
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 13 December 2023
Sec. Gynecological Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1289379
 Miriam R. Brezis1
Miriam R. Brezis1 Eliya Shachar1,2
Eliya Shachar1,2 Shira Peleg Hasson1,2
Shira Peleg Hasson1,2 Ido Laskov1,2
Ido Laskov1,2 Nadav Michaan1,2
Nadav Michaan1,2 Bar Levy1
Bar Levy1 Ido Wolf1,2
Ido Wolf1,2 Tamar Safra1,2*
Tamar Safra1,2*Objective: To evaluate the effectiveness and safety of standard chemotherapy administered to patients >70 years with advanced ovarian cancer (OC).
Methods: Medical records of 956 advanced-stage patients with OC treated between 2002-2020 with standard surgery and paclitaxel-carboplatin chemotherapy in a three-weekly (PC-3W) or weekly (PC-1W) regimen were reviewed. Treatment response and tolerability were compared between patients ≤70 years (N=723) and >70 years (N=233) with stratification to septuagenarians (>70-80 years) and octogenarians (>80 years).
Results: Median overall survival (mOS) in patients >70 was 41.26 months (95% confidence interval [Cl], 37.22-45.14) and median progression-free survival (mPFS) was 11.04 months (95% Cl, 8.97-15.74). No statistically significant differences in mPFS and mOS were observed between septuagenarians and octogenarians. Patients >70 treated with PC-1W versus PC-3W had significantly longer mOS (57.17 versus 30.00 months) and mPFS (19.09 versus 8.15 months). Toxicity rates were mostly similar between younger and older patients. Among patients >70 treated with PC-1W, the rate of neutropenia (75.7% versus 51.8%, p=0.0005), thrombocytopenia (41.0% versus 22.2%, p=0.0042) and anemia (78.1% versus 51.9%, p<0.0001) were significantly higher and the rate of grade 2 alopecia was statistically significantly lower compared with those >70 treated with PC-3W. Significantly more patients treated with PC-1W completed ≥6 chemotherapy cycles, suggesting better tolerability of this regimen.
Conclusions: Older patients with OC may benefit from improved OS with reasonable toxicity if treated with standard chemotherapy. Older patients treated with PC-1W are more likely to complete the full chemotherapy course and survive longer compared with those treated with conventional PC-3W.
About 50% of ovarian cancer cases are diagnosed in women >65 (1). Standard treatment of advanced ovarian cancer includes comprehensive cytoreductive surgery and six cycles of combination therapy with a platinum and taxane with, or without, maintenance bevacizumab and a poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitor. Neoadjuvant chemotherapy with interval debulking surgery is considered in patients with advanced-stage disease who are not good candidates for upfront primary debulking surgery (2). However, less than two-fifths of older women with advanced ovarian cancer received this modified treatment, usually due to poorer performance status or comorbidities (3, 4).
The outcomes of ovarian cancer worsen with age (5, 6). Cumulative analyses from 6 Gynecologic Oncology Group trials estimated that an interval increase of 10 years in age increases the risk for disease progression by 6% and for death by 12% (7). The EUROCARE study showed that relative survival among European women with ovarian cancer was much higher in middle-aged women than in older ones (8).
Several factors make the older population prone to a poorer prognosis, including delayed diagnosis, comorbidities, resistance to chemotherapy, poorer physical and cognitive performance, polypharmacy, depression and frailty (9, 10). Women >65 with advanced ovarian cancer are more likely to have incomplete surgery and more residual disease. These elements were prognostic factors for survival regardless of age (11). In the past, some clinical trials excluded older women, thus suboptimal oncological treatments provided to older patients were based on outcome data from a younger subsection (12).
In a previous analysis we have shown that weekly scheduling of paclitaxel 80 mg/m2 and carboplatin area under the curve (AUC) 2, administered on days 1, 8, and 15 in a 28-day cycle (PC-1W) for first-line therapy for advanced ovarian cancer, is as active and better tolerated than the standard regimen of carboplatin and paclitaxel (175 mg/m2) every 3 weeks (PC-3W) (13). To challenge the conventional assumption that older patients with advanced ovarian cancer do not tolerate standard therapy, we retrospectively analyzed and compared the effectiveness and tolerability of both regimens (PC-1W and PC-3W) in patients >70 years with advanced ovarian cancer.
