- 1Department of Radiation Medicine, MedStar Georgetown University Hospital, Washington, DC, United States
- 2Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, United States
- 3Medical Science Department, Myovant Sciences, Inc, United States
- 4Biotechnology Research Institute, North Carolina Central University, Durham, NC, United States
Introduction: Injectable GnRH receptor agonists have been shown to improve cancer control when combined with radiotherapy. Prostate SBRT offers an abbreviated treatment course with comparable efficacy to conventionally fractionated radiotherapy. Relugolix is a new oral GnRH receptor antagonist which achieves rapid, sustained testosterone suppression. This prospective study sought to evaluate early testosterone suppression and PSA response following relugolix and SBRT for intermediate to high prostate cancer.
Methods: Relugolix was initiated at least 2 months prior to SBRT. Interventions to improve adherence were not utilized. PSA and total testosterone levels were obtained prior to and 1-4 months post SBRT. Profound castration was defined as serum testosterone ≤ 20 ng/dL. Early PSA nadir was defined as the lowest PSA value within 4 months of completion of SBRT. Per prior trials, we examined the percentage of patients who achieved PSA level of ≤ 0.5 ng/mL and ≤ 0.2 ng/mL during the first 4 months post SBRT.
Results: Between July 2021 and January 2023, 52 men were treated at Georgetown with relugolix (4-6 months) and SBRT (36.25-40 Gy in 5 fractions) per an institutional protocol (IRB 12-1775). Median age was 71 years. 26.9% of patients were African American and 28.8% were obese (BMI ≥30 kg/m2). The median pretreatment PSA was 9.1 ng/ml. 67% of patients were ≥ Grade Group 3. 44 patients were intermediate- and 8 were high-risk. Patients initiated relugolix at a median of 3.6 months prior to SBRT with a median duration of 6.2 total months. 92.3% of patients achieved profound castration during relugolix treatment. Poor drug adherence was observed in 2 patients. A third patient chose to discontinue relugolix due to side effects. By post-SBRT month 4, 87.2% and 74.4% of patients achieved PSA levels ≤ 0.5 ng/ml and ≤ 0.2 ng/ml, respectively.
Discussion: Relugolix combined with SBRT allows for high rates of profound castration with low early PSA nadirs. We observed a 96% testosterone suppresion rate without the utilization of scheduled cues/reminders. This finding supports the notion that patients with localized prostate cancer can consistently and successfully follow an oral ADT protocol without daily reminders. Given relugolix’s potential benefits over injectable GnRH receptor agonists, its usage may be preferred in specific patient populations (fear of needles, prior cardiovascular events). Future studies should focus on boundaries to adherence in specific underserved populations.
1 Introduction
In 2022, there was an estimated 268,490 new cases of prostate cancer in the United States (1). Prostate cancer continues to be the leading cause of new cancer diagnoses, comprising 11% of all male cancer-related deaths in 2022 (1). For intermediate to high risk prostate cancer, the National Comprehensive Cancer Network (NCCN) guidelines endorse radiation therapy (RT) plus ADT (2). ADT in conjunction with conventionally fractionated radiation therapy significantly improves metastases-free and overall survival (3). Radiation dose escalation does not improve either of these important endpoints (4). However previous work has shown that SBRT with radiobiological dose escalation can achieve high rates of cancer control in unfavorable prostate cancer with minimal toxicity (5). As with external beam radiation therapy (EBRT), early data suggests that the addition of ADT to SBRT for high to intermediate risk prostate cancer may also reduce local persistence of disease and biochemical recurrence (6, 7). Unfortunately, ADT combined with RT remains underutilized possibly due to bothersome persistent side effects and its potentially negative impact on cardiovascular comorbidities (8).
In recent decades, the potential benefits of GnRH antagonists have been evaluated. Degarelix was the first readily available GnRH antagonist, exclusively offered in an injectable formulation (9). Although it is strongly efficacious in achieving testosterone suppression, Degarelix is associated with a high frequency of painful, injection site hypersensitivity reactions compared to GnRH agonist Leuprolide (40% versus <1%) (9). Approved by the FDA in 2020, relugolix is an oral ADT that suppresses gonadotropic release from the pituitary gland, thus decreasing concentrations of testosterone (10). The HERO study investigated the efficacy of this oral GnRH receptor antagonist compared to GnRH agonist Leuprolide. The randomized Phase 3 trial demonstrated the superiority of relugolix in achieving and maintaining castration, as well as a quicker testosterone recovery following discontinuation (11). On day 4 of use, 56.0% of patients receiving relugolix reached castrate level versus 0% with Leuprolide (11). Men treated with relugolix also maintained castration through 48 weeks at a rate of 96.7% compared to 88.8% with Leuprolide (11). These patients had a 54% lower risk of major adverse cardiovascular (CV) event after 12 months (11). The incidence of major adverse CV events favored relugolix at 2.9% versus 6.2% in the leuprolide group especially given similar distribution of CV risk factors between the two treatment arms (11). Importantly, the study exhibited a 99% adherence rate with oral relugolix using daily audible reminders (11).
Neoadjuvant/adjuvant relugolix (6 months) has been studied in intermediate to high risk prostate cancer in combination with conventionally fractionated radiation therapy (79.2 Gy in 44 fractions) (12). With relugolix, 95% achieved castration (total testosterone < 50 ng/dL, 1.73 nmol/L) and 82% reached profound castration (total testosterone < 20 ng/dL; 0.7 nmol/L). As with the HERO study, interventions to improve adherence were utilized (12). While oral ADTs have potential advantages, their real-world effectiveness is dependent on patient adherence (13). Bothersome side effects such as hot flashes, fatigue and decreased libido may lead to drug holidays and/or early cessation (14). This may be a bigger problem in minority and other underserved populations (15). In addition, patient characteristics such as obesity and unrecognized drug interactions could limit its relugolix real world effectiveness (13). Our investigation sought to evaluate real world early testosterone suppression and PSA response following relugolix and SBRT for intermediate to high risk prostate cancer.
