
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 15 December 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1289916
Background and aim: Standardized approach to postoperative adjuvant therapy for hepatocellular carcinoma (HCC) remains elusive. This study endeavors to examine the effects of postoperative PD-1 adjuvant therapy on the short-term and long-term prognosis of patients at a heightened risk of post-surgical recurrence.
Methods: The data of HCC patients who underwent hepatectomy at our center from June 2018 to March 2023 were collected from the hospital database. Propensity score matching (PSM) was employed to perform a 1:1 match between the postoperative anti-PD-1 antibody group and the postoperative non-anti-PD-1 antibody group. Kaplan-Meier method was utilized to compare the overall survival (OS) and recurrence-free survival (RFS) between the two groups. Cox regression analysis was conducted to identify the prognostic factors affecting patient outcomes. Subgroup analyses were performed for different high-risk factors.
Results: Among the 446 patients included in the study, 122 patients received adjuvant therapy with postoperative anti-PD-1 antibodies. After PSM, the PD-1 group had postoperative 1-year, 2-year, 3-year, and 4-year OS rates of 93.1%, 86.8%, 78.2%, and 51.1%, respectively, while the non-PD-1 group had rates of 85.3%, 70.2%, 47.7%, and 30.0%. The PD-1 group had postoperative 1-year, 2-year, 3-year, and 4-year RFS rates of 81.7%, 77.0%, 52.3%, and 23.1%, respectively, whereas the non-PD-1 group had rates of 68.4%, 47.7%, and 25.8% in 1-year, 2-year, 3-year. A multifactorial Cox regression analysis revealed that postoperative PD-1 use was a prognostic protective factor associated with OS and RFS. Subgroup analysis results indicated that HCC patients with high recurrence risks significantly benefited from postoperative anti-PD-1 antibody treatment in terms of OS and RFS.
Conclusion: For HCC patients with high-risk recurrence factors and undergoing hepatectomy, postoperative adjuvant therapy with anti-PD-1 antibodies can effectively improve their survival prognosis.
Hepatocellular carcinoma (HCC) accounts for over 80% of primary liver cancers, making it one of the most common cancer types worldwide. The incidence and mortality rates have been increasing year by year (1, 2). For the majority of HCC patients in many Asian countries, the primary treatment option has been hepatic resection (3, 4). However, for most patients undergoing hepatic resection, the five-year recurrence rate is as high as 50-70%, especially for those with high-risk recurrence factors such as microvascular invasion, portal vein tumor thrombosis, large tumor diameter, multiple tumor nodules, and so on (5, 6). Therefore, effective postoperative adjuvant treatments should be offered to HCC patients after hepatic resection to reduce recurrence and improve long-term survival rates.
Currently, immune checkpoint inhibitors (ICIs) have made significant strides in the treatment of unresectable HCC (7–9). Anti-PD-1 antibodies enhance the body’s immune recognition, enabling T cells to reidentify tumor cells within the body (10). Moreover, responses to ICIs can be sustained over a long duration. For certain other tumors, such as melanoma, esophageal cancer, and gastric cancer, anti-PD-1 antibodies have proven effective in extending patients’ overall survival (OS) and recurrence-free survival (RFS). Hence, the mechanisms of ICIs make them a promising approach for postoperative adjuvant therapy in HCC patients. For patients at high risk of postoperative recurrence, several phase III clinical trials are currently underway (11–13). These trials include postoperative combined therapy with anti-PD-1 antibodies and bevacizumab as adjuvant treatment (NCT03847428 and NCT04102098). Some preliminary results indicate that postoperative PD-1 antibodies can effectively extend the survival of high-risk patients. Researchers like Chen et al (14). believe that HCC patients with portal vein tumor thrombosis (PVTT) or tumors larger than or equal to 5 cm significantly benefit from anti-PD-1 antibody treatment in terms of and OS. After curative resection, HCC may leave behind small disseminated lesions, which often lead to postoperative recurrence and worsened prognosis. However, anti-PD-1 antibodies can modulate the immune environment and increase the number of T cells, eliminating these small lesions (15). Therefore, adjuvant therapy based on anti-PD-1 antibodies holds great promise in preventing recurrence and extending survival.
In this study, our research team aims to investigate whether the use of anti-PD-1 antibodies in postoperative HCC patients can improve prognosis, especially in patients with high-risk recurrence factors. This endeavor seeks to provide additional evidence regarding postoperative adjuvant therapy for HCC patients.
This retrospective study collected data from all patients who underwent curative hepatic resection at Zhongshan People Hospital from June 2018 to June 2023. The study adhered to the requirements of the Helsinki Declaration and received approval from the hospital’s ethics committee [ZS-20210807].
Strict inclusion and exclusion criteria were applied in this study. Inclusion criteria were as follows: (1) age greater than 18, (2) postoperative pathological diagnosis of HCC, (3) R0 resection, (4) tumor detected as the initial occurrence, (5) Child-Pugh class A or B, and (6) ECOG performance status 0-1. Exclusion criteria were as follows: (1) prior history of anticancer treatment, (2) incomplete follow-up records, and (3) non-compliance with drug therapy.
MVI (Microvascular Invasion) is typically defined as clusters of cancer cells observed within the lumens of vessels lined with endothelial cells under a microscope. It is graded pathologically as M1 and M2. PVTT (Portal Vein Tumor Thrombosis) refers to tumor emboli within the portal vein. In this study, PVTT is primarily categorized into the following types: Type I (vp1): involving the invasion of third-order portal vein branches, Type II (vp2): involving the invasion of second-order portal vein branches, and Type III (vp3): involving the invasion of the main portal vein (16). Satellite nodules are defined as small tumor foci appearing within the liver tissue adjacent to the primary tumor, with a distance of less than 2 centimeters between the tumor foci and the primary tumor. In this study, high-risk recurrence factors are defined as one or more of the following: tumor diameter greater than 5 cm, multifocal tumors, MVI, PVTT, or satellite nodules.