We retrospectively reviewed all medical records of consecutive patients with a diagnosis of advanced (stage III/IV) ovarian cancer, tubal carcinoma, or primary peritoneal carcinomatosis treated at our institution between January 2002 and December 2020. The study was approved by the institutional ethics committee (approval number 07-160). The requirement for informed consent was waived.
Collected data included demographics, background disease, BRCA status, mutation type, clinical staging, pathological data, surgical parameters, first treatment line, adverse events during treatment, tumor markers (serum cancer antigen [CA]-125) and survival data. Women with substantial missing treatment outcome data were excluded from the analysis.
Patients underwent primary debulking surgery followed by 6 cycles of carboplatin and paclitaxel, or were treated with 3-4 courses of neoadjuvant chemotherapy followed by interval debulking surgery and complementary 2-3 courses post-surgery. Most patients received 6 chemotherapy cycles. Patients with massive disease received >6 cycles.
Chemotherapy regimens included: conventional doublet paclitaxel 175 mg/m2 and carboplatin (AUC 6) every 21 days (PC-3W), or a modified weekly regimen (PC-1W) consisting of paclitaxel 80 mg/m2 and carboplatin (AUC 2) administered on days 1, 8, and 15 of a 28-day cycle, or single-agent carboplatin (AUC 6) administered every 21 days.
Treatment protocols remained mostly unchanged for the past 20 years, except for the addition of bevacizumab in 2013 and PARP inhibitors in 2017, following their authorization in Israel.
Treatment regimens were often chosen according to the patient’s performance status. Patients with poorer performance status, older women or women with extremely advanced disease and severe ascites, or those with severe comorbidities were treated with PC-1W. This regimen was also offered to those who preferred having better chances for grade 1 alopecia compared to grade 2 (i.e., complete hair loss). Some patients were treated with carboplatin monotherapy and others refused to receive any chemotherapy.
Treatment effectiveness was evaluated by computed tomography every 3 chemotherapy cycles. Complete blood count was performed before each treatment. CA-125 levels were assessed once every cycle. A confirmed increase in serum CA-125 levels to more than double the upper limit of normal values (35 U/dL) was considered as evidence for disease progression in patients with no corresponding imaging assessment.
Overall survival (OS) was calculated from the diagnosis date to either death or to the last known follow-up. Progression-free survival (PFS) was calculated from the end of the first platinum-based combination to either progression/recurrence, or death, or to the last known follow-up. Platinum sensitivity was defined as recurrence of disease, after more than 6 months from the end of the first platinum-based treatment.
Toxicity was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 2. At each follow-up visit (for the duration of patient follow-up) the patient was also asked if she had long-lasting (>1 year) peripheral sensory neuropathy.
Outcomes were analyzed by patient age: ≤70 years, >70 years, septuagenarians (>70-80), and octogenarians (>80 years). In addition, the outcomes were analyzed by chemotherapy regimen (PC-1W and PC-3W).
Continuous variables were summarized as median and range and compared by independent Student’s t-test. Categorical variables were summarized as number and percentage and compared by Fisher’s exact test or chi-squared test. Survival functions were demonstrated using the Kaplan-Meier method, and the effect of each subgroup was assessed using the Log-Rank test.
The Cox proportional hazards model was used for comparing the risk of death and the risk of a composite endpoint of death or recurrence between the 2 treatment regimens, adjusting for age, stage of disease, histology, BRCA status (carriers versus noncarriers), and debulking status. Results are presented as hazard ratio (HR) and 95% confidence interval (CI). All analyses were 2-tailed, and a p value of <0.05 was considered significant. Statistical analyses were performed using R software, version 4.05 (R Development Core Team, Vienna, Austria).
In total, 956 women with stage III/IV ovarian cancer were diagnosed and treated at our institution during the study period. They comprised 87.35% of all patients; the remaining 12.65% had stage I/II disease. As shown in Table 1, most patients (723, 75.62%) were >70 at diagnosis. About two-thirds had serous papillary histology (69.87% in patients ≤70 and 67.29% in patients >70; p=0.5893). Optimal cytoreduction (R0) was performed in 65.54% of patients ≤70 versus 51.76% of patients >70 (p=0.0016). Suboptimal cytoreduction (R1 and R2) was significantly more common in older patients. Approximately 70% of patients were tested for BRCA mutations; BRCA mutations were less common in patients >70. Older patients had significantly higher rates of hypertension and hypercholesterolemia than those ≤70 (p<0.0001).
