- 1Department of Pediatric Surgery, Capital Institute of Pediatrics, Beijing, China
- 2Graduate School of Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 3Research Unit of Minimally Invasive Pediatric Surgery on Diagnosis and Treatment, Chinese Academy of Medical Sciences, Beijing, China
Introduction: Inflammation is closely associated with tumor development and patient prognosis. The objective of this study is to assess the prognostic value of the preoperative inflammatory indexes in pediatric hepatoblastoma patients who receive neoadjuvant chemotherapy.
Methods:: A retrospective analysis was performed on clinical and pathological data of 199 hepatoblastoma patients who underwent hepatectomy with preoperative neoadjuvant chemotherapy from January 2015 to June 2020. The receiver operating characteristic curve was used to evaluate the prognostic value of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), and systemic inflammation response index (SIRI) in predicting OS and EFS. Patients were grouped based on optimal cutoff values of preoperative inflammatory indexes. Survival rates were calculated using the Kaplan-Meier method, and survival outcomes were compared between groups using the log-rank test. Univariate and multivariate Cox proportional hazards regression models were used to identify independent prognostic factors, and a nomogram was constructed using R software to predict the probability of OS.
Results: The receiver operating characteristic curve showed prognostic value for OS, not EFS, in preoperative inflammatory indexes. Patients were categorized into low/high groups: SII ≤ 266.70/higher, NLR ≤ 1.24/higher, PLR ≤ 85.25/higher, and SIRI ≤ 0.72/higher. High NLR, PLR, SII, and SIRI groups had significantly lower 5-year OS than their low counterparts (all p-value < 0.05). The Cox analysis identified four independent prognostic factors: SIRI (HR=2.997, 95% CI: 1.119-8.031), microvascular invasion (HR=2.556, 95% CI: 1.14-5.73), the post-treatment extent of disease (POSTTEXT) staging (IV vs. I: HR=244.204, 95% CI:11.306-5274.556), and alpha-fetoprotein (>100 ng/ml: HR=0.11, 95% CI: 0.032-0.381) for hepatoblastoma patients with neoadjuvant chemotherapy. High SIRI group had more patients with adverse NLR, SII, and POSTTEXT III (all p-value < 0.05). Independent prognostic factors led to an OS nomogram with a concordance index of 0.85 (95% CI: 0.78-0.91, p-value = 1.43e-27) and the calibration curve showed a good fit between the prediction curve and the true curve.
Conclusions: SIRI is an independent prognostic factor of hepatoblastoma patients receiving neoadjuvant chemotherapy. The OS nomogram based on SIRI, POSTTEXT staging, MiVI, and AFP can be used to assess the prognosis of those patients.
1 Introduction
Hepatoblastoma (HB) is the most common primary liver malignant tumor in children, ranking as the third most prevalent pediatric abdominal malignancy after Wilms’ tumor and neuroblastoma. It accounts for approximately 1% of all pediatric cancers, and its incidence is on the rise (1–3). HB typically occurs in children under 3 years old, with a male-to-female ratio of approximately 1.5:1. The disease is often associated with premature birth, low birth weight, heredity and chromosome abnormalities, although the specific etiology remains unclear (4, 5). HB is sensitive to chemotherapy, and preoperative neoadjuvant chemotherapy (NACT) plays a crucial role in down-grading the disease staging, improving the rate of complete tumor resection during surgery, and lowering tumor recurrence rates (6). Therefore, preoperative NACT has been widely adopted in clinical practice and gained significant recognition. The combination of surgery and NACT has significantly improved postoperative survival in high-risk children with HB (1). Currently, the pretreatment extent of disease (PRETEXT)/the post-treatment extent of disease (POSTTEXT) staging based on imaging is widely used in prognosis prediction and treatment decision-making of HB patients (7–9). The PRETEXT staging is determined by imaging at diagnosis and the POSTTEXT staging is determined after neoadjuvant chemotherapy. When feasible and safe, the Children’s Oncology Group (COG) strategy recommends upfront resection at diagnosis (mainly PRETEXT I and PRETEXT II) to minimize overall chemotherapy exposure (10–12), and preoperative NACT or liver transplantation for those patients with unresectable hepatoblastoma. However, in clinical practice, the prognosis of patients who do not clearly respond to neoadjuvant therapy may vary considerably despite having the same POSTTEXT staging. This disparity can be attributed to several underlying biological factors. Consequently, there exists an imperative clinical demand for the establishment of biomarkers capable of identifying HB patients with a bleak prognosis. Recently, several immunological and histological biomarkers have been identified for assessing the prognosis of HB patients (13–15). Nevertheless, these biomarkers frequently rely on primary tumor samples, entail technical complexity, and incur substantial costs. Therefore, it is of paramount importance to explore a convenient, cost-effective, and efficient preoperative biomarker that can assist surgeons in the identification of HB patients at a higher risk. Furthermore, this endeavor could facilitate the prognostic assessment of HB patients predicted to have poor survival outcomes, allowing the development of personalized follow-up plans to maximize benefit for these individuals.
The systemic inflammation response index (SIRI) is a hematological index established based on the counts of neutrophils, monocytes, and lymphocytes in peripheral blood to evaluate the inflammatory state of the body. It serves as a useful predictor of poor prognosis in the treatment of malignant tumors (16). Previous research has indicated that the inflammatory process can activate oncogenic signaling pathways, promoting tumor growth and metastasis, thereby leading to poor outcomes (17–19). SIRI effectively reflects the body’s inflammatory response and immune status. An elevated SIRI indicates increased neutrophil and monocyte counts, as well as decreased lymphocyte counts, which are conducive to tumor development and metastasis (20–23). Numerous studies in the field of adult oncology have confirmed the association between elevated preoperative SIRI and adverse clinical outcomes (24–27).
Therefore, the objective of this study is to analyze the correlation between preoperative SIRI and the prognosis of HB patients who received NACT. Additionally, we aim to evaluate the clinical value of SIRI as a preoperative biomarker for predicting the prognosis of these patients and to compare it with other common inflammatory indexes.
2 Materials and methods
2.1 Patients
This study retrospectively analyzed pediatric HB patients who underwent hepatectomy at our Institution from January 2015 to June 1, 2020. Ethical approval was obtained from the Ethics Committee of the Capital Institute of Pediatrics, and written informed consent was obtained from the parents before surgery.
Inclusion criteria were as follows:
1. Pathologically confirmed hepatoblastoma.
2. Completion of preoperative neoadjuvant chemotherapy including either the PLADO (cisplatin and doxorubicin) or C5 V (cisplatin, 5-flourouracil, and vincristine) regimen.
3. Primary radical resection of the tumor.
4. Age less than 18 years.
Exclusion criteria were as follows:
1. Coexisting malignancies in other systems.
2. Perioperative death (≤60 days).
3. Incomplete clinical and follow-up data.
4. Previous hepatectomy for other diseases.
2.2 Clinical and pathological data
Clinical information was collected from electronic medical records, including age, gender, initial alpha-fetoprotein (AFP) levels and preoperative imaging data. The preoperative imaging data included the PRETEXT/POSTTEXT staging, tumor location, multifocality (F+) including two or more tumor nodules separated by normal hepatic parenchyma, macrovascular involvement (MaVI) including either hepatic venous involvement (V+) or portal venous involvement (P+), extrahepatic intra-abdominal disease (E+), tumour rupture or intraperitoneal haemorrhage(H+) and distant metastasis. Based on the International Pediatric Liver Tumor Strategy Group (SIOPEL) guidelines, the POSTTEXT staging was used for those HB patients who received preoperative NACT (28, 29). Pathological data included tumor histological types and microvascular invasion (MiVI), which was defined as pathologic vascular invasion noted microscopically by the examining pathologist (30). According to the Chinese Guidelines for the Diagnosis and Treatment of Pediatric Hepatoblastoma (2019 edition) and the prognostic characteristics of different pathological types (31, 32), the HB histological types were classified into three categories: small cell undifferentiated (SCUD) HB, other epithelial HB excluding SCUD, and mixed epithelial-mesenchymal (MEM) HB. all tumor samples were reviewed in detail by at least two pathologists, and by three in case of uncertainty.
2.3 Preoperative inflammatory indexes
The preoperative inflammatory indexes (Collected within 1 week before surgery) included the Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), Systemic Immune-Inflammation Index (SII), and Systemic Inflammation Response Index (SIRI). NLR was calculated as neutrophil counts/lymphocyte counts, PLR as platelet counts/lymphocyte counts, SII as platelet counts × neutrophil counts/lymphocyte counts, and SIRI as monocyte counts × neutrophil counts/lymphocyte counts.
2.4 Follow-up
Patients were followed up regularly through outpatient visits and telephone contacts. Within the first year after surgery, liver function, AFP levels, and upper abdominal ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) examinations were performed every 3 months. Subsequently, follow-up occurred every 3 to 6 months after the first year. The follow-up period ended on July 1, 2023. The primary endpoints were overall survival (OS), defined as the time from the first hepatectomy to death, and event-free survival (EFS), defined as the first hepatectomy to the diagnosis of relapse, metastasis, or death.
2.5 Statistical analysis
Statistical analyses were performed using SPSS (version 26, Chicago, Illinois) and R software (version 4.3.1, http://www.r-project.org). Continuous data are reported as mean ± standard deviation or median (interquartile range [IQR]). Categorical variables were presented as frequencies (n) and percentages (%). Student’s t-test was used for comparison of normally distributed continuous variables and Wilcoxon rank sum test for non-normally distributed continuous variables. The Chi-square test was used to compare categorical variables. The Mann-Whitney U test was used to compare group-ranked data. Receiver operating characteristic (ROC) curve was used to calculate the area under the curve (AUC) of preoperative NLR, PLR, SIRI and SII for OS and EFS in HB patients. A minimum AUC > 0.7 was considered clinically significant (33).The optimal cut-off values for inflammatory indexes were determined using the Youden index to stratify patients for further analysis (34, 35). The Kaplan-Meier (KM) curve was used to calculate survival rates, and the log-rank test was used to compare survival outcomes between groups. The Cox proportional hazards regression model was used for univariate and multivariate analysis of prognostic factors. Based on the independent prognostic factors identified by the Cox proportional hazards regression model, the “rms” package in R was used to draw calibration plots for the prediction model and to calculate the concordance index (C-index) to determine the discrimination of the prediction model. Consistency analysis was performed by plotting calibration curves. The clinical utility of the nomogram was evaluated by decision curve analysis (DCA) using the “devtools” package. In all of the above analyses, p-value < 0.05 was considered statistically significant.
