- 1Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, TX, United States
- 2Department of Surgery, Dan L. Duncan Comprehensive Cancer Center, Houston, TX, United States
- 3Systems Onco-Immunology Laboratory, David J. Sugarbaker Division of Thoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, TX, United States
- 4Department of Surgery, Michael E. DeBakey VA Medical Center, Houston, TX, United States
- 5The Pelotonia Institute for Immuno-Oncology, Ohio State University Comprehensive Cancer Center, Columbus, OH, United States
- 6Department of Preventative Medicine, Northwestern University Clinical and Translational Sciences Institute, Chicago, IL, United States
Introduction: Pancreatic adenocarcinoma (PDAC) is an aggressive tumor with limited response to both chemotherapy and immunotherapy. Pre-treatment tumor features within the tumor immune microenvironment (TiME) may influence treatment response. We hypothesized that the pre-treatment TiME composition differs between metastatic and primary lesions and would be associated with response to modified FOLFIRINOX (mFFX) or gemcitabine-based (Gem-based) therapy.
Methods: Using RNAseq data from a cohort of treatment-naïve, advanced PDAC patients in the COMPASS trial, differential gene expression analysis of key immunomodulatory genes in were analyzed based on multiple parameters including tumor site, response to mFFX, and response to Gem-based treatment. The relative proportions of immune cell infiltration were defined using CIBERSORTx and Dirichlet regression.
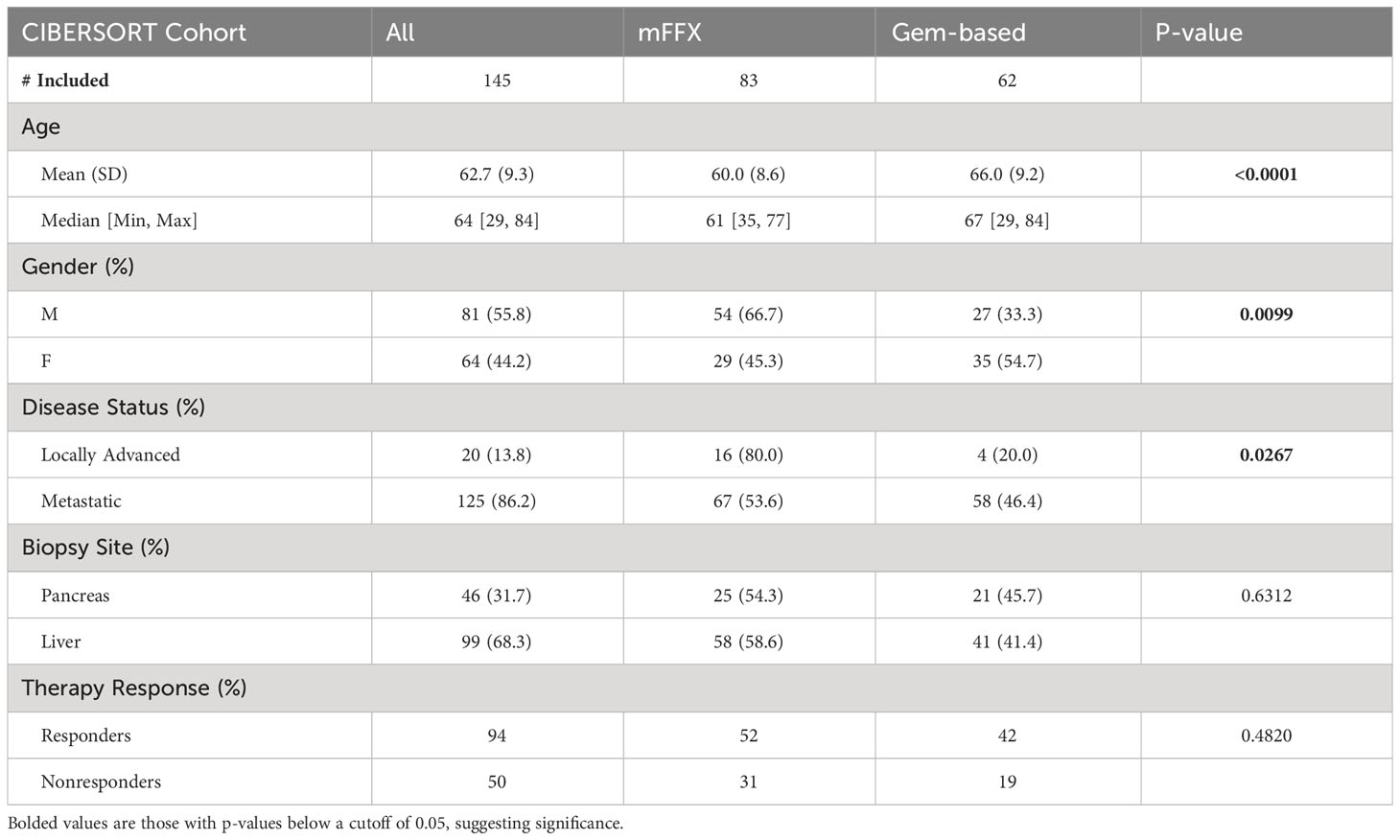
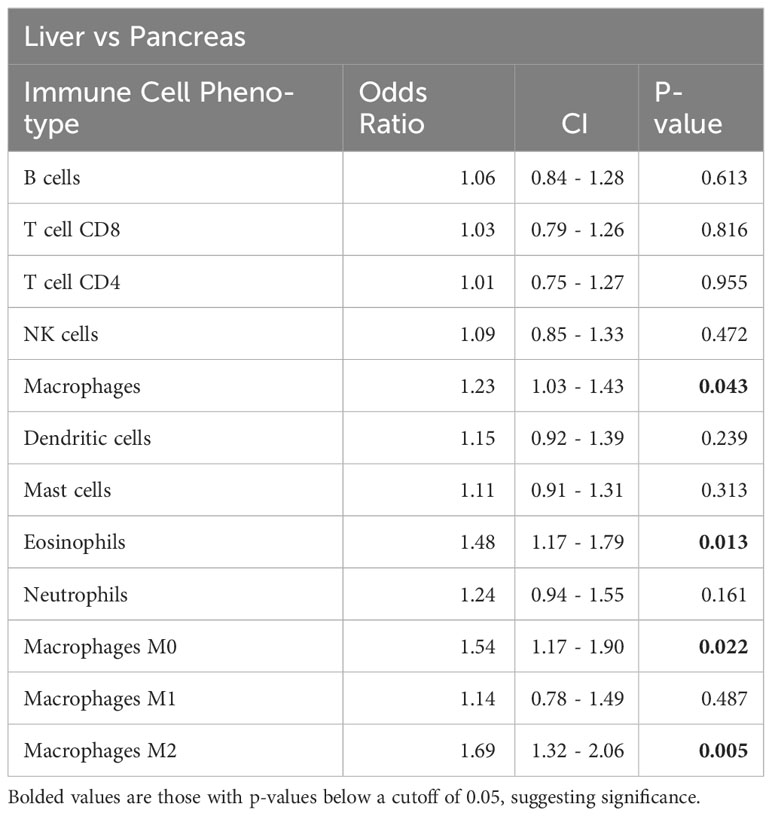
