a malignant gray soft tumor, which visibly invaded the surrounding bone tissues. Some cancer thrombi could be seen in the vasculature.
Histology and immunophenotypes
The lesion was composed of sheets or nests of poorly differentiated small cells, and most nuclei were round or oval-round in shape and medium in size (Figure 2A). In addition, vesicular nuclei were noted focally (Figure 2B). Moreover, focal well-differentiated cells were observed in Case 1 (Figure 2C). Abrupt keratinization with pale eosinophilic cytoplasm was commonly noted (Figure 2D). Nuclear atypia or pleomorphism was generally not prominent, but mitotic activity varied from 1/10 HPFs to 8/10 HPFs. Two tumors showed focal areas of hemorrhage and necrosis, characteristically surrounded or separated by fibrotic stroma.
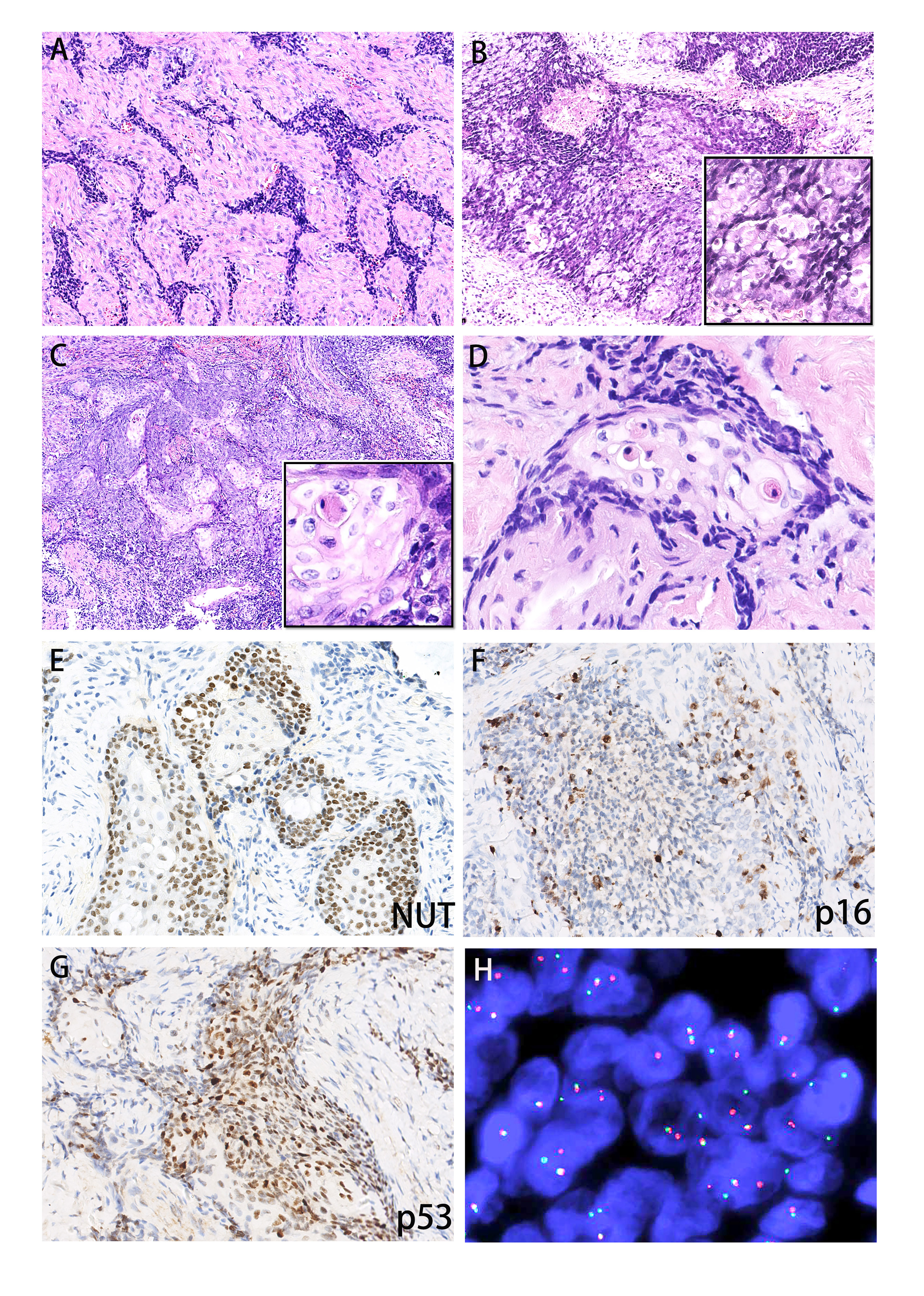
Figure 2 Histopathology and immunophenotypes of the two sinonasal NCs. Case 1 featured the BRD3::NUT fusion, and case 2 featured the BRD4::NUT fusion. (A) Poorly differentiated small squamous cells in both cases, (B) focal vesicular nuclei (right lower insert), (C) focal well differentiated small cells in Case 1 (right lower insert), (D) abrupt keratinization with pale eosinophilic cytoplasm, (E) diffuse expression of NUT was observed in both cases, (F) positive staining for p16 in Case 1, (G) positive staining for p53 in Case 1, (H) fluorescence in situ hybridization studies illustrating NUTM1 break-apart in both cases.