Preoperatively, a meticulous evaluation and discussion are carried out by the hospital’s Multidisciplinary Team (MDT) to determine the surgical approach. The EGOS (Eastern Cooperative Oncology Group) performance status is used to assess the general condition of patients. Preoperative assessments include abdominal CT and magnetic resonance imaging (MRI). Liver functional reserve is assessed using ICG-15 (Indocyanine Green clearance at 15 minutes). Intraoperatively, intraoperative ultrasound is performed for exploration. All surgeries are conducted by a team of experienced surgeons who have surpassed the learning curve. Major hepatectomy is defined as the resection of three or more liver segments, while minor hepatectomy is defined as the resection of one or two liver segments.
HCC patients with high-risk factors are recommended for adjuvant therapy following liver resection. However, the ultimate decision regarding treatment depends on the patient and their family. Treatment with anti-PD-1 antibodies begins four weeks after surgery at the recommended dosage. Patients receive intravenous anti-PD-1 antibody therapy at 21-day intervals. Patients continue to receive anti-PD-1 antibody treatment until HCC recurrence, the occurrence of severe adverse events, or voluntary withdrawal by the patient. Anti-PD-1 antibodies include camrelizumab, toripalimab, sintilimab, and pembrolizumab. Anti-PD-1 antibodies include camrelizumab, toripalimab, sintilimab, and pembrolizumab. The antibodies currently employed are not approved for postoperative adjuvant use and are considered off-label.
Tyrosine kinase inhibitors (TKIs), including sorafenib, lenvatinib, donafenib, regorafenib, and apatinib, are administered at recommended doses starting four weeks postoperatively. Treatment continues until hepatocellular carcinoma (HCC) recurrence, the onset of severe adverse reactions, or patient-initiated withdrawal. Typically, a treatment cycle spans three weeks, with patients in the Patient-Administered Therapy (PAT) group receiving a minimum of three treatment cycles. During the treatment period, drug interruptions or dose reductions are allowed to minimize drug-related toxicities. Adverse reactions are classified according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 developed by the National Cancer Institute.
The follow-up team comprises one general surgeon and two specialized follow-up coordinators. All patients who have undergone curative hepatic resection are required to undergo regular follow-up visits to monitor the recurrence of liver cancer, the patient’s survival status, and potential drug-related toxicities. In the first year after surgery, follow-up visits are scheduled every three months, including assessments of liver function and plasma alpha-fetoprotein (AFP) levels. Additionally, relevant imaging studies are conducted. In the second year, the follow-up frequency gradually decreases to semi-annual visits. The diagnosis of recurrence is typically based on the typical radiological manifestations of HCC and changes in relevant tumor markers.
Overall Survival (OS) refers to the time from the date of surgery to the patient’s death or the date of the last follow-up visit. Recurrence-Free Survival (RFS) is defined as the time interval between undergoing curative hepatic resection and the first diagnosis of recurrence or the date of the last follow-up visit. The date of the last follow-up was June 1, 2023.
Statistical analysis in this study was conducted using R software version 4.0.6, which is available at http://www.R-project.org. Continuous variables following a normal distribution were presented as mean ± standard deviation (SD), while non-normally distributed continuous variables were presented as median and interquartile range (IQR). To compare differences between continuous variables, we employed independent samples t-tests or Mann-Whitney U tests, depending on the specific circumstances. Categorical variables were described using numbers (n) or percentages (%), and comparisons were made using the appropriate chi-squared test or Fisher’s exact test.
To adjust for confounding factors between the two groups, we conducted 1:1 propensity score matching (PSM). Propensity scores are continuous values ranging from 0 to 1 and were generated using binary logistic regression with selected variables. We employed nearest-neighbor matching to match patients in the PD-1 group with those in the non-PD-1 group. Survival curves were constructed using the Kaplan-Meier method, and between-group comparisons were made using the log-rank test. Independent prognostic factors for RFS and OS were determined through univariate and multivariate Cox regression analyses. In the univariate Cox regression, factors with a p-value less than 0.05 were included in the multivariate Cox regression for further analysis.
After applying strict inclusion and exclusion criteria, our institution included a total of 446 patients who underwent liver resection surgery in the Department of Hepatobiliary and Pancreatic Surgery from June 2018 to March 2023. Among them, 122 patients received postoperative anti-PD-1 antibody treatment. In the PD-1 group, there were 110 males, accounting for 90.2% of the group. The majority of patients had solitary tumors (74.6%), and 104 patients (85.2%) were infected with the hepatitis B virus. Most patients had good preoperative liver function, with 97 patients (80.2%) classified as Child-Pugh class A. Baseline differences existed in several variables between the two groups, including AST, MVI, PVTT, and CSPH, while the other variables showed no statistical differences. In the group of patients who did not receive anti-PD-1 antibody treatment, 260 patients (80.2%) underwent TKI therapy. In the group receiving anti-PD-1 antibody treatment, 95 patients (77.9%) received TKI therapy.
To balance these baseline differences, a 1:1 PSM was performed on the two groups of patients. This resulted in 122 pairs of patients, with a median follow-up time of 32.6 months. Following PSM, there were no statistical differences in any variables between the two groups. Detailed data distribution can be found in (Table 1).
The results of the univariate Cox regression analysis showed that Tumor length (HR=1.284, 95% CI: 1.198-1.423), AFP (HR=1.784, 95% CI: 1.366-2.164), Differentiation (HR=1.664, 95% CI: 1.287-2.102), MVI (HR=2.172, 95% CI: 1.618-2.914), PVTT (HR=1.899, 95% CI: 1.573-2.223), and CSPH (HR=1.428, 95% CI: 1.132-1.921) were risk factors affecting patient OS. Postoperative use of anti-PD-1 antibodies (HR=0.448, 95% CI: 0.335-0.598) was a protective factor affecting patient OS. When these variables were included in a multivariate Cox regression model, the results of the multivariate Cox regression analysis still indicated that postoperative use of anti-PD-1 antibodies (HR=0.471, 95% CI: 0.367-0.613) remained a protective factor affecting patient OS (Table 2).
