
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 04 December 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1291479
 Li-Ming Huang1†
Li-Ming Huang1† Zhen-Xin Zeng1†
Zhen-Xin Zeng1† Jun-Yi Wu1,2
Jun-Yi Wu1,2 Yi-Nan Li1
Yi-Nan Li1 Jin-Xiu Wang1
Jin-Xiu Wang1 Yang-Kai Fu1
Yang-Kai Fu1 Jia-Yi Wu1,2
Jia-Yi Wu1,2 Shao-Ming Wei1,3
Shao-Ming Wei1,3 Jia-Hui Lv1
Jia-Hui Lv1 Wei-Zhao Chen1
Wei-Zhao Chen1 Rong-Fa Huang1
Rong-Fa Huang1 Shu-Qun Cheng4*
Shu-Qun Cheng4* Mao-Lin Yan1,2*
Mao-Lin Yan1,2*Background: The long-term prognosis after surgery of patients with hepatocellular carcinoma (HCC) and extrahepatic bile duct tumor thrombus (Ex-BDTT) remains unknown. We aimed to identify the surgical outcomes of patients with HCC and Ex-BDTT.
Methods: A total of 138 patients with Ex-BDTT who underwent hepatectomy with preservation of the extrahepatic bile duct from five large hospitals in China between January 2009 and December 2017 were included. The Cox proportional hazards model was used to analyze overall survival (OS) and recurrence-free survival (RFS).
Results: With a median follow-up of 60 months (range, 1–127.8 months), the median OS and RFS of the patients were 28.6 and 8.9 months, respectively. The 1-, 3-, and 5-year OS rates of HCC patients with Ex-BDTT were 71.7%, 41.2%, and 33.5%, respectively, and the corresponding RFS rates were 43.5%, 21.7%, and 20.0%, respectively. Multivariate analysis identified that major hepatectomy, R0 resection, and major vascular invasion were independent prognostic factors for OS and RFS. In addition, preoperative serum total bilirubin ≥ 4.2 mg/dL was an independent prognostic factor for RFS.
Conclusion: Major hepatectomy with preservation of the extrahepatic bile duct can provide favorable long-term survival for HCC patients with Ex-BDTT.
Liver cancer is the fourth most common cause of cancer deaths worldwide, of which approximately 75%–85% are hepatocellular carcinomas (HCCs) (1). Tumor cells invade the portal vein and its branches and can form a portal vein tumor thrombus, which has been demonstrated as a poor prognostic factor (2, 3). Similarly, HCC can spread into the bile duct and can cause a bile duct tumor thrombus (BDTT). Based on the location of the BDTT in the biliary tree, it can be categorized as intrahepatic or extrahepatic BDTT (Ex-BDTT). The latter can cause extrahepatic bile duct obstruction and cause hemobilia, cholangitis, obstructive jaundice, and other complications (4–6).
Surgical excision has been proven to be the first choice for treating HCC with BDTT in some studies (7–11). However, due to the low incidence rate, only a few studies focusing on a small number of patients have shown concern about this issue. There is no consensus on the best surgical methods for treating HCC with BDTT, especially for Ex-BDTT. Some studies have shown that extrahepatic bile duct resection (BDR) can decrease the local residual and recurrence rates (7, 8, 10). In contrast, other studies have shown that most extrahepatic bile ducts should be preserved because Ex-BDTT seldom invades the extrahepatic bile duct wall (9, 12–14). Therefore, the long-term prognosis after surgery of patients who underwent Ex-BDTT remains unknown. In this study, all cases collected postoperative pathological data, and all patients were diagnosed with HCC with BDTT by postoperative pathology.
The purpose of this study was to determine the surgical results of hepatectomy with preservation of extrahepatic bile ducts in patients with HCC and Ex-BDTT.
We collected the medical information of 138 patients with Ex-BDTT, who underwent hepatectomy with preservation of the extrahepatic bile duct, in five large hospitals in China between January 2009 and December 2017.
During this period, the total number of HCC patients in these institutions was 8815, and the number of HCC with BDTT patients was 442. These institutions included Fujian Provincial Hospital (Fuzhou, China), First Affiliated Hospital of Fujian Medical University (Fuzhou, China), Eastern Hepatobiliary Surgery Hospital of Second Military Medical University (Shanghai, China), West China Hospital of Sichuan University (Chengdu, China), and Zhongshan Hospital of Xiamen University (Xiamen, China). The original data were collected and then merged for analysis. The Institutional Review Committee of each cooperative hospital reviewed and approved our study. The patients themselves or their authorized persons signed a written informed consent to use their data for research purposes. This retrospective study did not contradict the principles of the Declaration of Helsinki.
The collected data included demographic, perioperative information, histopathological information, recurrence, and survival data. The diagnosis of Ex-BDTT was defined as tumor thrombus in the extrahepatic bile duct, which is detected by preoperative clinical imaging examination (such as computed tomography [CT], magnetic resonance cholangiopancreatography [MRCP] or magnetic resonance imaging [MRI]) and intraoperative findings, and diagnosed by postoperative pathological reports. The tumor thrombus invading the first-order branch or main trunk of the portal and/or hepatic vein can be detected by CT or MRI preoperatively. This is called a major vascular invasion (MVI). The greatest dimension of the largest lesion is regarded as the tumor size regardless of whether there are multiple tumors. The staging system was based on the American Joint Committee on Cancer (AJCC) 8th edition (15). The definition of post-hepatectomy liver failure (PHLF) and the classification of postoperative complications were carried out according to the recommendations of the International Study Group of Liver Surgery and the Clavien-Dindo classification (16, 17).