Both cases showed similar immunophenotypes. The poorly differentiated tumor cells exhibited strong and uniform nuclear immunoreactivity for NUT (Figure 2E). The tumor cells homogenously and robustly expressed p63, CK5/6, CK7, EMA, and p40. Additionally, Case 1 showed diffuse nuclear and cytoplasmic block positivity for EGFR, p16 (Figure 2F) and p53 (Figure 2G) in well-differentiated areas. No signal was found for chromogranin A, synaptophysin, S100 or EBV-ISH in either case. PD-L1 showed weak membranous positivity in less than 1% of tumor cells. The proliferation activity as determined by the Ki67-proliferation index in hot spot areas reached 60% and 40% in Cases 1 and 2, respectively.
Genetic results
In Case 1, the NUTM1 break-apart signal was observed in 84% of tumor cells (Figure 2H), whereas the tumor was negative for BRD4::NUTM1, SS18, and EWSR1 rearrangements. Consistent with the FISH results, Case 1 harbored a rare BRD3::NUTM1 fusion, with in-frame breakpoints in BRD3 exon 10 (NM_007371.4) and NUTM1 exon 2 (NM_001284292.2) (Figure 3A).
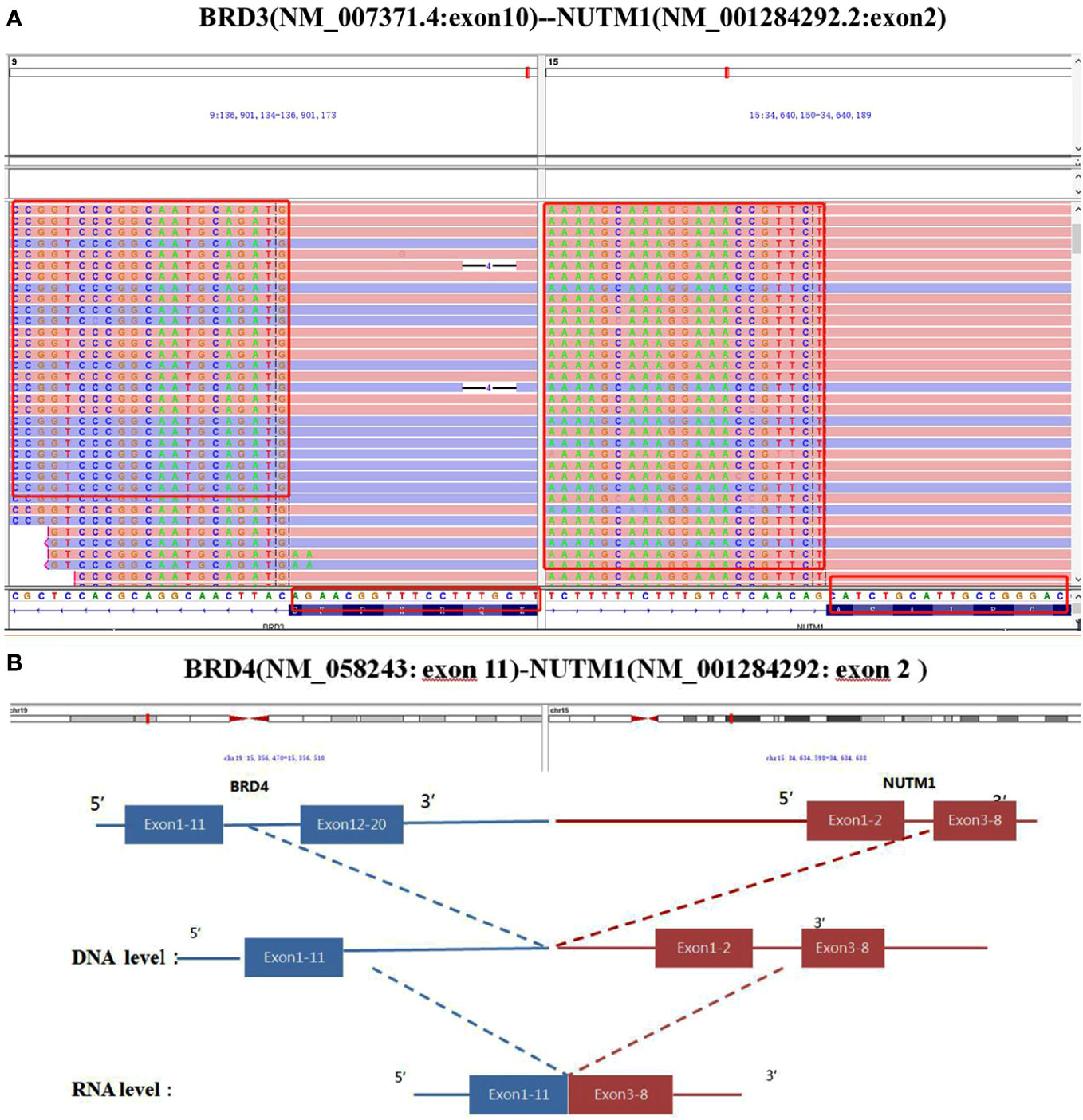
Figure 3 Partial nucleotide sequence of the BRD3::NUTM1 fusion transcript in Case 1 (A) and the BRD4::NUTM1 fusion transcript in Case 2 determined using next generation sequencing (B).