Results: 145 samples were included in the analysis; 83 received mFFX, 62 received Gem-based therapy. Metastatic liver samples had both increased macrophage (1.2 times more, p < 0.05) and increased eosinophil infiltration (1.4 times more, p < 0.05) compared to primary lesion samples. Further analysis of the specific macrophage phenotypes revealed an increased M2 macrophage fraction in the liver samples. The pre-treatment CD8 T-cell, dendritic cell, and neutrophil infiltration of metastatic samples were associated with therapy response to mFFX (p < 0.05), while mast cell infiltration was associated with response to Gem-based therapy (p < 0.05). Multiple immunoinhibitory genes such as ADORA2A, CSF1R, KDR/VEGFR2, LAG3, PDCD1LG2, and TGFB1 and immunostimulatory genes including C10orf54, CXCL12, and TNFSF14/LIGHT were significantly associated with worse survival in patients who received mFFX (p = 0.01). There were no immunomodulatory genes associated with survival in the Gem-based cohort.
Discussion: Our evidence implies that essential differences in the PDAC TiME exist between primary and metastatic tumors and an inflamed pretreatment TiME is associated with mFFX response. Defining components of the PDAC TiME that influence therapy response will provide opportunities for targeted therapeutic strategies that may need to be accounted for in designing personalized therapy to improve outcomes.
1 Introduction
Overall survival for pancreatic adenocarcinoma (PDAC) remains dismal with 5-year survival less than 10% (1, 2). Effective conventional cytotoxic chemotherapeutic regimens are limited; however, combinations such as FOLFIRINOX or gemcitabine/nab-paclitaxel have demonstrated efficacy and can prolong survival for PDAC patients by months (3, 4). Immune checkpoint inhibitors (ICI) have had dramatic success in malignancies such as melanoma (5) non-small cell lung cancer (6), and biliary tract cancer (7). Unfortunately, ICIs as monotherapy have essentially failed in PDAC (8), prompting investigations into strategies to potentiate PDAC immunotherapy.