Neoadjuvant chemotherapy was given to 51.56% and 63.68% of patients ≤70 and >70 patients, respectively (p=0.0029). Most patients were treated with ≥6 cycles (90.61% of patients ≤70 patients and 83.25% of patients >70, p=0.0316).
The standard PC-3W regimen was given to 60.1% and 45.2% of patients ≤70 and >70, respectively, (p<0.0001). The PC-1W regimen was given to 38.89% and 42.46% of patients ≤70 and >70, respectively (p<0.0001). Carboplatin alone was administered to 0.66% and 8.21% of patients ≤70 and >70, respectively (p<0.0001).
Bevacizumab as first-line therapy and maintenance treatment was administered to 30.55% of patients ≤70 and 22.94% of patients >70, (p=0.0848). PARP inhibitors were administered as first-line therapy to 4.5% of patients ≤70 patients and 4.59% of patients >70. After recurrence PARP inhibitors were administered to 11.41% of patients ≤70 and 2.3% of patients >70 (p=0.0003).
Median PFS, OS, and OS after recurrence were all statistically significantly longer in patients ≤70 compared to those >70 (Table 2 and Figure 1).
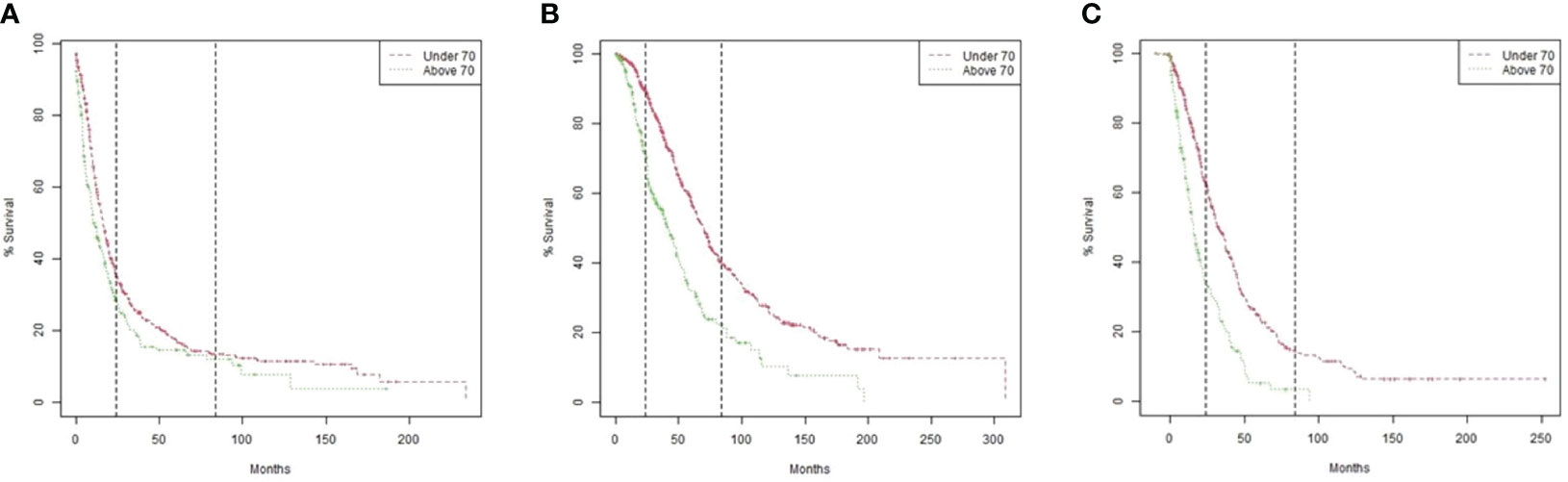
Figure 1 Kaplan Meier Survival models by age group (>70 versus ≤70). (A) Overall survival. P value by Log-rank test <0.0001. (B) Progression-free survival. P value by Log-rank test 0.0041. (C) Overall survival after recurrence according to the time interval from initiation of treatment after the first recurrence to death. P value by Log-rank test <0.0001.