In Case 2, NUTM1 break-apart and BRD4::NUTM1 fusion signals were observed in 84% and 75% of the tumor cells, respectively, with in-frame breakpoints in BRD4 exon 11 and NUTM1 exon 3 (Figure 3B). Additionally, this Case showed MCL1 gene copy number gain. Both cases exhibited microsatellite stability and a low TMB, with 0.7 and 0 mutations per megabase (mut/mb) in Case 1 and 2, respectively.
Treatment and outcomes
Two months after the surgery, the patient in Case 1 received TP chemotherapy (albumin paclitaxel-400 mg Day 1, cisplatin-40 mg, Day 1-day 2, 35 mg Day 3, intravenous glucose tolerance test, every 3 weeks). She was diagnosed with grade 4 high-frequency hearing loss as a result of cisplatin treatment. After completing 2 cycles of chemotherapy, CT/MRI revealed that the patient did not have lymph node involvement or distant metastases. She was referred for radiation therapy and subsequently developed treatment-associated radiation dermatitis of her central face. Unfortunately, local recurrence was noted within 5 months. Bone and lung metastases subsequently appeared, and the patient died 13 months later.
Despite chemotherapy with cisplatin and navelbine, the patient in Case 2 developed brain metastases and passed away 6 months after diagnosis.
Review of studies
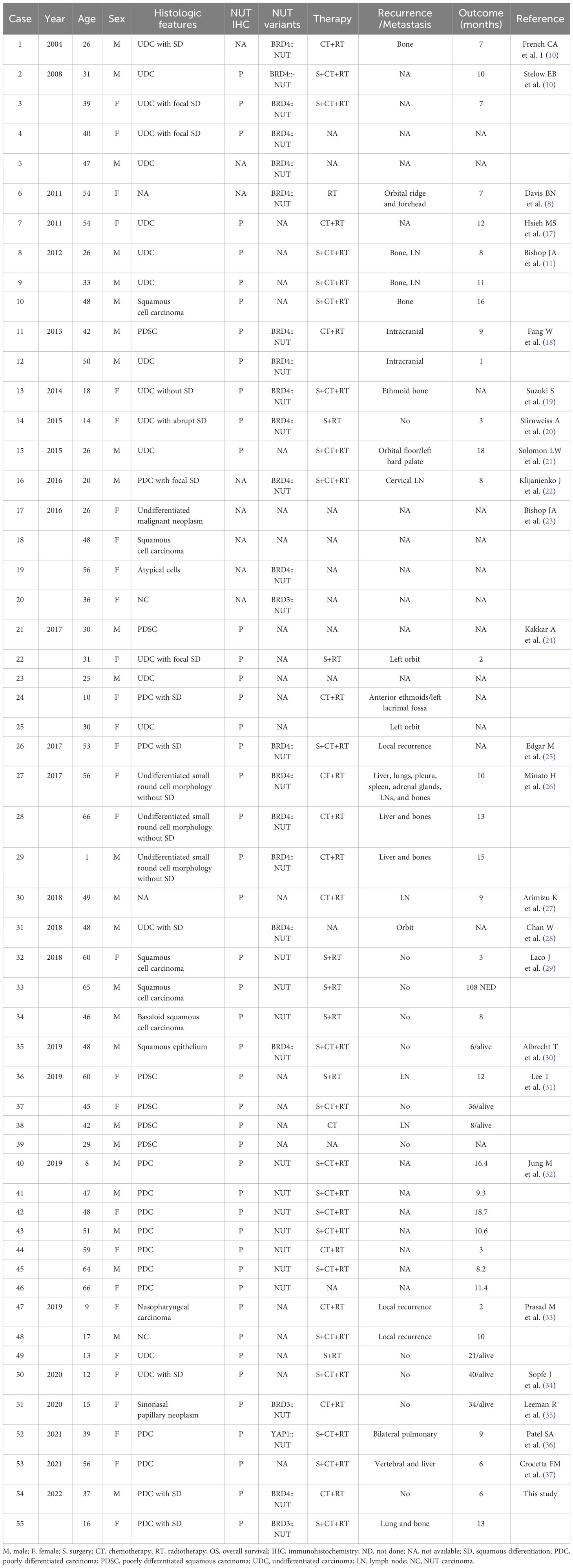
The Detailed clinical information about these patients is given in Table 2. Patient age at presentation ranged from 1 to 66 years (mean 37.9 years, median 39 years), among which 11 pediatric patients were identified (<18 years old). There were 26 males and 29 females. Interestingly, the sex predilection for pediatric sinonasal NC was prominent, with females constituting 72.3% (n=11, male: female =3:8). In contrast, the adult patients displayed an almost equal gender distribution (n=44, male: female =23:21). Furthermore, sinonasal NC tends to involve the frontal and ethmoidal sinuses more frequently than other sinonasal cancers.
