- 1Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Ministry of Education), Sichuan University, Chengdu, China
Background: Studies evaluating the prognostic significance of lymphovascular space invasion (LVSI) in early stage endometrial cancer (EC) are conflicting.
Objectives: To evaluate whether LVSI identified in stage I EC is associated with worse survival.
Search strategy: A comprehensive literature search of three databases (Embase, PubMed, and Cochrane) was performed up to April 30th 2023.
Selection criteria: Cohort studies that have evaluated the relationship between LVSI and prognosis in patients with stage I EC were included.
Data collection and analysis: Two authors independently assessed the studies for inclusion, extracted the data of recurrence and survival, and conducted meta-analysis using random effects model. Heterogeneity was evaluated by I2 test.
Main results: A total of 15 studies involving 6,705 patients were included in the meta-analysis. The overall pooled rate of LVSI was 14% [95% confidence interval (CI) CI 0.09-0.18] in stage I EC. LVSI was significantly associated with a higher risk of recurrence [odds ratio (OR) = 2.79, 95%CI 2.07-3.77], reduced overall survival (OS) [hazard ratio (HR)=5.19, 95%CI 3.33-8.07] and recurrence free survival (RFS) [HR = 5.26, 95%CI 3.45-8.02] in stage I EC patients. Similarly, LVSI was associated with an increased risk of recurrence [OR= 3.10, 95%CI 2.13-4.51], decreased OS [HR=5.52, 95%CI 2.16-14.09] and RFS [HR = 4.81, 95%CI 2.34-9.91] in stage IA grade 1 or 2 endometrioid carcinoma patients.
Conclusion: The presence of LVSI in stage I EC and in stage IA, grade 1 or 2 endometrioid carcinoma is associated with an increased risk of recurrence, lower OS and RFS.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier 42023425231.
1 Introduction
Endometrial cancer (EC) is the most common malignancy of the female reproductive system in developed countries, with more than 65,000 new cases reported in the United States each year (1). The prognosis of EC is influenced by various factors such as age, clinical stage, tumor differentiation, and pathological type (2). Lymphovascular space invasion (LVSI), defined as the presence of tumor cells in lymphatic or small blood vessels outside the tumor core. Up to 35% of EC patients are reported to have LVSI (3). The National Comprehensive Cancer Network (NCCN) guideliness (version 1.2023, Uterine Neoplasms) recommend quantifying LVSI to guide postoperative treatment (4). Besides, the 2023 International Federation of Gynecology and Obstetrics (FIGO) surgical staging system include LVSI in staging (5).
Nevertheless, conflicting results have been reported regarding the impact of LVSI on survival outcomes in early-stage EC. Many studies have shown that LVSI-positive is associated with lower overall survival (OS), higher recurrence, and increased risks of lymph node and distant metastasis, and is a poor prognostic factor for EC (6–8). LVSI has been reported as a risk factor, even in cases of stage I EC without lymph node metastasis (9–11). Conversely, several studies have concluded that LVSI does not significantly affect survival rates in patients with early-stage EC (12–14). For instance, Okugawa et al. (14) reported that the 5-year EC specific survival rates for tumors in LVSI-positive and LVSI-negative patients were 97.0% and 98.9%, which do not show significant difference. Furthermore, there is a lack of consensus on whether adjuvant therapy is necessary for stage I EC patients with LVSI after surgery. It is crucial to determine which EC patients with LVSI, especially those in the low-risk group (defined as stage IA, grade 1 or 2 endometrioid carcinoma), would benefit from postoperative adjuvant therapy.
Therefore, we aimed to address the knowledge gap regarding the independent prognostic significance of LVSI in early-stage EC, particularly in the low-risk group. We investigated the impact of LVSI on survival and recurrence in stage I EC. The findings of this study will contribute to improving the management and treatment decisions for stage I EC patients, aiding in the identification of those who would benefit from adjuvant therapy.
2 Methods
This meta-analysis was conducted in strict accordance with the Systematic Review and Meta-Analysis Preferred Reporting Project (PRISMA) statements (15), and registered in the International Register of Prospective Systems Evaluation (CRD: 42023425231).
2.1 Literature search
We performed a comprehensive literature search of three databases: Embase, PubMed, and Cochrane, from database inception to April 30th 2023. Cohort studies that have evaluated the relationship between LVSI and prognosis in patients with stage I EC were included. The predefined search string was the following: (endometrial cancer OR endometrial carcinoma OR uterine cancer OR uterine carcinoma of endometrium) AND (early stage OR stage IA OR stage I OR FIGO I OR FIGO IA) AND (lymphovascular space invasion OR LVSI). Other literature resources, such as the reference lists of eligible studies, were reviewed to identify additional studies. We applied language restriction that only publications in English were included. There were no restrictions on the publication time, publication status or article type.
2.2 Study selection
Two authors (ZJ Qin and YS Wang) independently screened the titles and abstracts based on the inclusion criteria to determine relevant studies. After initial screening, the full text of all potentially eligible articles were independently reviewed by two authors for further assessment. Any discrepancies were resolved through discussion with the corresponding author.