A wide body of preclinical data (9–16) supports the concept that chemotherapy favorably modifies the tumor immune microenvironment (TiME) through a variety of mechanisms. For example, one of the components of FOLFIRINOX, oxaliplatin, causes DNA damage (17) and can induce immunogenic cell death (ICD) via release of damage-associated molecular patterns in tumors, uptake of tumor debris and neoantigens by antigen-presenting cells, and ultimately, induction of an adaptive immune response and cytotoxic T cell activity (12, 13, 18, 19). Similarly, 5FU is thought to selectively kill myeloid-derived suppressor cells (MDSCs) to enhance T cell mediated anti-tumoral immunity (20). Results from phase III trials across a wide range of cancers have demonstrated that chemotherapy such as oxaliplatin combined with ICI (chemo-ICI) leads to improved overall survival and outcome compared with chemotherapy alone (21–35). Currently, the two main chemotherapy regimens for PDAC are FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, oxaliplatin) (3) and gemcitabine with nab-paclitaxel (4). These treatments have widespread applicability in the treatment of PDAC and are administered both as systemic chemotherapy in unresectable and metastatic PDAC (3, 36) as well as in a neoadjuvant fashion to improve cancer resectability and survival (37, 38). While FOLFIRINOX is typically favored as the initial chemotherapeutic strategy (3), it is associated with increased toxicity compared with Gem-based regimens (3, 4). In practice, there is currently no indications to guide clinicians in choosing between the two chemotherapy regimens beyond the patient’s performance status (39, 40). However, in the clinic, FOLFIRINOX delivery is associated with increased tumor-infiltrating CD8+ T lymphocytes (TILs), decreased circulating regulatory T cells (Tregs), and can increase tumoral PD-L1 expression (41–43). Thus, FOLFIRNOX holds potential to augment ICI therapy in PDAC patients. Studies of combination chemo-ICI in advanced PDAC patients demonstrate improved survival compared to chemotherapy alone (44). mRNA vaccines have also been used in combination with FOLFIRINOX and anti-PD1 therapy, demonstrating the presence of persistent vaccine-expanded tumor-specific T-cells (45). These recent developments underscore the importance of understanding the dynamic interplay of the PDAC TiME and chemotherapy.
Classically, PDAC has been described to have a “cold” TiME, including multiple immunosuppressive cell lines such as Tregs, MDSCs, and M2-phenotypic tumor-associated macrophages (TAMs) (46–52). However, a growing body of evidence supports that the PDAC TiME is heterogeneous, and represented by a diverse milieu of immune cell phenotypes (53). While such heterogeneity has been well-described across a variety of cancers and associated with survival, available data in PDAC is limited. For example, the Immunoscore, which is based on quantification of CD3+/CD8+ lymphocyte heterogeneity at the core and boundary of tumors (54), can outperform traditional TNM staging in predicting disease-free survival and overall survival in colorectal cancer (54, 55) and other cancers (56, 57). Recent investigations have also described significant differences in the TiME between metastatic and primary lesions (58–60). It has been shown that PD-L1 expression is decreased in immune cells of metastatic lesions of triple negative breast cancer (61) and differences exist in PD-1+ TIL infiltration between metastatic and primary lung cancer lesions (62).
The association between chemotherapy response and the PDAC TiME has not been well characterized and the influence of disease site has not been investigated thoroughly. We evaluated publicly available data from the COMPASS trial, a prospective study of treatment-naïve patients with a diagnosis of locally advanced or metastatic PDAC who had core needle biopsies obtained prior to treatment used for whole genome sequencing and RNA sequencing (63, 64). By analyzing the unique genomic dataset from patients with advanced disease, we investigated what molecular and cellular determinants are associated with chemotherapy response. A secondary goal was to characterize the TiME based on the primary versus metastatic site for PDAC. Considering the biologic differences between metastatic and primary lesions, as well as the established influence of chemotherapy on the TiME, we hypothesized that the components of the pre-treatment TiME would differ between metastatic and primary lesions and would be associated with therapy response and survival in a cohort of advanced PDAC patients. Going forward, these findings may have important implications for personalized therapies and for designing next-generation immunotherapy combination strategies.
2 Methods
2.1 COMPASS trial
Institutional Review Board approval and written consent for the COMPASS trial (63, 64) was obtained from participating institutions (University Health Network, Toronto, Ontario, Canada; MUHC Centre for Applied Ethics, Montreal, Quebec, Canada; and Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board, Kingston, Ontario, Canada) (63, 64), and a data use agreement was completed by Baylor College of Medicine with the Ontario Institute for Cancer Research for use of the data within this study. Briefly, image-guided percutaneous core needle biopsies were obtained, and patients then received modified FOLFIRINOX (mFFX), gemcitabine/nab-paclitaxel, or a combination of these along with investigational drugs as standard first line therapy and had therapy response data by RECIST 1.1 (65). For our analysis, chemotherapy response data was defined based on tumor size change to therapy. “Responders” were patients whose measured tumor decreased in size, while “nonresponders” no change or an increase in tumor size while on therapy (Figure 1A). Patients included in our subsequent analysis were comprised of those who had a confirmed diagnosis of PDAC, had a biopsy obtained from either the liver or pancreas, had longer than 30-day survival from time of trial enrollment, had received at least one cycle of either mFFX or gemcitabine-based (Gem-based) therapy as treatment, and had RECIST data available for evaluation of therapy response.