The most common histology of sinonasal NC was poorly differentiated or undifferentiated carcinoma (46 of 55 cases, 83%); however, abrupt keratinization was seen in 40% (22 of 55) of cases. Tumors lacking evidence of epithelial differentiation or where histologic classification was not specified composed the remaining (5 of 55, 9%) cases. This observation was consistent with previous studies. Sinonasal NC demonstrates a consistent immunoprofile similar to that seen at other anatomic sites. We found that 46 sinonasal NC cases with available data all stained positive for NUT. The tumor commonly expresses cytokeratins and squamous markers such as p63, with variable expression of p40 and CK proteins. Some reports described patchy staining of synaptophysin, p16, or even TTF-1 and tumor EBER-negative status.
Discussion
The head and neck region is the second most common primary site of NCs, comprising 40% of all NC cases (7). Sinonasal NC is undifferentiated malignant neoplasm (1). Recently, Wang et al. reported 3 sinonasal NCs identified from 145 sinonasal malignancies (38). Ramesh et al. found 12 sinonasal NCs, which was the largest single-institutional cohort reported to date (39). In this study, we reported 2 cases of sinonasal NC and reviewed 53 other cases reported in the literature (8, 10, 11, 17–37, 40).
The frequency of the NUTM1 variant in sinonasal tissue is consistent with that observed in NC overall. Of the NUTM1-fusion-positive cases, BRD4::NUTM1 fusion accounted for 83% (19/23), BRD3::NUTM1 accounted for 13% (3/23) and one rare YAP1::NUTM1 fusion was observed (36). The genetic basis of NC progression and transformation has been studied in several cohorts using next-generation sequencing technologies (41–43). Lee et al.’s findings suggest that this single catastrophic event involving NUTM1 rearrangement in proliferating normal cells could be sufficient for neoplastic transformation into NUT carcinoma (44). Stefano et al. first reported that metastatic NC patients carried other somatic mutations, including deletions in colorectal cancer (DCC), mixed lineage leukemia protein 3, and splicing factor 3B subunit genes in NC cells (41). Moreover, the most highly and recurrently mutated genes in NC are associated with the Wnt, MAPK, and PI3K signaling pathways (42). Additional frequently mutated genes have also been discovered in NC, including mutations in MYC, p63, and MED24 (43). MYC is a master regulator of cell proliferation and metabolism and is central to the pathogenesis of many human cancers (45, 46). Various types of MYC gene mutations are present in diffuse large B-cell lymphoma (DLBCL) and show different impacts on MYC function and clinical outcomes (47). Unlike MYC gene translocations and overexpression, most MYC gene mutations may not have a role in driving lymphomagenesis (47). Previous studies demonstrated that MYC is a downstream target of BRD-NUT, and targeting MYC was necessary and sufficient for the blockade of NUT midline carcinoma differentiation (48). However, there are few data on sinonasal NC, and most studies have focused on pulmonary NC. In the present study on sinonasal NC, we identified one additional genomic alteration in myeloid cell leukemia-1 protein (Mcl-1). Mcl-1 is an antiapoptotic protein in the Bcl-2 family that is essential for the survival of multiple cell lineages and is highly amplified in human cancer (49). Yasuda Y et al. suggested that MCL1 inhibition therapy be applied for high MCL1- and low BCL-X L-expressing small-cell lung cancer patients (50), but it is unclear whether direct inhibition of MCL1 is also useful for sinonasal NC.