The inclusion criteria were (1) surgical resection and (2) HCC with Ex-BDTT confirmed by histopathological examination. The exclusion criteria were (1) Hepatectomy with BDR (n = 18), (2) recurrent tumor (n = 3), (3) preoperative anticancer treatment (n = 2), (4) history of other cancers (n = 2), (5) Child-Pugh grade C (n = 2), (6) poorly tolerated hepatectomy (n = 1) and (7) incomplete data (n = 5). The flow chart for screening all cases of HCC with Ex-BDTT is shown in Supplementary Figure S1.
There is no unified standard for biliary drainage in this study. The level of serum total bilirubin (TBil) in all patients with biliary drainage is greater than 11.7 mg/L. A percutaneous transhepatic cholangial drainage tube is usually placed in the remnant intrahepatic bile duct of the liver. Before surgery, CT or MRI was used to evaluate the patient’s future liver reserve (FLR). Major hepatectomy was indicated only when the residual liver volume of patients with liver cirrhosis reduces to 40% of the liver parenchymal volume. Preoperative liver function evaluation was comprehensively evaluated by each center.
In this study, major hepatectomy was defined as liver resection greater than or equal to hemihepatectomy, including central hepatectomy (which greater than or equal to 3 liver segments). Negative bile duct and specimen margins on histology, no evidence of tumor recurrence on CT or MRI investigations, and normal serum alpha-fetoprotein (AFP) levels defined R0 resection at 90 days postoperatively (8).
Tumor size, the number of tumors, tumor position, and hepatic functional reserve were used to evaluate the extent of hepatectomy. The Pringle maneuver was performed, if necessary. Extrahepatic bile duct was preserved unless the Ex-BDTT could not be removed through thrombectomy or had invaded the extrahepatic bile duct wall. This was a multicenter retrospective study, and the surgical methods of each center were different. However, most of the tumor thrombectomy techniques in each center were similar to the “peeling-off technique” of Yamamoto et al. (12). We incised the bile duct, peeled off the BDTT, confirmed that the margin of the bile duct was negative by intraoperative frozen section analysis, and sutured the bile duct incision with 5-0 or 6-0 absorbable monofilament thread. In the Fujian Provincial Hospital, the tumor thrombectomy technique, called “q-shaped thrombectomy”, which we have previously reported, was also used in some patients (18). Moreover, choledochoscopy was performed routinely during the operation to confirm whether the tumor thrombus was removed neatly.
The first follow-up was conducted in the first month after the operation, every three months in the first year, and every six months thereafter until death. Laboratory examinations (including AFP, liver function, etc.) and imaging examinations (including CT or MRI enhancement, etc.) were performed at each re-examination. Recurrence was treated using a multidisciplinary approach that included surgical resection, systemic therapy, radiofrequency ablation, or transarterial chemoembolization, according to the recurrence site and liver functional reserves. The time between the date of the first curative hepatectomy and the date of death or last follow-up is called overall survival (OS). The time between the date of the first curative hepatectomy and the date of the first relapse or last follow-up is called recurrence-free survival (RFS). The last follow-up ended on June 1, 2021.
Continuous data are expressed as medians with ranges. Categorical variables are expressed as counts with percentages (%). The Kaplan–Meier method was used to calculate the rates of OS and RFS, and the log-rank test was used for comparison of survival between groups. The Kaplan–Meier curves were produced by R software (R Foundation for Statistical Computing, Vienna, Austria). The proportional hazards assumption was assessed by performing Schoenfeld’s tests and plots and was met for all analyses. The Cox proportional hazards regression model was performed for univariate and multivariate analyses. A two-tailed p-value of < 0.05 was considered statistically significant. IBM SPSS (version 24.0; IBM Corp, Armonk, NY, USA) was used for statistical analysis.
One hundred and thirty-eight HCC patients with Ex-BDTT were included in the present study. The clinicopathological features of patients having HCC with Ex-BDTT are presented in Table 1. At the first visit, 94 patients (68.1%) had obstructive jaundice, and 27 (19.6%) received preoperative biliary drainage. Among them, twenty-two patients (15.9%) underwent percutaneous transhepatic cholangial drainage, and five patients (3.6%) underwent endoscopic retrograde cholangiopancreatography. The level of TBil in all patients with biliary drainage is greater than 11.7 mg/L. In the biliary drainage group, the median serum TBil decreased from 17.5 mg/dl to 7.4 mg/dl. Twenty-six patients (18.8%) presented with MVI preoperatively. Sixty-five patients (47.1%) underwent major hepatectomy and 103 patients (74.6%) underwent R0 resection. The R0 resection rates were 53.4% and 46.6% in those who underwent major hepatectomy and those who did not, respectively (p = 0.011). Liver cirrhosis was confirmed in 74 patients (53.6%). According to the Clavien-Dindo classification of postoperative complications, 12 patients (8.7%) were classified as having grade IIIb-V. Sixty-one (44.2%) patients underwent transarterial chemoembolization, which is the most common postoperative adjuvant treatment.