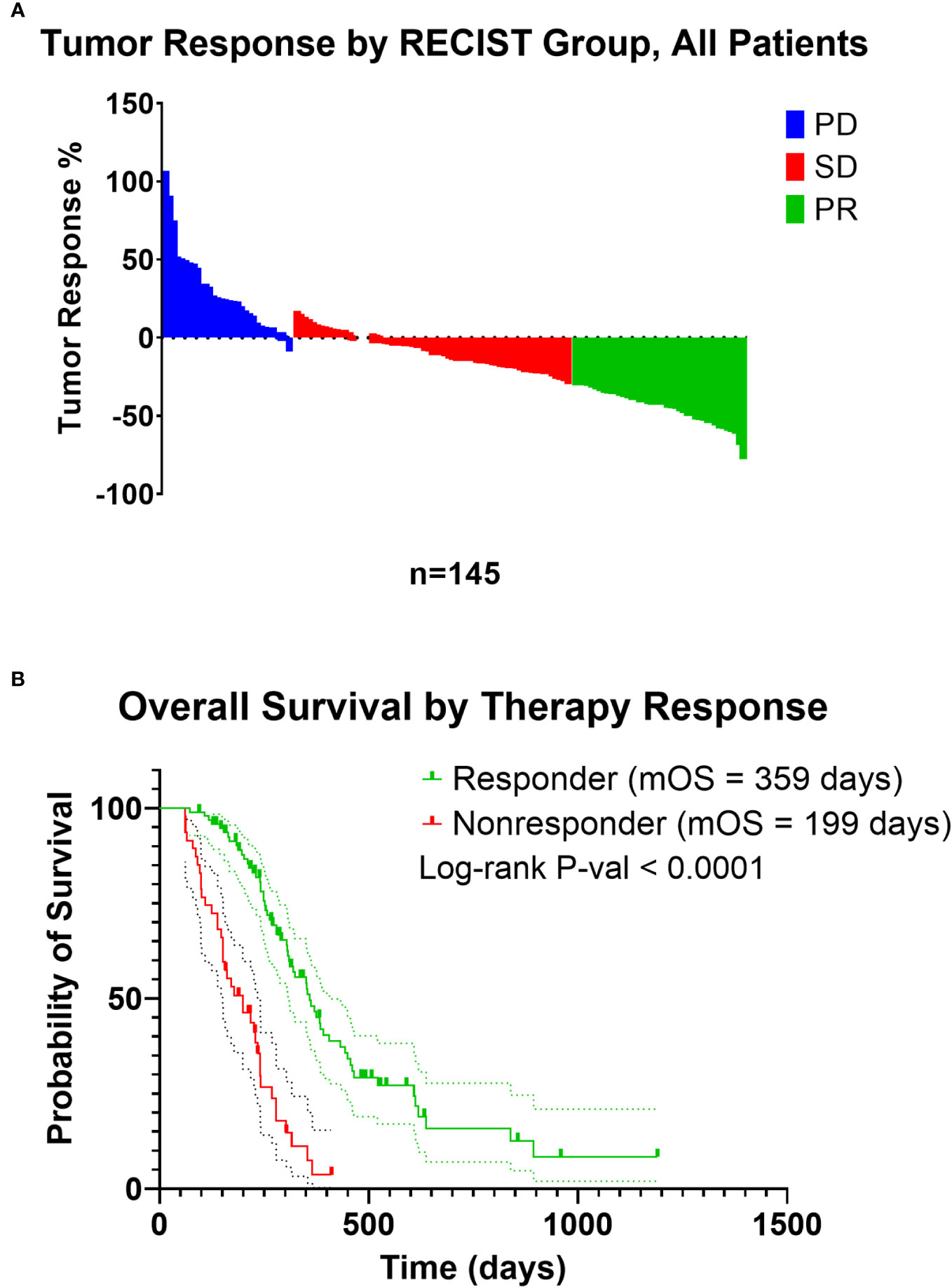
Figure 1 (A) Waterfall plot of tumor response of patients included in the analysis from the COMPASS trial. Patients were recoded from PD (Progressive Disease), SD (Stable Disease), and PR (Partial Response) to those with a decrease in tumor size on treatment as “responders”, and patients with an increase in tumor size on treatment as “nonresponders”. (B) Kaplan-Meier estimate of responders and nonresponders.