3 Results
3.1 Baseline data
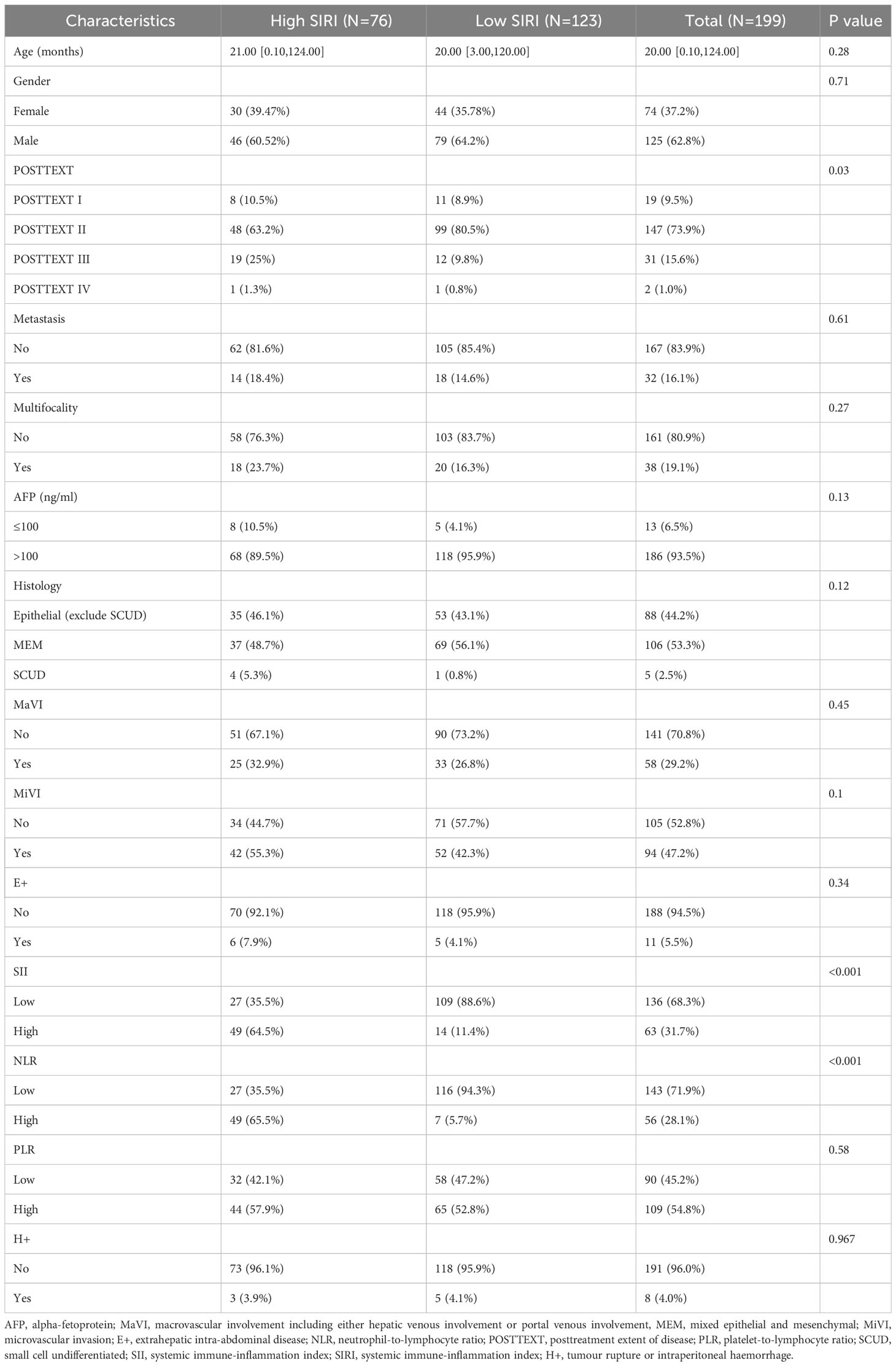
A total of 199 patients were enrolled in this study, including 125 males (62.8%) and 74 females (37.2%), with a median age of 20.0 months (range, 0.1-124.0 months) and an IQR of 21.0 months. According to SIOPEL criteria, before NACT, 5 patients were PRETEXT I (2.5%), 61 patients were PRETEXT II (30.6%), 102 patients were PRETEXT III (51.3%), and 31 patients were PRETEXT IV (15.6%). Prior to surgery, 19 patients were classified as POSTTEXT I (9.5%), 147 as POSTTEXT II (73.9%), 31 as POSTTEXT III (15.6%), and 2 as POSTTEXT IV (1%). Intrahepatic multifocality (F+) were identified in 38 patients (19.1%), extrahepatic intra-abdominal disease (E+) in 11 patients (5.5%) and tumour rupture or intraperitoneal haemorrhage (H+) in 8 patients (4.0%). 32 patients had distant metastasis (16.1%), and 58 cases of macrovascular involvement (29.1%). The results of the pathological analysis showed that there were 5 cases of SCUD HB (2.5%), 88 cases of epithelial HB other than SCUD (44.2%), mixed epithelial-mesenchymal HB in 106 cases (53.3%). Microscopic microvascular invasion was positive in 94 cases (47.2%). The median follow-up time for all patients was 