The median follow-up of 60 months (range, 1–127.8 months) and the median OS and RFS of the patients with Ex-BDTT were 28.6 and 8.9 months, respectively. The 1-, 3-, and 5-year OS rates of patients with HCC having Ex-BDTT were 71.7%, 41.2%, and 33.5%, respectively (Figure 1A), and the RFS rates were 43.5%, 21.7%, and 20.0%, respectively (Figure 1B).
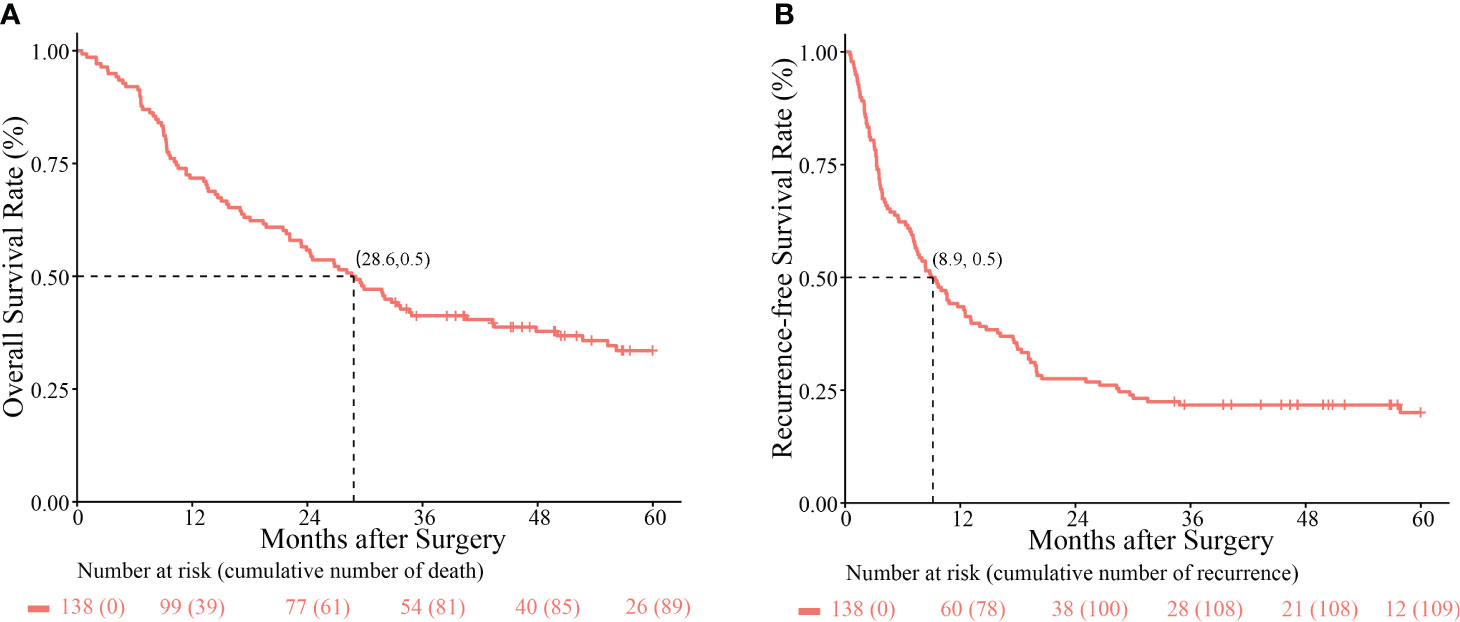
Figure 1 Kaplan–Meier survival curves comparing overall survival (A) and recurrence-free survival rates (B) among hepatocellular carcinoma patients with extrahepatic bile duct tumor thrombus who underwent hepatectomy.
As shown in Figure 2, better OS and RFS rates were observed following major hepatectomy. The 1-, 3-, and 5-year OS rates in patients with Ex-BDTT who underwent major hepatectomy and those who did not were 86.2%, 52.3%, and 43.8%, versus 58.9%, 31.4%, and 24.6%, respectively (p = 0.002) (Figure 2A). The 1-, 3-, and 5-year RFS rates for those who underwent major hepatectomy and those who did not were 58.9%, 32.2%, and 32.2% versus 34.2%, 12.3%, and 9.9%, respectively (p < 0.001) (Figure 2B). Moreover, the 1 -, 3 - and 5-year OS rates of patients with MVI (46.2%, 15.4%, and 15.4%, respectively) were significantly lower than those without MVI (77.7%, 47.3%, and 37.9%, respectively) (p = 0.001) (Figure 2C). Similarly, the 1-, 3-, and 5-year RFS rates of patients with MVI (19.2%, 7.7%, and 7.7%, respectively) were also significantly lower than those without MVI (49.1%, 25.0%, and 22.9%, respectively) (p < 0.001) (Figure 2D).
