
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 01 December 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1287555
This article is part of the Research Topic Updates on Radiation-induced Lymphopenia View all 10 articles
Background: Treatment-related lymphopenia (TRL) is common in patients with lung cancer, particularly in those with radiotherapy. However, the influence of TRL on the efficacy of immune checkpoint inhibitors (ICIs) for patients with lung cancer remains poorly understood. We performed a systematic review and meta-analysis to investigate the influence of TRL on survival of lung cancer patients on ICIs.
Methods: In order to accomplish the aim of the meta-analysis, a comprehensive search was conducted on databases including PubMed, Embase, Cochrane Library, and the Web of Science to identify observational studies with longitudinal follow-up. The Cochrane Q test was employed to evaluate heterogeneity among the included studies, while the I2 statistic was estimated. Random-effects models were utilized to merge the results, considering the potential impact of heterogeneity.
Results: Ten cohort studies with 1130 lung cancer patients who were treated with ICIs were included. Among them, 427 (37.8%) had TRL. Pooled results showed that compared to patients without TRL, patients with TRL were associated with poor progression-free survival (hazard ratio [HR]: 2.05, 95% confidence interval [CI]: 1.62 to 2.60, p < 0.001; I2 = 22%) and overall survival (HR: 2.69, 95% CI: 2.10 to 3.43, p < 0.001; I2 = 0%). Sensitivity analysis limited to patients with non-small cell lung cancer showed similar results (HR: 2.66 and 2.62, both p < 0.05). Moreover, subgroup analyses according to the diagnostic criteria of TRL, regression analysis model (univariate or multivariate), and indications of ICIs (for locally advanced or advanced lung cancer) showed consistent results (p for subgroup difference all > 0.05).
Conclusion: TRL was associated with poor survival of lung cancer patients who were treated with ICIs.
Lung cancer is a prevalent malignancy affecting the global population (1). According to the global cancer statistics in 2020, lung cancer constituted 11.4% of all cancer cases and accounted for 18.0% of cancer-related fatalities worldwide (2). Histologically, lung cancer can be categorized as non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), with therapeutic interventions primarily encompassing surgery, radiation therapy, chemotherapy, and targeted drug therapy (3, 4). A growing body of research underscores the significance of the immune system in cancer surveillance and anti-tumor activity (5, 6). Recent evidence has emphasized the significant role of immune checkpoint inhibitors (ICIs) as efficacious anticancer agents through the inhibition of programmed death-ligand 1 (PD-L1), programmed death 1 (PD-1), or cytotoxic t-lymphocyte-associated protein 4 (CTLA-4) receptors, thereby augmenting the cytotoxicity of T lymphocytes towards tumor cells (7). The overall effectiveness and safety of immunotherapy utilizing ICIs have been generally demonstrated in patients afflicted with metastatic and locally advanced non-small cell lung cancer (NSCLC) (8), as well as small cell lung cancer (SCLC) (9). However, subsequent observations suggest that the therapeutic response to ICIs may vary in individual patients with lung cancer (10). Accordingly, uncovering of the clinical factors that are related to the efficacy of ICIs in patients with lung cancer is of great clinical significance.
Lymphopenia is a prevalent occurrence in cancer patients, primarily attributed to the administration of anticancer treatments such as radiotherapy and chemotherapy, referred to as treatment-related lymphopenia (TRL) (11, 12). A recent meta-analysis encompassing 14 studies revealed that the average occurrence of severe lymphopenia (defined as an absolute lymphocyte count [ALC] < 500/ul) in lung cancer patients undergoing radiotherapy was 64.2% (13). Although sever TRL has been related to poor prognosis in patients with various solid tumors including lung cancer in early studies, patients with concurrent ICIs were rarely included in these studies (14). The potential impact of TRL on the effectiveness of ICIs, specifically by reducing the active T lymphocytes, has been postulated (15). However, the precise effect of TRL on the efficacy of ICIs in individuals with lung cancer has yet to be fully elucidated. Consequently, we conducted a comprehensive review and meta-analysis to examine the influence of TRL on the survival outcomes of lung cancer patients undergoing ICIs treatment.
The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (16, 17) and the Cochrane Handbook (18) throughout the stages of planning, conducting, and reporting.
The development of inclusion criteria adhered to the PICOS recommendations and aligned with the objective of the meta-analysis.
P (patients): Patients with pathologically confirmed diagnosis of lung cancer who were treated with ICIs.
I (exposure): Patients with TRL at the initiation or during ICIs treatment. Diagnostic criteria and cutoffs for defining TRL were consistent with those of the original studies. We included studies of patients with lymphopenia related to any anticancer treatment, not limited to those of patients received radiation only.
C (control): Patients without TRL.
O (outcomes): The study compared the progression-free survival (PFS) and/or overall survival (OS) outcomes between individuals with and without TRL. In essence, PFS denotes the time from the initiation of treatment to the occurrence of disease recurrence or progression, while OS represents the time from the initiation of treatment to the patient’s eventual demise.
S (study design): This study incorporated longitudinal follow-up studies, such as cohort and nested case-control studies, along with post-hoc analyses of clinical trials. Excluded from the meta-analysis were reviews, editorials, meta-analyses, preclinical studies, and studies that did not involve patients with lung cancer or with ICIs, failed to evaluate TRL, or did not report the survival outcomes of interest during follow-up. In cases where there was an overlap in patient populations, the study with the largest sample size was included in the meta-analysis.