60.1 months (time range 2-102 months), with an IQR of 42.9 months. During the follow-up period, 62 patients (31.1%) had tumor recurrence, of which 41 patients (20.6%) died as a result of tumor progression. The 1-, 3-, and 5-year OS rates were 81%, 80%, and 79%, respectively. The EFS rates at 1, 3, 5 years were 73%, 69% and 69%, respectively.
3.2 ROC analysis of inflammatory indexes
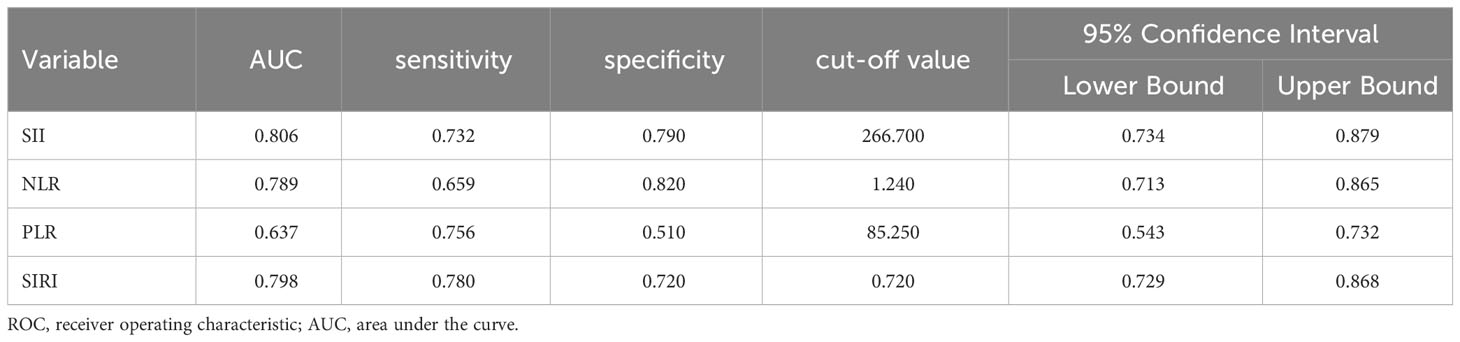
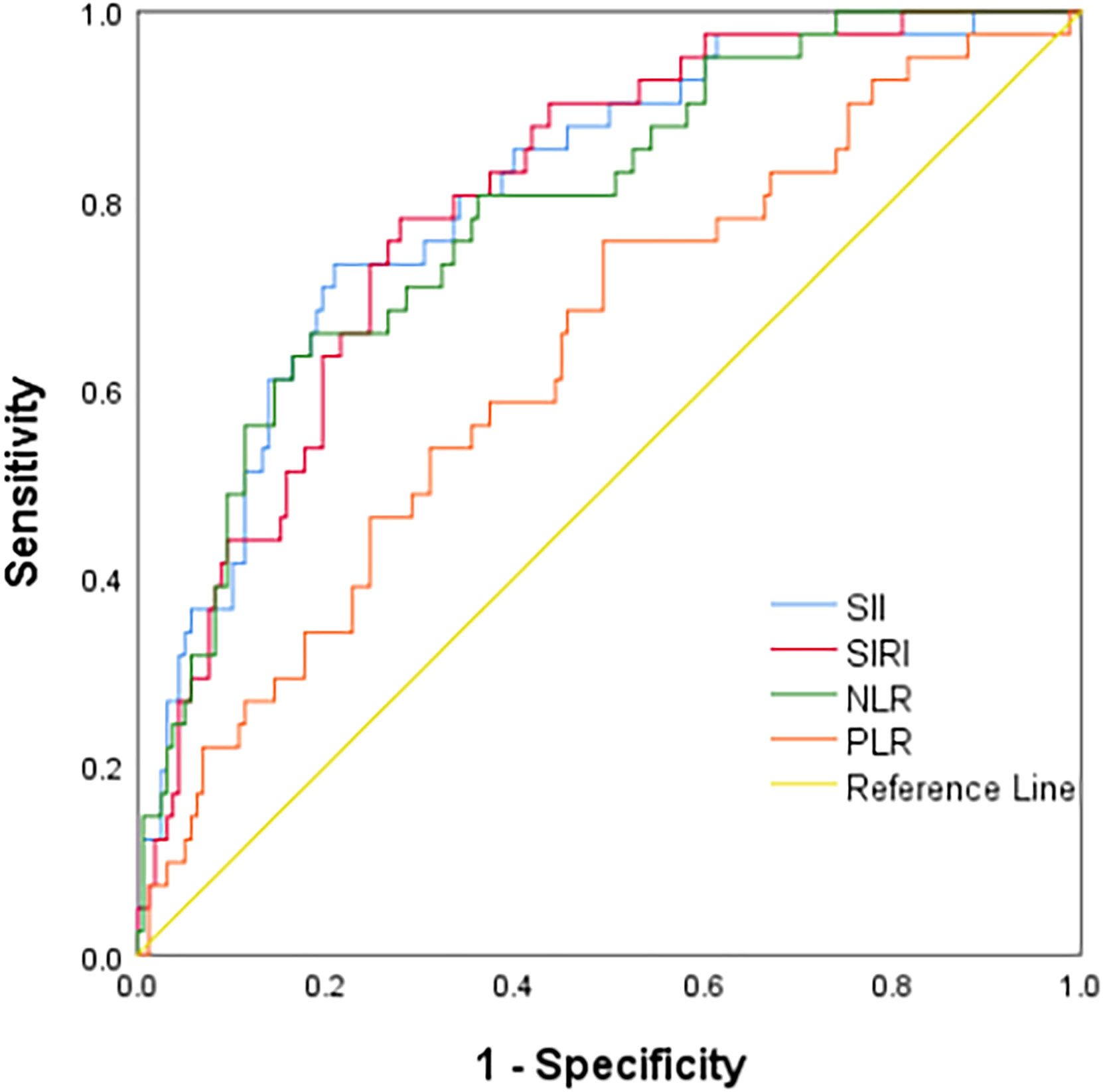
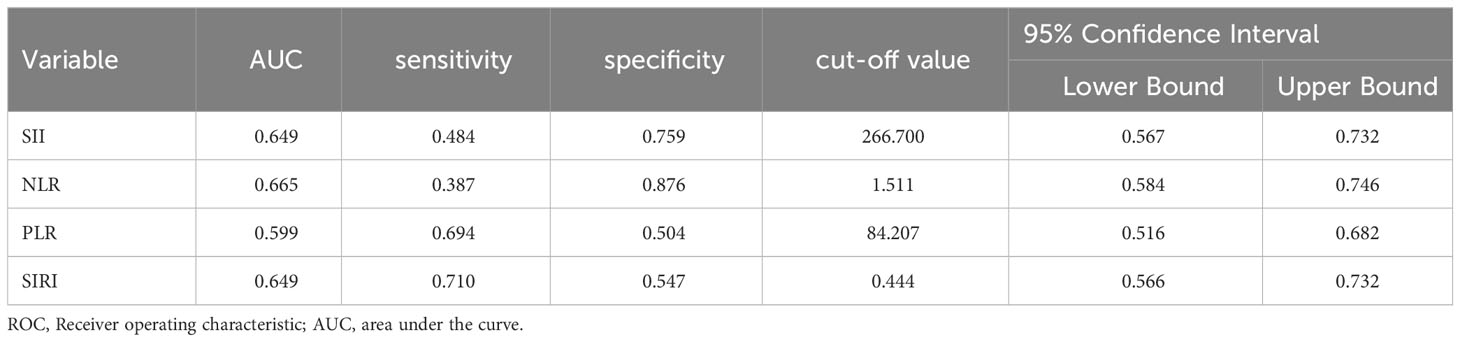
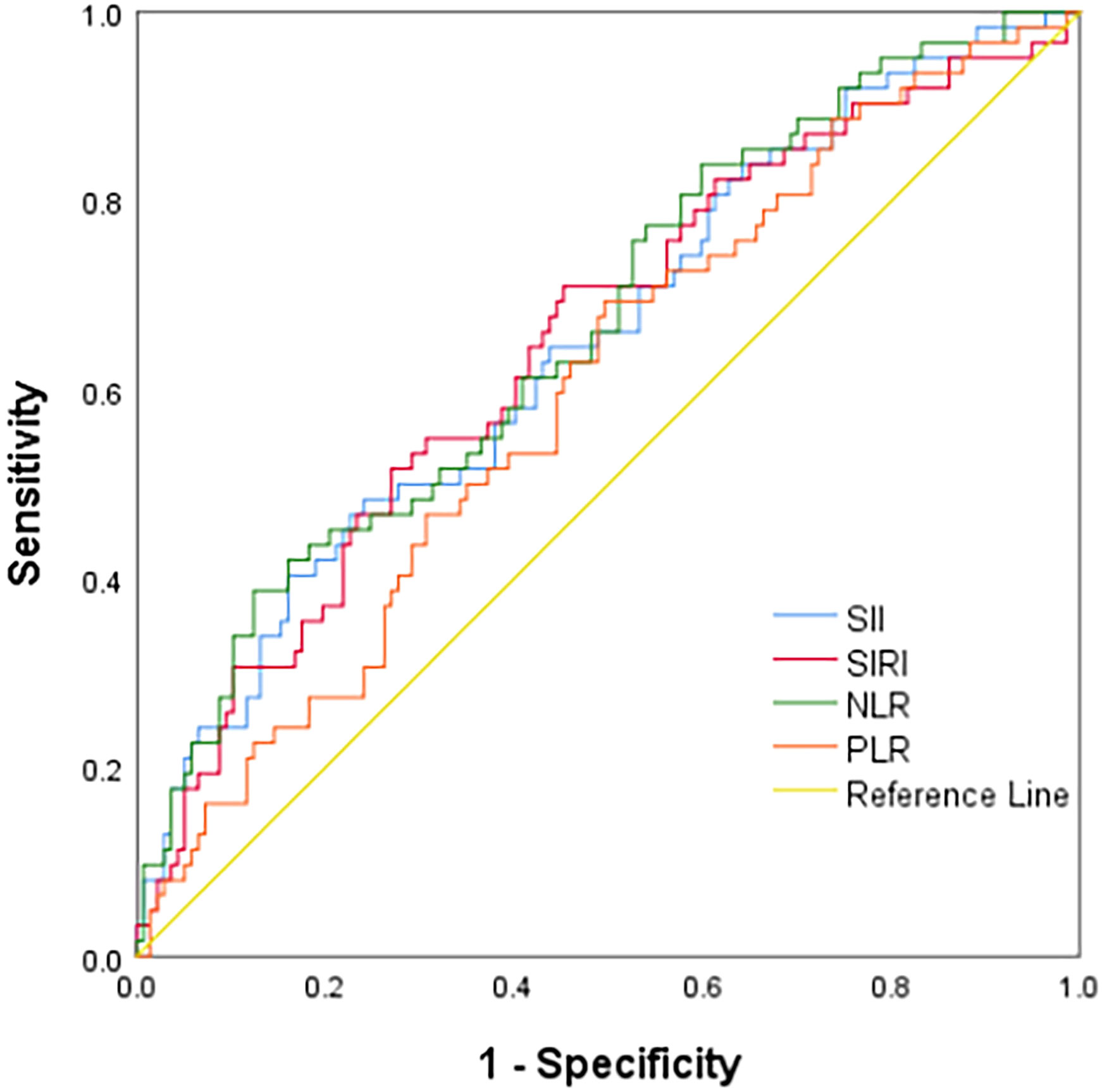
ROC curve analysis showed that the AUCs for predicting postoperative OS in HB patients were 78.9% (95% CI: 71.3%-86.5%) for NLR, 63.7% (95% CI: 54.3%-73.2%) for PLR, 80.6% (95% CI: 73.4%-87.9%) for SII, and 79.8% (95% CI: 72.9%-86.8%) for SIRI. The optimal cutoff values (Table 1) were 1.24 for NLR, 85.25 for PLR, 266.70 for SII, and 0.72 for SIRI. The AUCs for SIRI, SII, and NLR were all greater than 0.7, indicating that these preoperative inflammatory indexes had prognostic value for OS (Figure 1). The AUCs for NLR, PLR, SII, and SIRI for predicting postoperative EFS were 66.5% (95% CI: 58.4%-74.6%), 59.9% (95% CI: 51.6%-68.2%), 64.9% (95% CI: 56.7%-73.2%), and 64.9% (95% CI: 56.6%-73.2%), respectively, all less than 0.7 (Table 2), indicating poor prognostic performance for EFS (Figure 2).