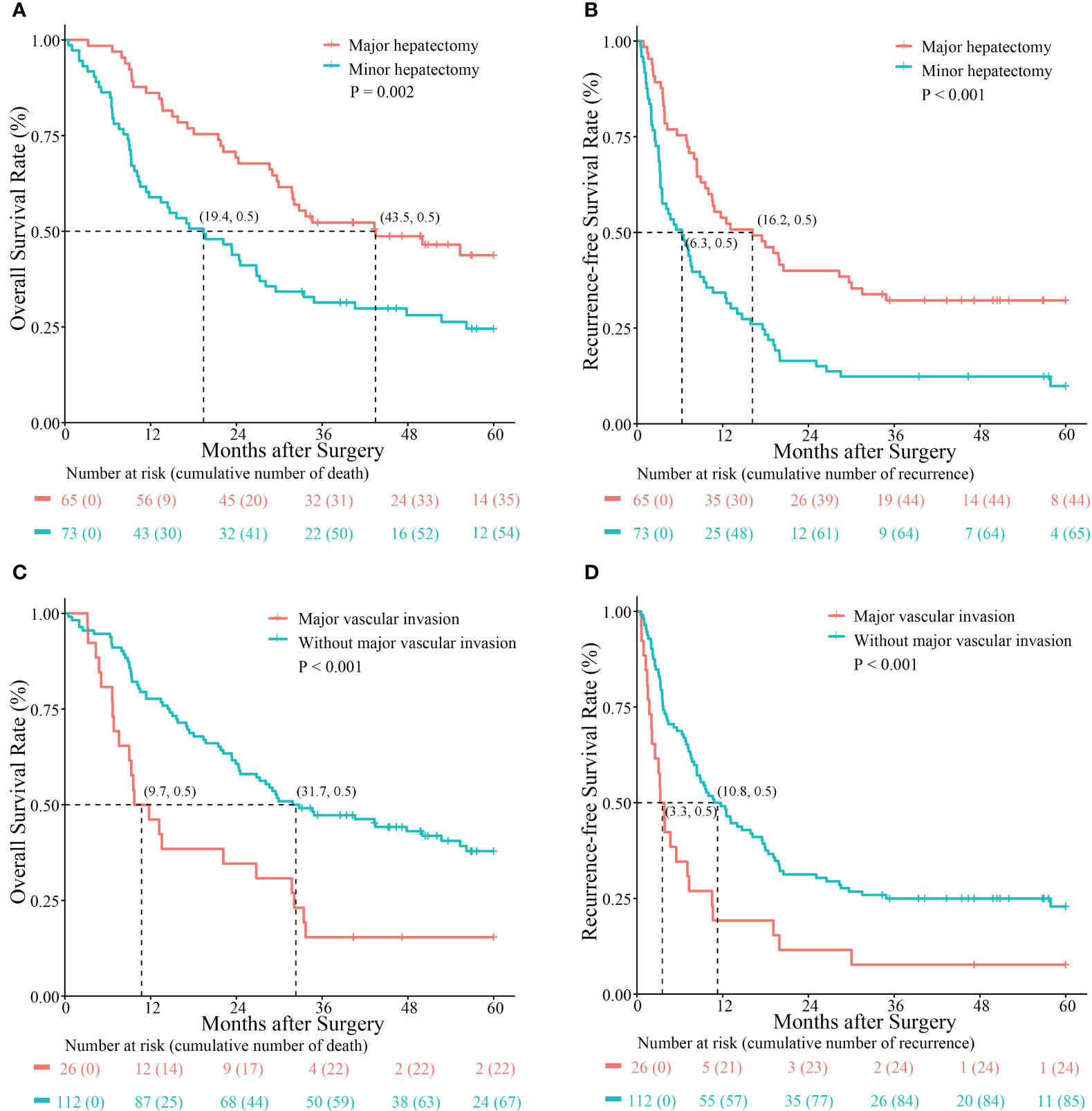
Figure 2 Kaplan–Meier survival curves comparing overall survival and recurrence-free survival rates between hepatocellular carcinoma patients with extrahepatic bile duct tumor thrombus who underwent major hepatectomy and those without major hepatectomy (A, B) and those with and without major vascular invasion (C, D).
As seen in Table 2, the results of the Cox multivariate analysis indicated that major hepatectomy, R0 resection, and MVI were independent prognostic factors for OS (hazard ratio [HR] 0.452, 95% confidence interval [CI] 0.295–0.695, p < 0.001; HR 0.292, 95% CI 0.185–0.460, p < 0.001; and HR 2.010, 95% CI 1.195–3.379, p = 0.008, respectively) and RFS (HR 0.518, 95% CI 0.344–0.779, p = 0.002; HR 0.176, 95% CI 0.108–0.287, p < 0.001; and HR 1.781, 95% CI 1.073–2.954, p = 0.026, respectively). Preoperative serum TBil ≥ 4.2 mg/dL was an independent prognostic factor for RFS (HR 1.803, 95% CI 1.204–2.700; p = 0.004).
