
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 07 December 2023
Sec. Cancer Genetics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1286610
This article is part of the Research Topic Tumorigenesis: Developmental and Homeostatic Signaling Gone Awry! View all 4 articles
Background: MAF transcription factor G antisense RNA 1 (MAFG-AS1), a novel long non-coding RNA discovered recently, was proved to be useful in predicting malignancy prognosis. Nevertheless, its association with cancer prognosis has been inconsistent. Therefore, this meta-analysis aimed to explore the clinicopathological and prognostic significance of MAFG-AS1 in diverse carcinomas.
Methods: Studies focused on MAFG-AS1 expression as a prognostic role in cancers were thoroughly searched in six electronic databases. The value of MAFG-AS1 in malignancies was assessed by hazard ratios (HRs) or odds ratios (ORs). Additionally, the GEPIA database was utilized to further strengthen our conclusion.
Results: A total of 15 studies involving 1187 cases and nine types of cancers were recruited into this meta-analysis. High MAFG-AS1 expression was significantly related to advanced tumor stage (OR = 0.52, 95%CI [0.39, 0.69], P < 0.00001), earlier lymph node metastasis (OR = 3.62, 95%CI [2.19, 5.99], P < 0.00001), worse tumor differentiation (OR = 0.64, 95%CI [0.43, 0.95], P = 0.03), and poor overall survival (HR = 1.94, 95%CI [1.72, 2.19], P < 0.00001). No significant heterogeneity and publication bias was detected across studies. Meanwhile, MAFG-AS1 was significantly elevated in ten kinds of cancers based on the validation of the GEPIA database.
Conclusion: The results of this meta-analysis indicated that high MAFG-AS1 expression is dramatically correlated with unfavorable prognosis in cancers. MAFG-AS1 may be served as a promising biomarker for malignancies.
Cancer is a major threat to human health worldwide. According to the cancer statistics published by the American Cancer Society recently, there were approximately 0.6 million cancer deaths and 1.95 million newly diagnosed cancer cases in the United States in 2023 (1). Furthermore, in China, an estimated 3 million cancer-related deaths and 4.5 million new cancer cases occurred during 2020, accounting for 30.2% of all cancer deaths in the world, which were significantly higher than those in the United States and thus remarkably increased the cancer and economic burden in China (2). Although breakthroughs in diagnostic techniques such as circulating tumor DNA, circulating tumor cells, and positron emission tomography/computed tomography have notably improved cancer surveillance, the mortality of many malignancies has not been considerably reduced (3–5). The GLOBOCAN 2020 estimated that there will be 28.4 million cancer cases in 2040 (6). Therefore, exploring new cancer biomarkers to detect early-stage cancer and determine the prognosis of cancer patients is rather imperative and rewarding (7).
Long non-coding RNAs (lncRNAs) which exceed 200 nucleotides in length and lack protein-coding potential have been identified in an extensive range of biological processes, like regulating gene expression and shaping nuclear structure (8). More recently, mounting evidence suggested that lcnRNAs were highly associated with prognosis in various malignancies and may be novel targets for cancer detection and therapy (9, 10). For example, the MAF transcription factor G antisense RNA 1 (MAFG-AS1), a novel lncRNA with a transcript size of 1914bp, is located on chromosome 17q25.3 (11, 12). Clinical studies had demonstrated that elevated expression of MAFG-AS1 could accelerate the progression of diverse kinds of cancers, including breast cancer, bladder cancer, hepatocellular carcinoma, lung cancer, gastric cancer, colorectal cancer, glioblastoma, ovarian cancer, prostate cancer, pancreatic cancer, and esophageal squamous cell cancer (ESCC) (12). Basically, overexpression of MAFG-AS1 is closely connected to higher histological grade, lymph node metastasis (LNM), larger tumor size, and shorter overall survival (OS) in many human cancers (13). Simultaneously, accumulating experiments indicated that MAFG-AS1 participated in various biological effects, such as promoting migration, proliferation, and epithelial-mesenchymal transition (EMT), along with inhibiting apoptosis of carcinoma (12). Collectively, MAFG-AS1 appeals to a wide range of clinicians and may serve as a potential predictor of carcinoma prognosis.
Although a considerable number of clinical studies have investigated the correlation between MAFG-AS1 and cancer prognosis, several variables concerning MAFG-AS1 in malignancies have generated controversial results. For example, Li et al. proved that high MAFG-AS1 expression in bladder cancer was obviously correlated with larger tumor size (≥ 3 cm), but not with tumor stage (14). Instead, Sun et al. also estimated MAFG-AS1 expression in bladder cancer and demonstrated that elevated MAFG-AS1 expression was closely associated with tumor stage (P < 0.05), but not with larger tumor size (≥ 3 cm) (15). Furthermore, regarding breast cancer, Di et al., based on 54 cases, confirmed that overexpression of MAFG-AS1 was significantly related to LNM (P < 0.05) (11), which was contrary to Feng’s study (16). Consequently, the significance of these associations may be insufficiently evaluated, due to the small sample sizes of individual study. Therefore, this meta-analysis was conducted to quantitatively estimate the prognostic significance of MAFG-AS1 in various cancers.
This study was performed following the preferred reporting program of the systematic review and meta-analysis (PRISMA) (17).
Six databases, including Web of Science, Springer, Cochrane Library, PubMed, Scopus, and EBSCO were thoroughly searched from inception up to August 22, 2023, for eligible studies. The search keywords were as follows: MAF transcription factor G antisense RNA 1, long non-coding RNA MAFG-AS1, lncRNA MAFG-AS1, and MAFG-AS1. Besides, the references of retrieved records were evaluated carefully to screen more potentially relevant records.