3.3 Prognostic factors for overall survival
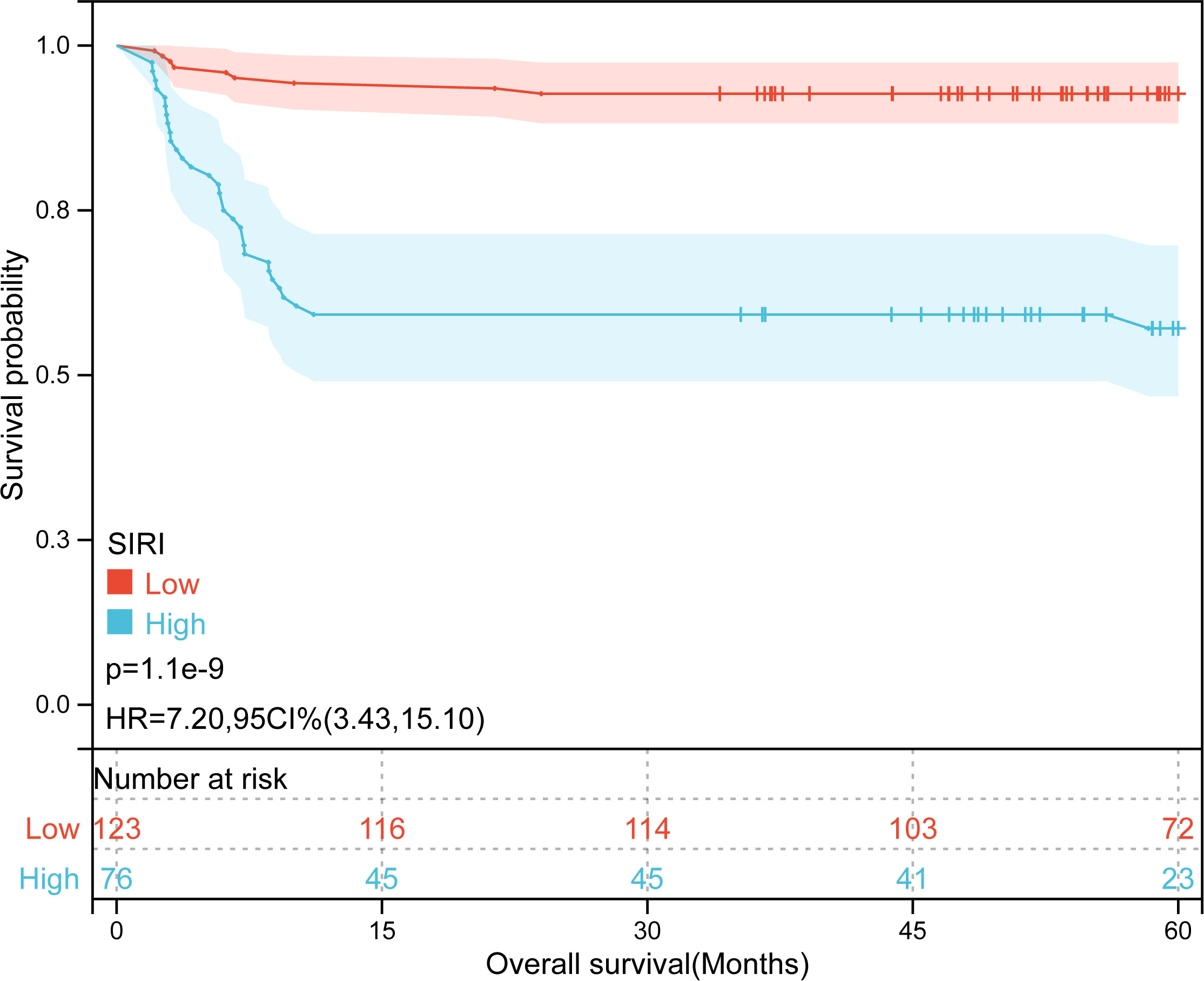
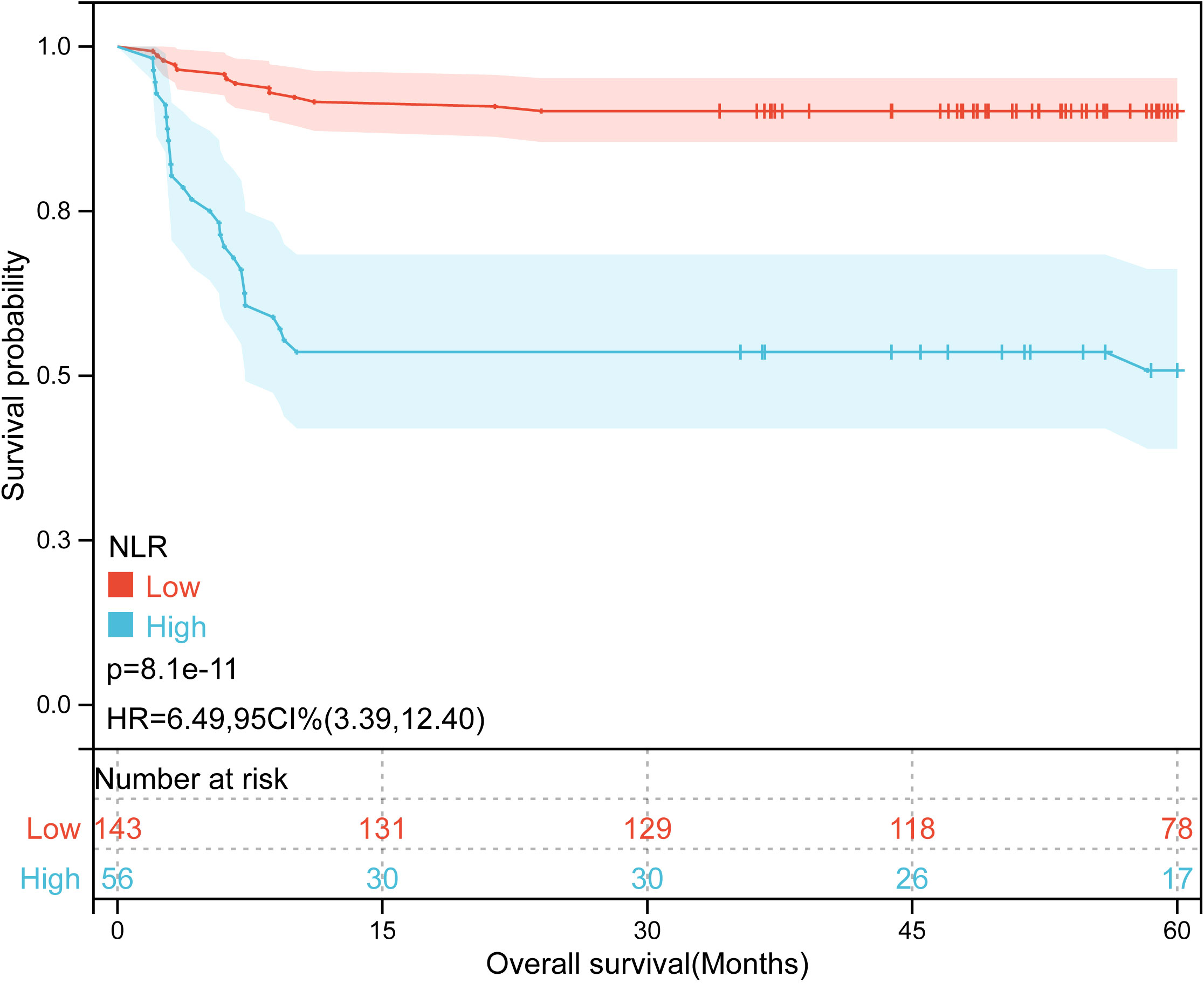
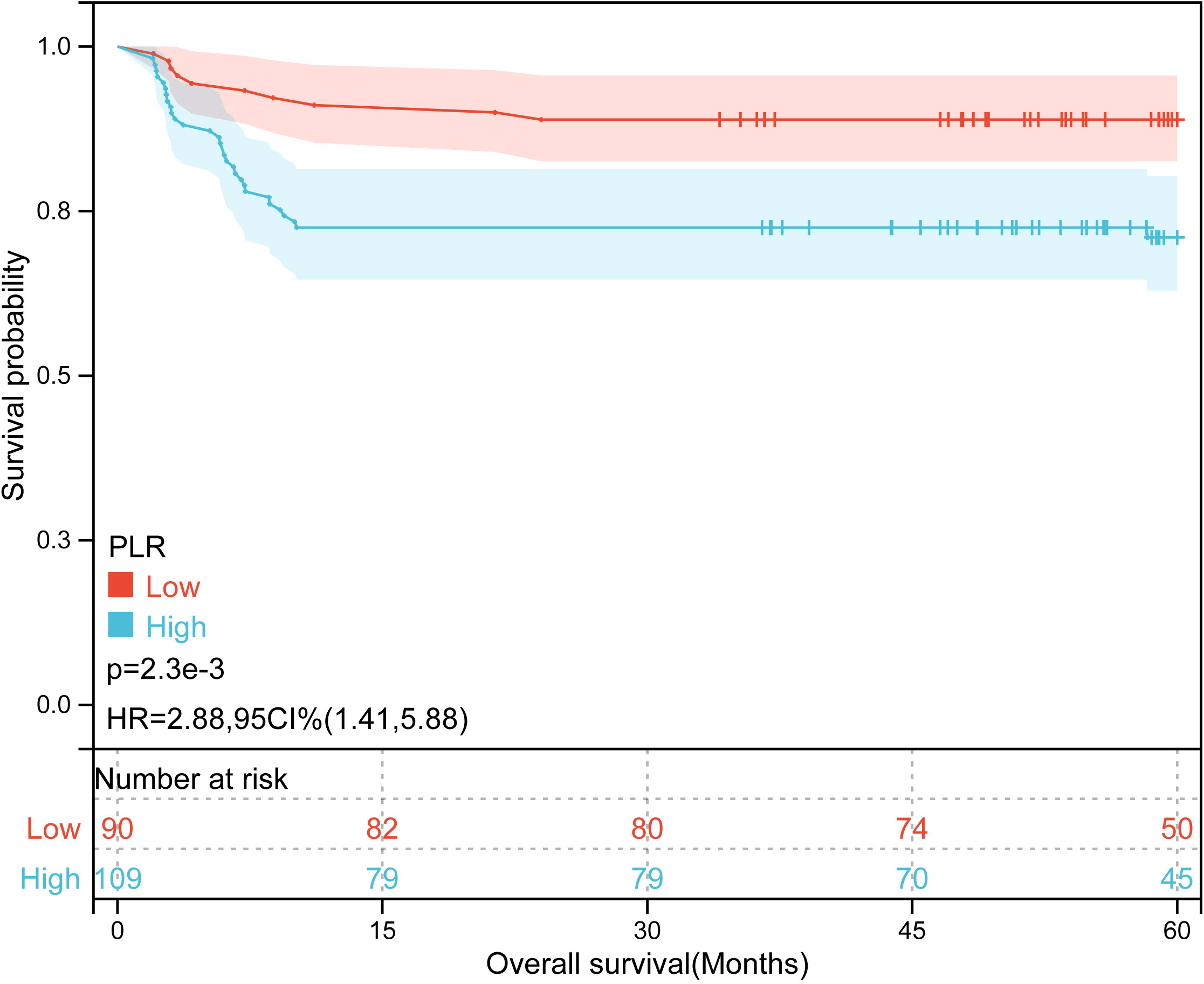
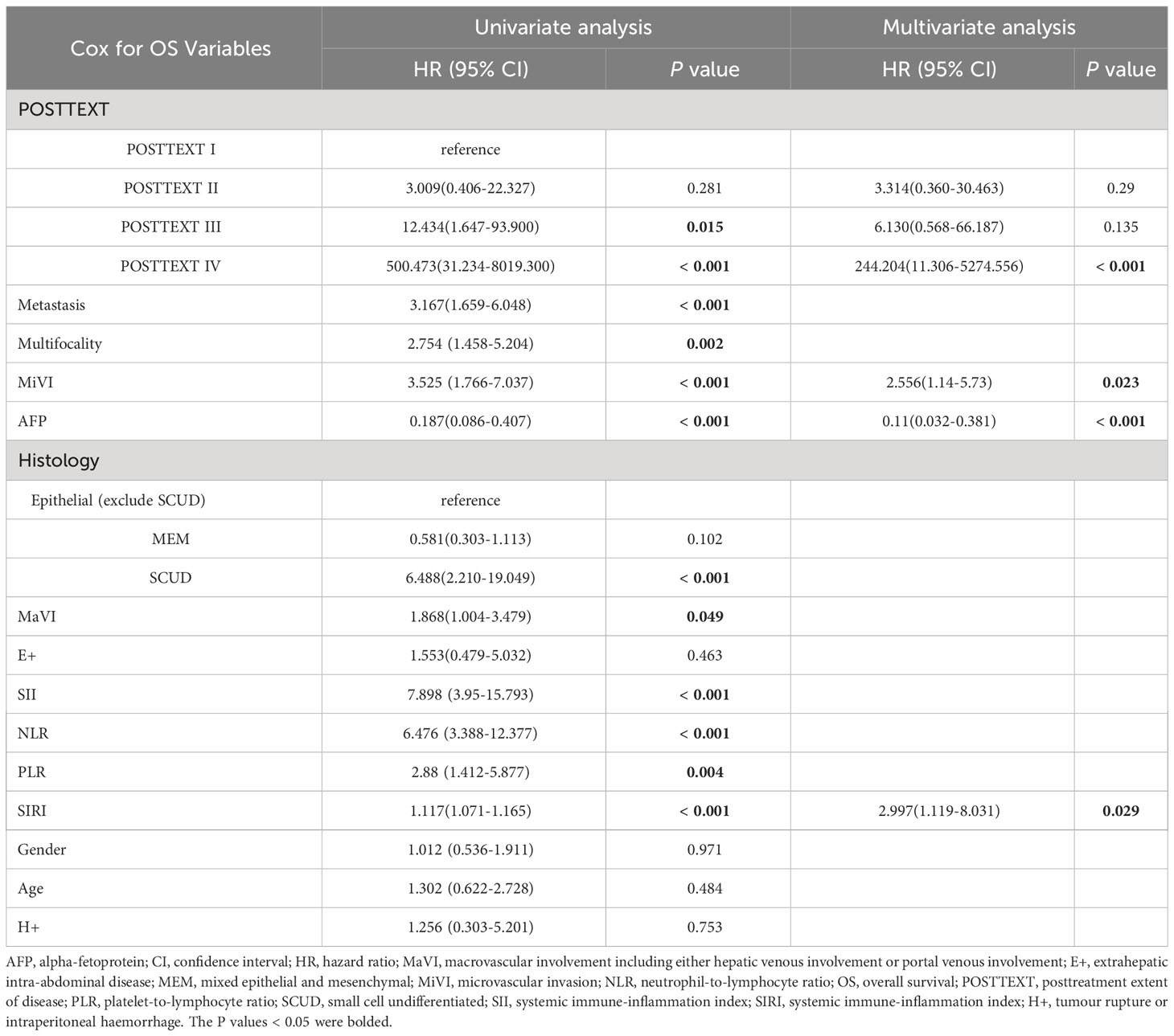
Kaplan-Meier plots (Figures 3–6) demonstrated that the 5-year OS of the high SII group, high NLR group, high PLR group, and high SIRI group was lower than that of the low SII group, low NLR group, low PLR group, and low SIRI group (all p-value < 0.05). Univariate Cox regression analysis revealed that NLR, PLR, SII, SIRI, POSTTEXT staging, AFP, metastasis, microvascular invasion, macrovascular involvement, and multifocality were associated with OS (all p-value < 0.05, Table 2). Multivariate Cox regression analysis indicated that SIRI (HR=2.997, 95% CI: 1.119-8.031), MiVI (HR=2.556, 95% CI: 1.14-5.73), POSTTEXT staging (IV vs. I: HR=244.204, 95% CI:11.306-5274.556), and AFP (>100 ng/ml: HR=0.11, 95% CI: 0.032-0.381) were independent prognostic factors for OS (all p-value < 0.05, Table 3). Among the preoperative inflammatory indexes, only SIRI was an independent prognostic factor for OS.