A comprehensive search was conducted in electronic databases, namely PubMed, Embase, Cochrane Library, and Web of Science, encompassing the period from inception to July 10, 2023. The search strategy employed relevant terms pertaining to the subject matter of our investigation, aiming to identify studies published within this timeframe, which included: (1) “lymphopenia” OR “lymphocytopenia”; (2) “lung cancer”; and (3) “immunotherapy” OR “immune checkpoint inhibitor” OR “PD-1” OR “PD-L1” OR “CTLA-4” OR “programmed death 1” OR “programmed death ligand 1” OR “pembrolizumab” OR “atezolizumab” OR “nivolumab” OR “ipilimumab” OR “durvalumab” OR “tremelimumab” OR “camrelizumab” OR “tislelizumab” OR “sintilimab” OR “cemiplimab” OR “toripalimab” OR “lambrolizumab” OR “pidilizumab” OR “avelumab”. Only studies that met the criteria of being published as full-length articles in English and appearing in peer-reviewed journals were included in our analysis. Additionally, during our manual screening process, we thoroughly examined the references cited in relevant original and review articles to identify any potentially relevant studies.
Two authors independently performed literature searches, data collection, and assessments of study quality. In cases where discrepancies emerged, a third author was consulted for deliberation, leading to a consensus. The analysis of studies encompassed the gathering of data related to study information, design characteristics, patient diagnosis, demographic factors, medications for immunotherapy, definition of TRL, number of patients with TRL, median follow-up durations, outcomes reported, and variables adjusted for the evaluation of the association between TRL and survival of lung cancer patients on ICIs. The quality of the study was assessed using the Newcastle-Ottawa Scale (NOS) (19), which evaluates participant selection, group comparability, and outcome validity. The scale consisted of nine stars, with a greater number of stars indicating a study of higher quality.
Hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were utilized as the variables to assess the relationship between TRL and the survival of lung cancer patients receiving immune ICIs. To stabilize and normalize the variance, a logarithmical transformation was applied to the HR and its corresponding standard error in each study (20). The Cochrane Q test and the I2 statistic (21) were employed to estimate between-study heterogeneity. A value of I2 greater than 50% indicates the presence of significant heterogeneity among the studies. The random-effects model was utilized to combine the findings, as it has been recognized to account for potential heterogeneity (18). Sensitivity analysis limited to patients with NSCLC was performed. Additionally, subgroup analysis was conducted to explore the influence of cutoffs for TRL, different regression analysis model (univariate or multivariate), and indications of ICIs (for locally advanced or advanced lung cancer) on the outcomes. The cutoffs for defining overall TRL (<1000 lymphocytes/ul) and severe TRL (<500 lymphocytes/ul) were in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) criteria (22). Publication bias was estimated using a funnel plot, which involved visual assessments of symmetry, as well as Egger’s regression asymmetry test (23). The statistical analyses were conducted using RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) and Stata software (version 12.0; Stata Corporation, College Station, TX).
Figure 1 illustrates the procedure employed for conducting the literature search and study retrieval. Initially, a total of 781 records were acquired from the designated database, and subsequently, 172 duplicate entries were eliminated. Upon scrutinizing the titles and abstracts, an additional 583 studies were excluded due to their incompatibility with the objectives of the meta-analysis. Following comprehensive evaluations of the full texts of 26 studies, 16 were excluded based on the rationales outlined in Figure 1. Consequently, ten studies were deemed suitable for the subsequent meta-analysis (24–33).
Overall, one prospective (27) and nine retrospective cohort studies (24–26, 28–33) were included in the meta-analysis. The characteristics of the studies incorporated in this analysis are concisely outlined in Table 1. These studies were conducted in the United States, Korea, and France, and were published within the timeframe of 2019 to 2023. All of the studies encompassed patients diagnosed with lung cancer who underwent treatment with ICIs. Among these studies, eight exclusively focused on patients with NSCLC (24, 25, 28–33), whereas the remaining two also encompassed patients with SCLC (26, 27). The drugs for ICIs varied among the included studies, which involved nivolumab, pembrolizumab, durvalumab, atezolizumab, ipilimumab, or a combination of nivolumab and ipilimumab. The cutoffs for the diagnosis of TRL also varied among the included studies, and accordingly, 427 patients (37.8%) were diagnosed as TRL. The follow-up durations varied from 4.7 months to 24.0 months, with the median follow-up duration of 13.0 months. Outcome of PFS was reported in nine studies (24, 26–33), while the outcome of OS was reported in eight studies (24–27, 29–31, 33). Univariate regression analysis was used in three studies when the association between TRL and survival of lung cancer patients on ICIs was reported (25, 26, 30), while in the other seven studies (24, 27–29, 31–33), multivariate regression analysis was used with the adjustment of confounding factors such as age, sex, performance status, histological type of cancer, and other concurrent anticancer treatments etc. The NOS of the included studies were six to nine, indicating that they were of moderate to good quality (Table 2).
Nine studies (24, 26–33) evaluated the association between TRL and PFS in lung cancer patients treated with ICIs. Since one study reported the outcome in two cohorts of patients with different concurrent radiotherapy strategies (27), these two datasets were included independently into the meta-analysis. Pooled results showed that compared to those without TRL, lung cancer patients with TRL was associated with poor PFS (HR: 2.05, 95% CI: 1.62 to 2.60, p < 0.001; Figure 2A) with mild heterogeneity (I2 = 22%). Sensitivity analysis limited to patients with NSCLC showed similar results (HR: 2.26, 95% CI: 1.76 to 2.91, p < 0.001; I2 = 0%). Further subgroup analysis showed consistent association between TRL and poor PFS in studies with TRL diagnosed as ALC < 1000 and < 500/ul (p for subgroup difference = 0.64; Figure 2B), in studies with univariate and multivariate analysis (p for subgroup difference = 0.69; Figure 2C), and in studies with ICIs for locally advanced or advanced lung cancer (p for subgroup difference = 0.73; Figure 2D).