The inclusion criteria for this study were as follows: (1) randomized controlled trials or retrospective studies; (2) patients were divided into high MAFG-AS1 expression group and low MAFG-AS1 expression group; (3) studies provided OS, disease-free survival (DFS), or clinicopathologic parameters (e.g., lymph node metastasis, tumor differentiation, tumor size, tumor stage, gender, and age of patients) at least; (3) malignancies were solid tumor; (4) patients had not been treated with chemotherapy or radiotherapy prior to surgery; (5) no ethnical and geographical restrictions.
The exclusion criteria were as follows: (1) studies failed to report sufficient data (e.g., without clinicopathologic parameters, OS, or DFS); (2) data from public databases, duplicate publications, cellular-based experiments, reviews, animal experiments, non-solid tumor, retracted articles; (3) studies were not published in English.
The first two authors independently summarized the major characteristics of eligible studies after being screened by our inclusion and exclusion criteria. The following items were recorded: the author’s last name, publication year, types of cancer, sample size capacity, number of patients from two groups, detection technique, prognostic variables (e.g., OS and DFS), corresponding hazard ratio (HR) and 95% confidence interval (CI), clinicopathologic parameters, and data extraction method for OS, along with follow-up time. If the study only showed Kaplan-Meier curves without listing accurate HR and 95% CI, then the HR and 95% CI were extrapolated based on Engauge Digitizer 4.1 software indirectly (18). Any disagreement was resolved after prompt discussion with the corresponding author.
We determined the expression of MAFG-AS1 in normal tissues with diverse tumor tissues utilizing Gene Expression Profiling Interactive Analysis (GEPIA) which comprised 9736 tumors clinical samples based on GTEx and TCGA data, and it has been widely adopted to validate meta-analysis results (19–21). P < 0.01 was regarded as significantly statistical.
Review Manager 5.3 software were adopted for statistical analysis. EndNote 20.2 software was utilized for document management. We calculated the association between MAFG-AS1 expression and clinicopathologic parameters across studies using pooled odds ratios (ORs) and 95% CIs. Besides, HR and 95% CIs were estimated to investigate the correlation between MAFG-AS1 expression and the OS of various malignancies. Moreover, the fixed- or random-effects models were applied to determine the summarized OR or HR and 95% corresponding CIs based on between-study heterogeneity. If I2 ≥ 50%, the random-effects model was selected. Otherwise, the fixed-effects model was utilized. P < 0.05 was deemed as statistically significant. Furthermore, sensitivity analysis was conducted by sequentially omitting individual studies to appraise whether the results were evidently impacted by individual study if at least five studies were involved. Subsequently, Begg’s and Egger’s tests were performed using Stata 15.1 software to objectively demonstrate publication bias, if at least ten studies were included, and P > 0.05 was supposed that there was no publication bias existing among studies.
Figure 1 depicts the literature selection process. After the preliminary screening search of six databases, 226 records concerning the association of MAFG-AS1 and cancer prognosis were retrieved. Subsequently, after removing 175 duplicate publications, the remaining 51 studies proceeded to further estimation. We then removed an additional 27 studies as they met the exclusion criteria. Thereafter, 9 records were excluded due to lack of sufficient data after the full-text screening. Finally, 15 studies published between 2018 and 2022 were recruited for the meta-analysis.
The characteristics of the 15 included publications are exhibited in Table 1. All studies were performed in China with sample sizes varying from 40 to 172 and published from 2018 to 2022. A total of 1187 cases were divided into low and high MAFG-AS1 expression groups. Additionally, qRT-PCR was utilized to determine MAFG-AS1 expression in tissues. Moreover, 12 studies reported clinicopathologic parameters and ten studies offered OS, five of which provided OS data directly. Besides, there were nine kinds of malignant tumors in this meta-analysis, including colorectal cancer, bladder carcinoma, breast cancer, gastric cancer, ESCC, gastric adenocarcinoma, ovarian cancer, hepatocellular carcinoma, and lung adenocarcinoma.
Ten studies estimated the potential association of MAFG-AS1 expression with tumor stage. Due to low heterogeneity among studies (I2 = 35%), the fixed-effects model was applied. As shown in Figure 2A, high MAFG-AS1 expression was noticeably associated with advanced tumor stage (P < 0.00001). Regarding the clinicopathologic parameter of LNM, elevated MAFG-AS1 expression substantially predicted LNM (P < 0.00001). Also, the fixed-effects model was used as no heterogeneity was detected (I2 = 0%) (Figure 2B). In addition, four studies enrolling 419 cancer patients indicated that high MAFG-AS1 expression was related to worse tumor differentiation (P = 0.03) (Figure 2C). Similarly, the fixed-effects model was utilized for low heterogeneity (I2 = 19%). Furthermore, the pooled results were robust after examining with sensitivity analysis.