As shown in Table 3, There was no significant difference between the two groups in terms of surgical duration, blood loss, postoperative hospital stay, 90-day mortality, PHLF, and postoperative complications. Meanwhile, the surgical duration of major hepatectomy is longer than that of minor hepatectomy, and the difference is statistically significant (p < 0.001). However, there was no significant difference in the other observation indicators. Recurrence sites after hepatectomy are described in Table 4. Intrahepatic recurrence is the most common of all recurrent sites (58.2%; 64/110).
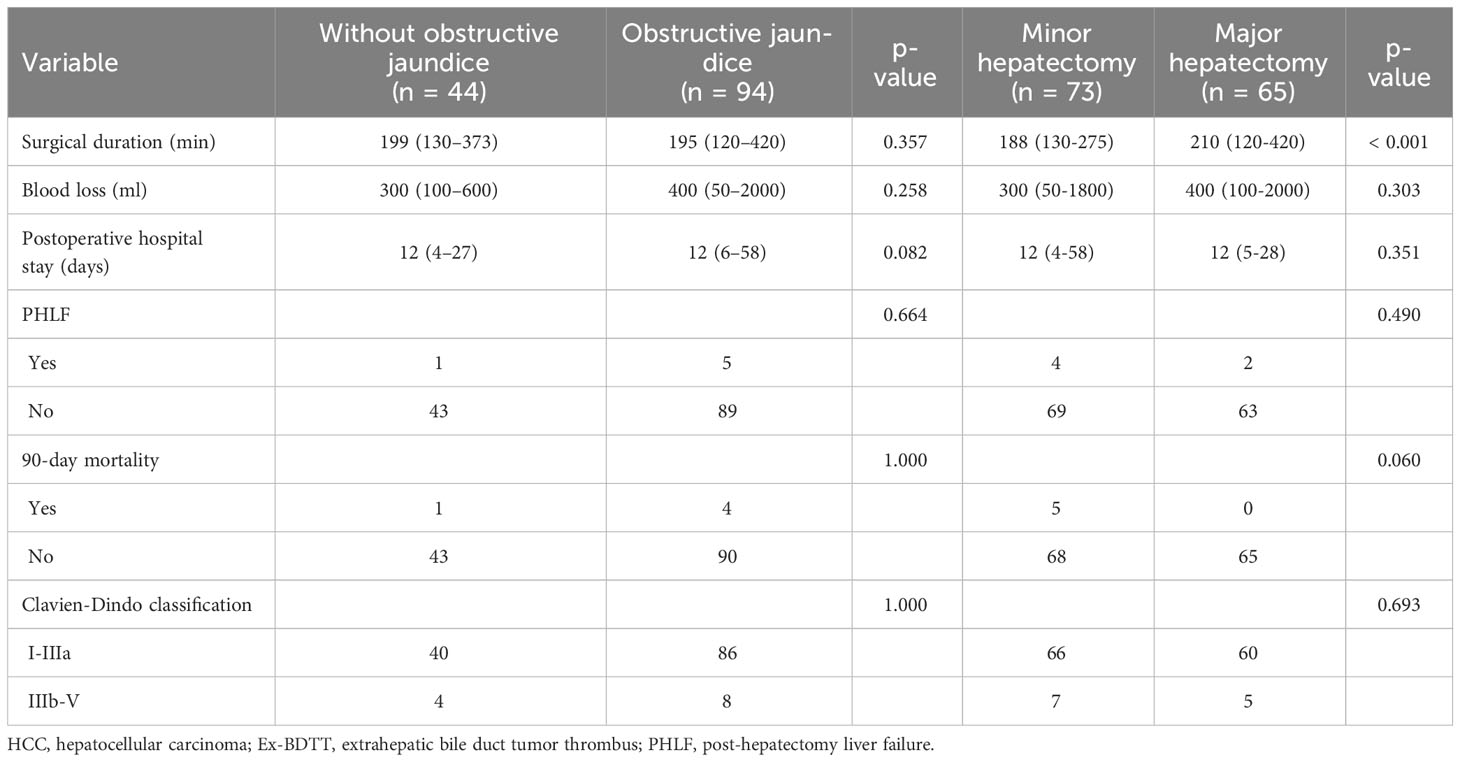
Table 3 Perioperative outcomes of obstructive jaundice group and without obstructive jaundice group, major hepatectomy group and minor hepatectomy group.