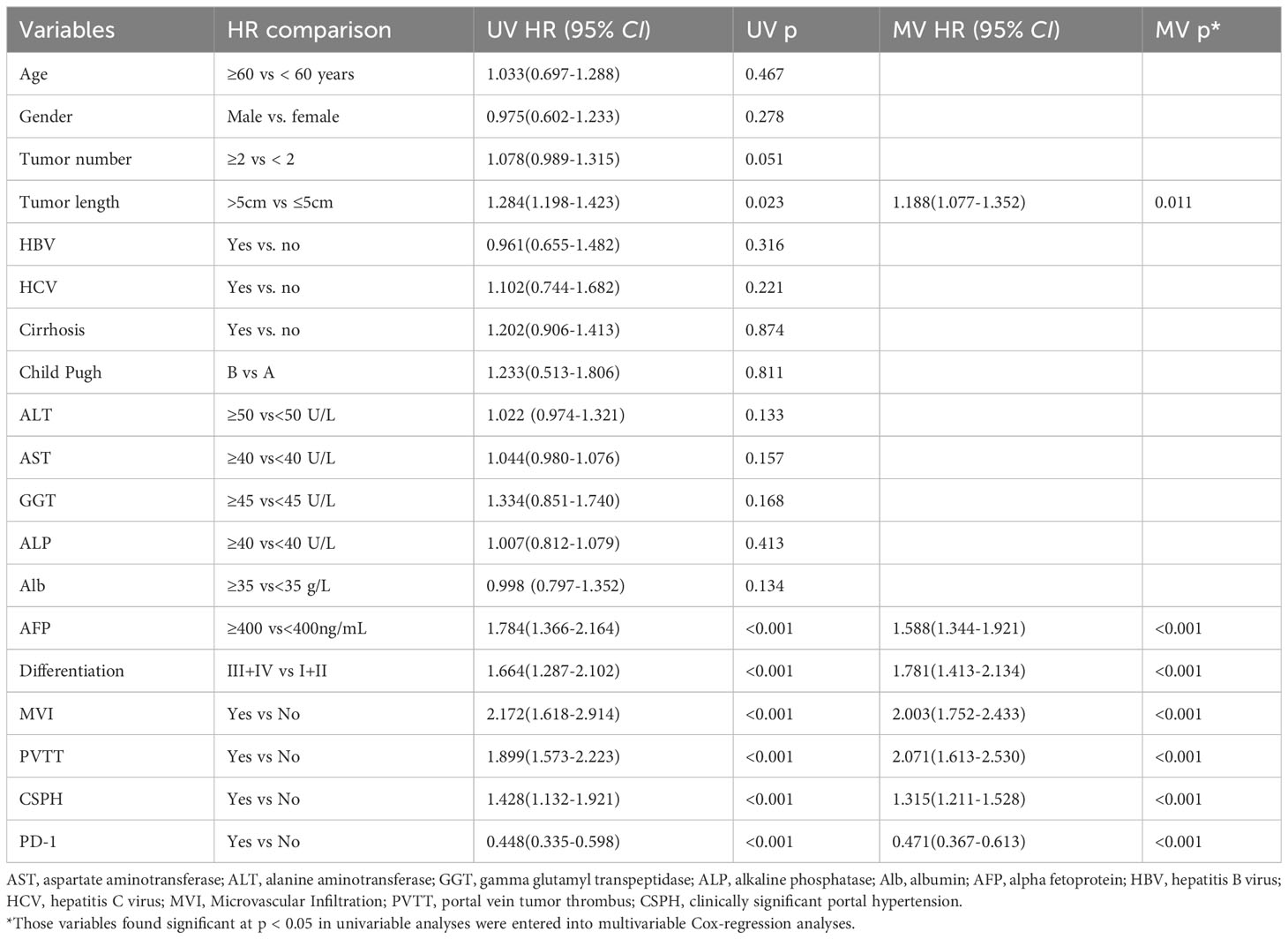
Table 2 Univariate and multivariate Cox regression analysis was used to identify independent risk factors for OS in overall patients.
The results of the univariate Cox regression analysis revealed that Tumor length (HR=1.319, 95% CI: 1.214-1.520), AFP (HR=1.578, 95% CI: 1.277-2.088), Differentiation (HR=1.563, 95% CI: 1.195-2.261), MVI (HR=2.061, 95% CI: 1.505-2.771), PVTT (HR=1.971, 95% CI: 1.711-2.199), and CSPH (HR=1.581, 95% CI: 1.261-1.986) were risk factors influencing patient RFS. Postoperative use of anti-PD-1 antibodies (HR=0.443, 95% CI: 0.322-0.598) was identified as a protective factor affecting patient RFS. When these variables were included in a multivariate Cox regression model, the results of the multivariate Cox regression analysis continued to show that postoperative use of anti-PD-1 antibodies (HR=0.503, 95% CI: 0.398-0.715) remained a protective factor influencing patient RFS (Table 3).

Table 3 Univariate and multivariate Cox regression analysis was used to identify independent risk factors for RFS in overall patients.
Before PSM, the patients in the PD-1 group had one-year, two-year, three-year, and four-year overall survival rates of 93.5%, 87.5%, 77.4%, and 48.8%, respectively. In contrast, the patients in the No-PD-1 group had one-year, two-year, three-year, and four-year overall survival rates of 84.9%, 69.9%, 43.9%, and 26.6%, respectively. There was a significant statistical difference between the two groups (P<0.001) (Figure 1A). Regarding RFS, before PSM, the PD-1 group had one-year, two-year, three-year, and four-year RFS rates of 82.5%, 77.9%, 51.0%, and 22.8%, respectively, while the No-PD-1 group had one-year, two-year, and three-year RFS rates of 69.0%, 48.5%, and 26.4%, respectively. There was a significant statistical difference between the two groups (P<0.001) (Figure 1B).