3.4 Relationship between clinicopathological features of different SIRI groups
According to the optimal cutoff value for predicting OS in HB patients using SIRI, patients were divided into the low SIRI group (SIRI ≤0.72) and the high SIRI group (SIRI > 0.72). The proportions of patients with NLR > 1.24, SII > 266.70, and POSTTEXT III were higher in the high SIRI group than in the low SII group (all p-value < 0.05, Table 4).
3.5 Nomogram predictive model for overall survival
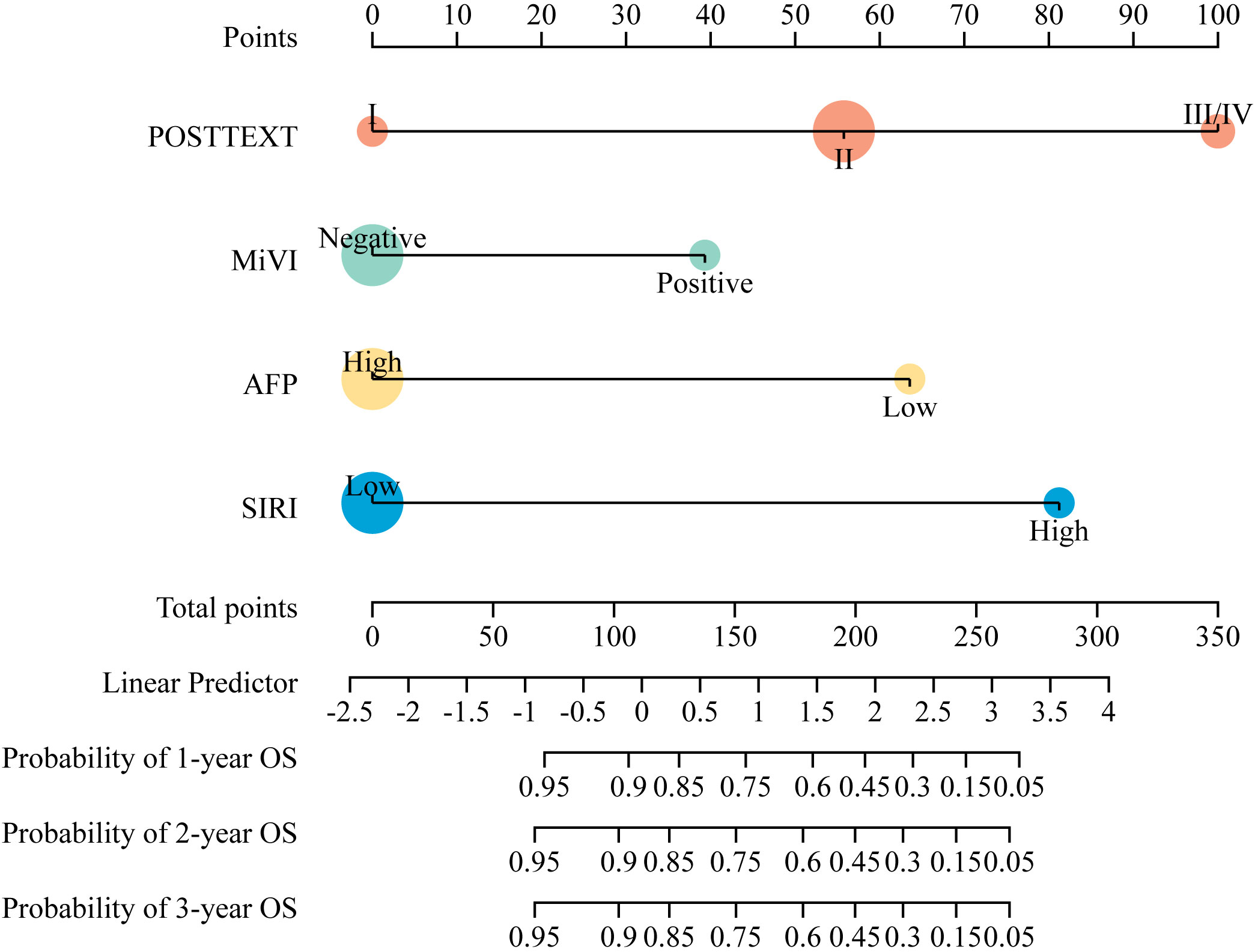
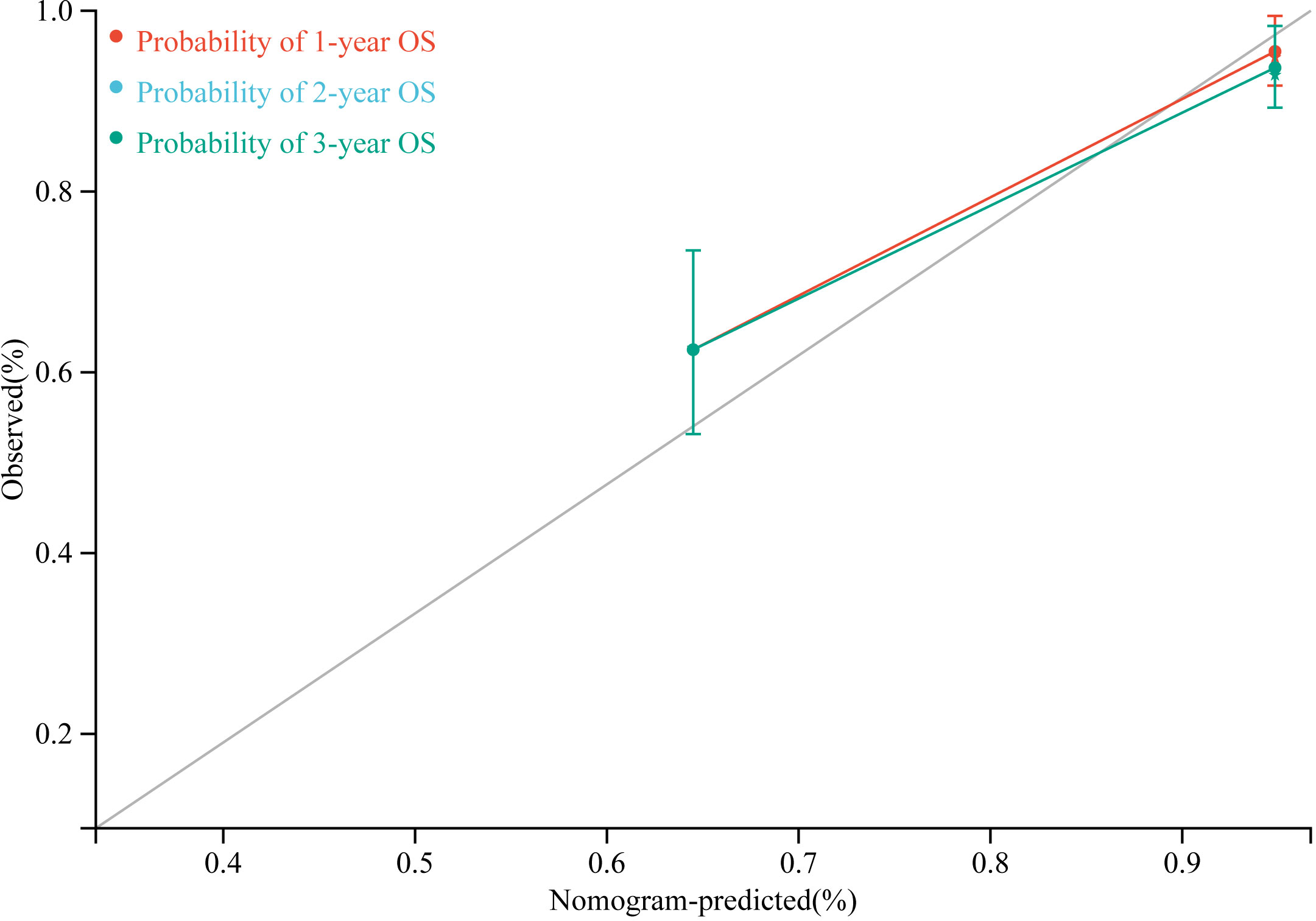
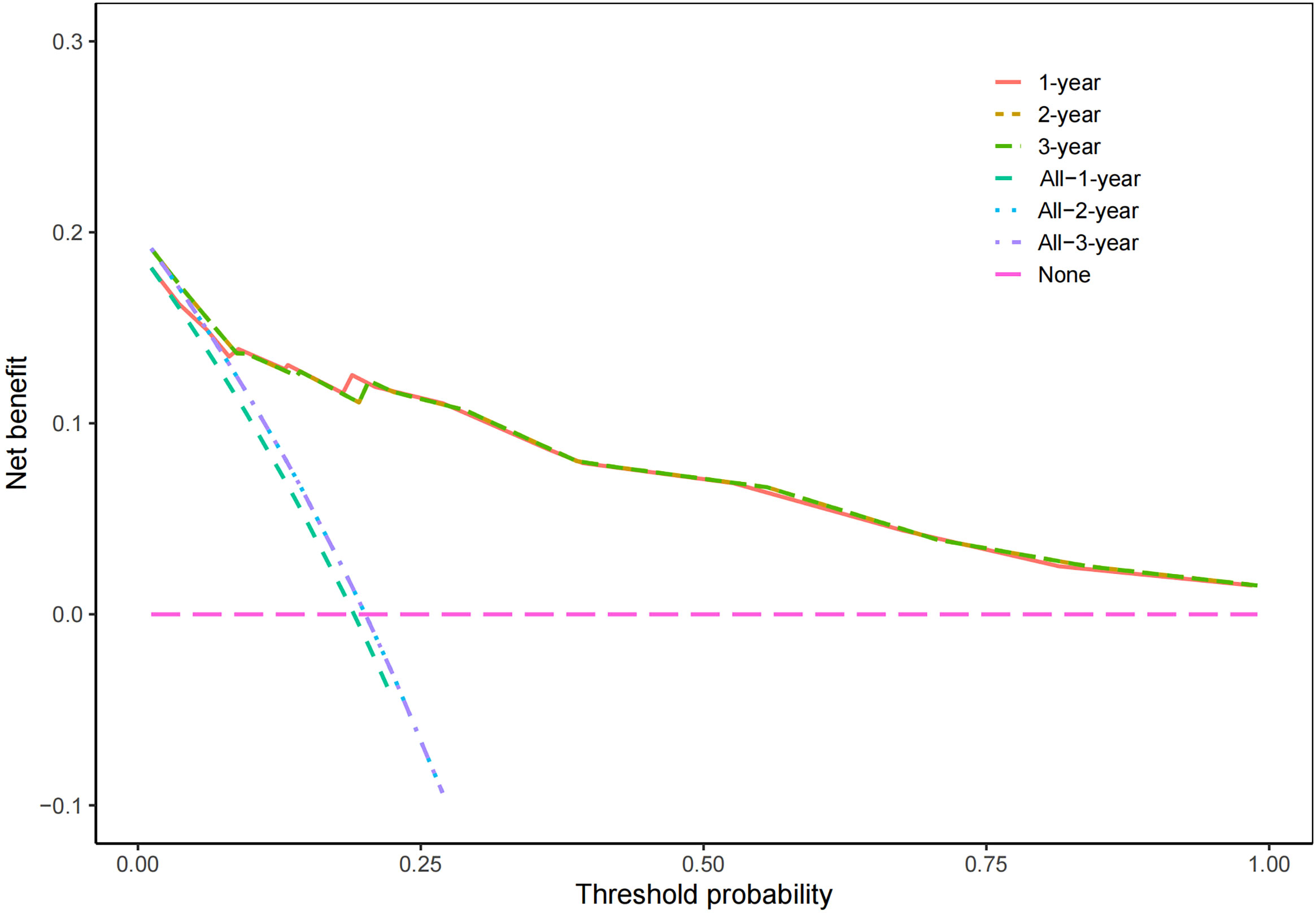
According to the results of multivariate Cox regression analysis, a nomogram for predicting OS of HB patients receiving preoperative NACT was constructed using SIRI, POSTTEXT staging, MiVI, and AFP (Figure 7). Based on the points assigned to the four independent prognostic factors in the nomogram, we can add up the points for each factor to obtain a total point and the corresponding probabilities of OS at 1, 2, and 3 years. A higher total point indicates a lower probability of OS. The C-index of the nomogram was 0.85 (95% CI: 0.78-0.91, p-value = 1.43e-27) and the calibration curves are shown in Figure 8, indicating good overall fit and predictive ability. The decision curve analysis (DCA) is presented in Figure 9, where the net benefit of the nomogram is greater than 0 over the entire threshold probability, indicating a significant clinical utility of this nomogram.
4 Discussion
Many studies have shown that tumor development and progression are closely related to inflammatory response (36). First of all, tumor cells can release a variety of inflammatory factors, such as interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor (TNF) and so on to functionally shape their microenvironment, and these factors can dilate blood vessels and attract innate immune cells and adaptive immune cells to the tumor site, which in turn cause local and systemic inflammatory responses (37). Inflammation, in turn, can promote tumorigenesis and progression, and numerous studies have shown that neutrophils, lymphocytes, monocytes and platelets play an important role in the tumor microenvironment. For example, neutrophils affect tumor development by secreting a variety of factors, such as matrix metalloproteinase-9 (MMP-9), reactive oxygen species (ROS) and reactive nitrogen species (RNS), which promote tumor growth, metastasis, angiogenesis, and immunosuppression (38–40), and monocytes can secrete a variety of growth factors and cytokines, such as IL-6, TNF-α, vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), which can stimulate tumor cell proliferation and metastasis. In addition, monocytes can mature into tumor-associated macrophages and promote angiogenesis by secreting various growth factors and cytokines (41–44). Platelets can directly interact with tumor cells, promote their proliferation and metastasis, and form a physical barrier around tumor cells, protecting them from immune-mediated lysis by natural killer cells (NK) (45). On the other hand, lymphocytes, especially CD8+ T lymphocytes, play a crucial role in the anti-tumor immune response. CD8+ T lymphocytes mediate cytotoxic responses by releasing inflammatory factors like Interferon-gamma (IFN-γ) and TNF-α, inhibiting tumor growth, proliferation, and metastasis (46). Therefore, under the interaction between cancer cells and inflammatory cells, patients with malignant tumors often exhibit systemic inflammatory responses.