NC is aggressive and the majority of NC patients have regional and/or distant metastases at the time of presentation (4–6). Imaging typically reveals an extensively infiltrative tumor with frequent involvement of the orbit and cranial cavity (1). Of the 55 reviewed sinonasal NCs in this study, disease progression was evaluable for 43 patients, including 14 patients (33%) who had isolated locoregional disease, 10 (23%) who had isolated distant disease, and 2 (5%) who developed both locoregional and distant disease. The remaining 17 patients did not develop local or distant metastases. The outcomes and responses to therapy vary based on the anatomical site. Compared to the median OS time for NC of all ages and locations (4.7–6.7 months) (4) and the head and neck region overall (9.7 months) (5), sinonasal NC appears to have a better prognosis (13.8 months). Pediatric patients with sinonasal NC have the longest OS duration (17 months). Similarly, Chau NG et al. proposed a survival tree regression and identified three statistically distinct risk groups among 124 patients classified by anatomical site and genetics (6). They found that nonthoracic primary, BRD3, or NSD3::NUTM1 NC patients had longer survival durations (3 years, n=12) than nonthoracic primary, BRD4::NUTM1 NC patients. Nonthoracic primary NC with nonBRD4::NUTM1 fusion conferred the best prognosis, followed by nonthoracic primary NC with BRD4::NUTM1. However, primary thoracic NC patients had an average OS duration of only 4.4 months. In this study, the patient in Case 1 had a BRD3::NUTM1 fusion and a longer survival duration than the patient in Case 2 with the classic BRD4::NUTM1 fusion (13 months vs. 6 months), which is in accordance with the findings of previous studies.
A standard treatment strategy has not been established for NC, but surgery or radiation was reported to significantly improve survival (6, 7). Chemotherapy is generally ineffective, although successful treatment and long-term survival have rarely been reported in cases treated with ifosfamide-based regimens (36). To date, the majority of sinonasal NC patients have received intensive traditional multimodality therapy, consisting of various combinations of surgery, chemotherapy, and radiotherapy. Recently, several promising classes of agents, including bromodomain and extraterminal motif (BET) inhibitors (7, 51), histone deacetylase inhibitors, and immune checkpoint inhibitors (ICIs) (52), have emerged as candidates for the treatment of NC. Currently, there is no single biomarker that can reliably predict the response to ICIs. The expression of PD-L1 by tumor cells (TPS) has been the most widely studied. Some patients with high PD-L1 expression do not respond to ICIs, whereas a small proportion of patients with no PD-L1 expression respond to ICIs (52). We previously reported two low PD-L1 expression NCs, presenting a diverse response to immunotherapy. Patient 1 exhibited a poor response and soon showed tumor progression and metastasis; however, patient 2 responded remarkably and achieved pathologic complete response (pCR) without uncontrollable adverse events (53).
Additional biomarkers that have been shown to predict ICI treatment response include TMB and microsatellite instability. He et al. first applied whole transcriptome RNA sequencing to determine TMB and microsatellite instability in NC (54). Their data demonstrate that TMB ranges from intermediate (between 5 and 20 mut/mb) in an adult case to low (<5 mut/mb) in pediatric cases. Other studies have reported low TMB and stable microsatellites in NC (55). Riess et al. described 31 solid tumor cases harboring a BRD4::NUT translocation. The cohort was all microsatellite stable and harbored a low TMB (mean 1.7 mut/mb, range 0–4) (56). In our previous study, pulmonary NCs also had stable microsatellites and a lower TMB, ranging from 0.5 to 1.7 mut/mb(median 1.25) (12). Consistent with these studies, the sinonasal NC patients in this study presented low TMB and stable microsatellites. Although the number of cases is limited, the results suggest that therapy with ICIs may be beneficial in some patients; and should be further studied in this patient population.
This study has some limitations. First, this was a retrospective study from a single center. Second, because of the rarity of NUT carcinoma cases, the number of samples was too small, and only two patients were included. Finally, because of insufficient tumor samples, we did not investigate the tumor immune microenvironment in the two patients.
In conclusion, sinonasal NC presents typical undifferentiated or poorly differentiated cells, abrupt keratinization features and heterogeneous genotypes, including BRD4::NUTM1 and BRD3::NUTM1 fusions, with low tumor mutation burden and stable microsatellites.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: DNA Data Bank of Japan (DDBJ) and BioSample accession(s): SAMD00665363, SAMD00665364.
Ethics statement
The studies involving humans were approved by West China hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MC: Data curation, Writing – original draft. SL: Formal Analysis, Writing – original draft. LJ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Sichuan University 1.3.5 Project for Disciplines of Excellence-Clinical Research Incubation (No. 2019HXFH002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. El-Naggar AK, Chan J, Takata T, Grandis J, Blootweg P. WHO classification of tumours. Pathology and genetics of head and neck tumours. 4th ed. Lyon: IARC Press (2017).
2. Kubonishi I, Takehara N, Iwata J, Sonobe H, Ohtsuki Y, Abe T, et al. Novel t (15,19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res (1991) 51(12):3327–28.