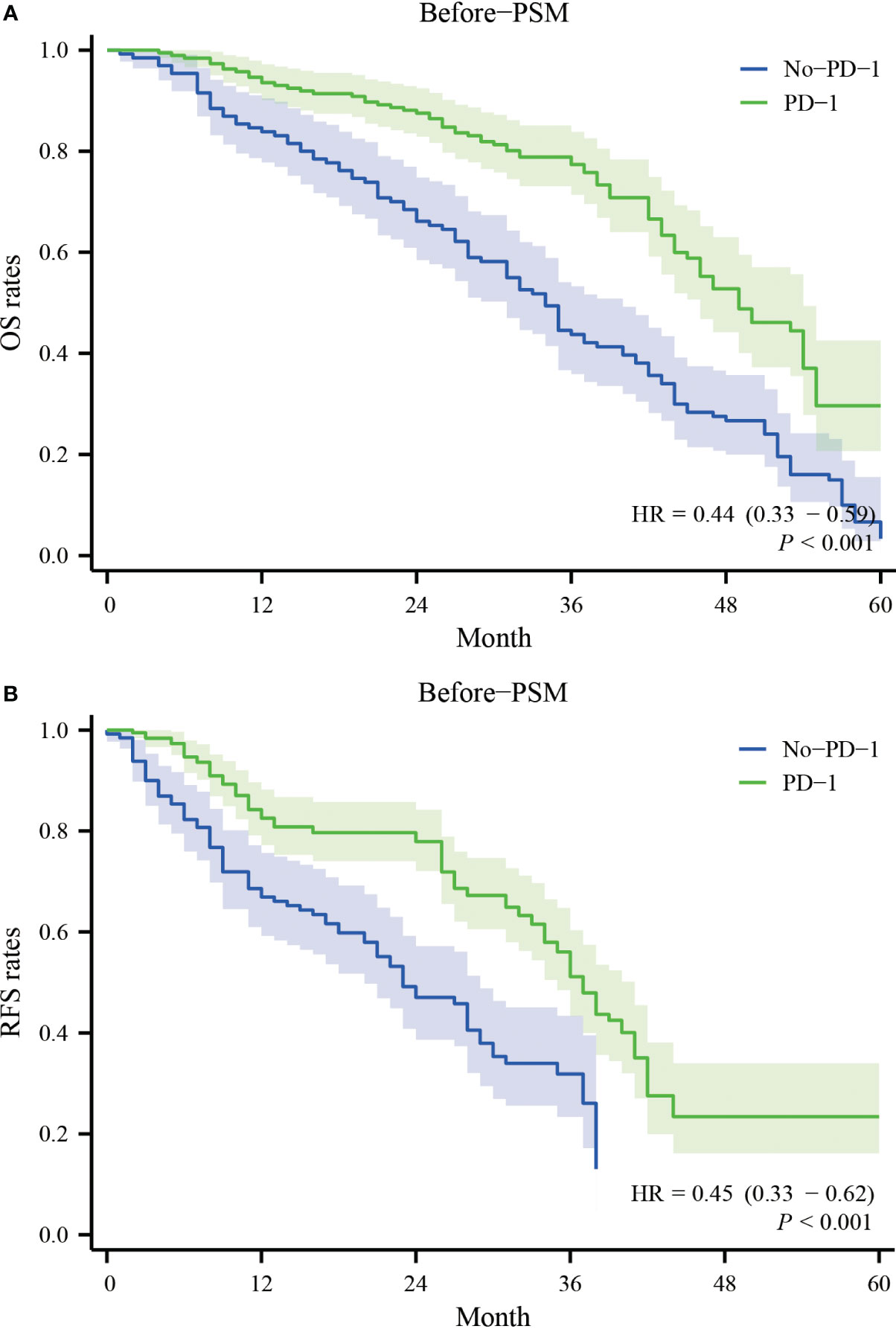
Figure 1 Survival curves for OS and RFS in the PD-1 and No-PD-1 groups before PSM. (A) represents the overall survival rate of the two groups of patients before PSM; (B) represents the recurrence-free survival rate of the two groups of patients before PSM.
After PSM, the patients in the PD-1 group had one-year, two-year, three-year, and four-year overall survival rates of 93.1%, 86.8%, 78.2%, and 51.1%, respectively. In contrast, the patients in the No-PD-1 group had one-year, two-year, three-year, and four-year overall survival rates of 85.3%, 70.2%, 47.7%, and 30.0%, respectively. There was a significant statistical difference between the two groups (P<0.001) (Figure 2A). Regarding RFS, after PSM, the PD-1 group had one-year, two-year, three-year, and four-year RFS rates of 81.7%, 77.0%, 52.3%, and 23.1%, respectively, while the No-PD-1 group had one-year, two-year, and three-year RFS rates of 68.4%, 47.7%, and 25.8%, respectively. There was a significant statistical difference between the two groups (P<0.001) (Figure 2B).

Figure 2 Survival curves for OS and RFS in the PD-1 and No-PD-1 groups after PSM. (A) represents the overall survival rate of the two groups of patients after PSM; (B) represents the recurrence-free survival rate of the two groups of patients after PSM.
An analysis of the causes of death for all deceased patients revealed no statistically significant differences in the proportions of different causes of death between the PD-1 group and the No-PD-1 group. The majority of patient deaths were attributed to the tumor itself, such as postoperative metastasis or recurrence (Supplementary Table 1).