2.2 Immunomodulatory differential gene expression analysis
Raw count data of RNA sequencing data of patients included in the COMPASS trial were downloaded from EGAD00001004548 (https://ega-archive.org/datasets/EGAD00001004548) and EGAD00001006081 (https://ega-archive.org/datasets/EGAD00001006081). Gene quantification was performed by TPMCalculator (66) and using GENCODE Human Release 43 version of gene annotation GTF file (67). As the immunomodulatory genes which influence immune cell infiltration into the TiME (68) can mediate chemoresistance to gemcitabine (69, 70) or platinum-based therapies (71), we evaluated the immunomodulatory genes within these samples as well. Tumor-Immune System Interactions Database (TISIDB) is an online repository of integrated data of tumor-immune interactions (72), including a curated list of genes encoding immunomodulators based on data from 30 non-hematologic cancer types from The Cancer Genome Atlas (TCGA). The raw count data were processed using edgeR v3.42.4 (73) by filtering to remove lowly expressed genes using the “filterByExpr” function, normalization by trimmed mean of M values (74), and dispersion estimation using the negative binomial distribution method. Differential gene expression analysis was calculated using the quasi-likelihood pipeline with a nominal log fold change threshold of 0.5 and a false discovery rate correction (73) set at a nominal value of 0.05 using genes of interest were obtained from TISIDB. Immunomodulatory genes were divided into immunoinhibitory, immunostimulatory, and MHC genes.
2.3 In silico cytometry based on transcriptomics
The leukocyte composition of each sample was then characterized as an immune cellular fraction using CIBERSORTx, which estimates proportions of immune cell populations from deconvoluted bulk transcriptomic data (67). CIBERSORTx analysis was performed using the following settings: the LM22 signature matrix was used, consisting of 547 genes to distinguish 22 mature immune cell populations; B-mode batch correction was used; quantile normalization was disabled; 1000 permutations were performed for significance analysis. Only CIBERSORTx results with a p-value < 0.05 were included in subsequent analyses. To increase abundance of more comprehensive immune cell phenotypes, immune cell fractions obtained through LM22 were aggregated as outlined in “Aggregate 2” of the Supplementary Materials in Thorsson et al. (75) to obtain 9 immune cell aggregate phenotypes. Differences in immune cell infiltration proportions were analyzed using Mann-Whitney tests.
2.4 Dirichlet regression and statistical analysis
As the output from CIBERSORTx is considered compositional data that carries relative information as proportions of the total amount of immune cell infiltration, summing to 1 for each sample, traditional data analysis may violate modeling assumptions, such as homoscedasticity (76, 77). Therefore, Dirichlet regression was also performed comparing immune cell fractions between groups of interest using DirichletReg v0.7-1 (76), R version 4.3.0 (78) using the common parametrization model. The regression estimate coefficients obtained using this method can be interpreted similarly to odds ratios if taken as exponentiated coefficients (76). Differences in clinical characteristics of patients were analyzed using Chi-square test or ANOVA, where appropriate. Survival probabilities for each RECIST group were estimated using the Kaplan-Meier method and the log-rank test. Univariate cox proportional hazards for immunomodulators was performed in R using the survival package v3.5-5 (79). Visualization was performed using GraphPad PRISM v9.5.0 and R 4.3.0.
3 Results
3.1 Clinical parameters of included PDAC patients from the COMPASS trial
In total, 145 of the 195 patients from the COMPASS trial dataset met inclusion criteria for our secondary analysis (Table 1). Compared with patients who received Gem-based therapy, mFFX treated patients were significantly younger, predominantly male, and were more likely to have locally advanced disease rather than metastatic disease. However, there was no statistically significant difference in terms of site of biopsy or chemotherapy response between patients receiving mFFX and Gem-based therapy. Similar to previous studies in the efficacy standard chemotherapy regimens in PDAC (3, 64), we observed an improvement in median overall survival (OS) in patients who received FOLFIRINOX compared to Gem-based therapy, although this did not reach significance (median OS 307 vs 254 days, p-value = 0.12, Supplemental Figure 1). For the entire cohort, chemotherapy response correlated with overall survival (Figure 1B, p-value < 0.0001), similar to previous studies utilizing RECIST in a metastatic PDAC setting (80, 81). As expected, patients with tumors that progressed or demonstrated no change with treatment (nonresponder) had significantly shorter median survival compared to the patients with tumors that decreased in size following treatment (responder) (199 vs 359 days).