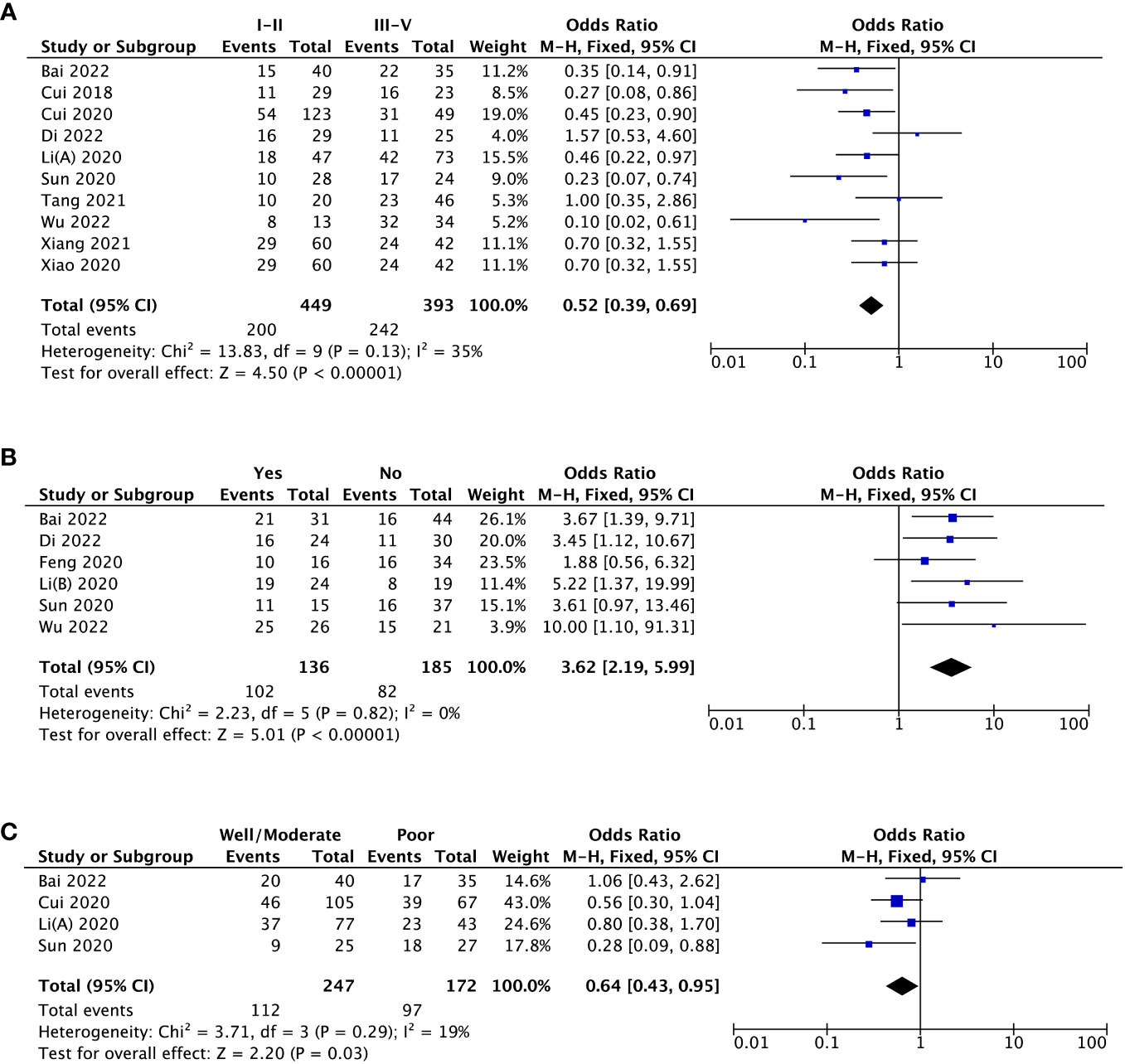
Figure 2 The forest plots assessing the association between MAFG-AS1 expression and clinicopathological parameters [(A), tumor stage; (B), lymph node metastasis; (C), tumor differentiation].
With respect to tumor size, data from four studies suggested that there was no striking association between MAFG-AS1 expression and tumor size (P = 0.53) (Figure 3A). Additionally, three trial studies in which all patients were female were removed in analyzing the covariate of gender. The result revealed that MAFG-AS1 expression was not correlated with patient gender (P = 0.47) (Figure 3B). Also, a total of three studies explored the relationship between MAFG-AS1 expression and patient age (≤ 60 or > 60). The result implied that there was no significant correlation between MAFG-AS1 expression and patient age (P = 0.93) (Figure 3C). The fixed-effects model was adopted for all pooled outcomes mentioned above. Simultaneously, the results were not affected by individual studies after sensitivity analysis. Furthermore, Begg’s and Egger’s tests also indicated that there was no publication bias across studies (P > 0.05) (Figure 4).