Based on the presence or absence of high-risk factors, two groups were formed: the high-risk factor group and the non-high-risk factor group. In the high-risk factor group, for overall survival, the PD-1 group had one-year, two-year, three-year, and four-year overall survival rates of 100.0%, 83.2%, 61.3%, and 52.7%, respectively, while the No-PD-1 group had one-year, two-year, three-year, and four-year overall survival rates of 78.2%, 43.0%, 38.8%, and 18.9%, respectively. There was a significant statistical difference between the two groups (P<0.001) (Figure 3A). For recurrence-free survival, in the high-risk factor group, the PD-1 group had one-year, two-year, three-year, and four-year recurrence-free survival rates of 88.1%, 78.6%, 50.3%, and 36.1%, respectively, while the No-PD-1 group had one-year, two-year, three-year, and four-year recurrence-free survival rates of 51.7%, 36.9%, 23.6%, and 23.6%, respectively. Again, there was a significant statistical difference between the two groups (P<0.001) (Figure 3B). In the non-high-risk factor group, for overall survival, the PD-1 group had one-year, two-year, three-year, and four-year overall survival rates of 93.2%, 86.9%, 80.8%, and 54.9%, respectively, while the No-PD-1 group had one-year, two-year, three-year, and four-year overall survival rates of 100.0%, 100.0%, 52.2%, and 40.1%, respectively. There was no statistically significant difference between the two groups (P=0.052) (Figure 3C). For recurrence-free survival, in the non-high-risk factor group, the PD-1 group had one-year, two-year, three-year, and four-year recurrence-free survival rates of 84.2%, 77.8%, 50.7%, and 35.5%, respectively, while the No-PD-1 group had one-year, two-year, three-year recurrence-free survival rates of 92.3%, 57.7%, and 38.0%, respectively. Similarly, there was no statistically significant difference between the two groups (P=0.060) (Figure 3D).
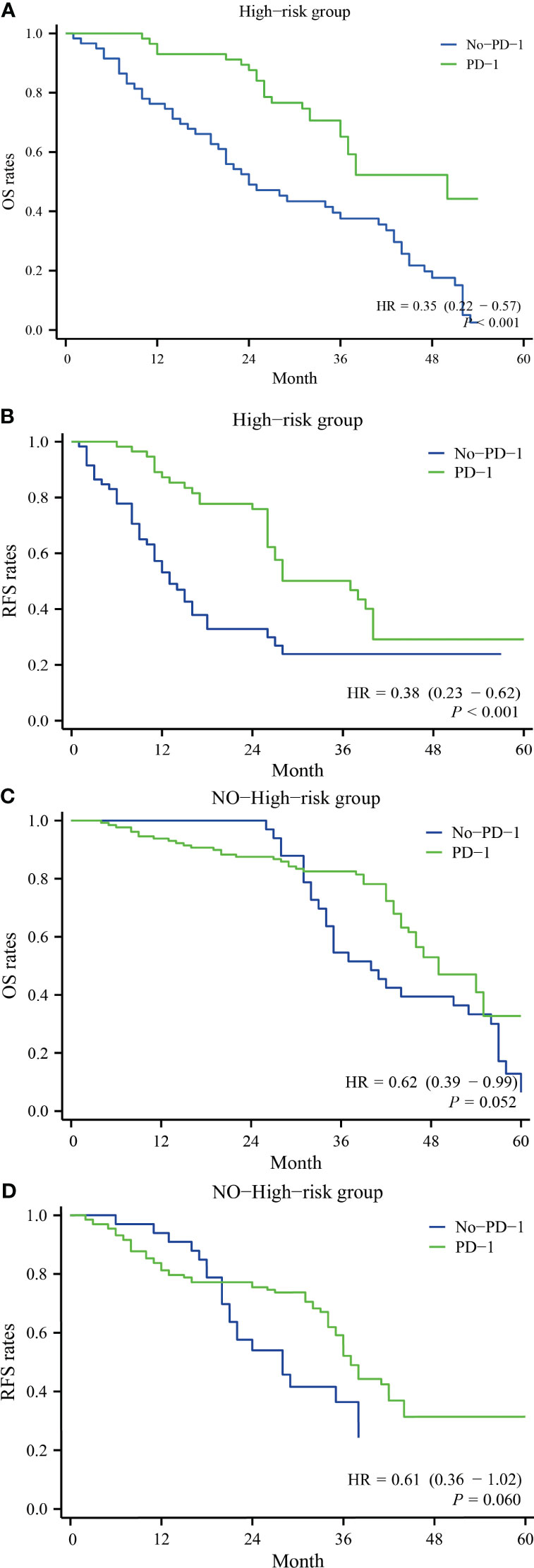
Figure 3 Comparison of survival with and without postoperative anti-PD-1 antibody use in subgroup analyses in the high recurrence risk group or in the non-high recurrence risk group. (A) represents the overall survival rate of patients with a high-risk factor in both groups, (B) represents the recurrence-free survival rate of patients with a high-risk factor in both groups; (C) represents the overall survival rate of patients without a high-risk factor in both groups, and (D) represents the recurrence-free survival rate of patients without a high-risk factor in both groups.
All postoperative 122 patients with anti-PD-1 antibodies had a very small number of grade 3-4 AEs, only the postoperative patients with grade 3-4 ALT/AST elevations were relatively higher at 14.8%, the rest had relatively few low-grade hyponatremia, and no patients had Rash, Pruritus, Decreased appetite. Pneumonia, Decreased weight, Nausea/vomiting (Table 4). After comparing postoperative complications between the PD-1 group and the No-PD-1 group, it was observed that the proportion of patients with elevated ALT/AST levels was higher in the PD-1 group. Additionally, a higher proportion of patients in the PD-1 group experienced a decrease in neutrophil count. There were no statistically significant differences in other complications (Supplementary Table 2).