The inclusion criteria for our research were as follows: (a) studies that are either prospective or retrospective cohort studies; (b) inclusion of EC patients who have undergone surgical pathological staging, FIGO stage I or IA, and any pathological type; (c) analysis of the prognosis of patients with and without LVSI; and (d) availability of the full text. The exclusion criteria were as follows: (a) inability to extract valid outcome indicators from the literature; (b) non-English literature; (c) low scores in quality assessment; and (d) sample size less than 50.
2.3 Data extraction
Two authors (ZJ Qin and YL Chen) independently extracted the following data from each study: first author name, publication year, country, inclusion of EC staging and pathological type, time of initial treatment, median follow-up time, LVSI incidence, administration of adjuvant therapy, and outcome data related to the potential relationship between LVSI and prognosis, including recurrence, OS, and recurrence-free survival (RFS). Any discrepancies in data extraction were resolved through discussion with the corresponding author.
2.4 Quality assessment
The methodological quality of the studies included in the research was independently assessed by two authors (ZJ Qin and A Zheng) according to the Newcastle-Ottawa Quality Assessment Scale (NOS) (16). For the ‘comparability of cohort based on design or analysis’ criterion, studies that controlled for histological type of endometrioid adenocarcinoma received 1 star. For the ‘adequacy of follow-up time in the cohort’ criterion, a median follow-up time of more than 40 months was rated 1 star. Studies scoring 6 stars or more were considered high-quality and were included in our meta-analysis.
2.5 Statistical analysis
Statistical analysis was conducted using the metan, metabias, and metaprop software packages in STATA 15.0 (Statacorp, College Station, TX, USA). We calculated the odds ratio (OR) of recurrence, the hazard ratio (HR) of RFS and OS, as well as their 95% confidence intervals (Cls). In studies that only reported OS and RFS as Kaplan-Meier curves and where HR and 95% intervals could not be obtained from the original text, the necessary data were extracted using engage Digitizer 4.1 (http://sourceforge.net/projects/digitizer/), and HR was calculated using the tool recommended by Tierney et al (17).
Heterogeneity was quantified using the I2 statistic: I2 <30% was considered low heterogeneity, 30% <I2 <50% was considered moderate heterogeneity, and I2 >50% was considered high heterogeneity (18). Given the clinical heterogeneity of the studies included, we used a random-effects model for all meta-analyses. We created Galbraith radial plots to explore potential causes of heterogeneity. We also conducted subgroup analyses based on FIGO stage, histological type, and grade of differentiation. The Begg’s test was used to assess the risk of publication bias, with a p-value of less than 0.1 considered as evidence of significant publication bias.
3 Results
3.1 Characteristics of the included studies
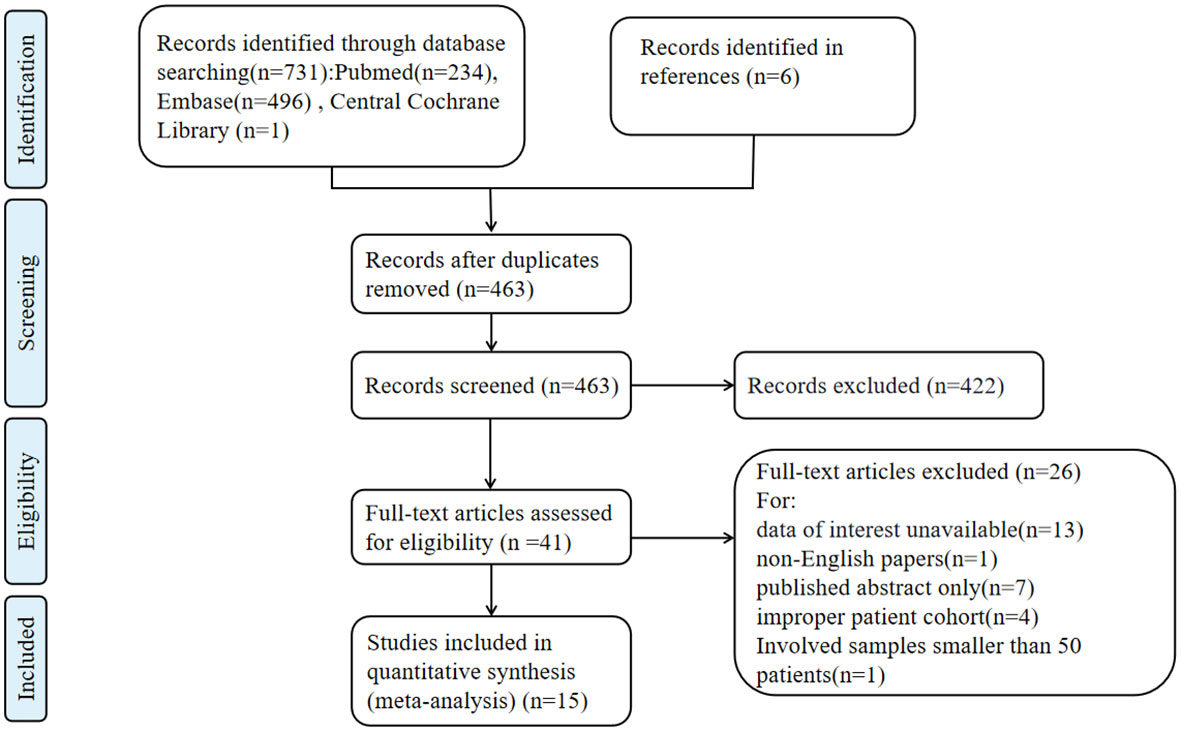
A total of 731 records were identified through the literature search. After removing duplicates, 463 articles underwent title and abstract screening, and 41 articles were selected for full-text screening. Ultimately, 15 studies (3, 6–14, 19–23) involving 6705 patients were included in the data analysis. The characteristics of the 15 included studies are shown in Table 1. The study selection process is illustrated in Figure 1. These studies were published between 2007 and 2022. All the included studies achieved a Newcastle-Ottawa Scale (NOS) score of 6 or higher, with the highest score being 8.