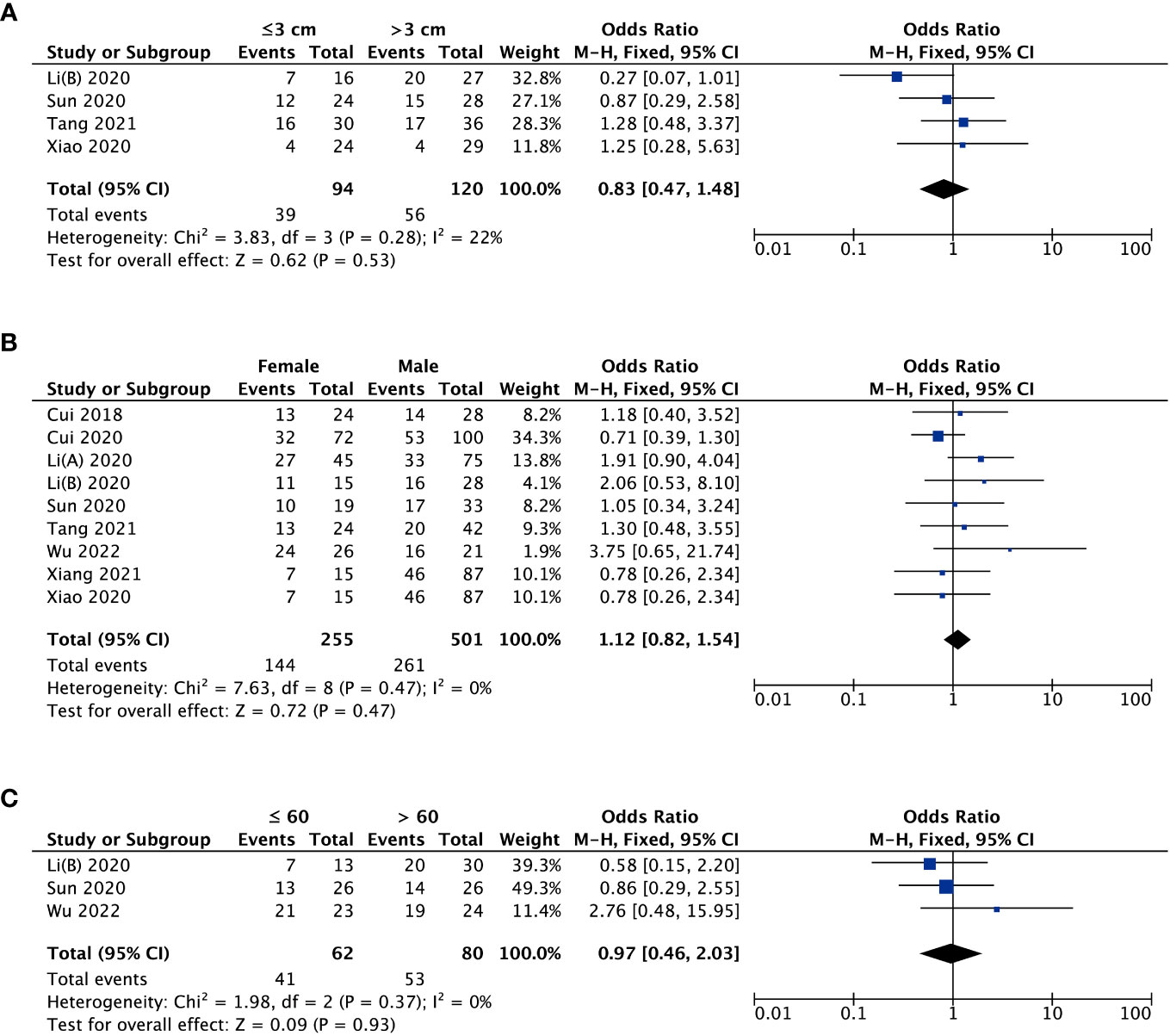
Figure 3 The forest plots assessing the association between MAFG-AS1 expression and clinicopathological parameters [(A), tumor size; (B), gender; (C), age].
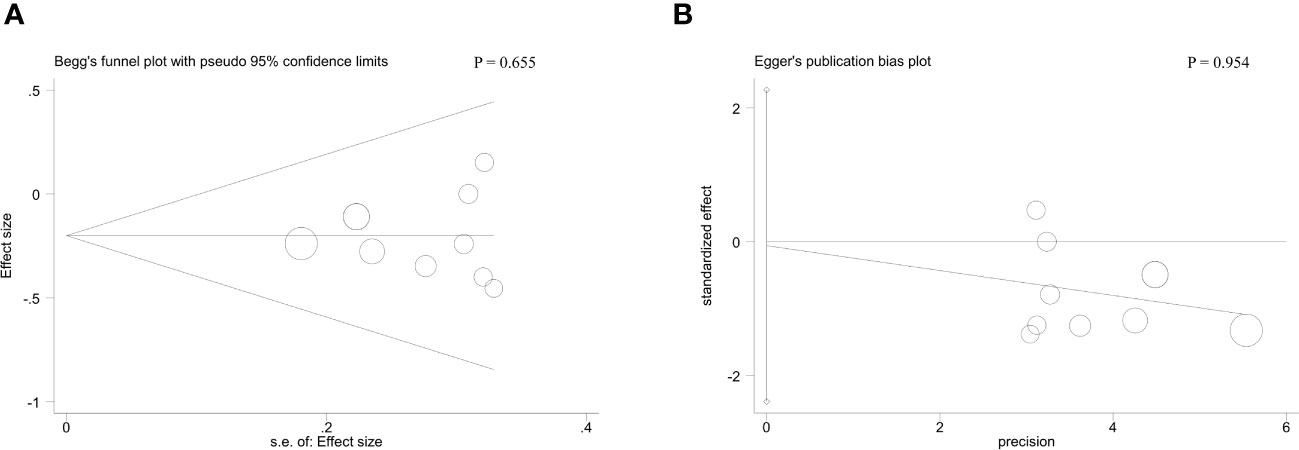
Figure 4 The Begg’s test (A) and Egger’s test (B) for the association between MAFG-AS1 expression with tumor stage.
A total of ten studies were enrolled to analyze MAFG-AS1 expression with OS. Because there was considerable heterogeneity among studies (I2 = 67%); thus, we removed one study (29) using sensitivity analysis, and the heterogeneity was reduced from 67% to 48%. Similarly, the fixed-effects model was conducted. Our results revealed that high MAFG-AS1 expression was significantly related to shorter OS (P < 0.00001) (Figure 5). Additionally, the subgroup analysis was performed based on cancer type, sample size, follow-up time, and extracted method. As illustrated in Table 2, high MAFG-AS1 expression predicted poor OS in patients with malignancy compared to low MAFG-AS1 expression, regardless of cancer type, sample size, follow-up time, and extracted method (P < 0.05).
To further strengthen our conclusion, we analyzed the MAFG-AS1 expression in diverse malignancies with the help of GEPIA online gene analysis tool. Figure 6 indicates that the expression of MAFG-AS1 was dramatically elevated in ten kinds of cancers (P < 0.01), such as bladder urothelial carcinoma (BLCA), pancreatic adenocarcinoma (PAAD), uterine carcinosarcoma (UCS), rectum adenocarcinoma (READ), lung squamous cell carcinoma (LUSC), breast invasive carcinoma (BRCA), lung adenocarcinoma (LUAD), thymoma (THYM), colon adenocarcinoma (COAD), and pheochromocytoma and paraganglioma (PCPG).