The effectiveness and tolerability of the PC-1W and PC-3W regimens were compared in patients >70. Age, stage at diagnosis, histological type, BRCA status and optimal cytoreduction were similar in both treatment groups (Table 3). A higher rate of patients treated with PC-3W versus PC-1W received 1-5 courses (17.93% versus 6.16%, p=0.0103), mainly due to toxicity/intolerance to chemotherapy. Additionally, a lower rate of patients treated with PC-1W versus PC-3W received ≥7 cycles (16.04% versus 29.9%, p=0.0103), mainly due to advanced disease, stage IV or ascites.
Patients treated with PC-1W versus PC-3W showed statistically significant longer median PFS (19.09 months [95% CI 14.55-23.85] versus 8.15 months [95% CI 5.85-10.05], p=0.001) and OS (57.17 months [95% CI 47.74-69.22] versus 30.00 months [95% CI 25.00-45.14], p=0.0092), (Table 4). A higher rate of patients treated with PC-1W had platinum-sensitive disease compared to those treated with PC-3W (77.78 versus 56.70%, p=0.0028).
Stage at diagnosis, histological subtype, induction, optimal cytoreduction, BRCA status, and platinum sensitivity were similar in septuagenarians (n=187) versus octogenarians (n=46), (Table 5).
PC-1W and single-agent carboplatin were more frequently administered to octogenarians compared to septuagenarians (p<0.0001). A higher percentage of octogenarians received 1-5 courses compared to septuagenarians (p<0.0001).
A trend for statistically significant longer median OS was observed in septuagenarians versus octogenarians (47.74 months [95% CI, 38.77-55.29] versus 37.22 months [95% CI, 24.61-65.35], p=0.0792). No statistically significant difference in median PFS was observed between the two age groups (12.45 months [95% CI, 9.82-17.91] versus 9.13 months [95% CI, 5.32-20.30], p=0.3746).
To evaluate the safety of chemotherapy protocols in older patients, we compared toxicities among all age groups (≤70, >70-80, >80). Only the rates of asthenia were statistically significantly different among the groups (Table 6).
Comparison of toxicities between patients treated with PC-1W and PC-3W (Table 7) showed a statistically significant higher rate of neutropenia (75.7% versus 51.8%, p=0.0005), thrombocytopenia (41.0% versus 22.2%, p=0.0042) and anemia (78.1% versus 51.9%, p<0.0001) in patients treated with PC-1W versus PC-3W. The rates of all-grade alopecia were similar between the 2 regimens, but the rate of grade 2 alopecia was statistically significantly lower among patients treated with PC-1W compared to PC-3W (26.3% versus 63.9%, p<0.0001). The rate of asthenia was also statistically significantly higher in patients treated with PC-1W compared to PC-3W (39.4% versus 15.7%, p-0.0022). The rates of short-term and long-term neuropathy were similar in both treatment regimens. The rates of grade 3 or higher toxicities were similar in both treatment regimens, except for neutropenia, which was statistically significantly higher in patients treated with PC-1W versus PC-3W (23.2% versus 7.4%, p=0.0017). Two women died within 30 days of surgery. No other records regarding morbidity or mortality after surgery were documented.
This retrospective study showed that patients >70 treated with the modified PC-1W regimen had significantly longer median OS (57.17 versus 30.00 months) and PFS (19.09 versus 8.15 months) compared to patients >70 treated with the standard PC-3W regimen. The weekly protocol was more tolerable, as reflected by the higher rate of patients who completed ≥6 cycles of treatment compared to those treated with PC-3W, with similar rates of grade 3 toxicities, except for neutropenia. No statistically significant differences in median PFS and median OS were observed between septuagenarians and octogenarians. Toxicity rates were mostly similar between younger and older patients.
Patients >70 had significantly higher rates of comorbidities and advanced disease (stage IV) compared to younger patients, thus, neoadjuvant chemotherapy was more common in older patients. Neoadjuvant chemotherapy with interval debulking surgery is associated with higher rates of optimal cytoreduction, lower perioperative morbidity and mortality rates and similar outcomes, compared to primary debulking surgery (14, 15).
Although there was no difference in BRCA testing frequency in the younger and older groups, the rate of BRCA1/2 mutations was significantly lower in the older population. This may be explained by diagnosis of BRCA cancers at a younger age, or an active medical follow-up in patients carrying BRCA1/2 mutations.