2 Materials and methods
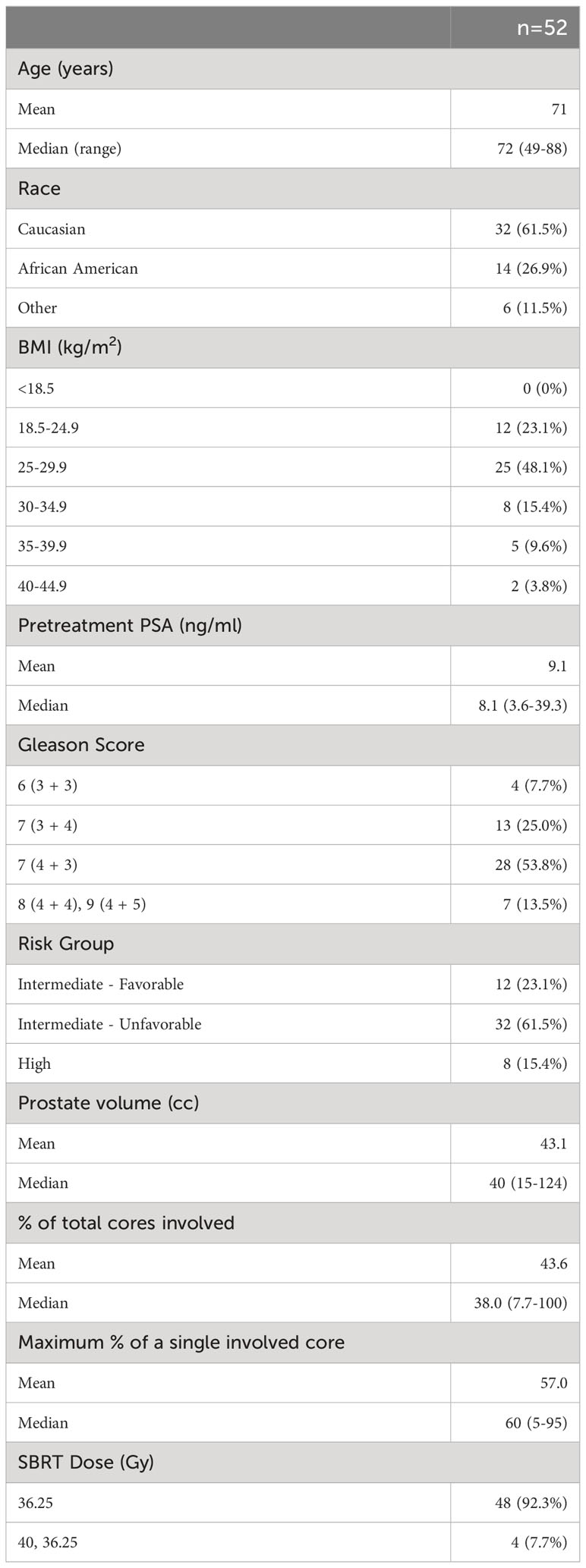
We conducted an IRB approved, prospective study (IRB 12-1175) of men with intermediate to high risk prostate cancer treated at MedStar Georgetown University Hospital. Patients were treated per institutional protocol with short-term relugolix (4-6 months) and SBRT (36.25-40 Gy in 5 fractions). Risk groups were defined using the D’Amico criteria. Other patient and treatment characteristics such as age, race, BMI, prostate volume, pretreatment PSA, T stage, Gleason score, and dose were acquired from the medical records.
2.1 Drug treatment
Neoadjuvant relugolix was initiated at least 2 months prior to SBRT with loading dose of 360 mg on the first day and continue treatment with a 120 mg dose taken orally once daily at approximately the same time each day. For patients with favorable intermediate risk disease, the decision to prescribe relugolix was made based on Decipher test results.
2.2 SBRT treatment planning and delivery
SBRT was delivered using the CyberKnife robotic radiosurgical system (Accuray Inc., Sunnyvale, CA) as previously described (16). Plans were inhomogeneous by design however prescription dose was prescribed to the 83% isodose line. Approximately two months following the initiation of relugolix, gold fiducials were transperineally placed into the prostate. One week after fiducial placement, CT and high-resolution MR images were obtained for treatment planning. The clinical target volume (CTV) included the prostate and proximal seminal vesicles. The planning target volume (PTV) included a uniform 3 mm expansion around the CTV. In general, each patient initiated treatment 2-4 weeks following treatment simulation. The prescription dose to the PTV was 36.25-40 Gy delivered in 5 fractions of 7.25-8 Gy over 1-2 weeks. Care was taken to avoid treatment beams that directly traversed the testes, and the testicular scatter dose was limited (D20% < 2 Gy).
2.3 Follow up and assessment
Early PSA and total testosterone levels were collected at two timepoints: immediately prior to SBRT initiation and 1-4 months after SBRT. We defined both effective and profound castration as a serum testosterone of ¾50 ng/dL (¾1.73 nmol/L) and ¾ 20 ng/dL (¾0.7 nmol/L) respectively as previously described (7). Early PSA nadir was demarcated as the lowest PSA value within 4 months of SBRT completion. We assessed the proportion of patients who achieved early PSA nadirs ≤ 1.0, ≤ 0.5, ≤ 0.2, ≤ 0.1, and <0.1 ng/mL at each of three timepoints: at SBRT, 1-4 months post-SBRT, and 5-8 months post-SBRT. In line with the efficacy endpoints defined in prior trials, we determined the percentage of patients reaching early PSA nadirs ≤ 0.5 ng/mL and ¾ 0.2 ng/mL during the first 4 months post SBRT (17, 18). Poor drug adherence was characterized as failure to reach profound castration, or testosterone ¾ 20 ng/dL, at any time point. Figures were obtained using R programming.