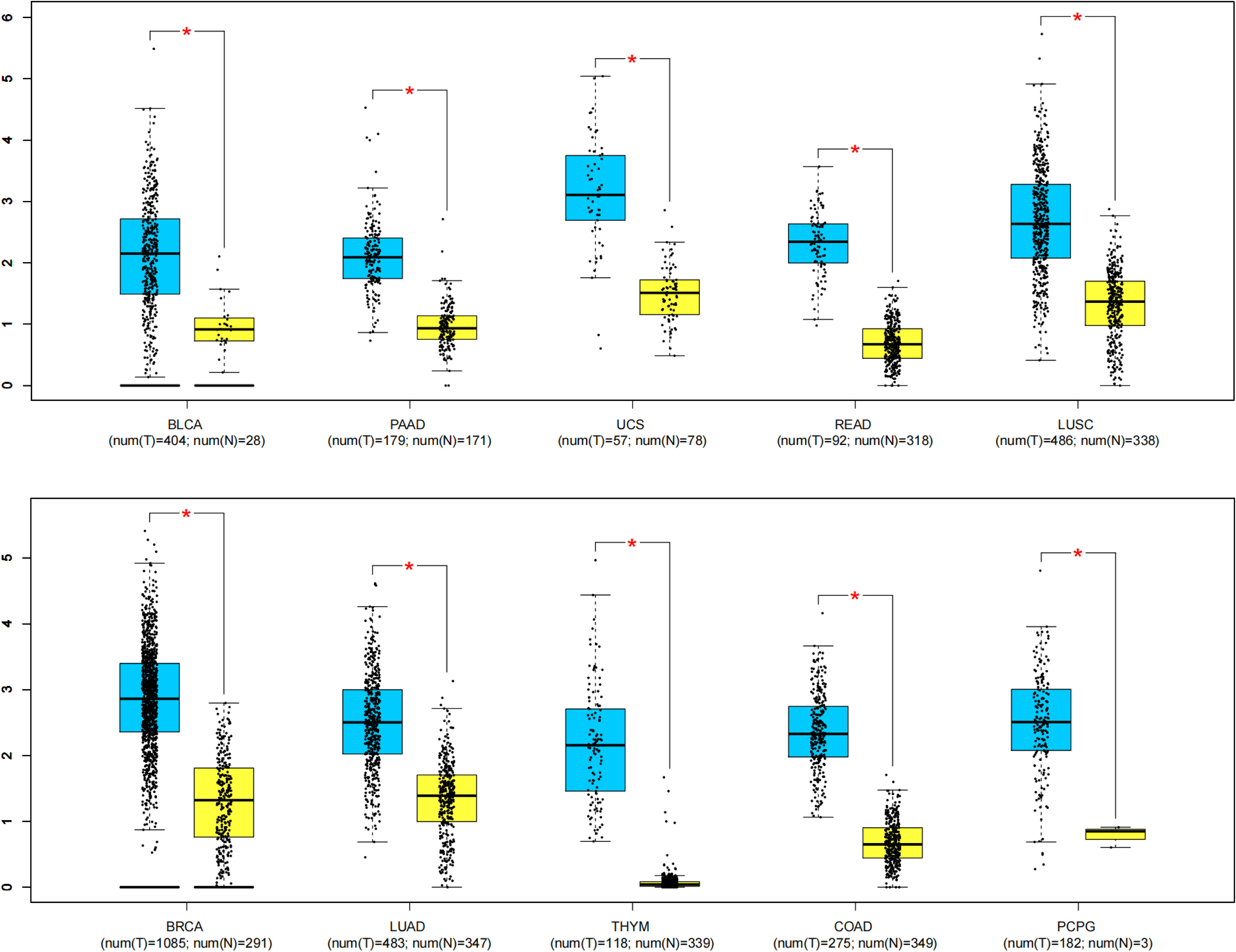
Figure 6 MAFG-AS1 expression in ten kinds of tumor tissues (blue) vs. normal tissues (yellow). “*” P < 0.01. T, tumor tissues; N, normal tissues.
With the rapid development of biomedicine, the role of lncRNAs in various physiological and pathological processes of cancer has been gradually clarified, which can even be explored as promising prognostic biomarkers and therapeutic targets for malignancies (33, 34). As a novel lncRNA, MAFG-AS1 is recognized as a vital oncogene since it has intimate terms with unfavorable clinicopathologic parameters and poor prognosis in cancer patients (12). Mechanistically, the underlying cell biological functions behind its role in carcinomas are extremely complex.