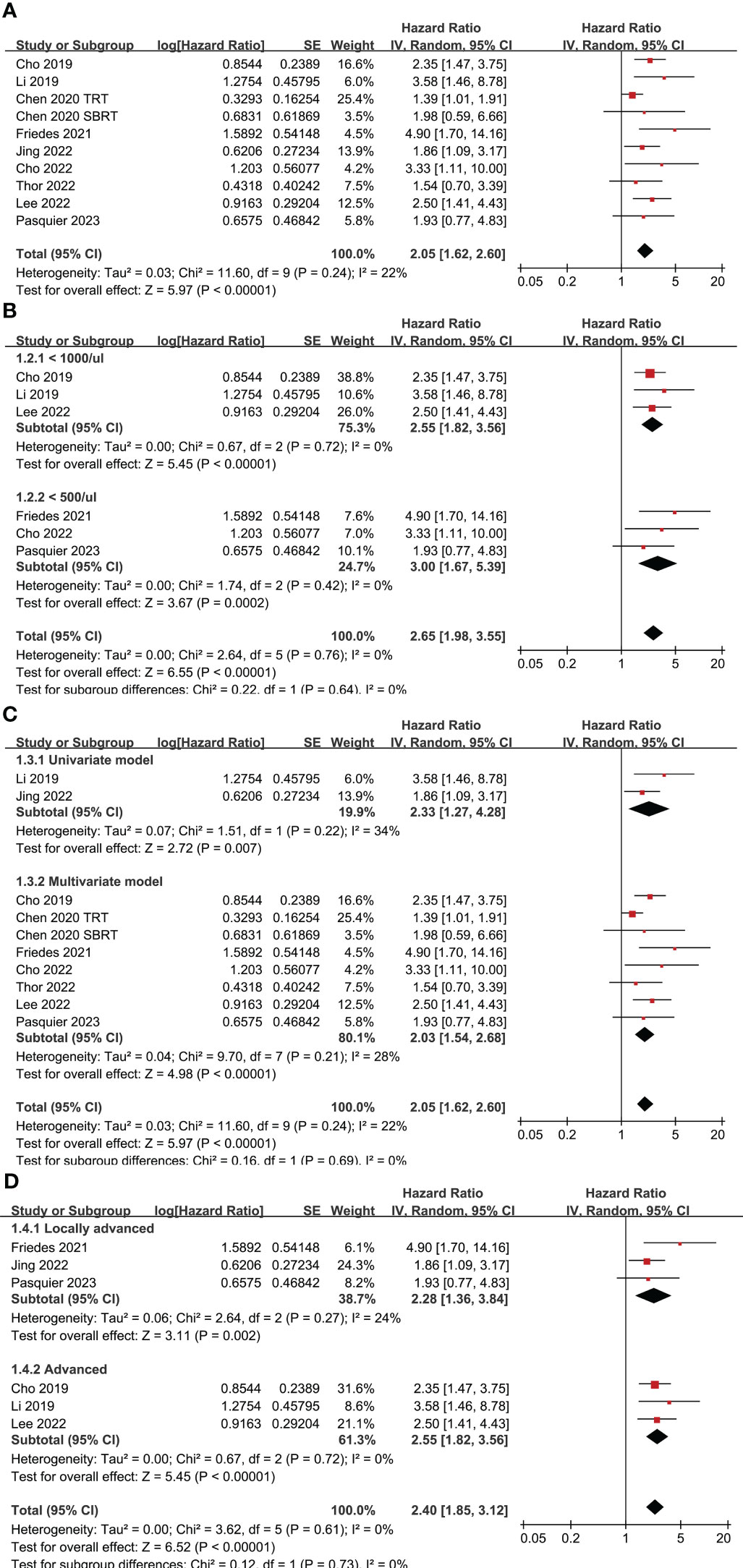
Figure 2 Forest plots for the meta-analyses regarding the association between TRL and PFS of lung cancer patients on ICIs; (A) overall meta-analysis; (B) subgroup analysis according to the cutoff for the diagnosis of TRL; (C) subgroup analysis according to the different analytic models used in the original studies; and (D) subgroup analysis according to the indications of ICIs.
Eight studies (24–27, 29–31, 33) with nine datasets were included for the meta-analysis of the association between TRL and OS in lung cancer patients on ICIs. Results of the meta-analysis showed that TRL was associated with poor OS (HR: 2.69, 95% CI: 2.10 to 3.43, p < 0.001; Figure 3A) with no significant heterogeneity (I2 = 0%). Consistent results were observed in sensitivity analysis limited to NSCLC only (HR: 2.62, 95% CI: 1.99 to 3.44, p < 0.001; I2 = 0%). Moreover, subgroup analyses according to the diagnostic criteria of TRL (p for subgroup difference = 0.80, Figure 3B), the analytic models (p for subgroup difference = 0.26, Figure 3C), and indications of ICIs (p for subgroup difference = 0.63, Figure 3D) also showed similar results.