3.2 Rate of LVSI
A total of 15 studies involving 6,705 patients investigated the incidence of LVSI in early-stage EC. The pooled incidence of LVSI was 14% (95% CI 0.09-0.18; I2 = 96.28%, p < 0.001) in stage I EC (Figure 2A). Given the high heterogeneity observed among the LVSI meta-analyses of all studies, subgroup analyses were conducted based on country, sample size, publication year, and pathological subtype. However, these subgroup analyses did not clearly identify the source of heterogeneity, and further analysis of individual study characteristics failed to explain the observed heterogeneity.
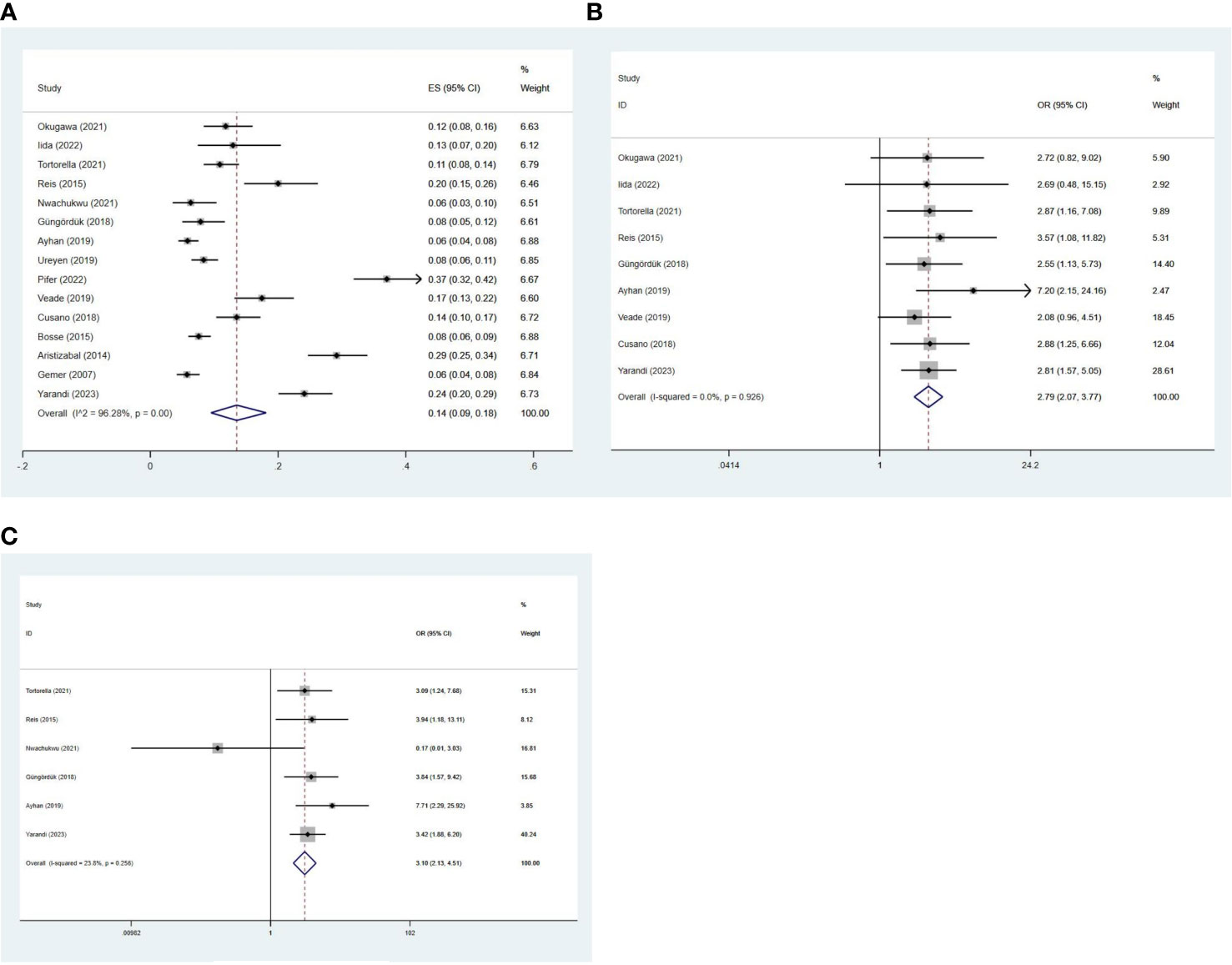
Figure 2 Forest plots of the (A) incidence of LVSI; potential relationships of LVSI with recurrence in (B) stage I and (C) stage IA G1/2 EC.