First, in urologic tumors, elevated MAFG-AS1 may promote invasion, metastasis, proliferation, and EMT of BLCA via regulation of the HUR/PTBP1 axis, and ultimately contribute to poor OS (23). Similarly, MAFG-AS1 was also shown to activate the PCBP2/FPN1 axis, which then inhibited ferroptosis in BUC cells and subsequently increased cisplatin resistance (29). Besides, Li et al. identified that MAFG-AS1 overexpression repressed miR-143-3p, which further facilitated the proliferation, metastasis, and invasion of bladder cancer cells (14). Also, the same results were confirmed by Sun’s study (15). Meanwhile, cellular experiments proved that MAFG-AS1 could promote the development of bladder cancer by regulating the miR-125b-5p/SphK1 axis (28). Clinically, a lot of patients with bladder cancer struggle to achieve satisfying treatment outcomes due to immunotherapy resistance (35–37). However, there was scarce study investigating the correlation between MAFG-AS1 and immunotherapy resistance. Therefore, it will be a novel direction to explore their relationship in the future. Second, as for gastrointestinal cancers, MAFG-AS1 regulated miR-505 and PLK1 to increase the proliferation rate of gastric cancer cells and decrease the OS of patients with gastric cancer (27). In colon cancer cells, MAFG-AS1 interacted with miR-147b and NDUFA4 and then contributed to cell glycolysis, which was closely associated with cell apoptosis, cycle progression, invasion, and cycle progression (22). In another study, downregulation of MAFG-AS1 could inhibit the invasion, proliferation, migration, and tumorigenesis of colorectal cancer cells via downregulating HOXB8 and upregulating miR-149-3p (38). Overexpression of MAFG-AS1 was established in hepatocellular carcinoma as well. Mechanistic outcomes discovered that MAFG-AS1 accelerated hepatocellular carcinoma cells EMT, migration, and proliferation via miR-3196/STRN4 (39). Third, in breast cancer, hyperactivation of MAFG-AS1 improved the proliferation and migration of tumor cells via modifying the miR-150-5p/MYB axis (40). For hormone receptor-positive breast cancer, MAFG-AS1 could inhibit tumor cell apoptosis and accelerate proliferation via miR-339-5p/CDK2 axis (16), which was consistent with the findings of Li’s study (41). Notably, Gao et al. observed that MAFG-AS1 contributed to the progression and autophagy of breast cancer by regulating miR-3612 and FKBP4 (42). For other types of tumors, Wu et al. recently demonstrated that augmented expression of MAFG-AS1 could promote lung adenocarcinoma cells EMT, proliferation, invasion, and migration by modulating the miR-3196/SOX12 pathway (32). Additionally, Zhao et al. verified that MAFG-AS1 was upregulated in glioblastoma, and that overexpression of MAFG-AS1 inhibited cell apoptosis and facilitated cell migration by affecting miR-34a (43). Taken together, mounting evidence suggested that MAFG-AS1 served an imperative role in tumor development and progression.
This meta-analysis involving 1187 cases and nine kinds of malignancies demonstrated that high MAFG-AS1 expression was obviously correlated with advanced tumor stage (OR = 0.52, 95%CI [0.39, 0.69], P < 0.00001), earlier LNM (OR = 3.62, 95%CI [2.19, 5.99], P < 0.00001), worse tumor differentiation (OR = 0.64, 95%CI [0.43, 0.95], P = 0.03), and poor OS (HR = 1.94, 95%CI [1.72, 2.19], P < 0.00001). Nevertheless, there was no remarkable association between MAFG-AS1 expression and tumor size, gender, along with age. Especially, GEPIA database was further adopted to strengthen our results as broadly as possible, and elevated MAFG-AS1 expression was also observed in ten types of cancers, in which six kinds of cancers such as PAAD, UCS, READ, LUSC, THYM, and PCPG were not reported in current published study exploring the significance of MAFG-AS1 with cancer prognosis. Meanwhile, the sensitivity analysis and Begg’s and Egger’s tests supported that our results were robust and reliable. Collectively, our study indicated that MAFG-AS1 can be considered as a novel biomarker for predicting cancer prognosis. Hence, we believe that this meta-analysis will inspire more researchers to investigate the correlation between MAFG-AS1 and malignancy prognosis.
This study has several limitations. First, some studies only provided Kaplan-Meier curves on OS, and thus we indirectly extracted the HR and corresponding 95%CI data utilizing Engauge Digitizer 4.1 software introduced by Tierney et al. (18), which might be inevitably influenced by subjective factors. Second, although we didn’t consider ethnical and geographical restrictions in our records screening procedure, all of the included studies were from China, which, to some extent, might confine the representativeness of the pooled results to other regions outside China. Third, all included studies were from single clinical research; thus, some cutoff values, such as age and tumor size, were inconsistent. Therefore, the most adopted cutoff values were selected for this meta-analysis, which limited our ability to estimate the association between MAFG-AS1 and cancer prognosis with insufficient statistical power. Fourth, though disease-free survival (DFS) is one of the imperative concerns for cancer patients, only one included study explored the correlation between MAFG-AS1 and DFS; therefore, we failed to assess their relationship, which might be an inherent deficiency of this study. Given these limitations above, more high-quality multicenter studies are required to further clarify the significance of MAFG-AS1 in future cancer prognosis.