3 Results
3.1 Patient, treatment, and tumor characteristics
Patient, treatment, and tumor characteristics are described in Table 1. Between July 2021 and January 2023, 52 men with intermediate to high risk prostate cancer were treated at Georgetown University Hospital. Patients ranged from 49 to 88 years old, with a median age of 71. 62% of cohort were Caucasian and 27% African American. The majority (48%) of men were characterized as overweight (BMI between 25 and 29.9 kg/m2) while 29% fell within the obese category (BMI ≥30 kg/m2). Median pretreatment prostate specific antigen (PSA) was 8.1 ng/ml and ranged from 3.6 up to 39.3 ng/ml. 67% of patients were ≥ Grade Group 3. 44 patients were classified as intermediate- and 8 were high-risk. Of the intermediate group, patients were predominantly diagnosed with unfavorable disease. Patients initiated relugolix at a median of 3.6 months (range 2.6-5.7 months) prior to SBRT with a median duration of 6.2 total months (range 3.9-9.1 months).
3.2 Total testosterone levels
See Table 2 for summary of testosterone responses following neoadjuvant relugolix. At the time of SBRT, 98.1% of patients achieved a testosterone level ≤50 ng/dl (effective castration) while 90.4% reached testosterone ≤20 ng/dl (profound castration). Following SBRT (1-4 months), 92.3% of patients reached profound castration. 48.7% achieved testosterone ≤3 ng/dl. Poor drug adherence was observed in two patients. At 1-4 months post SBRT, both patients fell within normal testosterone values of 291 and 478 ng/dl (Figure 1). A third patient chose to discontinue relugolix shortly after SBRT due to side effects, reaching a normal testosterone of 216 ng/dl within three months post-SBRT (Figure 1).
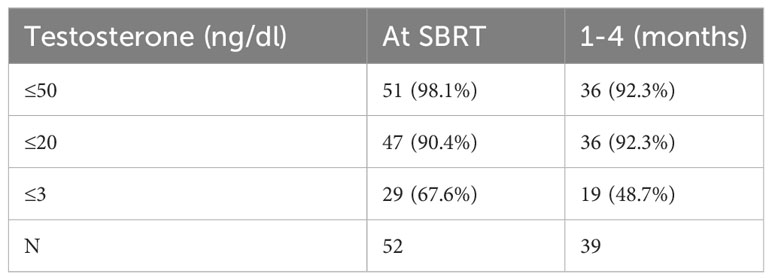
Table 2 Percentage of patients reaching given testosterone level in months following relugolix + SBRT treatment.
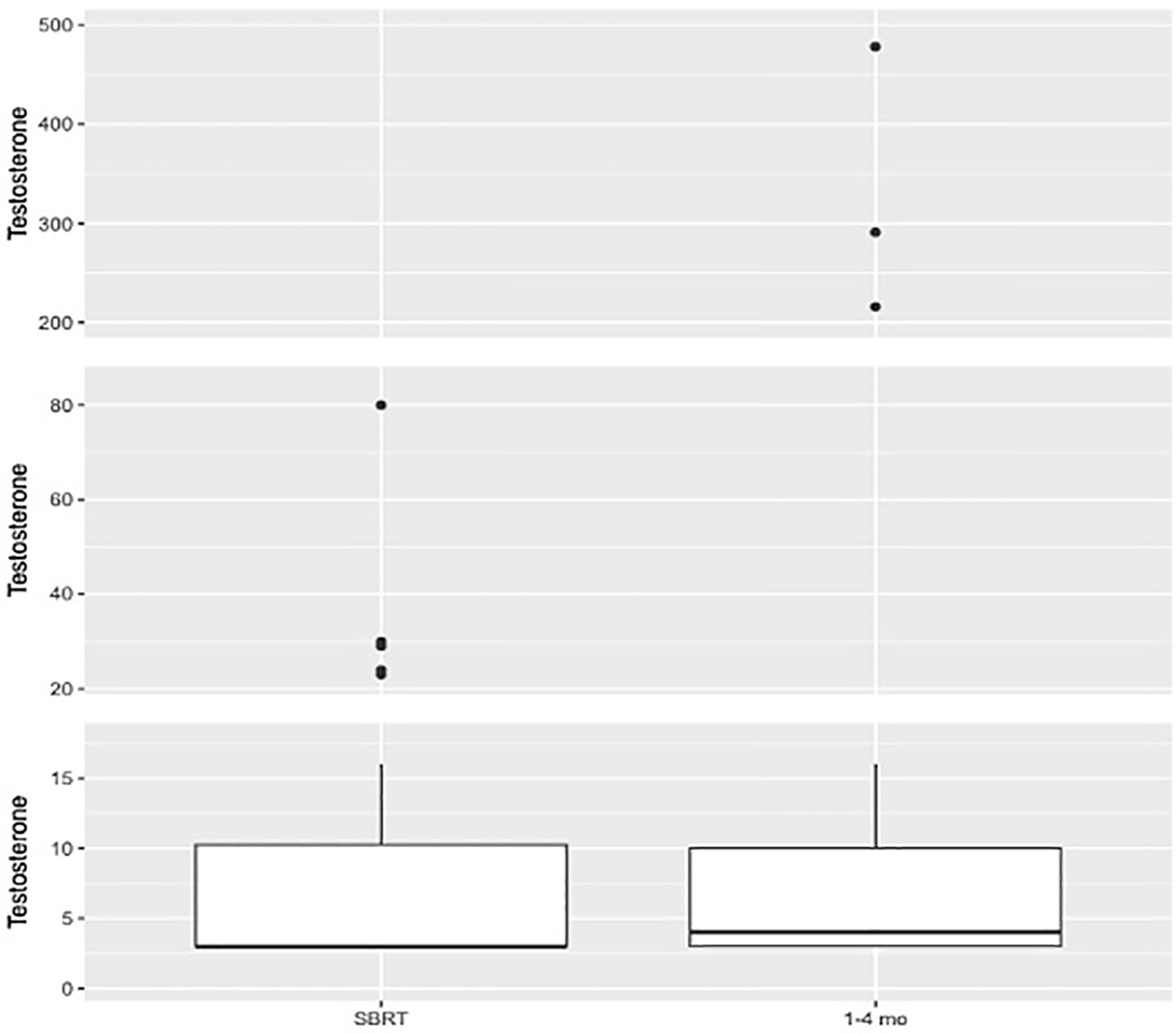
Figure 1 Box plot illustrating distribution of testosterone values (ng/dl) at SBRT (N=52. Mean=8.38. Median=3.0. Q1 = 3.0. Q3 = 10.25) and 1–4-month interval (N=39. Mean=31.05. Median=4.0. Q1 = 3.0. Q3 = 10.0).