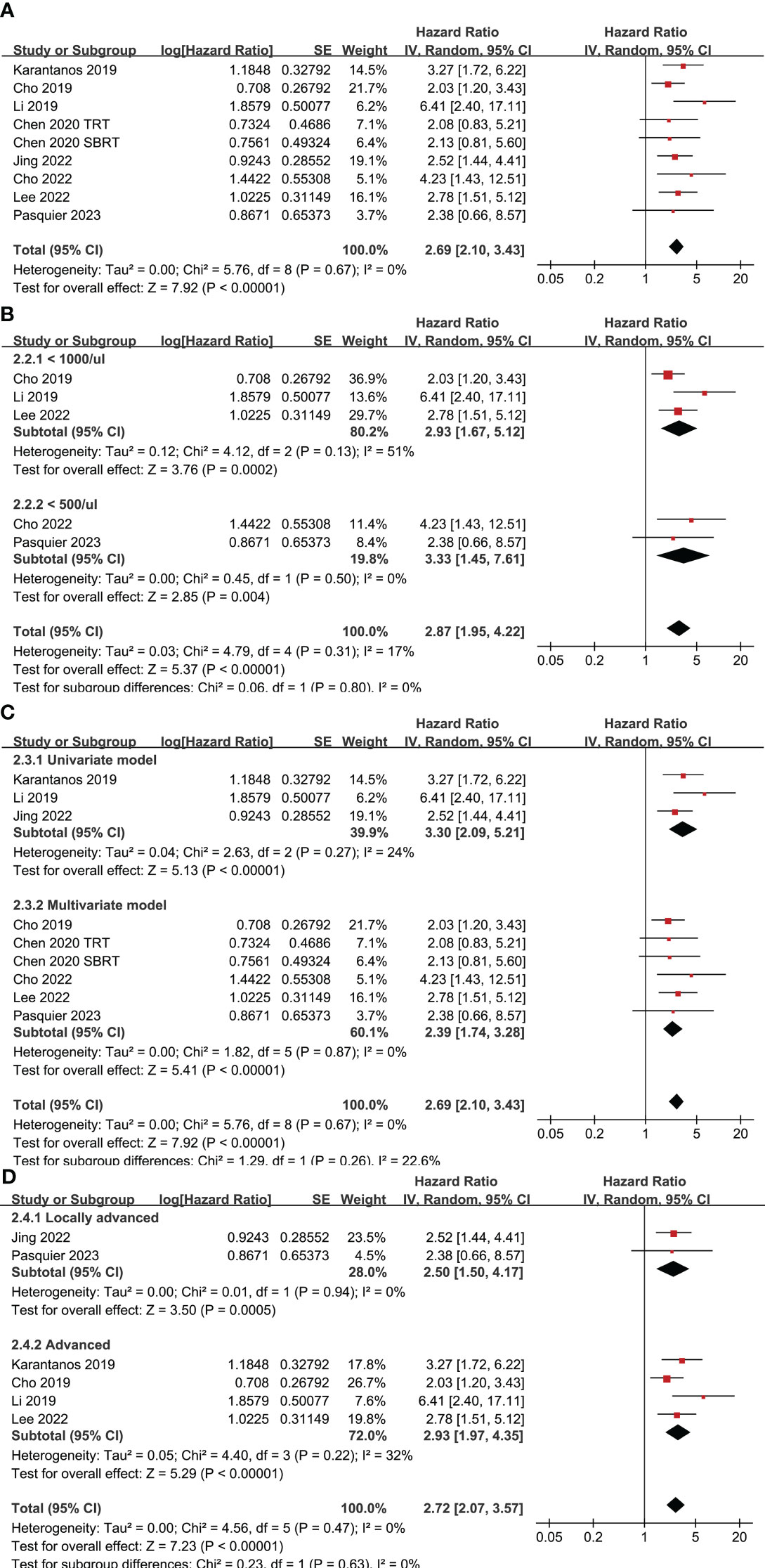
Figure 3 Forest plots for the meta-analyses regarding the association between TRL and OS of lung cancer patients on ICIs; (A) overall meta-analysis; (B) subgroup analysis according to the cutoff for the diagnosis of TRL; (C) subgroup analysis according to the different analytic models used in the original studies; and (D) subgroup analysis according to the indications of ICIs.
The funnel plots depicting the meta-analyses of the correlation between TRL and survival outcomes among lung cancer patients receiving ICIs are presented in Figure 4. Upon visual inspection, the plots exhibit symmetrical patterns, indicating a minimal presence of publication bias. Furthermore, the application of Egger’s regression tests yielded p-values of 0.37 and 0.49, further supporting the notion of a low probability of publication bias.