In conclusion, this meta-analysis confirmed that MAFG-AS1 is markedly elevated in various malignancies, and that high MAFG-AS1 expression is significantly correlated with advanced tumor stage, LNM, worse tumor differentiation, and poor OS when compared to low MAFG-AS1 expression. Therefore, MAFG-AS1 may be a potential biomarker and can be adopted to accelerate progression against cancer.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
LG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. LH: Conceptualization, Formal analysis, Investigation, Validation, Writing – original draft. LX: Funding acquisition, Methodology, Resources, Validation, Writing – original draft. LJ: Methodology, Resources, Visualization, Writing – review & editing. XL: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received funding from the Three-Year Action Plan on Promoting Clinical Skills and Clinical Innovation in Municipal Hospitals (grant numbers SHDC2020CR4058).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
2. Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond) (2021) 41(10):1037–48. doi: 10.1002/cac2.12197
3. Jones RP, Pugh SA, Graham J, Primrose JN, Barriuso J. Circulating tumour DNA as a biomarker in resectable and irresectable stage IV colorectal cancer; a systematic review and meta-analysis. Eur J Cancer (2021) 144:368–81. doi: 10.1016/j.ejca.2020.11.025
4. Lin D, Shen L, Luo M, Zhang K, Li J, Yang Q, et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther (2021) 6(1):404. doi: 10.1038/s41392-021-00817-8
5. Mertens LS, Meijer RP, Alfred Witjes J. Positron emission tomography/computed tomography for staging of bladder cancer: A continuing clinical controversy. Eur Urol (2023) 83(2):95–6. doi: 10.1016/j.eururo.2022.09.017
6. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
7. Duffy MJ, Sturgeon CM, Sölétormos G, Barak V, Molina R, Hayes DF, et al. Validation of new cancer biomarkers: a position statement from the European group on tumor markers. Clin Chem (2015) 61(6):809–20. doi: 10.1373/clinchem.2015.239863
8. Engreitz JM, Ollikainen N, Guttman M. Long non-coding RNAs: spatial amplifiers that control nuclear structure and gene expression. Nat Rev Mol Cell Biol (2016) 17(12):756–70. doi: 10.1038/nrm.2016.126
9. Barbieri I, Kouzarides T. Role of RNA modifications in cancer. Nat Rev Cancer (2020) 20(6):303–22. doi: 10.1038/s41568-020-0253-2
10. Serghiou S, Kyriakopoulou A, Ioannidis JPA. Long noncoding RNAs as novel predictors of survival in human cancer: a systematic review and meta-analysis. Mol Cancer (2016) 15(1):50. doi: 10.1186/s12943-016-0535-1
11. Di S, Bai R, Lu D, Chen C, Ma T, Zou Z, et al. Long non-coding RNA MAFG-AS1 promotes proliferation and metastasis of breast cancer by modulating STC2 pathway. Cell Death Discovery (2022) 8(1):249. doi: 10.1038/s41420-022-01043-z
12. Huang Z, Zhang M, Li J, Lou C. Long non-coding RNA MAFG-AS1: A promising therapeutic target for human cancers. BioMed Pharmacother (2023) 163:114756. doi: 10.1016/j.biopha.2023.114756
13. Ahmadi M, Morshedzadeh F, Ghaderian SMH, Mousavi P, Habibipour L, Peymani M, et al. Carcinogenic roles of MAFG-AS1 in human cancers. Clin Transl Oncol (2023). doi: 10.1007/s12094-023-03246-x
14. Li D, Zhong S, Zhu Z, Jiang X, Zhang J, Gu J, et al. LncRNA MAFG-AS1 promotes the progression of bladder cancer by targeting the miR-143-3p/COX-2 axis. Pathobiology (2020) 87(6):345–55. doi: 10.1159/000509957
15. Sun X, Cai Y, Hu X, Mo M, Zhao C, He W, et al. Long noncoding RNA MAFG-AS1 facilitates bladder cancer tumorigenesis via regulation of miR-143-3p/SERPINE1 axis. Transl Cancer Res (2020) 9(11):7214–26. doi: 10.21037/tcr-20-1971
16. Feng J, Wen T, Li Z, Feng L, Zhou L, Yang Z, et al. Cross-talk between the ER pathway and the lncRNA MAFG-AS1/miR-339-5p/CDK2 axis promotes progression of ER+ breast cancer and confers tamoxifen resistance. Aging (Albany NY) (2020) 12(20):20658–83. doi: 10.18632/aging.103966
17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339:b2535. doi: 10.1136/bmj.b2535
18. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
19. Li C, Tang Z, Zhang W, Ye Z, Liu F. GEPIA2021: integrating multiple deconvolution-based analysis into GEPIA. Nucleic Acids Res (2021) 49(W1):W242–6. doi: 10.1093/nar/gkab418
20. Lin G, Wang Y, Deng L, Ye T. Prognostic effect of lncRNA BBOX1-AS1 in Malignancies: a meta-analysis. Front Genet (2023) 14:1234040. doi: 10.3389/fgene.2023.1234040
21. Lin G, Liu X, Cong C, Xu L. Prognostic significance of long noncoding RNA TTN-AS1 in various Malignancies. Cancer Rep (Hoboken) (2023) 6(10):e1876. doi: 10.1002/cnr2.1876
22. Cui S, Yang X, Zhang L, Zhao Y, Yan W. LncRNA MAFG-AS1 promotes the progression of colorectal cancer by sponging miR-147b and activation of NDUFA4. Biochem Biophys Res Commun (2018) 506(1):251–8. doi: 10.1016/j.bbrc.2018.10.112
23. Xiao M, Liu J, Xiang L, Zhao K, He D, Zeng Q, et al. MAFG-AS1 promotes tumor progression via regulation of the HuR/PTBP1 axis in bladder urothelial carcinoma. Clin Transl Med (2020) 10(8):e241. doi: 10.1002/ctm2.241