3.3 Early PSA nadirs
See Table 3 for summary of early PSA nadir responses following neoadjuvant relugolix. Patients achieved a median PSA level of 0.58 ng/ml at the time of SBRT and 0.1 ng/ml 1-4 months after SBRT. At SBRT initiation, 71.2% of patients reached a PSA level ≤ 1.0 ng/ml. At this same time point, 46.2% and 13.5% of patients achieved PSA levels ≤ 0.5 ng/ml and ≤ 0.2 ng/ml, respectively. By 1-4 months post-SBRT, 94.9% reached early PSA nadir ≤ 1.0 ng/ml. 87.2% and 74.4% of men achieved PSA levels ≤ 0.5 ng/ml and ≤ 0.2 ng/ml respectively during the first 4 months following SBRT (Figure 2). We performed subgroup analyses of favorable intermediate, unfavorable intermediate, and high risk patients and found no significant differences in early PSA nadirs between disease risk groups.
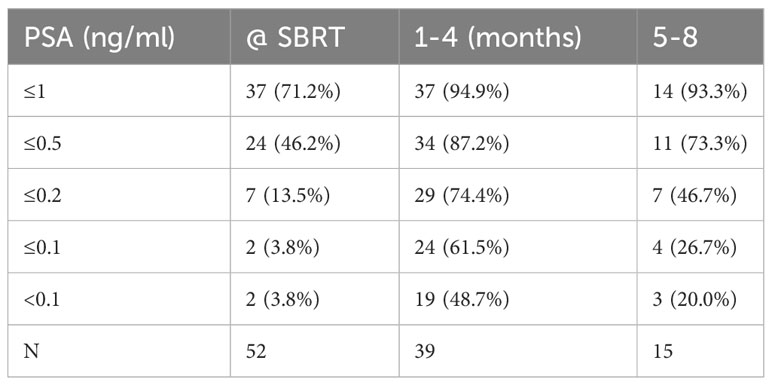
Table 3 Percentage of patients reaching given PSA level in months following relugolix + SBRT treatment.
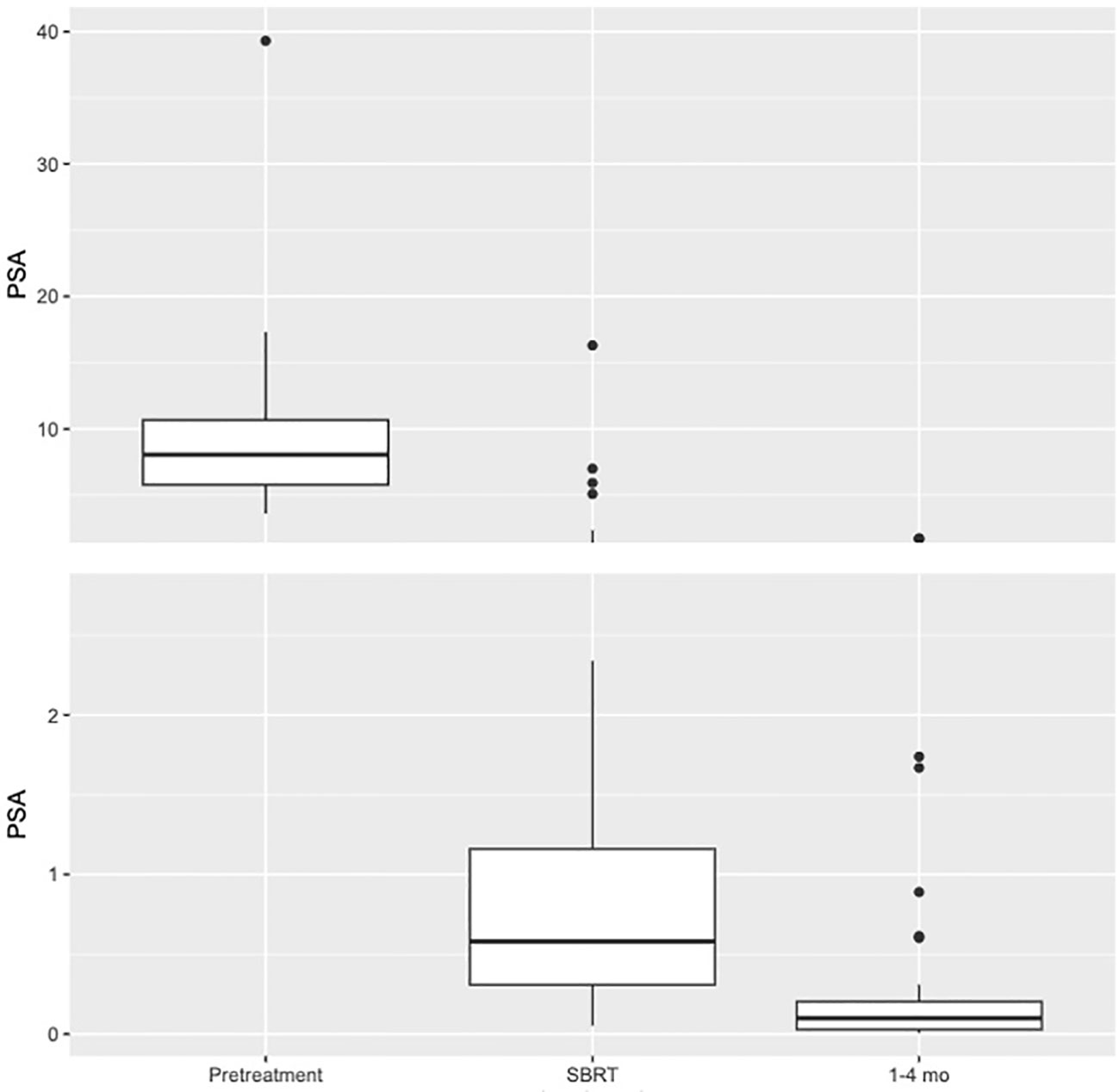
Figure 2 Box plot illustrating distribution of PSA values (ng/ml) at Pretreatment (N=52. Mean=9.09. Median=8.05. Q1 = 5.78. Q3 = 10.68), SBRT (N=52. Mean=1.29. Median=0.58. Q1 = 0.31. Q3 = 1.16), and 1–4-month interval (N=39. Mean=0.22. Median=0.1. Q1 = 0.03. Q3 = 0.2).
4 Discussion
Injectable GnRH agonists remain the most common choice for ADT in men with intermediate to high prostate cancer. However, patients face a variety of challenges and complications while undergoing treatment with these agents. First, they require regular injections that are inconvenient and painful. In addition, a clinically significant amount of patients report a fear of needles (19). Eek et al. found that oncology patients generally prefer oral over intravenous/injectable therapy due to convenience, increased efficacy perception, and prior experiences (20). Further difficulties present with GnRH agonists secondary to their intrinsic activating activity. Upon initiation of therapy, patients experience an early testosterone surge. This testosterone flare can lead to urinary retention/obstruction (9). Patients may also experience significant delays to castration (21). Likewise, testosterone recovery following discontinuation of GnRH agonists can be characterized as prolonged, uncontrolled, and unpredictable (22). An investigation of 307 prostate cancer patients by Nascimento et