3. Kees UR, Mulcahy MT, Willoughby ML. Intrathoracic carcinoma in an 11-year-old girl showing a translocation t (15,19). Am J Pediatr Hematol Oncol (1991) 13(4):459–64. doi: 10.1097/00043426-199124000-00011
4. Bauer DE, Mitchell CM, Strait KM, Lathan CS, Stelow EB, Lüer SC, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res (2012) 18(20):5773–79. doi: 10.1158/1078-0432.CCR-12-1153
5. Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, Kaplan L, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer (2016) 122(23):3632–40. doi: 10.1002/cncr.30242
6. Chau NG, Ma C, Danga K, Al-Sayegh H, Nardi V, Barrette R, et al. An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr (2019) 4(2):pkz094. doi: 10.1093/jncics/pkz094
7. French CA, Cheng ML, Hanna GJ, DuBois SG, Chau NG, Hann CL, et al. Report of the first international symposium on NUT carcinoma. Clin Cancer Res (2022) 28(12):2493–05. doi: 10.1158/1078-0432.CCR-22-0591
8. Davis BN, Karabakhtsian RG, Pettigrew AL, Arnold SM, French CA, Brill YM. Nuclear protein in testis midline carcinomas: a lethal and underrecognized entity. Arch Pathol Lab Med (2011) 135(11):1494–98. doi: 10.5858/arpa.2010-0389-CR
9. French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res (2003) 63(2):304–07.
10. Stelow EB, Bellizzi AM, Taneja K, Mills SE, Legallo RD, Kutok JL, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol (2008) 32(6):828–34. doi: 10.1097/PAS.0b013e31815a3900
11. Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol (2012) 36(8):1216–21. doi: 10.1097/PAS.0b013e318254ce54
12. Chen M, Zhao S, Liang Z, Wang W, Zhou P, Jiang L. NUT carcinoma of the parotid gland: report of two cases, one with a rare ZNF532-NUTM1 fusion. Virchows Arch (2022) 480(4):887–97. doi: 10.1007/s00428-021-03253-9
13. Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol (2009) 33(7):984–91. doi: 10.1097/PAS.0b013e318198d666
14. Wu Q, Wang W, Zhou P, Fu Y, Zhang Y, Shao YW, et al. Primary pulmonary lymphoepithelioma-like carcinoma is characterized by high PD-L1 expression, but low tumor mutation burden. Pathol Res Pract (2020) 216(8):153043. doi: 10.1016/j.prp.2020.153043
15. Tang Y, Li Y, Wang W, Lizaso A, Hou T, Jiang L, et al. Tumor mutation burden derived from small next generation sequencing targeted gene panel as an initial screening method. Transl Lung Cancer Res (2020) 9(1):71–81. doi: 10.21037/tlcr.2019.12.27
16. Yao T, Liu JJ, Zhao LJ, Zhou JY, Wang JQ, Wang Y, et al. Identification of new fusion genes and their clinical significance in endometrial cancer. Chin Med J (Engl) (2019) 132(11):1314–21. doi: 10.1097/CM9.0000000000000203
17. Hsieh MS, French CA, Liang CW, Hsiao CH. NUT midline carcinoma: case report and review of the literature. Int J Surg Pathol (2011) 19(6):808–12. doi: 10.1177/1066896909353600
18. Fang W, French CA, Cameron MJ, Han Y, Liu H. Clinicopathological significance of NUT rearrangements in poorly differentiated Malignant tumors of the upper respiratory tract. Int J Surg Pathol (2013) 21(2):102–10. doi: 10.1177/1066896912451651
19. Suzuki S, Kurabe N, Minato H, Ohkubo A, Ohnishi I, Tanioka F, et al. A rare Japanese case with a NUT midline carcinoma in the nasal cavity: a case report with immunohistochemical and genetic analyses. Pathol Res Pract (2014) 210(6):383–8. doi: 10.1016/j.prp.2014.01.013
20. Stirnweiss A, McCarthy K, Oommen J, Crook ML, Hardy K, Kees UR, et al. A novel BRD4-NUT fusion in an undifferentiated sinonasal tumor highlights alternative splicing as a contributing oncogenic factor in NUT midline carcinoma. Oncogenesis (2015) 4(11):e174. doi: 10.1038/oncsis.2015.33
21. Solomon LW, Magliocca KR, Cohen C, Müller S. Retrospective analysis of nuclear protein in testis (NUT) midline carcinoma in the upper aerodigestive tract and mediastinum. Oral Surg Oral Med Oral Pathol Oral Radiol (2015) 119(2):213–20. doi: 10.1016/j.oooo.2014.09.031
22. Klijanienko J, Le Tourneau C, Rodriguez J, Caly M, Theocharis S. Cytological features of NUT midline carcinoma arising in sino-nasal tract and parotid gland: Report of two new cases and review of the literature. Diagn Cytopathol (2016) 44(9):753–6. doi: 10.1002/dc.23506