3.3 Relationship between LVSI and recurrence
Nine studies (3, 6–9, 11, 12, 14, 21) involving 3,337 patients evaluated the relationship between LVSI and recurrence. The recurrence rate for patients with LVSI was 18.10% (72/398), compared to 6.16% (181/2939) for patients without LVSI in stage I EC. The meta-analysis showed that patients with LVSI had a significantly higher risk of recurrence (OR = 2.79, 95%CI 2.07-3.77; I2 = 0%, p = 0.926) in stage I EC (Figure 2B).
Further analysis revealed that the recurrence rate for patients with LVSI was 14.02% (30/214), compared to 5.03% (108/2148) for patients without LVSI in stage IA grade 1 or 2 endometrioid carcinoma. Similarly, patients with LVSI also had a significantly higher recurrence rate (OR = 3.10, 95%CI 2.13-4.51; I2 = 23.8%, p = 0.256) in stage IA grade 1 or 2 endometrioid carcinoma (Figure 2C).
3.4 Relationship between LVSI and survival
Five studies (8–10, 14, 20) reported on the OS of 2,880 patients. LVSI-positive patients in stage I EC had a 5.19-fold higher risk of death than LVSI-negative patients (95%CI 3.33-8.07; I2 = 0, p = 0.739; Figure 3A). In stage IA grade 1 or 2 endometrioid carcinoma