al. demonstrated that 24% of their cohort never regained normal testosterone values (>300 ng/dL) within 2 years of ADT cessation (23). Cardiovascular (CV) disease is currently recognized as the leading source of morbidity in males with prostate cancer, representing 27-34% of all-cause deaths (24). GnRH agonists have been long known to increase the risk of CV events particularly stroke and myocardial infarction (6). ADT with oral GnRH antagonist Relugolix may help ease these challenges and reduce the risk of serious complications.
Numerous studies have shown that PSA response to neoadjuvant ADT predicts long-term cancer control (25–29). In our study, greater than 90% of men achieved profound castration by the initiation of SBRT. This is comparable to previously reported studies of relugolix that utilized interventions to improve adherence (11, 12). With this high rate of profound castration in is not surprising that 71.2% and 46.2% achieved pre-SBRT PSA nadirs of ¾ 1 ng/ml and ¾ 0.5 ng/ml, respectively. This compares favorably with previous results utilizing 2-9 months neoadjuvant injectable GnRH agonists (¾ 1 ng/ml = 56%) (25). In this study, a post-neoadjuvant ADT PSA nadir of ¾ 1 ng/ml predicted a higher rate of biochemical control and a higher overall survival in men with unfavorable prostate cancer treated with EBRT (25). In a similar study, utilizing 3 months neoadjuvant injectable GnRH agonist with concurrent flutamide achieved an early PSA nadir of ¾ 0.5 ng/ml in 21.8% of men (26). Men with a post-neoadjuvant ADT PSA nadir of ¾ 0.5 ng/ml experienced a 5-year PSA relapse-free survival rate of 74%, as compared with 40% for patients with a higher PSA nadir (26). The etiology of these improved early PSA nadirs prior to SBRT is unknown but could be related to increases in sustained profound castration with relugolix (11).
Although guidelines define castrate testosterone levels of <50 ng/dL, there is evidence suggesting that serum testosterone levels of <20 ng/dL may improve clinical outcomes in certain clinical situations (30). For example, Klotz et al. ascertained that lower levels of testosterone during ADT (<0.7 nmol/L) in patients with biochemical recurrence correlated with an increase in cause-specific survival (CSS) (31). Still, it remains unclear how testosterone levels of <20 ng/dL affect the radio sensitizing ability of ADT.