23. Bishop JA, French CA, Ali SZ. Cytopathologic features of NUT midline carcinoma: A series of 26 specimens from 13 patients. Cancer Cytopathol (2016) 124(12):901–8. doi: 10.1002/cncy.21761
24. Kakkar A, Antony VM, Irugu DVK, Adhikari N, Jain D. NUT midline carcinoma: A series of five cases, including one with unusual clinical course. Head Neck Pathol (2018) 12(2):230–6. doi: 10.1007/s12105-017-0858-2
25. Edgar M, Caruso AM, Kim E, Foss RD. NUT midline carcinoma of the nasal cavity. Head Neck Pathol (2017) 11(3):389–92. doi: 10.1007/s12105-016-0763-0
26. Minato H, Kobayashi E, Nakada S, Kurose N, Tanaka M, Tanaka Y, et al. Sinonasal NUT carcinoma: clinicopathological and cytogenetic analysis with autopsy findings. Hum Pathol (2018) 71:157–65. doi: 10.1016/j.humpath.2017.10.011
27. Arimizu K, Hirano G, Makiyama C, Matsuo M, Sasaguri T, Makiyama A. NUT carcinoma of the nasal cavity that responded to a chemotherapy regimen for Ewing's sarcoma family of tumors: a case report. BMC Cancer (2018) 18(1):1134. doi: 10.1186/s12885-018-5087-x
28. Chan W, Bullock MJ, Samad AF, Archibald CW, Heathcote JG. NUT carcinoma of the sinonasal tract infiltrating the orbit in a man with birdshot chorioretinitis. Saudi J Ophthalmol (2018) 32(1):62–5. doi: 10.1016/j.sjopt.2018.02.018
29. Laco J, Kovarikova H, Chmelarova M, Vosmikova H, Sieglova K, Bubancova I, et al. Analysis of DNA methylation and microRNA expression in NUT (nuclear protein in testis) midline carcinoma of the sinonasal tract: a clinicopathological, immunohistochemical and molecular genetic study. Neoplasma (2018) 65(1):113–23. doi: 10.4149/neo_2018_161122N581
30. Albrecht T, Harms A, Roessler S, Goeppert B. NUT carcinoma in a nutshell: A diagnosis to be considered more frequently. Pathol Res Pract (2019) 215(6):152347. doi: 10.1016/j.prp.2019.01.043
31. Lee T, Cho J, Baek CH, Son YI, Jeong HS, Chung MK, et al. Prevalence of NUT carcinoma in head and neck: Analysis of 362 cases with literature review. Head Neck (2020) 42(5):924–38. doi: 10.1002/hed.26067
32. Jung M, Kim S, Lee JK, Yoon SO, Park HS, Hong SW, et al. Clinicopathological and preclinical findings of NUT carcinoma: A multicenter study. Oncologist (2019) 24(8):e740–8. doi: 10.1634/theoncologist.2018-0477
33. Prasad M, Baheti A, Ramadwar M, Chinnaswamy G, Vora T, Qureshi S. Pediatric NUT carcinoma is a rare and challenging tumor: single center experience of five children. Oncologist (2019) 24(11):e1232–35. doi: 10.1634/theoncologist.2019-0358
34. Sopfe J, Greffe B, Treece AL. Metastatic NUT midline carcinoma treated with aggressive neoadjuvant chemotherapy, radiation, and resection: A case report and review of the literature. J Pediatr Hematol Oncol (2021) 43(1):e73–5. doi: 10.1097/MPH.0000000000001860
35. Leeman R, Pinkney K, Bradley JA, Ruiz R, DuBois SG, French C, et al. NUT carcinoma without upfront surgical resection: A case report. J Pediatr Hematol Oncol (2021) 43(5):e707–10. doi: 10.1097/MPH.0000000000001865
36. Patel SA, Singer B, Shen C, Zanation AM, Yarbrough WG, Weiss J. A case of metastatic NUT carcinoma with prolonged response on gemcitabine and nab-paclitaxel. Clin Case Rep (2021) 9(8):e04616. doi: 10.1002/ccr3.4616
37. Crocetta FM, Botti C, Fornaciari M, Castellucci A, Murri D, Santandrea G, et al. Sinonasal NUT carcinoma: delayed diagnosis due to the COVID-19 pandemic and a review of the literature. Head Neck Pathol (2021) 15(4):1–6. doi: 10.1007/s12105-021-01311-x
38. Wang L, Zhu Z, Wang W, Zha Y, Wang X, Surita A, et al. Sinonasal NUT carcinoma: A retrospective case series from a single institution. Front Surg (2023) 10:1098704. doi: 10.3389/fsurg.2023.1098704
39. Ramesh U, Contrera KJ, Shakibai N, Su SY, Brahimaj B, Roberts D, et al. Sinonasal NUT carcinoma: A consecutive case series and systematic review. Head Neck (2023). doi: 10.1002/hed.27553
40. French CA, Kutok JL, Faquin WC, Toretsky JA, Antonescu CR, Griffin CA, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol (2004) 22(20):4135–39. doi: 10.1200/JCO.2004.02.107