3.2 Tumor site-specific variations in the PDAC tumor immune microenvironment
Considering the unique pre-treatment patient samples within our cohort, we initially sought to determine whether site-specific differences existed in the cellular components of the PDAC TiME (68) between primary pancreatic tumor biopsies and metastatic liver samples. Compared with pancreatic tumor samples, metastatic liver biopsies had significantly higher expression of multiple immunomodulatory genes. Immunoinhibitory genes such as ADORA2A (log fold-change 0.92, p-value < 0.0001) (82, 83), CSF1R (log fold-change 0.62, p-value = 0.003) (84, 85), and CD274/PD-L1 (log fold-change 0.72, p-value 0.03) had significantly higher expression in the liver biopsy samples compared with pancreatic samples (Figure 2). We also noted differences in immunostimulatory and MHC genes based on site of biopsy. A total of 12 immunostimulatory genes within the annotation (CD70, CD80, CD86, CD276, IL2RA, MICB, NT5E, PVR, TNFSF14, TNFRSF14, TNFRSF18, ULBP1) were significantly differentially expressed between liver and pancreatic biopsy samples. Of these, only TNFRSF14, a membrane-bound receptor (86) with both pro-inflammatory and anti-inflammatory immune signaling pathways (87), was downregulated in the liver biopsy samples compared to the pancreatic biopsy samples (log fold-change -0.43, p-value = 0.003), while the other immunostimulatory genes were upregulated. Similarly, of the MHC genes that were significantly differentially expressed (TAP2, HLA-DOB, TAP1, HLA-DQA1), all were upregulated in the liver biopsy samples compared to the pancreatic biopsy samples. Taken together, this data suggests that the TiME of liver metastases in PDAC undergoes more dynamic regulation compared to that of primary pancreatic lesions.
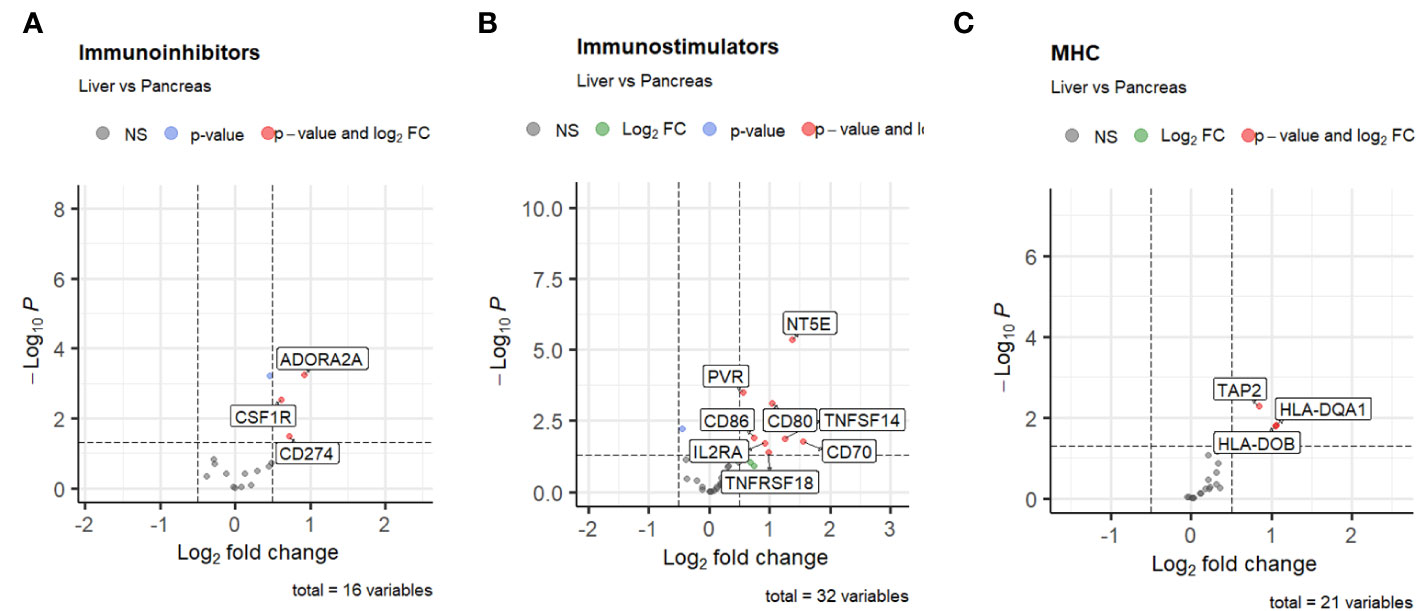
Figure 2 Volcano plots of differential expression of (A) immunoinhibitors, (B) immunostimulators, and (C) MHC genes from TISIDB based on site of biopsy. The threshold for log2 fold change is set at 0.5, and the threshold for false discovery rate is set at 0.05.