The early PSA nadir at the end of therapy (RT and ADT) has been shown to be a surrogate endpoint for prostate cancer specific mortality (18, 32, 33). DFCI 95-096 first established an overall survival advantage for short term GnRH agonist therapy (6 months) plus RT (70 Gy) for intermediate risk disease (34). Likewise, TROG 96.01 showed an improved all-cause mortality for short term GnRH agonist therapy (3-6 months)/flutamide plus RT (66 Gy) (35). 95% and 75% achieved an early PSA nadir ≤ 0.5 ng/ml at the end of therapy in the DFCI 95-096 and TROG 96.01 studies respectively (32). In our study, 87.2% of patients 1-4 months post SBRT achieving a PSA level ≤ 0.5 ng/ml. Given its reported prognostic value, Kaplan et al. used a PSA cutoff level ≤ 0.2 ng/ml in an open-label, phase 2 trial that assessed the effectiveness of Enzalutamide and external beam RT for intermediate risk prostate cancer (17, 32). It was hypothesized that 60% of patients would achieve PSA ≤ 0.2 ng/ml, with 77% of patients ultimately meeting the endpoint (17). Similarly in our study, 74.4% of patients reached early PSA nadirs ≤ 0.2 ng/ml 1-4 months after SBRT. The difference between early PSA nadirs between studies is likely multifactorial including variable grade/volume disease, length of ADT, follow-up timepoints and sporadic non-adherence.
Participants of the HERO trial received special pill bottles with audible reminders which likely improved compliance rates and ensured continued testosterone suppression (36). Given that such interactive containers are expensive and not commercially available to the average patient, we did not utilize any scheduled cues as part of our study. Nonetheless, we observed an excellent compliance rate of 96% without prompting. Relevantly, 38% of our study population was non-Caucasian and represented various socioeconomic and racial backgrounds (37). The high compliance rate seen with a socially diverse patient cohort further validates that patients can consistently and successfully follow an oral ADT protocol. It is also key to mention the average effective and elimination half-lives (t1/2) of relugolix are approximately 25 hours and 36-65 hours respectively, indicating that testosterone levels are unlikely to be impacted by a single missed dose (38, 39). Importantly, 97% and 86% of men remained at castrate levels upon temporary interruption of treatment for 7 and 14 days, respectively (39).
Of note, poor drug adherence was observed in two of our study participants. Both patients were non-English speaking which may have contributed to adherence difficulties. Such findings emphasize the need to consider patients’ functional status and level of support when planning for ADT especially with relugolix. In select patient populations, we propose the use of regular testosterone checks to monitor adherence. Given the negative impact of possible noncompliance, future research should focus on obstacles to medication adherence. In addition, one patient voluntarily discontinued relugolix prior to completion of treatment due to side effects. Shucheng et al. highlighted the diversity of needs in individuals with prostate cancer and importance of patient empowerment (40). We utilized a shared decision-making model and encouraged patient involvement throughout treatment. This one patient’s testosterone levels quickly returned to normal range within weeks of stopping relugolix. The cessation responses align with the swift testosterone recoveries noted in the HERO study.
Limitations of our investigation are secondary to its small size and minor variations in treatment scheduling. Although we aimed to treat all patients for a total of 4-6 months with initiation 2 months prior to SBRT, there was heterogeneity in the timing of relugolix therapy. We did not examine whether there were any differences in outcomes depending on the timeliness of relugolix schedule parameters.
5 Conclusions
This study supports the use of relugolix and SBRT for the treatment of intermediate to high risk prostate cancer. High rates of profound castration and low early PSA nadirs were observed through combination treatment with relugolix and SBRT. With the known advantages of relugolix over injectable GnRH receptor agonists, its usage may be preferred especially in patients with a fear of needles or history of prior cardiovascular events. Further follow up relating to medication compliance and cost are needed to address potential real-world barriers. Patient reported quality of life outcomes on relugolix are also an active area of investigation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Georgetown-MedStar IRB Systemic. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. JX: Formal Analysis, Visualization, Writing – review & editing. JH: Data curation, Writing – review & editing. SSh: Writing – review & editing. MD: Conceptualization, Data curation, Writing – review & editing. AZ: Conceptualization, Data curation, Resources, Writing – review & editing. MA: Resources, Writing – review & editing. TY: Conceptualization, Data curation, Writing – review & editing. TS: Data curation, Writing – review & editing. MF: Conceptualization, Formal Analysis, Investigation, Project administration, Writing – review & editing. DK: Data curation, Investigation, Methodology, Writing – review & editing. PL: Conceptualization, Data curation, Writing – review & editing. ND: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. SSu: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – review & editing. SC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Department of Radiation Medicine at Georgetown University Hospital receives a grant from Accuray to support a research coordinator. This work was supported by The James and Theodore Pedas Family Foundation. SC and DK acknowledge the grant R01MD012767 from the National Institute on Minority Health and Health Disparities.