In addition to promoting the occurrence and development of tumors, numerous clinical studies have demonstrated that various inflammatory indexes based on different combinations of inflammatory cells can be used to predict the prognosis of cancer patients. Common indexes such as SIRI, NLR, PLR, and SII can reflect systemic inflammatory responses, and higher levels of these indexes have been associated with worse prognosis in cancer patients (16). Among them, SIRI as a prognostic factor for tumor patients has been extensively studied only in recent years (25, 47–49). It can effectively reflect the inflammatory status of the body as well as the severity of the tumor. Therefore, SIRI has been used to evaluate the prognosis of patients with malignant tumors. For example, a study conducted by Cristina Valero et al. involving 824 patients with head and neck squamous cell carcinoma (HNSCC) found that SIRI is an independent prognostic factor in HNSCC. Patients with higher SIRI experienced a significant decrease in disease-specific survival (50). However, it is worth noting that SIRI is still relatively understudied in the field of pediatric tumors (51). According to available literature searches, there is currently no relevant study on the association between SIRI and pediatric abdominal tumors. Also, there are few studies on the correlation between preoperative inflammatory indexes and the prognosis of hepatoblastoma. In a recent study, Tan Xie analyzed data from 101 HB patients and 101 children with indirect inguinal hernia, and found that both NLR and PLR were prognostic factors for HB. Additionally, NLR was found to be an independent prognostic factor for OS among HB patients (52). However, the study did not differentiate between patients undergoing primary hepatic resection and those receiving preoperative neoadjuvant chemotherapy, which tends to have a large difference in survival (53, 54).
Our study divided HB patients who received preoperative NACT into different groups based on the optimal cutoff values of the inflammation indexes. KM curve of 5-year OS rates in different groups showed that all groups with higher preoperative inflammation indexes had significantly lower survival rates than the low group, and these differences were statistically significant. This suggests that higher preoperative inflammatory indexes are associated with adverse prognosis in HB patients. Furthermore, univariate Cox regression analysis showed that NLR, PLR, SII, and SIRI were all associated with the OS of the patients. However, when considering these inflammatory indexes together in a multivariate Cox regression analysis, only SIRI emerged as an independent prognostic factor, while NLR, PLR, and SII were not. This finding indicates that SIRI has a better predictive value than the other preoperative inflammatory indexes in terms of assessing the OS of HB patients who received preoperative NACT. Further analysis of the relationship between preoperative SIRI and the clinicopathologic characteristics of those patients revealed significant differences in the NLR, SII, POSTTEXT staging among different SIRI groups. A higher preoperative SIRI value indicates a more severe inflammatory state and a higher tumor staging in patients. Moreover, apart from SIRI and POSTTEXT staging, this study identified AFP and MiVI as independent factors influencing the prognosis of pediatric HB patients, which is consistent with previous literature. According to the results of the Children’s Hepatic Tumors International Collaboration (CHIC), a low level of AFP (< 100 ng/ml) was associated with the worst outcome (7, 55). De Ioris, Maretta found that HB patients with low level of AFP were characterized by a high-risk subgroup with extensive disease, poor chemotherapy response and a poor outcome (56). Additionally, MiVI, which may indicate occult micrometastasis of the tumor, was found to be associated with poor prognosis in patients with HB (57). Our study also found no statistically significant difference in MiVI and AFP values between patients in the high SIRI group and those in the low SIRI group. These results suggest that SIRI can serve as a clinical tool to identify patients with a potentially poor prognosis who have high AFP and negative MiVI values.