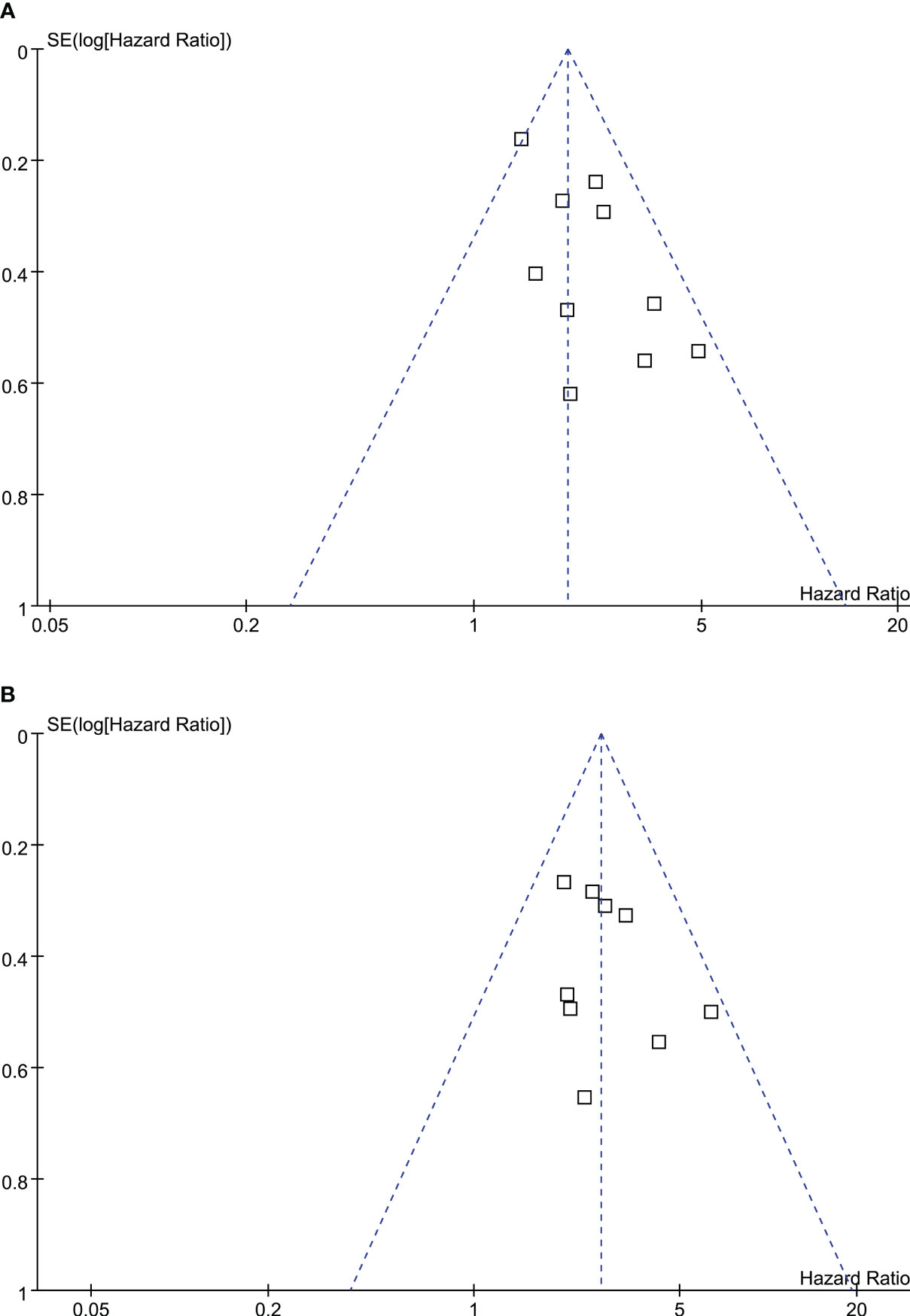
Figure 4 Funnel plots for the publication bias underlying the meta-analyses of the association between TRL and survival outcomes of lung cancer patients on ICIs; (A) funnel plots for the meta-analysis of the association between TRL and PFS; and (B) funnel plots for the meta-analysis of the association between TRL and OS.
This study conducted a systematic review and meta-analysis, incorporating data from ten cohort studies, to examine the correlation between TRL and survival outcomes in lung cancer patients undergoing ICIs treatment. The results of our analysis suggest that the presence of TRL at the start or during ICIs treatment is linked to unfavorable PFS and OS in lung cancer patients. Additionally, when focusing solely on studies involving patients with NSCLC, the findings remained consistent. Furthermore, subgroup analyses based on the cutoff value for diagnosing TRL, the chosen analytical model, and indications of ICIs also yielded consistent results. Taken together, these results suggest that TRL may be a risk factor of poor survival of lung cancer patients on the treatment of ICIs.
To the best of our knowledge, this study represents a potentially pioneering meta-analysis that examines the impact of TRL on the effectiveness of ICIs in individuals diagnosed with lung cancer. It is worth highlighting several notable advantages inherent in the employed meta-analysis methodologies. For instance, an extensive search of four widely utilized databases was conducted, thereby yielding up-to-date evidence pertaining to the association between TRL and the survival outcomes of lung cancer patients undergoing ICIs treatment. Moreover, it is worth mentioning that all the studies included in this analysis were cohort studies, indicating a possible longitudinal association between TRL and heightened risk of disease progression and mortality in these individuals. Furthermore, subgroup analysis based on the threshold values of ALC for diagnosing TRL yielded consistent findings, implying that even a mild TRL of ALC < 1000/ul may have a detrimental impact on the prognosis of lung cancer patients receiving ICIs. Finally, consistent results were obtained for subgroups of univariate and multivariate regression analyses, which suggested that the association between TRL and poor survival of lung cancer patients on ICIs may be independent of variables such as age, sex, functional status, and previous anticancer treatments. Collectively, these findings highly suggest the importance of monitoring lymphocyte count in peripheral circulating during the treatment with ICIs for patients with lung cancer.
The negative consequences observed in patients experiencing lymphopenia may be ascribed to modifications in the tumor microenvironment. These alterations can be linked to the accumulation of myeloid-derived suppressor cells, type-2 macrophages, or regulatory T cells, along with the generation of suppressive cytokines and metabolites, which can foster tumor advancement (15). Additionally, it has been postulated that neoantigen-specific T cells can be detected in the peripheral blood of patients with NSCLC undergoing anti-PD-L1 therapy. Notably, patients who exhibited an objective response demonstrated an increased presence of neoantigen-reactive T cells, which exhibited distinct phenotypic characteristics compared to non-responsive patients (34). The migration of these T cells to metastatic sites following immune checkpoint blockade stimulation is believed to play a crucial role in eliciting an effective antitumor response. However, the inhibition of T-cell function and subsequent lymphopenia in the peripheral blood may impede the transfer of T cells to the tumor site. In this particular scenario, lymphopenia serves as a surrogate marker indicating resistance to ICIs, necessitating the need for treatment modifications to overcome this resistance (35, 36). Notably, a recent investigation involving lung cancer patients with TRL who received ICIs revealed that inadequate lymphocyte recovery correlated with a shorter PFS, an increase in regulatory T cells, and a depletion of CD8+ T cells in the peripheral blood. These findings suggest that prompt recovery from TRL may hold significance in enhancing the prognosis of these patients (37).