Conflict of interest
Author MF was employed at the company Myovant Sciences, Inc., United States.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. National Comprehensive Cancer Network. Prostate Cancer (2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
3. Kishan AU, Sun Y, Hartman H, Pisansky TM, Bolla M, Neven A, et al. MARCAP Consortium group.Androgen deprivation therapy use and duration with definitive radiotherapy for localised prostate cancer: an individual patient data meta-analysis. Lancet Oncol (2022) 23(2):304–16. doi: 10.1016/S1470-2045(21)00705-1
4. Kishan AU, Wang X, Sun Y, Romero T, Michalski JM, Ma TM, et al. High-dose radiotherapy or androgen deprivation therapy (HEAT) as treatment intensification for localized prostate cancer: an individual patient-data network meta-analysis from the MARCAP consortium. Eur Urol (2022) 82(1):106–14. doi: 10.1016/j.eururo.2022.04.003
5. Kishan AU, Dang A, Katz AJ, Mantz CA, Collins SP, Aghdam N, et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw Open (2019) 2:e188006. doi: 10.1001/jamanetworkopen.2018.8006
6. Gorovets D, Wibmer AG, Moore A, Lobaugh S, Zhang Z, Kollmeier M, et al. Local failure after prostate SBRT predominantly occurs in the PI-RADS 4 or 5 dominant intraprostatic lesion. Eur Urol Oncol (2022) 6:275–81. doi: 10.1016/j.euo.2022.02.005
7. van Dams R, Jiang NY, Fuller DB, Loblaw A, Jiang T, Katz AJ, et al. Stereotactic body radiotherapy for high-risk localized carcinoma of the prostate (SHARP) consortium: analysis of 344 prospectively treated patients. Int J Radiat Oncol Biol Phys (2021) 110(3):731–7. doi: 10.1016/j.ijrobp.2021.01.016
8. Falchook AD, Basak R, Chen RC. Androgen deprivation therapy and dose-escalated radiotherapy for intermediate- and high-risk prostate cancer-reply. JAMA Oncol (2017) 3(2):281. doi: 10.1001/jamaoncol.2016.3974
9. Van Poppel H, Klotz L. Gonadotropin-releasing hormone: an update review of the antagonists versus agonists: GnRH agonists vs antagonists. Int J Urol (2012) 19:594–601. doi: 10.1111/j.1442-2042.2012.02997.x
10. Markham A. Relugolix: First global approval. Drugs (2019) 79:675–9. doi: 10.1007/s40265-019-01105-0
11. Shore ND, Saad F, Cookson MS, George DJ, Saltzstein DR, Tutrone R, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med (2020) 382:2187–96. doi: 10.1056/NEJMoa2004325
12. Dearnaley DP, Saltzstein DR, Sylvester JE, Karsh L, Mehlhaff BA, Pieczonka C, et al. The oral gonadotropin-releasing hormone receptor antagonist relugolix as neoadjuvant/adjuvant androgen deprivation therapy to external beam radiotherapy in patients with localised intermediate-risk prostate cancer: A randomised, open-label, parallel-group phase 2 trial. Eur Urol (2020) 78:184–92. doi: 10.1016/j.eururo.2020.03.001
13. Sachdev S, Zhang H, Hussain M. Relugolix: Early promise for a novel oral androgen deprivation therapy with radiation therapy for prostate cancer. Eur Urol (2020) 78:193–4. doi: 10.1016/j.eururo.2020.03.053
14. Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai W-Y, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol (2010) 28:4120–8. doi: 10.1200/jco.2009.25.9655
15. Wassermann J, Gelber SI, Rosenberg SM, Ruddy KJ, Tamimi RM, Schapira L, et al. Nonadherent behaviors among young women on adjuvant endocrine therapy for breast cancer. Cancer (2019) 125:3266–74. doi: 10.1002/cncr.32192
16. Chen LN, Suy S, Uhn S, Oermann EK, Ju AW, Chen V, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the georgetown university experience. Radiat Oncol (2013) 8:58. doi: 10.1186/1748-717X-8-58
17. Kaplan I, Bubley GJ, Bhatt RS, Taplin M-E, Dowling S, Mahoney K, et al. Enzalutamide with radiation therapy for intermediate-risk prostate cancer: A phase 2 study. Int J Radiat Oncol Biol Phys (2021) 110:1416–22. doi: 10.1016/j.ijrobp.2021.02.027
18. Royce TJ, Chen M-H, Wu J, Loffredo M, Renshaw AA, Kantoff PW, et al. Surrogate end points for all-cause mortality in men with localized unfavorable-risk prostate cancer treated with radiation therapy vs radiation therapy plus androgen deprivation therapy: A secondary analysis of a randomized clinical trial. JAMA Oncol (2017) 3:652–8. doi: 10.1001/jamaoncol.2016.5983
19. McLenon J, Rogers MAM. The fear of needles: A systematic review and meta-analysis. J Adv Nurs (2019) 75:30–42. doi: 10.1111/jan.13818
20. Eek D, Krohe M, Mazar I, Horsfield A, Pompilus F, Friebe R, et al. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer Adherence (2016) 10:1609–21. doi: 10.2147/PPA.S106629
21. Labrie F, Bélanger A, Luu-The V, Labrie C, Simard J, Cusan L, et al. Gonadotropin-releasing hormone agonists in the treatment of prostate cancer. Endocr Rev (2005) 26:361–79. doi: 10.1210/er.2004-0017
22. Crawford ED, Hou AH. The role of LHRH antagonists in the treatment of prostate cancer. Oncol (Williston Park) (2009) 23:626–30.