41. Cavalieri S, Stathis A, Fabbri A, Sonzogni A, Perrone F, Tamborini E, et al. Uncommon somatic mutations in metastatic NUT midline carcinoma. Tumori (2017) 103(Suppl. 1):e5–8. doi: 10.5301/tj.5000685
42. Xie M, Fu X, Wang W. Clinicopathological and molecular characterizations of pulmonary NUT midline carcinoma. Cancer Med (2021) 10(17):5757–64. doi: 10.1002/cam4.4096
43. Zhang Y, Han K, Dong X, Hou Q, Li T, Li L, et al. Case report and literature review: primary pulmonary NUT-midline carcinoma. Front Oncol (2021) 11:700781. doi: 10.3389/fonc.2021.700781
44. Lee JK, Louzada S, An Y, Kim SY, Kim S, Youk J, et al. Complex chromosomal rearrangements by single catastrophic pathogenesis in NUT midline carcinoma. Ann Oncol (2017) 28(4):890–97. doi: 10.1093/annonc/mdw686
45. Dang CV. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med (2013) 3(8):a014217. doi: 10.1101/cshperspect.a014217
46. Lüscher B, Vervoorts J. Regulation of gene transcription by the oncoprotein MYC. Gene (2012) 494(2):145–60. doi: 10.1016/j.gene.2011.12.027
47. Xu-Monette ZY, Deng Q, Manyam GC, Tzankov A, Li L, Xia Y, et al. Clinical and biologic significance of MYC genetic mutations in de novo diffuse large B-cell lymphoma. Clin Cancer Res (2016) 22(14):3593–05. doi: 10.1158/1078-0432
48. Grayson AR, Walsh EM, Cameron MJ, Godec J, Ashworth T, Ambrose JM, et al. MYC, a downstream target of BRD-NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene (2014) 33(13):1736–42. doi: 10.1038/onc.2013.126
49. Mojsa B, Lassot I, Desagher S. Mcl-1 ubiquitination: unique regulation of an essential survival protein. Cells (2014) 3(2):418–37. doi: 10.3390/cells3020418
50. Yasuda Y, Ozasa H, Kim YH, Yamazoe M, Ajimizu H, Yamamoto Funazo T, et al. MCL1 inhibition is effective against a subset of small-cell lung cancer with high MCL1 and low BCL-XL expression. Cell Death Dis (2020) 11(3):177. doi: 10.1038/s41419-020-2379-2
51. Lewin J, Soria JC, Stathis A, Delord JP, Peters S, Awada A, et al. a small-molecule inhibitor of bromodomain and extraterminal proteins, in patients with selected advanced solid tumors. J Clin Oncol (2018) 36(30):3007–14. doi: 10.1200/JCO.2018.78.2292
52. Li X, Shi H, Zhang W, Bai C, He M, Ta N, et al. Immunotherapy and targeting the tumor microenvironment: current place and new insights in primary pulmonary NUT carcinoma. Front Oncol (2021) 11:690115. doi: 10.3389/fonc.2021.690115
53. Chen M, Chen X, Zhang Y, Wang W, Jiang L. Clinical and molecular features of pulmonary NUT carcinoma characterizes diverse responses to immunotherapy, with a pathologic complete response case. J Cancer Res Clin Oncol (2023) 149(9):6361–70. doi: 10.1007/s00432-023-04621-5
54. Gupta R, Mumaw D, Antonios B, Anusim N, Dhulipalla SP, Stender M, et al. NUT midline lung cancer: a rare case report with literature review. AME Case Rep (2022) 6:2. doi: 10.21037/acr-21-35
55. Riess JW, Rahman S, Kian W, Edgerly C, Heilmann AM, Madison R, et al. Genomic profiling of solid tumors harboring BRD4-NUT and response to immune checkpoint inhibitors. Transl Oncol (2021) 14(10):101184. doi: 10.1016/j.tranon.2021.101184
Keywords: NUT carcinoma, BRD4::NUTM1 fusion, BRD3::NUTM1 fusion, next generation sequencing (NGS), sinonasal malignancies
Citation: Chen M, Li S and Jiang L (2024) Clinicopathological molecular characterizations of sinonasal NUT carcinoma: a report of two cases and a literature review. Front. Oncol. 13:1296862. doi: 10.3389/fonc.2023.1296862
Received: 19 September 2023; Accepted: 01 December 2023;
Published: 04 January 2024.
Edited by:
Sandra Nuyts, KU Leuven, BelgiumReviewed by:
Shweta Agarwal, University of New Mexico, United StatesJohn Charles Rotondo, University of Ferrara, Italy
Ab Zulkiflee, University Malaya Medical Centre, Malaysia
Copyright © 2024 Chen, Li and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Jiang, ODc5ODc2MDQ3QHFxLmNvbQ==, amlhbmdsaWxpQHNjdS5lZHUuY24=
 Min Chen
Min Chen Shuang Li
Shuang Li Lili Jiang
Lili Jiang