Currently, there is no standardized approach for adjuvant therapy following curative resection in HCC patients (17). Previous studies have suggested that the efficacy of postoperative adjuvant treatments may be limited. A global multicenter RCT in 2016 demonstrated that sorafenib, for example, did not effectively improve the prognosis of HCC patients after surgery (18). However, the recent performance of anti-PD-1 antibody inhibitors has shown promising results. These inhibitors have the ability to enhance the patient’s immune system and restore its ability to eliminate tumor cells. Given their excellent performance in multiple tumor types, investigating their role in HCC patients after surgery, particularly in those with high-risk recurrence factors, is crucial. The high recurrence rate of HCC remains a significant factor affecting postoperative survival (19, 20). Therefore, it is essential to provide appropriate postoperative adjuvant therapy to improve the survival of high-risk recurrence patients.
In previous studies, anti-PD-1 antibodies have shown promising efficacy in the treatment and downstaging of unresectable HCC patients. Researchers such as Zhu et al (21). demonstrated that a combination therapy of TKI (Tyrosine Kinase Inhibitor) and anti-PD-1 antibodies resulted in 10 out of 63 unresectable HCC patients (15.9%) achieving downstaging and undergoing R0 resection. Simultaneously, Xin et al (22). provided evidence that for unresectable patients, a combination of lenvatinib with PD-1 inhibitors, along with TACE (Transarterial Chemoembolization) triple therapy, could lead to favorable survival outcomes with a lower proportion of severe complications post-treatment, ensuring manageable safety. However, there is currently limited research on adjuvant therapy for HCC post-surgery. In our study, both before and after 1:1 PSM, the prognosis of HCC patients who received postoperative anti-PD-1 antibody treatment was significantly better than those who did not. This conclusion aligns with the findings of Chen and colleagues (14), who discovered that postoperative use of anti-PD-1 antibodies could effectively improve the one-year and two-year overall survival and recurrence-free survival rates of HCC patients at high risk of recurrence. Our study, with a larger sample size, also demonstrated improved three-year overall survival and recurrence-free survival rates in patients who received postoperative anti-PD-1 antibodies. Additionally, Li et al (23). showed that postoperative combination therapy of anti-PD-1 antibodies with TKI could effectively enhance the prognosis of HCC patients with high-risk recurrence factors, and overall, it was found to be a safe treatment approach without severe Grade 4/5 toxicities or adverse events. Therefore, in summary, the postoperative use of anti-PD-1 antibodies in HCC patients can effectively improve their prognosis (24, 25). Furthermore, the results of the multivariable Cox regression analysis suggest that the use of anti-PD-1 antibodies is a protective factor for both OS and RFS in patients.
Subsequently, we stratified the patients into high-risk and low-risk groups, and the results clearly demonstrate that in the high-risk group, anti-PD-1 antibodies can significantly improve both OS (Overall Survival) and RFS (Recurrence-Free Survival) with HRs (Hazard Ratios) of 0.35 and 0.38, respectively. The high-risk group had a notably high postoperative recurrence rate, which may be associated with the potential microscopic dissemination and multicentric development that is often not visible before surgery. The use of immune checkpoint inhibitors (ICI) as adjuvant therapy after surgery can help eliminate these disseminated lesions as much as possible. Additionally, the high-risk group included patients with PVTT (Portal Vein Tumor Thrombosis). In countries like China and other Asian regions, a significant number of cancer patients are diagnosed at an advanced stage, and surgical resection remains a common treatment approach. Therefore, current guidelines in these regions suggest that a relatively aggressive approach, including resection or removal of the tumor thrombus, can be considered during treatment (16). Previous research from Asian centers has also indicated that even in the presence of PVTT, surgical treatment can achieve favorable outcomes. To reflect real-world effectiveness, we included PVTT patients who underwent surgery in our study. It’s worth noting that differences in guidelines between Western and Asian countries may impact the generalizability of the results. Chen and colleagues conducted a separate analysis of the PVTT group and found that the use of ICI resulted in a remarkable HR of 0.15 (14), indicating a highly significant effect. However, in the non-high-risk group, the effect of postoperative ICI was not significant, with p-values around 0.050. This may be related to the relatively smaller sample size. In conclusion, we believe that postoperative anti-PD-1 antibodies hold promise as a therapy for HCC patients at high risk of recurrence following liver resection.
Anti-PD-1 antibody therapy symbolizes the forefront of newer research in HCC. Currently, due to the tumor heterogeneity of HCC, different combination therapies are targeted for different types of HCC. A meta-analysis by Li et al (26). indicates that external beam radiotherapy combined with sorafenib shows improved efficacy in the treatment of unresectable hepatocellular carcinoma (HCC). This combined therapy may guide future selections of sorafenib and local treatment. In a multicenter study conducted by Su et al (27)., the effectiveness of external beam radiotherapy (EBRT) versus transcatheter arterial chemoembolization (TACE) for HCC with a tumor diameter ≥ 5 cm was evaluated. They suggest that EBRT, as the primary local treatment for HCC with a diameter ≥ 5 cm, is more effective than TACE. Li et al (28). propose that the combination of PD-1 inhibitors with TACE has a significant impact on HCC. In summary, the treatment landscape for HCC is continually evolving, with combination therapies becoming a standard approach that can further extend the survival period of patients. At the same time, certain biomarkers can effectively predict patient prognosis, such as ALP, ALBI, and ALR scores, among others. They can further assist researchers in assessing prognosis (29–31). Therefore, there will be a greater focus on immunotherapy combinations and response markers in the future (32–37).