patients, LVSI-positive had a 5.52-fold higher risk of death than LVSI-negative patients (95%CI 2.16-14.09; I2 = 0, p = 0.729; Figure 3B). Additionally, six studies (8–10, 20, 23) reported on the RFS of 2,595 patients. LVSI significantly influenced RFS in stage I EC (HR = 5.26, 95%CI 3.45-8.02; I2 = 0, p = 0.537; Figure 3C) and stage IA grade 1 or 2 endometrioid carcinoma patients (HR = 4.81, 95%CI 2.34-9.91; I2 = 0, p = 0.596; Figure 3D).
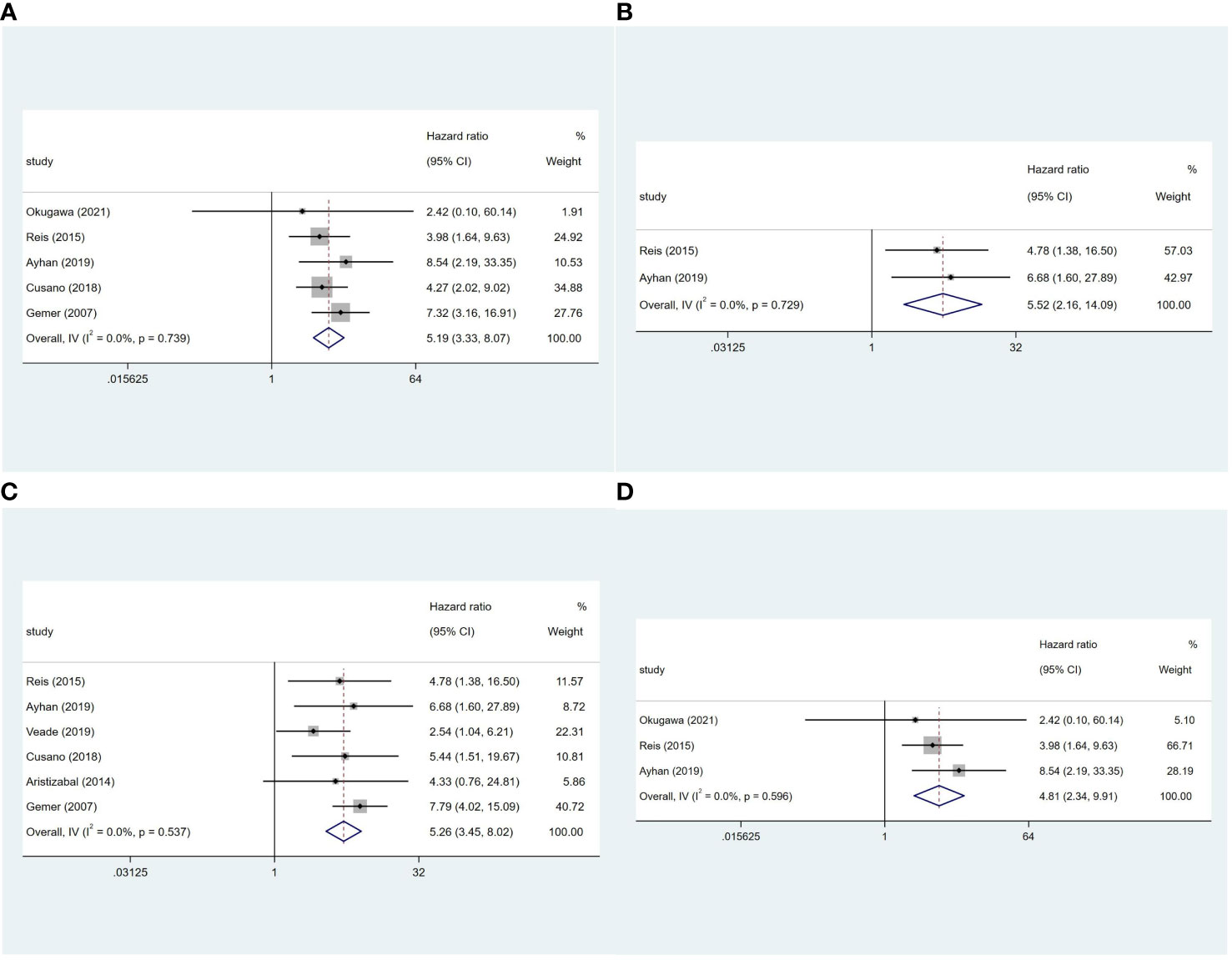
Figure 3 Forest plots of the potential relationships of LVSI with (A) overall survival in stage I and (B) stage IA G1/2 EC; (C) recurrence-free survival in stage I and (D) stage IA G1/2 EC.
3.5 Publication bias
Publication bias was assessed based on the LVSI rate and was reported in most of the included studies. Ultimately, 15 studies were included, and the Begg’s test (p=0.067) showed a potential source of publication bias. According to our funnel plot, studies with a lower LVSI rate are more likely to be published (Figure 4).
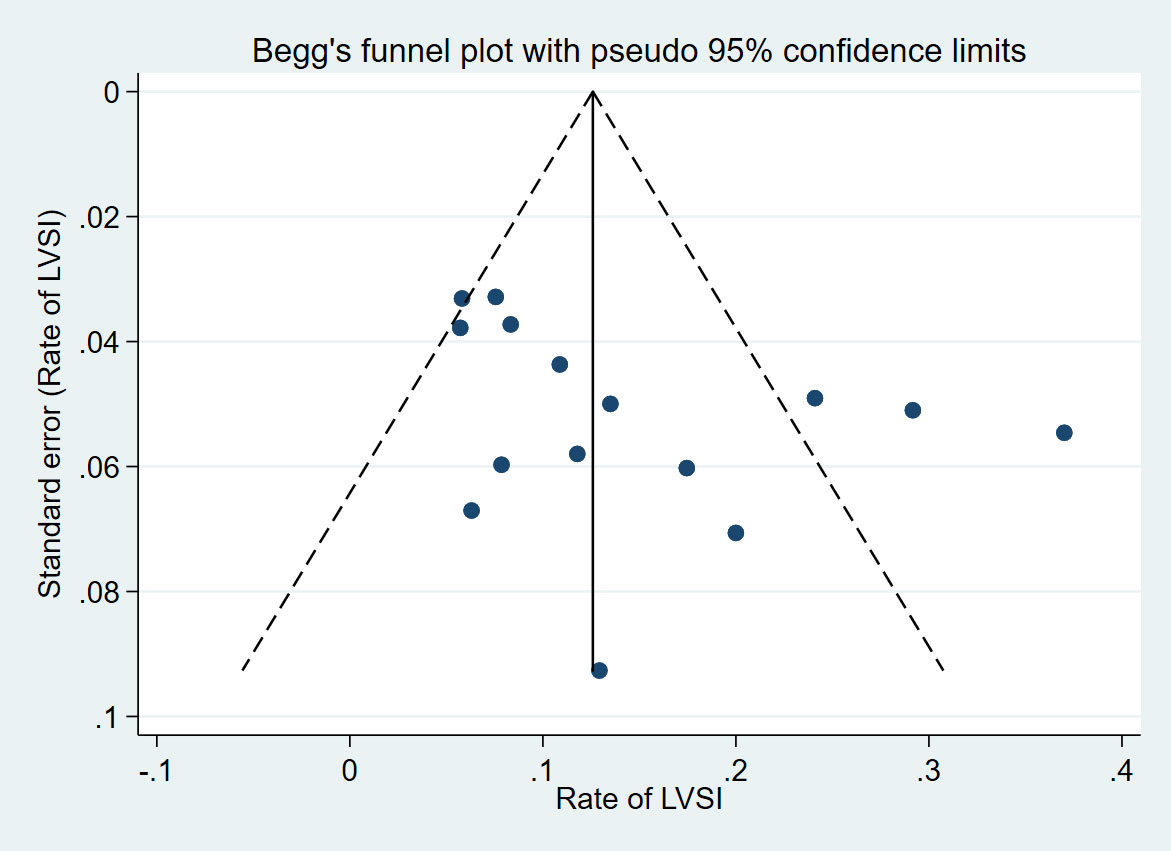
Figure 4 Funnel plot with pseudo 95% confidence limits of 15 included studies reporting the rate of LVSI.