For patients with cancer, radiation is a common cause of TRL, which is called radiation-induced lymphopenia (RIL). In lung cancer patients, the risk factors of RIL include advanced age, ALC before treatment, higher mean lung dose, larger volume of lung and heart receiving low dose (V5), longer treatment duration, and longer total beam-on time etc. (38–40). Among the studies included in the current meta-analysis, TRL caused by radiation therapy most frequently occurred at or within the six months after the initiation of the ICIs therapy. Results of the meta-analysis suggested that TRL may be associated with poor survival of lung cancer patients receiving ICIs, which is consistent with the results of studies in other types of tumors. A previous study of 105 patients with recurrent metastatic esophageal cancer receiving immunotherapy showed that lymphopenia is associated with a poorer immunotherapy prognosis in these patients (41). In another study of patients treated with nivolumab for recurrent/metastatic head and neck cancer, head and neck cancer, persistence of lymphopenia during immunotherapy was shown to be a predictor of worse OS (42). One attractive question at current stage is whether a prompt recovery from TRL may hold significance in enhancing the prognosis of patients with lung cancer on ICIs. A recently published pilot observational study in stage III NSCLC patients undergoing durvalumab consolidation therapy have suggested that recovery from TRL at the initiation of ICIs were associated with improved PFS and OS as compared to those without lymphocyte recovery (43). From a clinical standpoint, the restriction of radiotherapy dosage to the lungs, heart, and vertebrae presents modifiable risk factors that could potentially decrease the occurrence of RIL and PFS and OS, particularly in patients with non-modifiable risk factors such as advanced age, lower pre-radiotherapy ALC, and larger tumor size. However, modifying Lung V5 may pose technical challenges as it could lead to an increase in V20, thereby exacerbating fibrosis. Alternatively, shorter treatment duration may prove to be a more effective strategy when combined with immunotherapy to mitigate lymphopenia (44). Moreover, in the case of metastatic lung cancers, the utilization of stereotactic body radiotherapy to irradiate a specific portion of the tumor, as opposed to conventional radiotherapy targeting the entire tumor, has the potential to reduce RIL, while simultaneously preserving lung function and promoting antigen release. This, in turn, can contribute to the abscopal response (45). To comprehensively comprehend the relationship between TRL and unfavorable survival, as well as to devise efficacious approaches for improving the prognosis of patients experiencing lymphopenia during ICIs therapy, further translational investigations and clinical trials are imperative.
This study has several limitations that should be acknowledged. Firstly, the protocol of the meta-analysis was not prospectively registered, which is acknowledged as a limitation. Secondly, majority of the studies included in this analysis were retrospective, which introduces the possibility of selection and recall biases. To validate the findings, it is necessary to conduct large-scale prospective studies. Thirdly, among eight of the ten included studies, patients with NSCLC were included, while the other two studies included patients with NSCLC and SCLC. Accordingly, we could only observe the association between TRL and survival in NSCLC patients with ICIs via a sensitivity analysis limited to studies of patients with NSCLC only, instead of a subgroup analysis of NSCLC versus SCLC. It is important to considering subgroup analysis in NSCLC versus SCLC, because compared to NSCLC, SCLC is characterized by an exceptionally high proliferative rate, strong tendency for early widespread metastasis, and acquired chemoresistance (46). Currently, we could not determine if the association between TRL and survival of patients with SCLC on ICIS could be different from those of NSCLC, and studies are needed to address this association in patients with SCLC in the future. Furthermore, although the subset of studies employing multivariate regression analyses yielded comparable findings, it is important to acknowledge that the influence of residual factors on the correlation between TRL and unfavorable survival outcomes in these patients cannot be definitively dismissed. Ultimately, due to the nature of this meta-analysis being based on observational studies, it is not possible to establish a causal relationship between TRL and the heightened risk of cancer progression and mortality in lung cancer patients undergoing ICIs. Therefore, it is imperative to conduct clinical studies to ascertain if effective prevention or recovery of TRL could favorably influence the survival of lung cancer patients with ICIs treatment.