As per our knowledge, this study represents the largest sample size and longest follow-up duration in a single-center study of postoperative adjuvant therapy with anti-PD-1 antibodies. It further substantiates that HCC patients with high-risk recurrence factors after surgery can significantly improve their prognosis through immunotherapeutic interventions. Notably, there was a substantial improvement in OS and RFS at one and two years postoperatively. Furthermore, the adverse events associated with this adjuvant therapy were relatively mild, with very few patients experiencing grade 3-4 Adverse Events (AEs), and there were no deaths attributed to post-treatment AEs. However, it is essential to pay attention to patients who undergo certain combined treatments postoperatively, such as postoperative TACE or postoperative TKI therapy. This subgroup of patients may be more prone to experiencing grade 3-4 AEs. Therefore, close monitoring is required during postoperative adjuvant therapy, particularly in the context of combination treatments (38, 39).
This study has several limitations that should be acknowledged. Firstly, it is an observational single-center study, and the PSM cohort is not equivalent to an RCT cohort. The conclusions of this study need further confirmation through future randomized controlled trials. Additionally, the postoperative use of anti-PD-1 antibodies in this study comes from multiple companies, and there may be differences in treatment efficacy between them. Secondly, differences in patient selection criteria between Western and Eastern countries for those undergoing liver resection may impact the generalizability of our results. Lastly, given the higher proportion of hepatitis B virus infection among Asian HCC patients, postoperative antiviral therapy can influence patient outcomes. Therefore, further studies involving a larger number of patients with longer follow-up periods are needed to validate our findings.
The use of anti-PD-1 antibodies after hepatocellular carcinoma surgery is an important new and effective intervention that significantly improves short- and long-term survival outcomes in HCC patients with high recurrence factors after hepatic resection, while the therapy is safe and reliable.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of Zhongshan People Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
W-QZ: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. QZ: Conceptualization, Data curation, Project administration, Writing – original draft. LT: Software, Supervision, Writing – review & editing. Z-FG: Investigation, Methodology, Project administration, Writing – review & editing. FT: Investigation, Methodology, Project administration, Writing – review & editing. H-TT: Data curation, Investigation, Project administration, Writing – original draft. KH: Formal Analysis, Methodology, Project administration, Software, Writing – review & editing. W-QC: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Thanks to the nurses in the department for their help with the project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1289916/full#supplementary-material
1. Ayuso C, Rimola J, Vilana R, Burrel M, Darnell A, García-Criado Á, et al. Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol (2018) 101:72–81. doi: 10.1016/j.ejrad.2018.01.025
2. Xing R, Gao J, Cui Q, Wang Q. Strategies to improve the antitumor effect of immunotherapy for hepatocellular carcinoma. Front Immunol (2021) 12:783236. doi: 10.3389/fimmu.2021.783236
3. Allaire M, Goumard C, Lim C, Cleach Le A, Wagner M, Scatton O. New frontiers in liver resection for hepatocellular carcinoma. JHEP Rep Innovation Hepatol (2020) 2:100134. doi: 10.1016/j.jhepr.2020.100134
4. Gilles H, Garbutt T, Landrum J. Hepatocellular carcinoma. Crit Care Nurs Clinics North America (2022) 34:289–301. doi: 10.1016/j.cnc.2022.04.004
5. Kim E, Viatour P. Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med (2020) 52:1898–907. doi: 10.1038/s12276-020-00527-1
6. Nevola R, Ruocco R, Criscuolo L, Villani A, Alfano M, Beccia D, et al. Predictors of early and late hepatocellular carcinoma recurrence. World J Gastroenterol (2023) 29:1243–60. doi: 10.3748/wjg.v29.i8.1243
7. de Castria TB, Khalil DN, Harding JJ, EM, Abou-Alfa GK. Tremelimumab and durvalumab in the treatment of unresectable, advanced hepatocellular carcinoma. Future Oncol (London England) (2022) 18:3769–82. doi: 10.2217/fon-2022-0652
8. Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol (2023) 79:506–15. doi: 10.1016/j.jhep.2023.03.003
9. Sun L, Xu X, Meng F, Liu Q, Wang H, Li X, et al. Lenvatinib plus transarterial chemoembolization with or without immune checkpoint inhibitors for unresectable hepatocellular carcinoma: A review. Front Oncol (2022) 12:980214. doi: 10.3389/fonc.2022.980214
10. Liu X, Lu Y, Qin S. Atezolizumab and bevacizumab for hepatocellular carcinoma: mechanism, pharmacokinetics and future treatment strategies. Future Oncol (London England) (2021) 17:2243–56. doi: 10.2217/fon-2020-1290
11. Hack SP, Spahn J, Chen M, Cheng AL, Kaseb A, Kudo M, et al. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol (London England) (2020) 16:975–89. doi: 10.2217/fon-2020-0162
12. Qi W, Peng W, Qi X, Qiu Z, Wen T, Li C. TIDE: adjuvant tislelizumab plus donafenib combined with transarterial chemoembolization for high-risk hepatocellular carcinoma after surgery: protocol for a prospective, single-arm, phase II trial. Front Oncol (2023) 13:1138570. doi: 10.3389/fonc.2023.1138570
13. Zhang W, Zhang B, Chen XP. Adjuvant treatment strategy after curative resection for hepatocellular carcinoma. Front Med (2021) 15:155–69. doi: 10.1007/s11684-021-0848-3
14. Chen W, Hu S, Liu Z, Sun Y, Wu J, Shen S, et al. Adjuvant anti-PD-1 antibody for hepatocellular carcinoma with high recurrence risks after hepatectomy. Hepatol Int (2023) 17:406–16. doi: 10.1007/s12072-022-10478-6
15. Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res (2020) 10:727–42.