4 Discussion
Our analysis revealed that approximately 14% (95% CI 0.09-0.18) of stage I EC patients had LVSI, consistent with the reported range of LVSI frequency in the literature ranging from 6% (8) to 37% (13). This variability may be attributed to the inclusion criteria that did not limit the pathological type of EC, encompassing both endometrioid adenocarcinoma and other types of EC. Additionally, the clinical stages included both stage IA and stage I, introducing clinical heterogeneity in LVSI positive rates.
Importantly, our findings demonstrate that LVSI is significantly associated with a higher risk of recurrence, not only in stage I EC (OR= 2.79, 95%CI 2.07-3.77) but also in stage IA grade 1 or 2 endometrioid carcinoma patients (OR= 3.10, 95%CI 2.13-4.51). These findings are in line with previous investigations (3, 9, 10, 24), suggesting that the presence of LVSI increases the risk of tumor recurrence. Additionally, LVSI was found to significantly influence RFS in both stage I EC (HR = 5.26, 95%CI 3.45-8.02) and stage IA grade 1 or 2 endometrioid carcinoma patients (HR = 4.81, 95%CI 2.34-9.91). The presence of LVSI in early endometrial cancer patients increases the risk of tumor recurrence, Bosse et al. (22) and Güngördük et al. (21) illustrated the presence of LVSI was an independent risk factor for recurrence. Besides, LVSI is independently associated with lymph node metastasis in women with early-stage endometrial cancer (25).
Furthermore, our meta-analysis demonstrated that patients with LVSI have worse OS in both stage I EC (HR=5.19, 95%CI 3.33-8.07) and stage IA grade 1 or 2 endometrioid carcinoma patients (HR=5.52, 95%CI 2.16-14.09) compared to patients without LVSI. Our research findings are in concordance with previous studies reporting a significant association between LVSI and decreased OS (3, 6, 7). However, it is important to note that there have been conflicting reports regarding the impact of LVSI on prognosis. For instance, Iida et al. (12) found no difference in prognosis between patients in stage IA and type 1 EC with and without LVSI, Okugawa et al. (14) reported that LVSI did not significantly affect cancer-specific survival or serve as a prognostic factor in stage IA endometrial cancer. The reason for this difference may be the specific study design that included the stage of early EC, pathological type, and the proportion of lymph nodes evaluated. The discrepancies in these findings highlight the need for further research to explore the prognostic value of LVSI in early EC.
It is crucial to determine which EC patients, especially those in the low-risk group, would benefit from postoperative adjuvant therapy. There is a lack of consensus on whether adjuvant therapy is necessary for stage I EC patients with LVSI after surgery. Notably, studies by Beavis et al. (26) and Son et al. (27) reported improved progression-free survival with adjuvant therapy compared to observation alone in stage I endometrioid EC patients with LVSI. However, none of the four randomized studies (28–31) showed that adjuvant radiotherapy improved survival in stage I EC patients. In fact, Johnson et al. (32) reported that external beam radiation therapy (EBRT) after surgery reduced the risk of local recurrence, but did not reduce the risk of distant metastasis or OS in stage I EC. Thus, the role of adjuvant therapy in LVSI subgroups of early-stage EC remains uncertain and requires further investigation. Our study shows that LVSI increases the risk of early EC recurrence and death. Postoperative observation and follow-up can be selected for endometrioid adenocarcinoma with LVSI in the low-risk group of stage IA, but EBRT supplementation may be considered when combined with other high-risk factors such as age over 60 years old, lesion diameter greater than 2cm or molecular classification indicating poor prognosis. Other stage I EC patients with LVSI are advised to consider EBRT.
This study also has certain limitations. First, clinical heterogeneity exists among the included studies due to differences in pathology types, surgical procedures, and adjuvant treatment methods. Second, the retrospective nature of the included studies introduces a risk of selection bias. Peters et al (33). have studied the correlation between the extent of LVSI and prognosis in patients with EC using samples from PORTEC trials. Their quantitative analysis revealed a significant correlation between the extent of LVSI and the risk of pelvic lymph node recurrence in EC patients. They propose defining clinically relevant LVSI in EC as the involvement of more than 4 LVSI-positive vessels in at least one H&E slide. However, due to insufficient LVSI grading data in the included literature, we were unable to perform subgroup analysis based on this parameter. Additionally, there may also be inconsistent diagnostic criteria for LVSI positivity, leading to potential confounding bias in relevant studies. We only included English-language literature and publications and may have a publication bias risk.