The results of the meta-analysis suggest a correlation between TRL and decreased survival rates among lung cancer patients receiving ICIs, particularly in those with NSCLC. However, further prospective studies are necessary to confirm these findings. On the other hand, the meta-analysis underscores the significance of monitoring peripheral lymphocyte counts in lung cancer patients undergoing ICI treatment. Additionally, it is crucial to investigate whether interventions aimed at preventing or expediting the recovery of TRL are linked to improved survival outcomes for lung cancer patients undergoing ICIs treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
YZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. CH: Data curation, Formal Analysis, Investigation, Validation, Writing – review & editing. SL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Alduais Y, Zhang H, Fan F, Chen J, Chen B. Non-small cell lung cancer (NSCLC): A review of risk factors, diagnosis, and treatment. Med (Baltimore) (2023) 102(8):e32899. doi: 10.1097/MD.0000000000032899
4. Megyesfalvi Z, Gay CM, Popper H, Pirker R, Ostoros G, Heeke S, et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J Clin (2023) 73(6):620–52. doi: 10.3322/caac.21785
5. Muenst S, Laubli H, Soysal SD, Zippelius A, Tzankov A, Hoeller S. The immune system and cancer evasion strategies: therapeutic concepts. J Intern Med (2016) 279(6):541–62. doi: 10.1111/joim.12470
6. Abbott M, Ustoyev Y. Cancer and the immune system: the history and background of immunotherapy. Semin Oncol Nurs (2019) 35(5):150923. doi: 10.1016/j.soncn.2019.08.002
7. Lee J, Kim EH. Mechanisms underlying response and resistance to immune checkpoint blockade in cancer immunotherapy. Front Oncol (2023) 13:1233376. doi: 10.3389/fonc.2023.1233376
8. Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol (2022) 40(6):586–97. doi: 10.1200/JCO.21.01497
9. Carlisle JW, Leal T. Advancing immunotherapy in small cell lung cancer. Cancer (2023) 129(22):3525–34. doi: 10.1002/cncr.34977
10. Xiao Q, Yu X, Shuai Z, Yao T, Yang X, Zhang Y. The influence of baseline characteristics on the efficacy of immune checkpoint inhibitors for advanced lung cancer: A systematic review and meta-analysis. Front Pharmacol (2022) 13:956788. doi: 10.3389/fphar.2022.956788
11. Koukourakis MI, Giatromanolaki A. Lymphopenia and intratumoral lymphocytic balance in the era of cancer immuno-radiotherapy. Crit Rev Oncol Hematol (2021) 159:103226. doi: 10.1016/j.critrevonc.2021.103226
12. de Kermenguy F, Meziani L, Mondini M, Clemenson C, Morel D, Deutsch E, et al. Radio-induced lymphopenia in the era of anti-cancer immunotherapy. Int Rev Cell Mol Biol (2023) 378:1–30. doi: 10.1016/bs.ircmb.2023.03.002
13. Upadhyay R, Venkatesulu BP, Giridhar P, Kim BK, Sharma A, Elghazawy H, et al. Risk and impact of radiation related lymphopenia in lung cancer: A systematic review and meta-analysis. Radiother Oncol (2021) 157:225–33. doi: 10.1016/j.radonc.2021.01.034
14. Damen PJJ, Kroese TE, van Hillegersberg R, Schuit E, Peters M, Verhoeff JJC, et al. The influence of severe radiation-induced lymphopenia on overall survival in solid tumors: A systematic review and meta-analysis. Int J Radiat Oncol Biol Phys (2021) 111(4):936–48. doi: 10.1016/j.ijrobp.2021.07.1695
15. Menetrier-Caux C, Ray-Coquard I, Blay JY, Caux C. Lymphopenia in Cancer Patients and its Effects on Response to Immunotherapy: an opportunity for combination with Cytokines? J Immunother Cancer (2019) 7(1):85. doi: 10.1186/s40425-019-0549-5
16. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
18. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. London, UK: The Cochrane Collaboration (2021). Available at: www.training.cochrane.org/handbook.
19. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2010). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
20. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, UK: The Cochrane Collaboration (2011). Available at: www.cochranehandbook.org.
21. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
22. Zhong X, Lim EA, Hershman DL, Moinpour CM, Unger J, Lee SM. Identifying severe adverse event clusters using the national cancer institute's common terminology criteria for adverse events. J Oncol Pract (2016) 12(3):e270–80, 45-6. doi: 10.1200/JOP.2015.006106
23. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
24. Cho Y, Park S, Byun HK, Lee CG, Cho J, Hong MH, et al. Impact of treatment-related lymphopenia on immunotherapy for advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys (2019) 105(5):1065–73. doi: 10.1016/j.ijrobp.2019.08.047
25. Karantanos T, Karanika S, Seth B, Gignac G. The absolute lymphocyte count can predict the overall survival of patients with non-small cell lung cancer on nivolumab: a clinical study. Clin Transl Oncol (2019) 21(2):206–12. doi: 10.1007/s12094-018-1908-2
26. Li YD, Lamano JB, Kaur G, Veliceasa D, Biyashev D, Kruser T, et al. Lymphopenia predicts response to stereotactic radiosurgery in lung cancer patients with brain metastases. J Neurooncol (2019) 143(2):337–47. doi: 10.1007/s11060-019-03169-0
27. Chen D, Patel RR, Verma V, Ramapriyan R, Barsoumian HB, Cortez MA, et al. Interaction between lymphopenia, radiotherapy technique, dosimetry, and survival outcomes in lung cancer patients receiving combined immunotherapy and radiotherapy. Radiother Oncol (2020) 150:114–20. doi: 10.1016/j.radonc.2020.05.051