23. Nascimento B, Miranda EP, Jenkins LC, Benfante N, Schofield EA, Mulhall JP. Testosterone recovery profiles after cessation of androgen deprivation therapy for prostate cancer. J Sex Med (2019) 16:872–9. doi: 10.1016/j.jsxm.2019.03.273
24. Melloni C, Roe MT. Androgen deprivation therapy and cardiovascular disease. Urol Oncol (2020) 38:45–52. doi: 10.1016/j.urolonc.2019.02.010
25. Mitchell DM, McAleese J, Park RM, Stewart DP, Stranex S, Eakin RL, et al. Failure to achieve a PSA level ≤1 ng/mL after neoadjuvant LHRHa therapy predicts for lower biochemical control rate and overall survival in localized prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys (2007) 69:1467–71. doi: 10.1016/j.ijrobp.2007.05.008
26. Zelefsky MJ, Lyass O, Fuks Z, Wolfe T, Burman C, Ling CC, et al. Predictors of improved outcome for patients with localized prostate cancer treated with neoadjuvant androgen ablation therapy and three-dimensional conformal radiotherapy. J Clin Oncol (1998) 16:3380–5. doi: 10.1200/jco.1998.16.10.3380
27. Geara FB, Bulbul M, Khauli RB, Andraos TY, Abboud M, Al Mousa A, et al. Nadir PSA is a strong predictor of treatment outcome in intermediate and high risk localized prostate cancer patients treated by definitive external beam radiotherapy and androgen deprivation. Radiat Oncol (2017) 12:149. doi: 10.1186/s13014-017-0884-y
28. Foo M, Lavieri M, Pickles T. Impact of neoadjuvant prostate-specific antigen kinetics on biochemical failure and prostate cancer mortality: Results from a prospective patient database. Int J Radiat Oncol Biol Phys (2013) 85:385–92. doi: 10.1016/j.ijrobp.2012.04.009
29. Malik R, Jani AB, Liauw SL. External beam radiotherapy for prostate cancer: Urinary outcomes for men with high international prostate symptom scores (IPSS). Int J Radiat Oncol Biol Phys (2011) 80:1080–6. doi: 10.1016/j.ijrobp.2010.03.040
30. Djavan B, Eastham J, Gomella L, Tombal B, Taneja S, Dianat SS, et al. Testosterone in prostate cancer: the Bethesda consensus: ROLE OF TESTOSTERONE IN PROSTATE CANCER. BJU Int (2012) 110:344–52. doi: 10.1111/j.1464-410X.2011.10719.x
31. Klotz L, O’Callaghan C, Ding K, Toren P, Dearnaley D, Higano CS, et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: A secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J Clin Oncol (2015) 33:1151–6. doi: 10.1200/jco.2014.58.2973
32. D’Amico AV, Chen M-H, de Castro M, Loffredo M, Lamb DS, Steigler A, et al. Surrogate endpoints for prostate cancer-specific mortality after radiotherapy and androgen suppression therapy in men with localised or locally advanced prostate cancer: an analysis of two randomised trials. Lancet Oncol (2012) 13:189–95. doi: 10.1016/S1470-2045(11)70295-9
33. Lamb DS, Denham JW, Joseph D, Matthews J, Atkinson C, Spry NA, et al. A comparison of the prognostic value of early PSA test-based variables following external beam radiotherapy, with or without preceding androgen deprivation: Analysis of data from the TROG 96.01 randomized trial. Int J Radiat Oncol Biol Phys (2011) 79:385–91. doi: 10.1016/j.ijrobp.2009.10.071
34. D’Amico AV, Chen M-H, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: A randomized trial. JAMA (2008) 299:289–95. doi: 10.1001/jama.299.3.289
35. Denham JW, Steigler A, Lamb DS, Joseph D, Turner S, Matthews J, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol (2011) 12:451–9. doi: 10.1016/S1470-2045(11)70063-8
36. Kasparian S, Wei O, Tsai N-C, Palmer J, Pal S, Lyou Y, et al. A practical guide to relugolix: Early experience with oral androgen deprivation therapy. Oncologist (2023) 28:oyad036. doi: 10.1093/oncolo/oyad036
37. Sholklapper TN, Creswell ML, Payne AT, Markel M, Pepin A, Carrasquilla M, et al. Patient-reported financial burden following stereotactic body radiation therapy for localized prostate cancer. Front Oncol (2022) 12:852844. doi: 10.3389/fonc.2022.852844
38. MacLean DB, Shi H, Faessel HM, Saad F. Medical castration using the investigational oral GnRH antagonist TAK-385 (relugolix): Phase 1 study in healthy males. J Clin Endocrinol Metab (2015) 100:4579–87. doi: 10.1210/jc.2015-2770
39. Lee T-Y, Pierrillas PB, Lin Y-W, de Greef R, Zandvliet AS, Schindler E, et al. Population PK and semimechanistic PK/PD modeling and simulation of relugolix effects on testosterone suppression in men with prostate cancer. Clin Pharmacol Ther (2023) 113:124–34. doi: 10.1002/cpt.2743
Keywords: prostate adenocarcinoma, relugolix, stereotactic body radiation therapy (SBRT), androgen deprivation therapy (ADT), testosterone suppression
Citation: Gallagher L, Xiao J, Hsueh J, Shah S, Danner M, Zwart A, Ayoob M, Yung T, Simpson T, Fallick M, Kumar D, Leger P, Dawson NA, Suy S and Collins SP (2023) Early biochemical outcomes following neoadjuvant/adjuvant relugolix with stereotactic body radiation therapy for intermediate to high risk prostate cancer. Front. Oncol. 13:1289249. doi: 10.3389/fonc.2023.1289249
Received: 05 September 2023; Accepted: 02 October 2023;
Published: 17 October 2023.
Edited by:
Raphael Pfeffer, Assuta Medical Center, IsraelReviewed by:
Raees Tonse, Baptist Hospital of Miami, United StatesPaul Riviere, University of California, San Diego, United States
Copyright © 2023 Gallagher, Xiao, Hsueh, Shah, Danner, Zwart, Ayoob, Yung, Simpson, Fallick, Kumar, Leger, Dawson, Suy and Collins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lindsey Gallagher, bGc5MzhAZ2VvcmdldG93bi5lZHU=
 Lindsey Gallagher
Lindsey Gallagher Jerry Xiao1
Jerry Xiao1 Jessica Hsueh
Jessica Hsueh Sarthak Shah
Sarthak Shah Malika Danner
Malika Danner Sean P. Collins
Sean P. Collins