Median PFS and median OS were 11.04 months and 41.26 months, respectively, among patients >70 treated with a platinum-taxane doublet. Liontos et al. (16) reported similar findings with a median PFS of 11.3 months and a median OS of 30.2 months among patients >70 years treated with a platinum-taxane doublet (84% of patients) or carboplatin monotherapy (16%). Although these values are significantly shorter than for younger patients, they suggested that older patients can still benefit from substantial lengthening of their lives.
In a previous study, we have found significantly longer median PFS and OS in patients with ovarian cancer (all stages) treated with PC-1W compared to PC-3W with a survival HR of 0.54 (95% CI, 0.43–0.67; p<0.001) (13). Our current analysis corroborates this finding in older patients. The MITO-7 study showed that carboplatin and paclitaxel given once a week versus every 3 weeks did not significantly prolong PFS in patients >70 years versus ≤70 years but it was associated with better quality of life and fewer toxic effects (17). The EWOC-1 trial provided evidence that the paclitaxel-carboplatin combination should remain the standard of care even for older and frailer patients with ovarian cancer because survival was significantly worse in the carboplatin monotherapy arm (18). In a pan-cancer meta-analysis of 19 randomized clinical trials that included 5 ovarian cancer trials, 6 breast cancer trials, 6 non-small cell lung cancer trials, 1 head and neck squamous cell carcinoma trial and 1 cervical cancer trial, the weekly paclitaxel regimen showed statistically significant better PFS than the 3-week paclitaxel regimen, with a HR of 0.90 (95% CI 0.82–0.99, p=0.02), but no significant difference in OS was found between the 2 paclitaxel regimens. Interestingly, PFS was affected by ethnicity with the weekly paclitaxel regimen showing improved PFS compared to the 3-weekly paclitaxel regimen in North American and Asia, but not in Europe (19).
In contrast to other reports (20), except for higher rates of asthenia in the older population, which is expected, no statistically significantly different rates of toxicities were observed in older patients (>70-80 and >80) compared to younger ones (≤70).
Patients >70 treated with PC-1W had statistically significantly higher hematological toxicities (neutropenia, thrombocytopenia and anemia) as well as asthenia; however, statistically significant difference in grade 3 toxicities was only observed for neutropenia. The higher rate of hematological malignancies in patients treated with PC-1W, may be due to the weekly complete blood count monitoring that these patients undergo prior to receiving weekly treatment, which may lead to the identification of hematological changes that would not have been detected with a longer interval between treatments. In contrast, a lower risk for grade 3/4 toxicities was reported for the weekly paclitaxel schedule compared to the 3-weekly one in the pan-cancer meta-analysis (19). In the current study, no difference in toxicity was observed when comparing septuagenarians to octogenarians, as had been suggested in prior studies (21).
The PC-1W protocol, which is administered over 28 days instead of 21-day cycles, includes a week without treatment, allowing older patients to recuperate. The fact that more patients treated with the PC-1W regimen were able to complete ≥6 cycles of therapy may also be attributed to the 6-week delay in course completion of this regimen (24 weeks instead of 18 weeks), allowing highly frail patients to recover between cycles and increasing the completion rate. Hence, the PC-1W regimen may offer advantages for older patients in terms of tolerance while retaining efficacy, perhaps by allowing more carboplatin to be given.
We further tried to evaluate differences in the characteristics and prognosis of older patients. Treatment guidelines for women >80 are undetermined; some research suggests that octogenarians may not tolerate combined surgery and chemotherapy (22). Octogenarians received more PC-1W in addition to carboplatin monotherapy compared to the septuagenarians. These subgroups had similar median OS and PFS, and no statistically significant differences in toxicities.
The strengths of this study include the assessment of a large patient population in a real-life clinical setting, in addition to a long ~20-year follow-up. The study findings are limited by its retrospective design. Our analysis only included a population of women diagnosed at stage III+IV. Surgical mortality and morbidity were not analyzed in our study. However, only 2 women died within 30 days of surgery in the entire cohort.
This study extends the literature describing treatment of older women with ovarian cancer, demonstrating that chemotherapy may be used with appropriate caution in this population with significantly improved survival rates and moderate toxicities. In recent years it has been suggested that patient frailty should be considered alongside their chronological age when making therapeutic decisions, as these are not synonymous and may allow a considerable number of women to benefit from an optimal treatment (23, 24). While we had no data on patient frailty, we emphasize the importance of increasing the use of a comprehensive geriatric assessment to formally assess the physiological, cognitive, functional and social support that older women receive during treatment planning. Frailer patients may benefit from the milder weekly paclitaxel carboplatin 28-day protocol.