The treatment decisions for HB primarily rely on preoperative imaging and histopathologic assessment, without considering the potential impact of systemic inflammation on the formulation of appropriate treatment strategies. This is particularly pertinent for children with advanced HB, such as those with POSTTEXT IV and POSTTEXT III tumors with macrovascular involvement, where controversies still exist regarding the most suitable treatment plan. Early studies more recommend total hepatectomy with liver transplantation (58), as this approach has been shown to significantly improve their survival rate (59, 60). However, accumulating evidence from standardized chemotherapy regimens and surgical experience suggests that the prognosis after surgical resection is comparable to that of liver transplantation, while also avoiding long-term complications associated with postoperative immunosuppressive therapy (61–64). On the other hand, studies have demonstrated poor survival outcomes in HB patients undergoing salvage liver transplantation after tumor recurrence (65, 66). These conflicting findings highlight the need for careful consideration by clinicians when deciding between surgical resection and liver transplantation for children with advanced HB. In this context, preoperative SIRI, AFP,and MiVI, in combination with POSTTEXT staging, can offer valuable insights to clinicians, enabling them to comprehensively assess the prognosis of pediatric HB patients from different perspectives. The nomogram prediction model, which incorporates SIRI and clinicopathologic factors, has shown good predictive ability, suggesting its potential usefulness as a valuable tool for clinicians in formulating appropriate personalized treatment strategies. Moreover, alternative techniques, such as transarterial chemoembolization (TACE), radiofrequency ablation (RFA), and high-intensity focused ultrasound (HIFU), have been utilized in pediatric oncology (67–69). These techniques hold promise for patients with high preoperative inflammatory indexes, as they may effectively control tumor growth and allow for postponement of surgical treatment, leading to potentially improved outcomes for these children. However, as there is no relevant literature on this specific aspect, the feasibility and efficacy of such alternative treatments in the context of high preoperative inflammatory indexes would require further investigation through well-designed clinical trials.
Although this study demonstrates the predictive value of preoperative SIRI for the prognosis of HB patients after radical resection, some limitations must be acknowledged. The relatively small sample size and retrospective single-center design may have introduced some confounding factors and biases. To overcome these limitations and provide more definitive evidence, larger, well-designed multicenter prospective studies are warranted. Recently, the COG trial AHEP0731 indicated that patients with unresectable HB had the best (to date) published outcomes with neoadjuvant C5VD (70, 71). Whether our findings are relevant in the C5VD context has not been determined, which minimizes the clinical impact of these findings. Also, the prognostic value of SIRI for those HB patients who have liver transplantation remains to be further investigated. Finally, our study did not demonstrate the prognostic value of preoperative inflammatory indexes for EFS, which is an important factor in the prediction of relapse. We hope that future researchers will identify improved indexes to address this deficiency.
5 Conclusion
For pediatric HB patients who undergo preoperative NACT, the preoperative SIRI level can serve as a simple, effective, low-cost, and non-invasive prognostic indicator for predicting OS. Its predictive value is superior to NLR, SII, and PLR. The OS nomogram prediction model constructed based on SIRI, POSTTEXT staging, MiVI, and AFP, can help guide clinicians in formulating personalized treatment plans.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Capital Institute of Pediatrics. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
CZ: Data curation, Formal Analysis, Methodology, Writing – original draft. SY: Data curation, Writing – original draft. WL: Data curation, Visualization, Writing – review & editing. MD: Methodology, Project administration, Supervision, Writing – review & editing. LL: Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Research Unit of Minimally Invasive Pediatric Surgery on Diagnosis and Treatment, Chinese Academy of Medical Sciences (2021RU015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schnater JM, Kohler SE, Lamers WH, von Schweinitz D, Aronson DC. Where do we stand with hepatoblastoma? Cancer (2003) 98(4):668–78. doi: 10.1002/cncr.11585
2. Lim IIP, Bondoc AJ, Geller JI, Tiao GM. Hepatoblastoma-the evolution of biology, surgery, and transplantation. Children-Basel (2019) 6(1):1–11. doi: 10.3390/children6010001
3. Jha P, Chawla SC, Tavri S, Patel C, Gooding C, Daldrup-Link H. Pediatric liver tumors - a pictorial review. Eur radiology (2009) 19(1):209–19. doi: 10.1007/s00330-008-1106-7
4. Allan BJ, Parikh PP, Diaz S, Perez EA, Neville HL, Sola JE. Predictors of survival and incidence of hepatoblastoma in the paediatric population. HPB (2013) 15(10):741–6. doi: 10.1111/hpb.12112
5. Schooler GR, Infante JC, Acord M, Alazraki A, Chavhan GB, Davis JC, et al. Imaging of pediatric liver tumors: A COG Diagnostic Imaging Committee/SPR Oncology Committee White Paper. Pediatr Blood cancer (2023) 70 Suppl 4:e29965. doi: 10.1002/pbc.29965
6. Hafberg E, Borinstein SC, Alexopoulos SP. Contemporary management of hepatoblastoma. Curr Opin Organ Transplantation (2019) 24(2):113–7. doi: 10.1097/mot.0000000000000618
7. Meyers RL, Maibach R, Hiyama E, Haberle B, Krailo M, Rangaswami A, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol (2017) 18(1):122–31. doi: 10.1016/s1470-2045(16)30598-8
8. Czauderna P, Haeberle B, Hiyama E, Rangaswami A, Krailo M, Maibach R, et al. The (C)under-barhildren’s (H)under-barepatic tumors (I)under-barnternational (C)under-barollaboration (CHIC): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer (2016) 52:92–101. doi: 10.1016/j.ejca.2015.09.023
9. O’Neill AF, Meyers RL, Katzenstein HM, Geller JI, Tiao GM, López-Terrada D, et al. Children’s Oncology Group’s 2023 blueprint for research: Liver tumors. Pediatr Blood cancer (2023) 70 Suppl 6:e30576. doi: 10.1002/pbc.30576
10. Malogolowkin MH, Katzenstein HM, Meyers RL, Krailo MD, Rowland JM, Haas J, et al. Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children’s Oncology Group. J Clin Oncol (2011) 29(24):3301–6. doi: 10.1200/jco.2010.29.3837
11. Ortega JA, Douglass EC, Feusner JH, Reynolds M, Quinn JJ, Finegold MJ, et al. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children’s Cancer Group and the Pediatric Oncology Group. J Clin Oncol (2000) 18(14):2665–75. doi: 10.1200/jco.2000.18.14.2665
12. Katzenstein HM, Langham MR, Malogolowkin MH, Krailo MD, Towbin AJ, McCarville MB, et al. Minimal adjuvant chemotherapy for children with hepatoblastoma resected at diagnosis (AHEP0731): a Children’s Oncology Group, multicentre, phase 3 trial. Lancet Oncol (2019) 20(5):719–27. doi: 10.1016/s1470-2045(18)30895-7
13. Guo F, Ru Q, Zhang J, He S, Yu J, Zheng S, et al. Inflammation factors in hepatoblastoma and their clinical significance as diagnostic and prognostic biomarkers. J Pediatr Surg (2017) 52(9):1496–502. doi: 10.1016/j.jpedsurg.2017.01.059
14. Archana B, D’Cruze L, Nazneen S, Thanka J, Scott JX. Immunohistochemical expression of beta-catenin in hepatoblastoma and its clinical significance. J Cancer Res Ther (2022) 18(3):677–80. doi: 10.4103/jcrt.jcrt_1575_21
15. Jiao C, Jiao X, Zhu A, Ge J, Xu X. Exosomal miR-34s panel as potential novel diagnostic and prognostic biomarker in patients with hepatoblastoma. J Pediatr Surg (2017) 52(4):618–24. doi: 10.1016/j.jpedsurg.2016.09.070
16. Wei LS, Xie HL, Yan P. Prognostic value of the systemic inflammation response index in human malignancy: a meta-analysis. Medicine (Baltimore) (2020) 99(50):e23486. doi: 10.1097/md.0000000000023486
17. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal transduction targeted Ther (2021) 6(1):263. doi: 10.1038/s41392-021-00658-5
18. Villegas SN, Gombos R, García-López L, Gutiérrez-Pérez I, García-Castillo J, Vallejo DM, et al. PI3K/akt cooperates with oncogenic notch by inducing nitric oxide-dependent inflammation. Cell Rep (2018) 22(10):2541–9. doi: 10.1016/j.celrep.2018.02.049
19. Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J cancer (2010) 102(4):639–44. doi: 10.1038/sj.bjc.6605530
20. Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res (2016) 4(2):83–91. doi: 10.1158/2326-6066.Cir-15-0313
21. Yu Q, Dong L, Li Y, Liu G. SIRT1 and HIF1α signaling in metabolism and immune responses. Cancer letters (2018) 418:20–6. doi: 10.1016/j.canlet.2017.12.035
22. Murray PJ. Immune regulation by monocytes. Semin Immunol (2018) 35:12–8. doi: 10.1016/j.smim.2017.12.005
23. Alissafi T, Hatzioannou A, Legaki AI, Varveri A, Verginis P. Balancing cancer immunotherapy and immune-related adverse events: The emerging role of regulatory T cells. J autoimmunity (2019) 104:102310. doi: 10.1016/j.jaut.2019.102310
24. Cui S, Cao S, Chen Q, He Q, Lang R. Preoperative systemic inflammatory response index predicts the prognosis of patients with hepatocellular carcinoma after liver transplantation. Front Immunol (2023) 14:1118053. doi: 10.3389/fimmu.2023.1118053
25. Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer (2016) 122(14):2158–67. doi: 10.1002/cncr.30057
26. Zhu M, Chen L, Kong X, Wang X, Fang Y, Li X, et al. The systemic inflammation response index as an independent predictor of survival in breast cancer patients: A retrospective study. Front Mol biosciences (2022) 9:856064. doi: 10.3389/fmolb.2022.856064
27. Zhou Q, Su S, You W, Wang T, Ren T, Zhu L. Systemic inflammation response index as a prognostic marker in cancer patients: A systematic review and meta-analysis of 38 cohorts. Dose-Response (2021) 19(4):1–14. doi: 10.1177/15593258211064744