24. Li C, Wu R, Xing Y. MAFG-AS1 is a novel clinical biomarker for clinical progression and unfavorable prognosis in gastric cancer. Cell Cycle (2020) 19(5):601–9. doi: 10.1080/15384101.2020.1728017
25. Qian CJ, Xu ZR, Chen LY, Wang YC, Yao J. LncRNA MAFG-AS1 Accelerates Cell Migration, Invasion and Aerobic Glycolysis of Esophageal Squamous Cell Carcinoma Cells via miR-765/PDX1 Axis. Cancer Manag Res (2020) 12:6895–908. doi: 10.2147/cmar.S262075
26. Cui W, Wang Y, Shen X, Wu X, Liu H, Xu X. High-expression of LncRNA MAFG-AS1 is associated with the prognostic of patients with colorectal cancer. Rev Assoc Med Bras (1992) (2020) 66(11):1530–5. doi: 10.1590/1806-9282.66.11.1530
27. Fu Y, Wen J, Li X, Gong M, Guo Z, Wang G. LncRNA MAFG-AS1 upregulates polo-like kinase-1 by sponging miR-505 to promote gastric adenocarcinoma cell proliferation. Crit Rev Eukaryot Gene Expr (2021) 31(5):27–32. doi: 10.1615/CritRevEukaryotGeneExpr.2021038813
28. Tang C, Wu Y, Wang X, Chen K, Tang Z, Guo X. LncRNA MAFG-AS1 regulates miR-125b-5p/SphK1 axis to promote the proliferation, migration, and invasion of bladder cancer cells. Hum Cell (2021) 34(2):588–97. doi: 10.1007/s13577-020-00470-3
29. Xiang L, Zeng Q, Liu J, Xiao M, He D, Zhang Q, et al. MAFG-AS1/MAFG positive feedback loop contributes to cisplatin resistance in bladder urothelial carcinoma through antagonistic ferroptosis. Sci Bull (Beijing) (2021) 66(17):1773–88. doi: 10.1016/j.scib.2021.01.027
30. Bai Y, Ren C, Wang B, Xue J, Li F, Liu J, et al. LncRNA MAFG-AS1 promotes the Malignant phenotype of ovarian cancer by upregulating NFKB1-dependent IGF1. Cancer Gene Ther (2022) 29(3-4):277–91. doi: 10.1038/s41417-021-00306-8
31. Tian Y, Wang J, Tian G, Li B, Chen M, Sun X. Long non-coding RNA MAFG-AS1 as a potential biomarker for hepatocellular carcinoma: linkage with tumor features, markers, liver functions, and survival profile. Front Surg (2022) 9:848831. doi: 10.3389/fsurg.2022.848831
32. Wu Q, Jiang J. LncRNA MAFG-AS1 promotes lung adenocarcinoma cell migration and invasion by targeting miR-3196 and regulating SOX12 expression. Mol Biotechnol (2022) 64(9):970–83. doi: 10.1007/s12033-022-00455-7
33. Boriack-Sjodin PA, Ribich S, Copeland RA. RNA-modifying proteins as anticancer drug targets. Nat Rev Drug Discovery (2018) 17(6):435–53. doi: 10.1038/nrd.2018.71
34. Goodall GJ, Wickramasinghe VO. RNA in cancer. Nat Rev Cancer (2021) 21(1):22–36. doi: 10.1038/s41568-020-00306-0
35. Yu A, Hu J, Fu L, Huang G, Deng D, Zhang M, et al. Bladder cancer intrinsic LRFN2 drives anticancer immunotherapy resistance by attenuating CD8+ T cell infiltration and functional transition. J Immunother Cancer (2023) 11(10):e007230. doi: 10.1136/jitc-2023-007230
36. Li H, Chen J, Li Z, Chen M, Ou Z, Mo M, et al. S100A5 attenuates efficiency of anti-PD-L1/PD-1 immunotherapy by inhibiting CD8+ T cell-mediated anti-cancer immunity in bladder carcinoma. Adv Sci (Weinh) (2023) 10(25):e2300110. doi: 10.1002/advs.202300110
37. Cai Z, Chen J, Yu Z, Li H, Liu Z, Deng D, et al. BCAT2 shapes a noninflamed tumor microenvironment and induces resistance to anti-PD-1/PD-L1 immunotherapy by negatively regulating proinflammatory chemokines and anticancer immunity. Adv Sci (Weinh) (2023) 10(8):e2207155. doi: 10.1002/advs.202207155
38. Ruan Z, Deng H, Liang M, Xu Z, Lai M, Ren H, et al. Downregulation of long non-coding RNA MAFG-AS1 represses tumorigenesis of colorectal cancer cells through the microRNA-149-3p-dependent inhibition of HOXB8. Cancer Cell Int (2020) 20:511. doi: 10.1186/s12935-020-01485-4
39. Chen T, Huang B, Pan Y. Long non-coding RNA MAFG-AS1 promotes cell proliferation, migration, and EMT by miR-3196/STRN4 in drug-resistant cells of liver cancer. Front Cell Dev Biol (2021) 9:688603. doi: 10.3389/fcell.2021.688603
40. Jia H, Wu D, Zhang Z, Li S. Regulatory effect of the MAFG-AS1/miR-150-5p/MYB axis on the proliferation and migration of breast cancer cells. Int J Oncol (2021) 58(1):33–44. doi: 10.3892/ijo.2020.5150
41. Li H, Zhang GY, Pan CH, Zhang XY, Su XY. LncRNA MAFG-AS1 promotes the aggressiveness of breast carcinoma through regulating miR-339-5p/MMP15. Eur Rev Med Pharmacol Sci (2019) 23(7):2838–46. doi: 10.26355/eurrev_201904_17561
42. Gao Z, Zheng G, Gong X, Hu H, Shao L, Pang Y, et al. LncRNA MAFG-AS1 deregulated in breast cancer affects autophagy and progression of breast cancer by interacting with miR-3612 and FKBP4 invitro. Biochem Biophys Res Commun (2022) 616:95–103. doi: 10.1016/j.bbrc.2022.05.020
Keywords: lncRNA MAFG-AS1, malignancies, meta-analysis, prognosis, review
Citation: Lin G, Liu H, Lin J, Liu X and Xu L (2023) Correlation between long non-coding RNA MAFG-AS1 and cancer prognosis: a meta-analysis. Front. Oncol. 13:1286610. doi: 10.3389/fonc.2023.1286610
Received: 31 August 2023; Accepted: 21 November 2023;
Published: 07 December 2023.
Edited by:
Mina Tabrizi, Tehran University of Medical Sciences, IranReviewed by:
Jiao Hu, Central South University, ChinaCopyright © 2023 Lin, Liu, Lin, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lianwei Xu, eHVfbGlhbndlaTI4MDBAc2h1dGNtLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.