In conclusion, our analyses demonstrates that the presence of LVSI in stage I EC and in stage IA, grade 1 or 2 endometrioid carcinoma is associated with an increased risk of recurrence, reduced RFS and OS. LVSI status should be considered as an important prognostic factor in early stage EC, and its presence should prompt closer postoperative clinical follow-up. The role of adjuvant therapy in LVSI subgroups of early-stage EC remains controversial and requires further investigation. Future research should focus on evaluating the survival benefits and potential risks of adjuvant radiotherapy in patients with LVSI to determine its optimal use in clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/supplementary files, further inquiries can be directed to the corresponding author/s.
Author contributions
Z-JQ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft. Y-SW: Data curation, Investigation, Methodology, Software, Writing – original draft. Y-LC: Formal Analysis, Investigation, Methodology, Software, Writing – original draft. AZ: Formal Analysis, Supervision, Visualization, Writing – review & editing. LH: Conceptualization, Formal Analysis, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Singh N, Hirschowitz L, Zaino R, Alvarado-Cabrero I, Duggan MA, Ali-Fehmi R, et al. Pathologic prognostic factors in endometrial carcinoma (Other than tumor type and grade). Int J Gynecol Pathol (2019) 38 Suppl 1(Iss 1 Suppl;1):S93–S113. doi: 10.1097/PGP.0000000000000524
3. Tortorella L, Restaino S, Zannoni GF, Vizzielli G, Chiantera V, Cappuccio S, et al. Substantial lymph-vascular space invasion (LVSI) as predictor of distant relapse and poor prognosis in low-risk early-stage endometrial cancer. J Gynecol Oncol (2021) 32(2):e11. doi: 10.3802/jgo.2021.32.e11
4. Abu-Rustum N, Yashar C, Arend R, Barber E, Bradley K, Brooks R, et al. Uterine neoplasms, version 1.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2023) 21(2):181–209. doi: 10.6004/jnccn.2023.0006
5. Berek JS, Matias-Guiu X, Creutzberg C, Fotopoulou C, Gaffney D, Kehoe S, et al. Endometrial Cancer Staging Subcommittee, FIGO Women's Cancer Committee. FIGO staging of endometrial cancer: 2023. J Gynecol Oncol (2023) 34(5):e85. doi: 10.3802/jgo.2023.34.e85
6. Yarandi F, Shirali E, Akhavan S, Nili F, Ramhormozian S. The impact of lymphovascular space invasion on survival in early stage low-grade endometrioid endometrial cancer. Eur J Med Res (2023) 28(1):118. doi: 10.1186/s40001-023-01084-9
7. Veade AE, Foote J, Ehrisman J, Broadwater G, Davidson BA, Lee PS, et al. Associations between lymphovascular space invasion, nodal recurrence, and survival in patients with surgical stage I endometrioid endometrial adenocarcinoma. World J Surg Oncol (2019) 17(1):80. doi: 10.1186/s12957-019-1620-x
8. Ayhan A, Şahin H, Sari ME, Yalçin I, Haberal A, Meydanli MM. Prognostic significance of lymphovascular space invasion in low-risk endometrial cancer. Int J Gynecol Cancer (2019) 29(3):505–12. doi: 10.1136/ijgc-2018-000069
9. Cusano E, Myers V, Samant R, Sudai T, Keller A, Le T, et al. Prognostic significance of lymphovascular space invasion in the absence of lymph node metastases in early-stage endometrial cancer. Int J Gynecol Cancer (2018) 28(5):890–4. doi: 10.1097/IGC.0000000000001229
10. Gemer O, Arie AB, Levy T, Gdalevich M, Lorian M, Barak F, et al. Lymphvascular space involvement compromises the survival of patients with stage I endometrial cancer: results of a multicenter study. Eur J Surg Oncol (2007) 33(5):644–7. doi: 10.1016/j.ejso.2007.01.009
11. Nwachukwu C, Baskovic M, Von Eyben R, Fujimoto D, Giaretta S, English D, et al. Recurrence risk factors in stage IA grade 1 endometrial cancer. J Gynecol Oncol (2021) 32(2):e22. doi: 10.3802/jgo.2021.32.e22
12. Iida Y, Komatsu H, Okawa M, Osaku D, Nosaka K, Sato S, et al. Is adjuvant chemotherapy necessary in patients with early endometrial cancer? Yonago Acta Med (2022) 65(1):82–7. doi: 10.33160/yam.2022.02.014
13. Pifer PM, Jaishankar S, Bhargava R, SChad MD, Keller A, Musunuru HB, et al. Is substantial lymphovascular space invasion prognostic in patients with pathologically lymph node-negative endometrial cancer? Int J Radiat Oncol Biol Phys (2023) 117(1):148–53. doi: 10.1016/j.ijrobp.2023.02.053
14. Okugawa K, Yahata H, Hachisuga K, Tomonobe H, Yasutake N, Kodama K, et al. Evaluation of clinical significance of lymphovascular space invasion in stage IA endometrial cancer. Oncology (2022) 100(4):195–202. doi: 10.1159/000521382
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
16. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
17. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
18. Higgins J, Thomas J. Cochrane handbook for systematic reviews of interventions (2021). The Cochrane Collaboration. Available at: https://training.cochrane.org/handbook (Accessed 3rd May 2023).