3.3 Pre-treatment PDAC TiME differences associated with chemotherapy response
Next, we investigated the association of pre-treatment PDAC tumor immune cell infiltration with response to either mFFX or Gem-based therapy. We first compared the initial immune cell infiltration of each treatment group to determine if there were upfront differences in immune populations that may bias downstream analyses. While patients who received mFFX had decreased infiltration by the CD4 T cell aggregate compared to the Gem-based group, there were no statistical differences in immune infiltration by the individual CD4 T cell phenotypes included in the aggregate (Supplementary Table 1), suggesting a similar initial immune cell phenotypic infiltration between treatment groups.Chemotherapy response was associated with variations in expression of tumor pre-treatment immunomodulating genes (Figures 3A, B). The immunoinhibitory gene TGFB1 was downregulated in responders to mFFX compared to nonresponders (log fold-change -0.55, p-value < 0.005), while the immunostimulator CD70 (88) was significantly upregulated (log fold-change 2.2, p-value < 0.05). In the metastatic liver-cohort, only the upregulation of CD70 remained significantly increased among responders (log fold-change 2.8, p-value < 0.05). There were no significantly upregulated or downregulated genes associated with Gem-based therapy response (Supplementary Figures 2A, B).
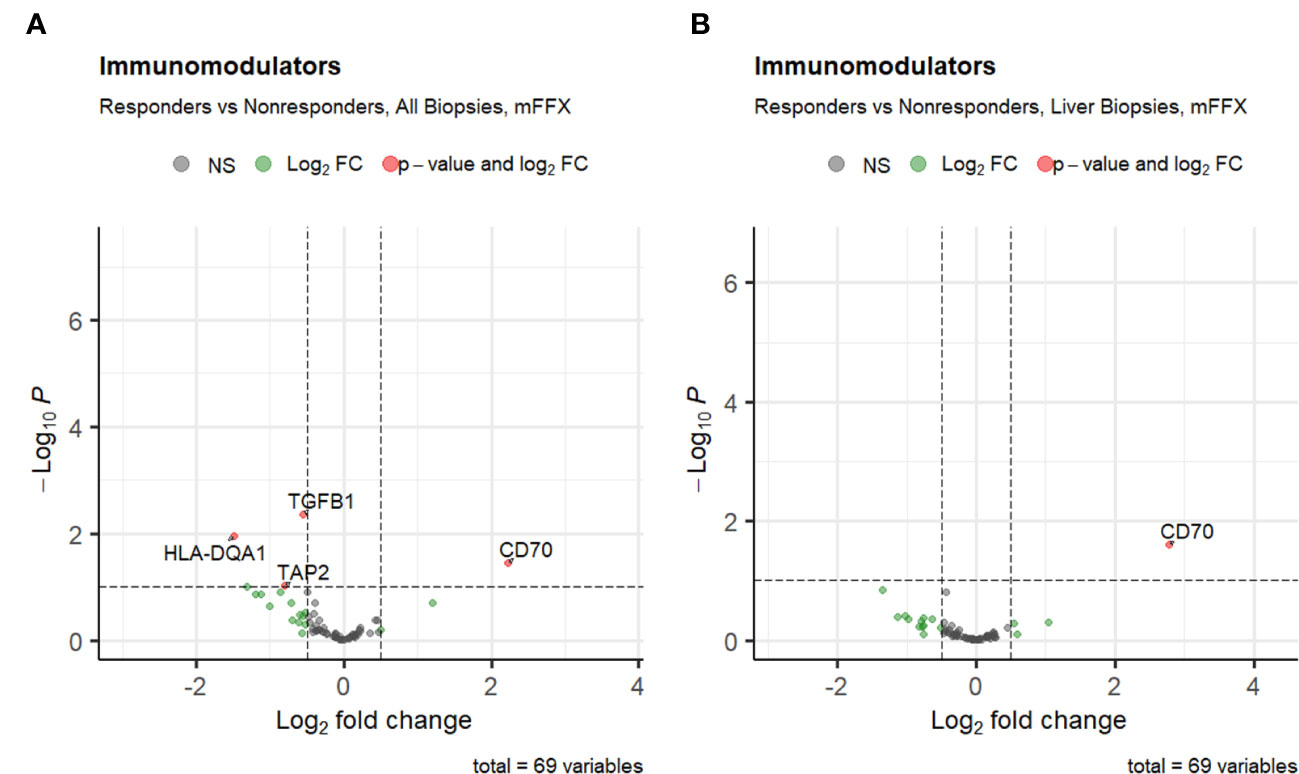
Figure 3 Volcano plots of differential expression of immunomodulatory genes from TISIDB for (A) patients who received mFFX, and (B) patients who received mFFX and had metastatic liver biopsies.
3.4 Clinical impact of immunomodulatory genes between biopsy sites
We wanted to evaluate the impact of immunomodulator gene expression on clinical outcomes within our patient cohort. As therapy response strongly correlated with overall survival in this patient cohort from the COMPASS trial, we investigated the association between immunomodulatory gene expression and survival in the different therapy cohorts based on treatment response (Figures 4A-D). Across all biopsies, the immunoinhibitors ADORA2A, CSF1R, KDR/VEGFR2, LAG3, PDCD1LG2, and TGFB1 were significantly associated with worse survival in patients who received mFFX (Figures 4A, C). A subset of these immunoinhibitors including ADORA2A, CSF1R, LAG3, and TGFB1 were also significantly associated with worse survival in the subset of liver biopsies from patients who received mFFX. There were no immunoinhibitors associated with either improved or worsened survival in the Gem-based cohort for all samples and the liver subset.