16. Sun J, Guo R, Bi X, Wu M, Tang Z, Lau WY, et al. Guidelines for diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus in China (2021 edition). Liver Cancer (2022) 11:315–28. doi: 10.1159/000523997
17. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatol (Baltimore Md) (2018) 68:723–50. doi: 10.1002/hep.29913
18. Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol (2015) 16:1344–54. doi: 10.1016/S1470-2045(15)00198-9
19. Rajendran L, Ivanics T, Claasen MP, Muaddi H, Sapisochin G. The management of post-transplantation recurrence of hepatocellular carcinoma. Clin Mol Hepatol (2022) 28:1–16. doi: 10.3350/cmh.2021.0217
20. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg (2015) 261:947–55. doi: 10.1097/SLA.0000000000000710
21. Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Sun Qu XD, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer (2021) 10:320–9. doi: 10.1159/000514313
22. Xin Y, Zhang X, Liu N, Peng G, Huang X, Cao X, et al. Efficacy and safety of lenvatinib plus PD-1 inhibitor with or without transarterial chemoembolization in unresectable hepatocellular carcinoma. Hepatol Int (2023) 17:753–64. doi: 10.1007/s12072-023-10502-3
23. Li J, Wang WQ, Zhu RH, Lv X, Wang JL, Liang BY, et al. Postoperative adjuvant tyrosine kinase inhibitors combined with anti-PD-1 antibodies improves surgical outcomes for hepatocellular carcinoma with high-risk recurrent factors. Front Immunol (2023) 14:1202039. doi: 10.3389/fimmu.2023.1202039
24. Li ZX, Zhang QF, Huang JM, Huang SJ, Liang HB, Chen H, et al. Safety and efficacy of postoperative adjuvant therapy with atezolizumab and bevacizumab after radical resection of hepatocellular carcinoma. Clinics Res Hepatol Gastroenterol (2023) 47:102165. doi: 10.1016/j.clinre.2023.102165
25. Yang J, Jiang S, Chen Y, Zhang J, Deng Y. Adjuvant ICIs plus targeted therapies reduce HCC recurrence after hepatectomy in patients with high risk of recurrence. Curr Oncol (Toronto Ont) (2023) 30:1708–19. doi: 10.3390/curroncol30020132
26. Li H, Wu Z, Chen J, Su K, Guo L, Xu K, et al. External radiotherapy combined with sorafenib has better efficacy in unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Exp Med (2023) 23:1537–49. doi: 10.1007/s10238-022-00972-4
27. Su K, Wang F, Li X, Chi H, Zhang J, He K, et al. Effect of external beam radiation therapy versus transcatheter arterial chemoembolization for non-diffuse hepatocellular carcinoma (≥ 5 cm): a multicenter experience over a ten-year period. Front Immunol (2023) 14:1265959. doi: 10.3389/fimmu.2023.1265959
28. Li H, Su K, Guo L, Jiang Y, Xu K, Gu T, et al. PD-1 inhibitors combined with antiangiogenic therapy with or without transarterial chemoembolization in the treatment of hepatocellular carcinoma: A propensity matching analysis. J Hepatocellular Carcinoma (2023) 10:1257–66. doi: 10.2147/JHC.S415843
29. Li H, Guo L, Su K, Li C, Jiang Y, Wang P, et al. Construction and validation of TACE therapeutic efficacy by ALR score and nomogram: A large, multicenter study. J Hepatocellular Carcinoma (2023) 10:1009–17. doi: 10.2147/JHC.S414926
30. Su K, Huang W, Li X, Xu K, Gu T, Liu Y, et al. Evaluation of lactate dehydrogenase and alkaline phosphatase as predictive biomarkers in the prognosis of hepatocellular carcinoma and development of a new nomogram. J hepatocellular carcinoma (2023) 10:69–79. doi: 10.2147/JHC.S398632
31. Su K, Shen Q, Tong J, Gu T, Xu K, Li H, et al. Construction and validation of a nomogram for HBV-related hepatocellular carcinoma: A large, multicenter study. Ann Hepatol (2023) 28:101109. doi: 10.1016/j.aohep.2023.101109
32. Appleman LJ. Multifactorial, biomarker-based predictive models for immunotherapy response enter the arena. J Natl Cancer Institute (2021) 113:7–8. doi: 10.1093/jnci/djaa077
33. Lemaire V, Shemesh CS, Rotte A. Pharmacology-based ranking of anti-cancer drugs to guide clinical development of cancer immunotherapy combinations. J Exp Clin Cancer Res CR (2021) 40:311. doi: 10.1186/s13046-021-02111-5
34. Looi CK, Chung FF, Leong CO, Wong SF, Rosli R, Mai CW. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J Exp Clin Cancer Res CR (2019) 38:162. doi: 10.1186/s13046-019-1153-8
35. Luo XY, Wu KM, He XX. Advances in drug development for hepatocellular carcinoma: clinical trials and potential therapeutic targets. J Exp Clin Cancer Res CR (2021) 40:172. doi: 10.1186/s13046-021-01968-w
36. Rotte A. Predictive models for response and survival in patients treated with anti-PD-1 monotherapy or with anti-PD-1 and ipilimumab combination: editorial commentary. Ann Trans Med (2023) 11:227. doi: 10.21037/atm-22-6564
37. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer (2022) 21:28. doi: 10.1186/s12943-021-01489-2
38. Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ (Clinical Res ed) (2018) 360:k793. doi: 10.1136/bmj.k793
Keywords: HCC, high-risk recurrence factors, anti-PD-1 antibodies, postoperative adjuvant therapy, biomark
Citation: Zhang W-Q, Zhang Q, Tan L, Guan Z-F, Tian F, Tang H-T, He K and Chen W-Q (2023) Postoperative adjuvant immunotherapy for high-risk hepatocellular carcinoma patients. Front. Oncol. 13:1289916. doi: 10.3389/fonc.2023.1289916
Received: 06 September 2023; Accepted: 29 November 2023;
Published: 15 December 2023.
Edited by:
Alberto Brolese, Department of General Surgery and HPB Unit - APSS, ItalyReviewed by:
Yunwei Han, The Affiliated Hospital of Southwest Medical University, ChinaCopyright © 2023 Zhang, Zhang, Tan, Guan, Tian, Tang, He and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Qiang Chen, Y3dxMjAxMzhAYWxpeXVuLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.