19. Ureyen I, Karalok A, Turkmen O, Kimyon G, Akdas YR, Akyol A, et al. Factors predicting recurrence in patients with stage IA endometrioid endometrial cancer: what is the importance of LVSI? Arch Gynecol Obstet (2020) 301(3):737–44. doi: 10.1007/s00404-019-05418-z
20. dos Reis R, Burzawa JK, Tsunoda AT, Hosaka M, Frumovitz M, Westin SN, et al. Lymphovascular space invasion portends poor prognosis in low-risk endometrial cancer. Int J Gynecol Cancer (2015) 25(7):1292–9. doi: 10.1097/IGC.0000000000000490
21. Güngördük K, Firat Cüylan Z, Kahramanoglu I, Oge T, Akbayir O, Dede M, et al. Risk factors for recurrence in low-risk endometrial cancer: A case-control study. Oncol Res Treat (2018) 41(7-8):466–70. doi: 10.1159/000488112
22. Bosse T, Peters EE, Creutzberg CL, Jürgenliemk-Schulz IM, Jobsen JJ, Mens JW, et al. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer–A pooled analysis of PORTEC 1 and 2 trials. Eur J Cancer (2015) 51(13):1742–50. doi: 10.1016/j.ejca.2015.05.015
23. Aristizabal P, Graesslin O, Barranger E, Clavel-Chapelon F, Haddad B, Luton D, et al. A suggested modification to FIGO stage I endometrial cancer. Gynecol Oncol (2014) 133(2):192–6. doi: 10.1016/j.ygyno.2014.03.009
24. O'Brien DJ, Flannelly G, Mooney EE, Foley M. Lymphovascular space involvement in early stage well-differentiated endometrial cancer is associated with increased mortality. BJOG (2009) 116(7):991–4. doi: 10.1111/j.1471-0528.2009.02162.x
25. Jorge S, Hou JY, Tergas AI, Burke WM, Huang Y, Hu JC, et al. Magnitude of risk for nodal metastasis associated with lymphvascular space invasion for endometrial cancer. Gynecol Oncol (2016) 140(3):387–93. doi: 10.1016/j.ygyno.2016.01.002
26. Beavis AL, Yen TT, Stone RL, Wethington SL, Carr C, Son J, et al. Adjuvant therapy for early stage, endometrial cancer with lymphovascular space invasion: Is there a role for chemotherapy? Gynecol Oncol (2020) 156(3):568–74. doi: 10.1016/j.ygyno.2019.12.028
27. Son J, Chambers LM, Carr C, Michener CM, Yao M, Beavis A, et al. Adjuvant treatment improves overall survival in women with high-intermediate risk early-stage endometrial cancer with lymphovascular space invasion. Int J Gynecol Cancer (2020) 30(11):1738–47. doi: 10.1136/ijgc-2020-001454
28. Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol (2004) 92(3):744–51. doi: 10.1016/j.ygyno.2003.11.048
29. Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet (2000) 355(9213):1404–11. doi: 10.1016/s0140-6736(00)02139-5
30. Wortman BG, Creutzberg CL, Putter H, Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LCHW, et al. PORTEC Study Group. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br J Cancer (2018) 119(9):1067–74. doi: 10.1038/s41416-018-0310-8
31. ASTEC/EN.5 Study Group, Blake P, Swart AM, Orton J, Kitchener H, Whelan T, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet (2009) 373(9658):137–46. doi: 10.1016/S0140-6736(08)61767-5
32. Johnson N, Cornes P. Survival and recurrent disease after postoperative radiotherapy for early endometrial cancer: systematic review and meta-analysis. BJOG (2007) 114(11):1313–20. doi: 10.1111/j.1471-0528.2007.01332.x
Keywords: endometrial cancer, lymphovascular space invasion, recurrence, overall survival, recurrence free survival
Citation: Qin Z-j, Wang Y-s, Chen Y-l, Zheng A and Han L (2024) Evaluation of prognostic significance of lymphovascular space invasion in early stage endometrial cancer: a systematic review and meta-analysis. Front. Oncol. 13:1286221. doi: 10.3389/fonc.2023.1286221
Received: 31 August 2023; Accepted: 22 December 2023;
Published: 11 January 2024.
Edited by:
Anthony Taylor, University of Leicester, United KingdomReviewed by:
Lucia Tortorella, Agostino Gemelli University Polyclinic (IRCCS), ItalyDmitry Aleksandrovich Zinovkin, Gomel State Medical University, Belarus
Copyright © 2024 Qin, Wang, Chen, Zheng and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ai Zheng, emhlbmdhaXdlc3RjaGluYUAxNjMuY29t; Ling Han, aGFubGluZ2x1b2JvQHNpbmEuY29t
 Zhao-juan Qin
Zhao-juan Qin Yi-si Wang
Yi-si Wang Ya-li Chen1,2
Ya-li Chen1,2 Ai Zheng
Ai Zheng Ling Han
Ling Han