28. Friedes C, Chakrabarti T, Olson S, Prichett L, Brahmer JR, Forde PM, et al. Association of severe lymphopenia and disease progression in unresectable locally advanced non-small cell lung cancer treated with definitive chemoradiation and immunotherapy. Lung Cancer (2021) 154:36–43. doi: 10.1016/j.lungcan.2021.01.022
29. Cho Y, Kim Y, Chamseddine I, Lee WH, Kim HR, Lee IJ, et al. Lymphocyte dynamics during and after chemo-radiation correlate to dose and outcome in stage III NSCLC patients undergoing maintenance immunotherapy. Radiother Oncol (2022) 168:1–7. doi: 10.1016/j.radonc.2022.01.007
30. Jing W, Xu T, Wu L, Lopez PB, Grassberger C, Ellsworth SG, et al. Severe radiation-induced lymphopenia attenuates the benefit of durvalumab after concurrent chemoradiotherapy for NSCLC. JTO Clin Res Rep (2022) 3(9):100391. doi: 10.1016/j.jtocrr.2022.100391
31. Lee YJ, Park YS, Lee HW, Park TY, Lee JK, Heo EY. Peripheral lymphocyte count as a surrogate marker of immune checkpoint inhibitor therapy outcomes in patients with non-small-cell lung cancer. Sci Rep (2022) 12(1):626. doi: 10.1038/s41598-021-04630-9
32. Thor M, Shepherd AF, Preeshagul I, Offin M, Gelblum DY, Wu AJ, et al. Pre-treatment immune status predicts disease control in NSCLCs treated with chemoradiation and durvalumab. Radiother Oncol (2022) 167:158–64. doi: 10.1016/j.radonc.2021.12.016
33. Pasquier C, Chaltiel L, Massabeau C, Rabeau A, Lebas L, Lusque A, et al. Impact of radiation on host immune system in patients treated with chemoradiotherapy and durvalumab consolidation for unresectable locally advanced non-small cell lung cancer. Front Oncol (2023) 13:1186479. doi: 10.3389/fonc.2023.1186479
34. Fehlings M, Jhunjhunwala S, Kowanetz M, O'Gorman WE, Hegde PS, Sumatoh H, et al. Late-differentiated effector neoantigen-specific CD8+ T cells are enriched in peripheral blood of non-small cell lung carcinoma patients responding to atezolizumab treatment. J Immunother Cancer (2019) 7(1):249. doi: 10.1186/s40425-019-0695-9
35. Li S, Simoni Y, Zhuang S, Gabel A, Ma S, Chee J, et al. Characterization of neoantigen-specific T cells in cancer resistant to immune checkpoint therapies. Proc Natl Acad Sci U.S.A. (2021) 118(30). doi: 10.1073/pnas.2025570118
36. Caushi JX, Zhang J, Ji Z, Vaghasia A, Zhang B, Hsiue EH, et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature (2021) 596(7870):126–32. doi: 10.1038/s41586-021-03752-4
37. Cheng B, Ding K, Chen P, Ji J, Luo T, Guo X, et al. Anti-PD-L1/TGF-betaR fusion protein (SHR-1701) overcomes disrupted lymphocyte recovery-induced resistance to PD-1/PD-L1 inhibitors in lung cancer. Cancer Commun (Lond) (2022) 42(1):17–36. doi: 10.1002/cac2.12244
38. Zhao Q, Li T, Chen G, Zeng Z, He J. Prognosis and risk factors of radiation-induced lymphopenia in early-stage lung cancer treated with stereotactic body radiation therapy. Front Oncol (2019) 9:1488. doi: 10.3389/fonc.2019.01488
39. Xie X, Lin SH, Welsh JW, Wei X, Jin H, Mohan R, et al. Radiation-induced lymphopenia during chemoradiation therapy for non-small cell lung cancer is linked with age, lung V5, and XRCC1 rs25487 genotypes in lymphocytes. Radiother Oncol (2021) 154:187–93. doi: 10.1016/j.radonc.2020.09.002
40. Kim Y, Chamseddine I, Cho Y, Kim JS, Mohan R, Shusharina N, et al. Neural network based ensemble model to predict radiation induced lymphopenia after concurrent chemo-radiotherapy for non-small cell lung cancer from two institutions. Neoplasia (2023) 39:100889. doi: 10.1016/j.neo.2023.100889
41. Zhao Q, Bi Y, Xue J, Liu Y, Zhu J, Qin S. Prognostic value of absolute lymphocyte count in patients with advanced esophageal cancer treated with immunotherapy: a retrospective analysis. Ann Transl Med (2022) 10(13):744. doi: 10.21037/atm-22-2669
42. Cesaire M, Rambeau A, Clatot F, Johnson A, Heutte N, Thariat J. Impact of lymphopenia on efficacy of nivolumab in head and neck cancer patients. Eur Arch Otorhinolaryngol (2023) 280(5):2453–61. doi: 10.1007/s00405-022-07800-1
43. Kuge T, Shiroyama T, Tamiya A, Tamiya M, Kanazu M, Kinehara Y, et al. Impact of lymphopenia recovery after chemoradiotherapy on durvalumab consolidation therapy in stage III NSCLC. JTO Clin Res Rep (2023) 4(5):100505. doi: 10.1016/j.jtocrr.2023.100505
44. Zhao Q, Li T, Du S, He J, Zeng Z. Shortened radiation time promotes recovery from radiation-induced lymphopenia in early-stage non-small cell lung cancer patients treated with stereotactic body radiation therapy. Technol Cancer Res Treat (2022) 21:15330338221112287. doi: 10.1177/15330338221112287
45. Tubin S, Khan MK, Salerno G, Mourad WF, Yan W, Jeremic B. Mono-institutional phase 2 study of innovative Stereotactic Body RadioTherapy targeting PArtial Tumor HYpoxic (SBRT-PATHY) clonogenic cells in unresectable bulky non-small cell lung cancer: profound non-targeted effects by sparing peri-tumoral immune microenvironment. Radiat Oncol (2019) 14(1):212. doi: 10.1186/s13014-019-1227-y
Keywords: lung cancer, lymphopenia, immunotherapy, survival, meta-analysis
Citation: Zhang Y, Huang C and Li S (2023) Influence of treatment-related lymphopenia on the efficacy of immune checkpoint inhibitors in lung cancer: a meta-analysis. Front. Oncol. 13:1287555. doi: 10.3389/fonc.2023.1287555
Received: 01 September 2023; Accepted: 07 November 2023;
Published: 01 December 2023.
Edited by:
Peter Sylvain Nicolas van Rossum, Amsterdam University Medical Center, NetherlandsReviewed by:
Pim Damen, Erasmus Medical Center, NetherlandsCopyright © 2023 Zhang, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shanqing Li, c2hhbnFpbmdsaV9wdW1jQDIxY24uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.