Similar percentages of older patients underwent comprehensive genomic profile testing, but the numbers are too small to evaluate its prognostic contribution. Future studies should evaluate the use of this test in older patients, to improve tailored therapy, particularly in this population. The effects and toxicities of PARP inhibitors and bevacizumab in this population should also be further researched. As the use of PARP inhibitors was only approved for use in Israel for recurrent ovarian cancer in 2017, its use in our cohort was somewhat limited.
Older women, have substantial prognosis with conservative therapies with tolerable toxicity. Moreover, weekly intermittent therapy with carboplatin and paclitaxel seems less toxic and more effective in the older population and patients are more likely to complete the full chemotherapy course and survive longer. As we rapidly advance our understanding of the response to treatment in older patients, we urge care providers to carefully give them a complete treatment that would offer them an improved probability for longer survival.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Tel Aviv Sourasky Medical Center Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study was a retrospective review of medical records.
MB: Data curation, Writing – review & editing. ES: Writing – review & editing. SP: Data curation, Writing – review & editing. IL: Data curation, Writing – review & editing. NM: Data curation, Writing – review & editing. BL: Data curation, Writing – review & editing. IW: Writing – review & editing. TS: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Oberaigner W, Minicozzi P, Bielska-Lasota M, Allemani C, de Angelis R, Mangone L, et al. Survival for ovarian cancer in europe: the across-country variation did not shrink in the past decade. Acta Oncol (2012) 51(4):441–53. doi: 10.3109/0284186X.2011.653437
2. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, nccn clinical practice guidelines in oncology. J Natl Compr Cancer Network (2021) 19(2):191–226. doi: 10.6004/jnccn.2021.0007
3. Lin JJ, Egorova N, Franco R, Prasad-Hayes M, Bickell NA. Ovarian cancer treatment and survival trends among women older than 65 years of age in the United States, 1995-2008. Obstet Gynecol (2016) 127(1):81–9. doi: 10.1097/AOG.0000000000001196
4. Thrall MM, Gray HJ, Symons RG, Weiss NS, Flum DR, Goff BA. Trends in treatment of advanced epithelial ovarian cancer in the medicare population. Gynecol Oncol (2011) 122(1):100–6. doi: 10.1016/j.ygyno.2011.03.022
5. Pectasides D, Fountzilas G, Aravantinos G, Bamias A, Kalofonos HP, Skarlos D, et al. Epithelial ovarian carcinoma in younger vs older women: is age an independent prognostic factor? The hellenic oncology cooperative group experience. Int J Gynecol Cancer (2007) 17(5):1003–10. doi: 10.1111/j.1525-1438.2007.00912.x
6. Wright JD, Chen L, Tergas AI, Patankar S, Burke WM, Hou JY, et al. Trends in relative survival for ovarian cancer from 1975 to 2011. Obstet Gynecol (2015) 125(6):1345–52. doi: 10.1097/AOG.0000000000000854
7. Winter WE 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage iii epithelial ovarian cancer: A gynecologic oncology group study. J Clin Oncol (2007) 25(24):3621–7. doi: 10.1200/JCO.2006.10.2517
8. Quaglia A, Tavilla A, Shack L, Brenner H, Janssen-Heijnen M, Allemani C, et al. The cancer survival gap between elderly and middle-aged patients in Europe is widening. Eur J Cancer (2009) 45(6):1006–16. doi: 10.1016/j.ejca.2008.11.028
9. Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs (2019) 35(2):151–6. doi: 10.1016/j.soncn.2019.02.001
10. Chi DS, Liao JB, Leon LF, Venkatraman ES, Hensley ML, Bhaskaran D, et al. Identification of prognostic factors in advanced epithelial ovarian carcinoma. Gynecol Oncol (2001) 82(3):532–7. doi: 10.1006/gyno.2001.6328