28. Towbin AJ, Meyers RL, Woodley H, Miyazaki O, Weldon CB, Morland B, et al. PRETEXT: radiologic staging system for primary hepatic Malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT). Pediatr Radiol (2017) 48(4):536–54. doi: 10.1007/s00247-018-4078-z
29. Roebuck DJ, Aronson D, Clapuyt P, Czauderna P, de Ville de Goyet J, Gauthier F, et al. PRETEXT: a revised staging system for primary Malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol (2005) 37(2):123–32. doi: 10.1007/s00247-006-0361-5
30. Shi Y, Commander SJ, Masand PM, Heczey A, Goss JA, Vasudevan SA. Vascular invasion is a prognostic indicator in hepatoblastoma. J Pediatr Surg (2017) 52(6):956–61. doi: 10.1016/j.jpedsurg.2017.03.017
31. Pediatric Diseases Group of Chinese Society of Pathology. Specialty Committee of Pathology Futang Research Center of Pediatric Development. Chinese expert group consensus on pathological diagnosis of hepatoblastoma. Zhonghua Bing Li Xue Za Zhi (2019) 48(3):176–81. doi: 10.3760/cma.j.issn.0529-5807.2019.03.002
32. Tanaka Y, Inoue T, Horie H. International pediatric liver cancer pathological classification: current trend. Int J Clin Oncol (2013) 18(6):946–54. doi: 10.1007/s10147-013-0624-8
33. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol (2010) 5(9):1315–6. doi: 10.1097/JTO.0b013e3181ec173d
34. Youden WJ. Index for rating diagnostic tests. Cancer (1950) 3(1):32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3
35. Hughes G. Youden’s index and the weight of evidence. Methods Inf Med (2015) 54(2):198–9. doi: 10.3414/me14-04-0003
36. Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res (2019) 79(18):4557–66. doi: 10.1158/0008-5472.Can-18-3962
37. Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res (2008) 14(21):6735–41. doi: 10.1158/1078-0432.Ccr-07-4843
38. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer (2016) 16(7):431–46. doi: 10.1038/nrc.2016.52
39. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest (2013) 123(8):3446–58. doi: 10.1172/jci67484
40. Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflammation (2016) 2016:6058147. doi: 10.1155/2016/6058147
41. Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature (2014) 515(7525):130–3. doi: 10.1038/nature13862
42. Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J Allergy Clin Immunol (2016) 138(4):984–1010. doi: 10.1016/j.jaci.2016.06.033
43. Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell (2012) 11(6):812–24. doi: 10.1016/j.stem.2012.08.013
44. Ramello MC, Tosello Boari J, Canale FP, Mena HA, Negrotto S, Gastman B, et al. Tumor-induced senescent T cells promote the secretion of pro-inflammatory cytokines and angiogenic factors by human monocytes/macrophages through a mechanism that involves Tim-3 and CD40L. Cell Death disease (2014) 5(11):e1507. doi: 10.1038/cddis.2014.451
45. Tesfamariam B. Involvement of platelets in tumor cell metastasis. Pharmacol Ther (2016) 157:112–9. doi: 10.1016/j.pharmthera.2015.11.005
46. Tsukumo SI, Yasutomo K. Regulation of CD8(+) T cells and antitumor immunity by notch signaling. Front Immunol (2018) 9:101. doi: 10.3389/fimmu.2018.00101
47. Wang T, Zhang D, Tang D, Heng Y, Lu LM, Tao L. The role of systemic inflammatory response index (SIRI) and tumor-infiltrating lymphocytes (TILs) in the prognosis of patients with laryngeal squamous cell carcinoma. J Cancer Res Clin Oncol (2023) 149(9):5627–36. doi: 10.1007/s00432-022-04469-1
48. Chen Y, Jiang W, Xi D, Chen J, Xu G, Yin W, et al. Development and validation of nomogram based on SIRI for predicting the clinical outcome in patients with nasopharyngeal carcinomas. J Invest Med (2019) 67(3):691–8. doi: 10.1136/jim-2018-000801
49. Li S, Lan X, Gao H, Li Z, Chen L, Wang W, et al. Systemic Inflammation Response Index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol (2017) 143(12):2455–68. doi: 10.1007/s00432-017-2506-3
50. Valero C, Pardo L, Sansa A, Garcia Lorenzo J, López M, Quer M, et al. Prognostic capacity of Systemic Inflammation Response Index (SIRI) in patients with head and neck squamous cell carcinoma. Head neck (2020) 42(2):336–43. doi: 10.1002/hed.26010
51. Jin YN, Liu BQ, Peng KW, Ou XQ, Zeng WS, Zhang WJ, et al. The prognostic value of adding systemic inflammation response index to Epstein-Barr virus DNA in childhood nasopharyngeal carcinoma: A real-world study. Head neck (2022) 44(6):1404–13. doi: 10.1002/hed.27033
52. Xie T, Hou D, Wang J, Zhao S. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as predictive markers in hepatoblastoma. Front pediatrics (2023) 11:904730. doi: 10.3389/fped.2023.904730
53. Tian Y, Chen XH, Yu F, Feng JY, Huang GM, Ren XH, et al. Neoadjuvant chemotherapy or upfront surgery in hepatoblastoma: A multicenter retrospective study. Pediatr Blood Cancer (2023) e30470. doi: 10.1002/pbc.30470
54. Hiyama E, Hishiki T, Watanabe K, Ida K, Ueda Y, Kurihara S, et al. Outcome and late complications of hepatoblastomas treated using the Japanese study group for pediatric liver tumor 2 protocol. J Clin Oncol (2020) 38(22):2488–+. doi: 10.1200/jco.19.01067
55. Meyers RL, Rowland JR, Krailo M, Chen Z, Katzenstein HM, Malogolowkin MH. Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children’s Oncology Group. Pediatr Blood cancer (2009) 53(6):1016–22. doi: 10.1002/pbc.22088
56. De Ioris M, Brugieres L, Zimmermann A, Keeling J, Brock P, Maibach R, et al. Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: The SIOPEL group experience. Eur J Cancer (2008) 44(4):545–50. doi: 10.1016/j.ejca.2007.11.022
57. Ke M, Zhou Y, Chang-Zhen Y, Li L, Diao M. A nomogram model to predict prognosis of patients with hepatoblastoma. Pediatr Blood cancer (2022) 69(12):e29932. doi: 10.1002/pbc.29932
58. Otte JB, de Ville de Goyet J. The contribution of transplantation to the treatment of liver tumors in children. Semin Pediatr surgery (2005) 14(4):233–8. doi: 10.1053/j.sempedsurg.2005.06.006
59. Zsíros J, Maibach R, Shafford E, Brugieres L, Brock P, Czauderna P, et al. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol (2010) 28(15):2584–90. doi: 10.1200/jco.2009.22.4857
60. Meyers RL, Tiao G, de Ville de Goyet J, Superina R, Aronson DC. Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr Opin pediatrics (2014) 26(1):29–36. doi: 10.1097/mop.0000000000000042
61. Lautz TB, Ben-Ami T, Tantemsapya N, Gosiengfiao Y, Superina RA. Successful nontransplant resection of POST-TEXT III and IV hepatoblastoma. Cancer (2011) 117(9):1976–83. doi: 10.1002/cncr.25722
62. Fuchs J, Cavdar S, Blumenstock G, Ebinger M, Schäfer JF, Sipos B, et al. and IV hepatoblastoma: extended hepatic resection avoids liver transplantation in selected cases. Ann surgery (2017) 266(2):318–23. doi: 10.1097/sla.0000000000001936
63. El-Gendi A, Fadel S, El-Shafei M, Shawky A. Avoiding liver transplantation in post-treatment extent of disease III and IV hepatoblastoma. Pediatr Int (2018) 60(9):862–8. doi: 10.1111/ped.13634
64. de Freitas Paganoti G, Tannuri ACA, Dantas Marques AC, Torres RR, Mendes Gibelli NE, Tannuri U. Extensive hepatectomy as an alternative to liver transplant in advanced hepatoblastoma: A new protocol used in a pediatric liver transplantation center. Transplant Proc (2019) 51(5):1605–10. doi: 10.1016/j.transproceed.2019.03.004
65. Leiskau C, Junge N, Mutschler FE, Laue T, Ohlendorf J, Richter N, et al. Long-term outcome following liver transplantation for primary hepatic tumors-A single centre observational study over 40 years. Children (Basel) (2023) 10(2):202. doi: 10.3390/children10020202
66. Lake CM, Tiao GM, Bondoc AJ. Surgical management of locally-advanced and metastatic hepatoblastoma. Semin Pediatr surgery (2019) 28(6):150856. doi: 10.1016/j.sempedsurg.2019.150856
67. Tang X, He X, Jiang H. Efficacy and safety of HIFU in combination with TACE in unresectable pediatric HB: A randomized, controlled, single-center clinical trial. Medicine (2022) 101(48):e32022. doi: 10.1097/md.0000000000032022
68. Vogl TJ, Scheller A, Jakob U, Zangos S, Ahmed M, Nabil M. Transarterial chemoembolization in the treatment of hepatoblastoma in children. Eur radiology (2006) 16(6):1393–6. doi: 10.1007/s00330-005-2827-5
69. van Laarhoven S, van Baren R, Tamminga RY, de Jong KP. Radiofrequency ablation in the treatment of liver tumors in children. J Pediatr Surg (2012) 47(3):e7–e12. doi: 10.1016/j.jpedsurg.2011.10.075
70. Vasudevan SA, Meyers RL, Finegold MJ, López-Terrada D, Ranganathan S, Dunn SP, et al. Outcomes of children with well-differentiated fetal hepatoblastoma treated with surgery only: Report from Children’s Oncology Group Trial, AHEP0731. J Pediatr Surg (2022) 57(10):251–6. doi: 10.1016/j.jpedsurg.2022.05.022
71. Katzenstein HM, Malogolowkin MH, Krailo MD, Piao J, Towbin AJ, McCarville MB, et al. Doxorubicin in combination with cisplatin, 5-flourouracil, and vincristine is feasible and effective in unresectable hepatoblastoma: A Children’s Oncology Group study. Cancer (2022) 128(5):1057–65. doi: 10.1002/cncr.34014
Keywords: systemic inflammation response index, hepatoblastoma, neoadjuvant chemotherapy, prognosis, nomogram
Citation: Zheng C, Ye S, Liu W, Diao M and Li L (2023) Prognostic value of systemic inflammation response index in hepatoblastoma patients receiving preoperative neoadjuvant chemotherapy. Front. Oncol. 13:1276175. doi: 10.3389/fonc.2023.1276175
Received: 11 August 2023; Accepted: 02 October 2023;
Published: 13 October 2023.
Edited by:
Harold "Bo" Newton Lovvorn, III, Vanderbilt University Medical Center, United StatesReviewed by:
Wei Xu, The First Affiliated Hospital of Soochow University, ChinaAndrew Murphy, St. Jude Children’s Research Hospital, United States
Daniel J. Benedetti, Vanderbilt University Medical Center, United States
Copyright © 2023 Zheng, Ye, Liu, Diao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long Li, bGlsb25nMjNAMTI2LmNvbQ==; Mei Diao, cHNwczMwMDFAaG90bWFpbC5jb20=
 Chen Zheng
Chen Zheng Shiru Ye1
Shiru Ye1 Wei Liu
Wei Liu Long Li
Long Li