HCC with BDTT can be classified into different types based on the extent of tumor thrombus invasion into the bile duct. Cheng et al. proposed a classification: Type I: intrahepatic BTT; and Type II: extrahepatic BTT involving a common bile duct or common hepatic duct (19). This study referred to the classification proposed by Cheng et al., where the EX-BDTT is equivalent to Type II. HCC presenting with obstructive jaundice due to BDTT, especially Ex-BDTT, has been classified as “icteric-type hepatoma” (20). Hyperbilirubinemia increases postoperative complications, such as PHLF and intraoperative bleeding, and leads to a poor prognosis (4–6, 21). This is probably ascribed to the insufficient liver reserve caused by obstructive jaundice. Thus, HCC patients with jaundice caused by advanced tumor infiltration, advanced liver cirrhosis, or progressive end-stage liver failure have no chance of achieving remission or cure. However, patients with HCC having Ex-BDTT often have reversible hyperbilirubinemia and hypoalbuminemia, which is caused by biliary obstruction. Previous research reported that biliary drainage procedures, including percutaneous transhepatic cholangial drainage and endoscopic retrograde cholangiopancreatography, are beneficial to the prognosis of patients with HCC having obstructive jaundice (9, 10). Preoperative biliary drainage can improve coagulation function, liver function, and reduce postoperative complications (10, 22–26). However, there is a debate about whether the liver function of patients with jaundice is reduced to a normal level before surgery. Some scholars have advocated reducing serum TBil to < 2 mg/dL before surgery (9–11). In our data, the serum TBil in the biliary drainage group did not decrease to normal levels. The median serum TBil in the biliary drainage group decreased from 17.5 mg/dL to 7.4 mg/dL. However, the 5-year OS rate in our research was almost the same as in the previous study in which TBil was reduced to 2 mg/L (9–11). In addition, it did not increase complications, such as PHLF and intraoperative bleeding. We also found that preoperative serum TBil ≥ 4.2 mg/dL was an independent prognostic factor for RFS, and reducing serum TBil to < 4.2 mg/dL before surgery was also feasible. However, the optimal TBil level before surgery requires further investigation.
Several studies have emphasized the importance of major hepatectomy for HCC with BDTT (10, 13, 27). Major hepatectomy can reduce the tumor residual in the extrahepatic bile ducts and the liver parenchyma. In addition, it could achieve anatomic resection to increase the R0 resection rate (10, 28, 29). Although some studies reported that major hepatectomy was associated with relatively high postoperative mortality, others indicated that major hepatectomy could reduce the risk of PHLF in HCC patients with adequate FLR (30–32). In the present study, major hepatectomy was an independent favorable prognostic factor for both OS and RFS. In addition, major hepatectomy did not increase postoperative complications, such as PHLF compared with the minor hepatectomy group, because all patients in the major hepatectomy group had sufficient FLR. Therefore, we believe that major hepatectomy is safe and feasible for HCC patients with BDTT when the liver function and FLR are carefully evaluated before surgery. However, it still needs further research.
The preservation of the extrahepatic bile duct for Ex-BDTT cases is still inconclusive. These controversies are possibly owing to the low incidence of BDTT and the lack of randomized controlled trials. Several studies have supported the use of hepatectomy with BDR, which can avoid residual tumors in the extrahepatic bile ducts and reduce postoperative recurrences (7, 8, 10, 27, 33). However, we adopted major hepatectomy with preservation of the extrahepatic bile duct in the present study. First, BDTT rarely invades the extrahepatic bile duct walls and can be easily peeled off (12–14, 34, 35). Second, bile duct recurrence lesions can be successfully treated by regional treatment, and BDR is a risk factor for liver abscesses after regional therapy due to retrograde infection through cholangiojejunostomy (12, 36–40). In the current research, the 5-year OS and RFS rates of Ex-BDTT patients who underwent major hepatectomy were similar to those of patients who underwent BDR in other studies (45.1% and 33.0%) (33). Therefore, preservation of extrahepatic bile ducts combined with major hepatectomy can be recommended in patients with Ex-BDTT, unless the BDTT cannot be removed by tumor thrombectomy or it invades the extrahepatic bile duct wall.
Although the number of Ex-BDTT cases in our study was the largest worldwide, there were some limitations. This was a retrospective study and lacked a control group (patients with HCC but without BDTT). In the future, well-designed multicenter randomized controlled trials are needed for further investigation. Moreover, the present study was carried out in China, which has a high incidence rate of hepatitis B virus-related HCC. Thus, these results may not be applicable to patients with HCC infected with other hepatitis viruses.
In conclusion, for patients with HCC having Ex-BDTT, major hepatectomy with preservation of the extrahepatic bile duct can provide an opportunity for favorable long-term survival.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Institutional Review Board of Fujian Provincial Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LH: Conceptualization, Data curation, Investigation, Validation, Writing – original draft. ZZ: Conceptualization, Data curation, Investigation, Writing – original draft, Visualization. JuW: Data curation, Investigation, Writing – original draft. YL: Data curation, Investigation, Writing – original draft. JW: Data curation, Investigation, Visualization, Writing – review & editing. YF: Data curation, Investigation, Visualization, Writing – review & editing. JiW: Investigation, Writing – review & editing, Project administration. SW: Investigation, Project administration, Writing – review & editing. JL: Investigation, Project administration, Writing – review & editing. WC: Investigation, Project administration, Writing – review & editing. RH: Investigation, Project administration, Writing – review & editing. SC: Writing – review & editing, Conceptualization, Resources, Supervision. MY: Conceptualization, Resources, Supervision, Writing – review & editing, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Natural Science Foundation of Fujian Province (2020J011105).