11. Joueidi Y, Dion L, Bendifallah S, Mimoun C, Bricou A, Nyangoh Timoh K, et al. Management and survival of elderly and very elderly patients with ovarian cancer: an age-stratified study of 1123 women from the Francogyn Group. J Clin Med (2020) 9(5):1451. doi: 10.3390/jcm9051451
12. Gibson SJ, Fleming GF, Temkin SM, Chase DM. The application and outcome of standard of care treatment in elderly women with ovarian cancer: A literature review over the last 10 years. Front Oncol (2016) 6:63. doi: 10.3389/fonc.2016.00063
13. Safra T, Waissengrin B, Levy T, Leidner E, Merose R, Matceyevsky D, et al. Weekly carboplatin and paclitaxel: A retrospective comparison with the three-weekly schedule in first-line treatment of ovarian cancer. Oncologist (2021) 26(1):30–9. doi: 10.1634/theoncologist.2020-0196
14. Piedimonte S, Kessous R, Laskov I, Abitbol J, Kogan L, Yasmeen A, et al. Similar overall survival using neoadjuvant chemotherapy or primary debulking surgery in patients aged over 75 years with high-grade ovarian cancer. J Obstet Gynaecol Can (2020) 42(11):1339–45. doi: 10.1016/j.jogc.2020.04.014
15. Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New Engl J Med (2010) 363(10):943–53. doi: 10.1056/NEJMoa0908806
16. Liontos M, Papatheodoridi A, Andrikopoulou A, Thomakos N, Haidopoulos D, Rodolakis A, et al. Management of the elderly patients with high-grade serous ovarian cancer in the real-world setting. Curr Oncol (2021) 28(2):1143–52. doi: 10.3390/curroncol28020110
17. Pignata S, Scambia G, Katsaros D, Gallo C, Pujade-Lauraine E, De Placido S, et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (Mito-7): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol (2014) 15(4):396–405. doi: 10.1016/S1470-2045(14)70049-X
18. Falandry C, Rousseau F, Mouret-Reynier MA, Tinquaut F, Lorusso D, Herrstedt J, et al. Efficacy and safety of first-line single-agent carboplatin vs carboplatin plus paclitaxel for vulnerable older adult women with ovarian cancer: A gineco/gcig randomized clinical trial. JAMA Oncol (2021) 7(6):853–61. doi: 10.1001/jamaoncol.2021.0696
19. Lin S, Peng T, Meng Y, Cao C, Gao P, Wu P, et al. Comparison of one-week versus three-week paclitaxel for advanced pan-carcinomas: systematic review and meta-analysis. Aging (Albany NY) (2022) 14(4):1959–82. doi: 10.18632/aging.203919
20. Repetto L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol (2003) 1(4 Suppl 2):18–24.
21. Susini T, Amunni G, Busi E, Villanucci A, Carriero C, Taddei G, et al. Ovarian cancer in the elderly: feasibility of surgery and chemotherapy in 89 geriatric patients. Int J Gynecol Cancer (2007) 17(3):581–8. doi: 10.1111/j.1525-1438.2007.00836.x
22. Moore KN, Reid MS, Fong DN, Myers TK, Landrum LM, Moxley KM, et al. Ovarian cancer in the octogenarian: does the paradigm of aggressive cytoreductive surgery and chemotherapy still apply? Gynecol Oncol (2008) 110(2):133–9. doi: 10.1016/j.ygyno.2008.03.008
23. Wethington SL, Armstrong DK, Gaillard SL. Vulnerable older adults with ovarian cancer-time to stop undertreating. JAMA Oncol (2021) 7(6):831–3. doi: 10.1001/jamaoncol.2021.0468
Keywords: older patients, ovarian cancer, chemotherapy, survival, tolerability, toxicity, neoadjuvant chemotherapy
Citation: Brezis MR, Shachar E, Peleg Hasson S, Laskov I, Michaan N, Levy B, Wolf I and Safra T (2023) Effectiveness and safety of standard chemotherapy in older patients with ovarian cancer: a retrospective analysis by age group and treatment regimen. Front. Oncol. 13:1289379. doi: 10.3389/fonc.2023.1289379
Received: 05 September 2023; Accepted: 29 November 2023;
Published: 13 December 2023.
Edited by:
Xiang-Xi Mike Xu, University of Miami, United StatesReviewed by:
Shinya Suzuki, National Cancer Center Hospital East, JapanCopyright © 2023 Brezis, Shachar, Peleg Hasson, Laskov, Michaan, Levy, Wolf and Safra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamar Safra, cHJvZi50YW1hcnNhZnJhQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.