The authors thank the staff of the participating hospitals for their efforts and the patients for their participation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1291479/full#supplementary-material
Supplementary Figure 1 | Flowchart for screening all HCC with Ex-BDTT cases. HCC, hepatocellular carcinoma; Ex-BDTT, extrahepatic bile duct tumor thrombus; BDR, bile duct resection.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg (2007) 245:909–22. doi: 10.1097/01.sla.0000254368.65878.da
3. Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol (2013) 20:325–39. doi: 10.1245/s10434-012-2513-1
4. Suh YG, Kim DY, Han KH, Seong J. Effective biliary drainage and proper treatment improve outcomes of hepatocellular carcinoma with obstructive jaundice. Gut Liver (2014) 8:526–35. doi: 10.5009/gnl13370
5. Sugiyama G, Okabe Y, Ishida Y, Saitou F, Kawahara R, Ishikawa H, et al. Evaluation of endoscopic biliary stenting for obstructive jaundice caused by hepatocellular carcinoma. World J Gastroenterol (2014) 20:6968–73. doi: 10.3748/wjg.v20.i22.6968
6. Minami Y, Kudo M. Hepatocellular carcinoma with obstructive jaundice: endoscopic and percutaneous biliary drainage. Dig Dis (2012) 30:592–7. doi: 10.1159/000343087
7. Wong TC, Cheung TT, Chok KS, Chan AC, Dai WC, Chan SC, et al. Outcomes of hepatectomy for hepatocellular carcinoma with bile duct tumour thrombus. HPB (Oxford) (2015) 17:401–8. doi: 10.1111/hpb.12368
8. Zeng H, Xu LB, Wen JM, Zhang R, Zhu MS, Shi XD, et al. Hepatocellular carcinoma with bile duct tumor thrombus: a clinicopathological analysis of factors predictive of recurrence and outcome after surgery. Med (Baltimore) (2015) 94:e364. doi: 10.1097/MD.0000000000000364
9. Orimo T, Kamiyama T, Yokoo H, Wakayama K, Shimada S, Tsuruga Y, et al. Hepatectomy for hepatocellular carcinoma with bile duct tumor thrombus, Including cases with obstructive jaundice. Ann Surg Oncol (2016) 23:2627–34. doi: 10.1245/s10434-016-5174-7
10. Kim DS, Kim BW, Hatano E, Hwang S, Hasegawa K, Kudo A, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a Korea-Japan multicenter study. Ann Surg (2020) 271:913–21. doi: 10.1097/SLA.0000000000003014
11. Kasai Y, Hatano E, Seo S, Taura K, Yasuchika K, Uemoto S. Hepatocellular carcinoma with bile duct tumor thrombus: surgical outcomes and the prognostic impact of concomitant major vascular invasion. World J Surg (2015) 39:1485–93. doi: 10.1007/s00268-015-2985-9
12. Yamamoto S, Hasegawa K, Inoue Y, Shindoh J, Aoki T, Sakamoto Y, et al. Bile duct preserving surgery for hepatocellular carcinoma with bile duct tumor thrombus. Ann Surg (2015) 261:e123–125. doi: 10.1097/SLA.0000000000001209
13. Yu XH, Xu LB, Liu C, Zhang R, Wang J. Clinicopathological characteristics of 20 cases of hepatocellular carcinoma with bile duct tumor thrombi. Dig Dis Sci (2011) 56:252–9. doi: 10.1007/s10620-010-1256-8
14. Moon DB, Hwang S, Wang HJ, Yun SS, Kim KS, Lee YJ, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a Korean multicenter study. World J Surg (2013) 37:443–51. doi: 10.1007/s00268-012-1845-0
15. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin (2017) 67:93–9. doi: 10.3322/caac.21388
16. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery (2011) 149:713–24. doi: 10.1016/j.surg.2010.10.001
17. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
18. Wu JY, Sun JX, Lau WY, Cheng SQ, Yan ML. Surgical resection for hepatocellular carcinoma with bile duct tumor thrombus. Surgery (2021) 169:1424–6. doi: 10.1016/j.surg.2020.12.045
19. Sun J, Wu J, Liu C, Shi J, Wei Y, Zhou J, et al. Typing of biliary tumor thrombus influences the prognoses of patients with hepatocellular carcinoma. Cancer Biol Med (2021) 18:808–15. doi: 10.20892/j.issn.2095-3941.2020.0202
20. Lin TY, Chen KM, Chen YR, Lin WS, Wang TH, Sung JL. Icteric type hepatoma. Med Chir Dig (1975) 4:267–70.
21. Umeda J, Itoi T. Current status of preoperative biliary drainage. J Gastroenterol (2015) 50:940–54. doi: 10.1007/s00535-015-1096-6
22. Guiu B, Bize P, Demartines N, Lesurtel M, Denys A. Simultaneous biliary drainage and portal vein embolization before extended hepatectomy for hilar cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol (2014) 37:698–704. doi: 10.1007/s00270-013-0699-7
23. Moole H, Bechtold M, Puli SR. Efficacy of preoperative biliary drainage in Malignant obstructive jaundice: a meta-analysis and systematic review. World J Surg Oncol (2016) 14:182. doi: 10.1186/s12957-016-0933-2
24. Chen P, Li B, Zhu Y, Chen W, Liu X, Li M, et al. Establishment and validation of a prognostic nomogram for patients with resectable perihilar cholangiocarcinoma. Oncotarget (2016) 7:37319–30. doi: 10.18632/oncotarget.9104
25. Yoshida Y, Ajiki T, Ueno K, Shinozaki K, Murakami S, Okazaki T, et al. Preoperative bile replacement improves immune function for jaundiced patients treated with external biliary drainage. J Gastrointest Surg (2014) 18:2095–104. doi: 10.1007/s11605-014-2674-2
26. Soares KC, Kamel I, Cosgrove DP, Herman JM, Pawlik TM. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobiliary Surg Nutr (2014) 3:18–34. doi: 10.3978/j.issn.2304-3881.2014.02.05
27. Hu XG, Mao W, Hong SY, Kim BW, Xu WG, Wang HJ. Surgical treatment for hepatocellular carcinoma with bile duct invasion. Ann Surg Treat Res (2016) 90:139–46. doi: 10.4174/astr.2016.90.3.139
28. Zheng-Rong L, Hai-Bo Y, Xin C, Chuan-Xin W, Zuo-Jin L, Bing T, et al. Resection and drainage of hilar cholangiocarcinoma: an 11-year experience of a single center in mainland China. Am Surg (2011) 77:627–33. doi: 10.1177/000313481107700525
29. Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg (2001) 234:507–517; discussion 517-509. doi: 10.1097/00000658-200110000-00010
30. Wiederkehr JC, Avilla SG, Mattos E, Coelho IM, Ledesma JA, Conceição AF, et al. Associating liver partition with portal vein ligation and staged hepatectomy (ALPPS) for the treatment of liver tumors in children. J Pediatr Surg (2015) 50:1227–31. doi: 10.1016/j.jpedsurg.2014.10.019
31. Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg (2009) 250:540–8. doi: 10.1097/SLA.0b013e3181b674df
32. Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) (2013) 15:91–103. doi: 10.1111/j.1477-2574.2012.00557.x
33. Feng JK, Chen ZH, Wu YX, Wang K, Sun JX, Chai ZT, et al. Comparison of different surgical interventions for hepatocellular carcinoma with bile duct tumor thrombus: a systematic review and meta-analysis. Ann Transl Med (2020) 8:1567. doi: 10.21037/atm-20-3935
34. Peng SY, Wang JW, Liu YB, Cai XJ, Deng GL, Xu B, et al. Surgical intervention for obstructive jaundice due to biliary tumor thrombus in hepatocellular carcinoma. World J Surg (2004) 28:43–6. doi: 10.1007/s00268-003-7079-4
35. Shiomi M, Kamiya J, Nagino M, Uesaka K, Sano T, Hayakawa N, et al. Hepatocellular carcinoma with biliary tumor thrombi: aggressive operative approach after appropriate preoperative management. Surgery (2001) 129:692–8. doi: 10.1067/msy.2001.113889
36. Woo S, Chung JW, Hur S, Joo SM, Kim HC, Jae HJ, et al. Liver abscess after transarterial chemoembolization in patients with bilioenteric anastomosis: frequency and risk factors. AJR Am J Roentgenol (2013) 200:1370–7. doi: 10.2214/AJR.12.9630
37. Hoffmann R, Rempp H, Schmidt D, Pereira PL, Claussen CD, Clasen S. Prolonged antibiotic prophylaxis in patients with bilioenteric anastomosis undergoing percutaneous radiofrequency ablation. J Vasc Interv Radiol (2012) 23:545–51. doi: 10.1016/j.jvir.2011.12.025
38. Spies JB, Rosen RJ, Lebowitz AS. Antibiotic prophylaxis in vascular and interventional radiology: a rational approach. Radiology (1988) 166:381–7. doi: 10.1148/radiology.166.2.3275979
39. Taura K, Ikai I, Hatano E, Fujii H, Uyama N, Shimahara Y. Implication of frequent local ablation therapy for intrahepatic recurrence in prolonged survival of patients with hepatocellular carcinoma undergoing hepatic resection: an analysis of 610 patients over 16 years old. Ann Surg (2006) 244:265–73. doi: 10.1097/01.sla.0000217921.28563.55
Keywords: hepatocellular carcinoma, hepatectomy, bile duct resection, R0 resection, obstructive jaundice, major vascular invasion, overall survival, recurrence-free survival
Citation: Huang L-M, Zeng Z-X, Wu J-Y, Li Y-N, Wang J-X, Fu Y-K, Wu J-Y, Wei S-M, Lv J-H, Chen W-Z, Huang R-F, Cheng S-Q and Yan M-L (2023) Surgical outcomes of hepatocellular carcinoma with extrahepatic bile duct tumor thrombus: a multicenter study. Front. Oncol. 13:1291479. doi: 10.3389/fonc.2023.1291479
Received: 09 September 2023; Accepted: 21 November 2023;
Published: 04 December 2023.
Edited by:
Wenqing Cao, New York University, United StatesReviewed by:
Beat Moeckli, University of Geneva, SwitzerlandCopyright © 2023 Huang, Zeng, Wu, Li, Wang, Fu, Wu, Wei, Lv, Chen, Huang, Cheng and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-Qun Cheng, Y2hlbmdzaHVxdW5AYWxpeXVuLmNvbQ==; Mao-Lin Yan, eWFubWFvbGluNzRAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.