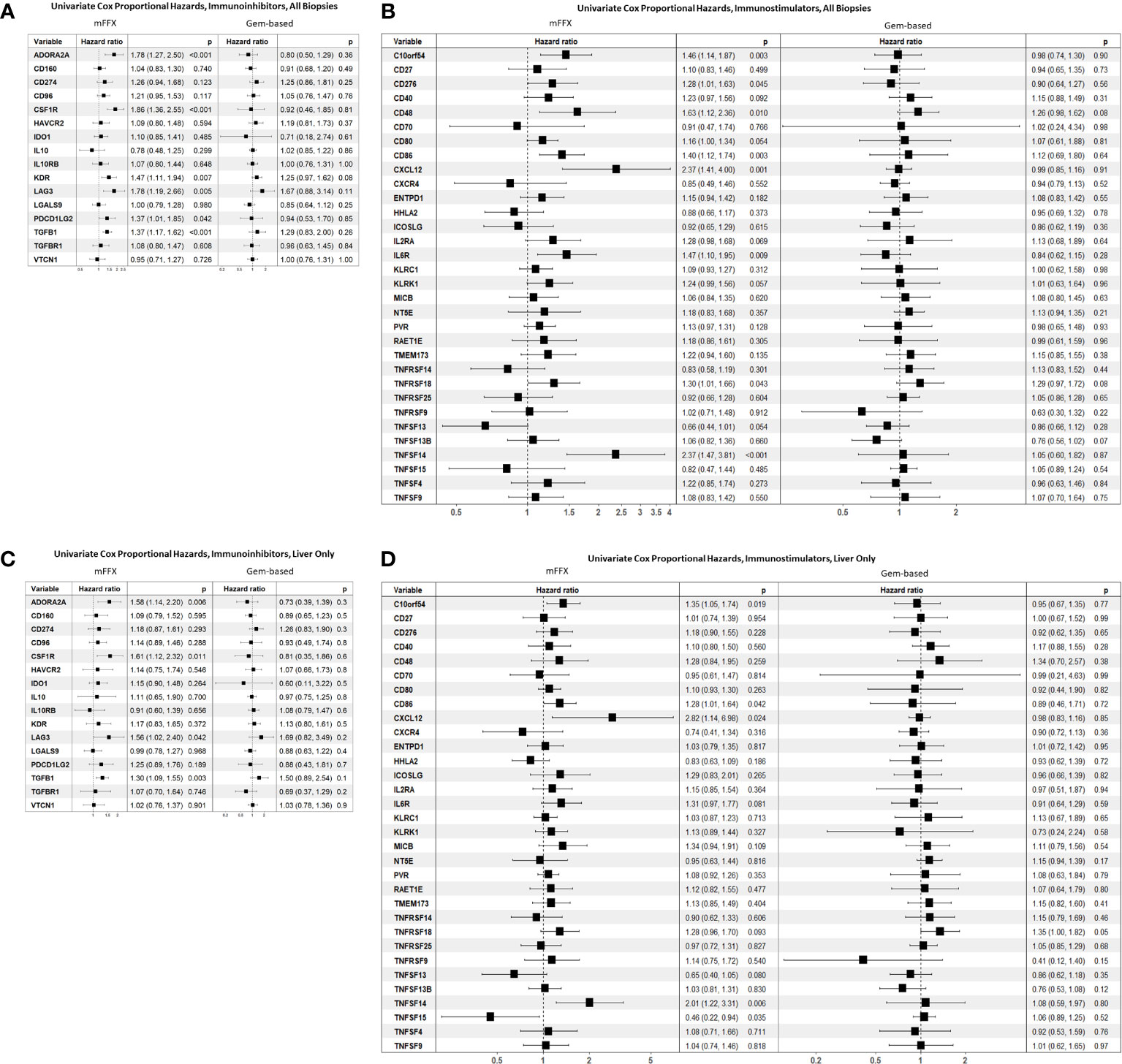
Figure 4 Forest plot of univariate Cox proportional hazard analyses based on chemotherapy received. (A) Reports hazard ratios for immunoinhibitors in all biopsies, (B) reports hazard ratios for immunostimulators in all biopsies. (C) Reports hazard ratios for immunoinhibitors in metastatic liver biopsies, and (D) reports hazard ratios for immunostimulators in metastatic liver biopsies.
Multiple immunostimulatory genes were also associated with worse survival in the mFFX cohort across all samples (Figure 4B), including C10orf54, CXCL12, and TNFSF14/LIGHT. While there was an overlap in significant immunostimulators between all samples and the liver sample subset (Figure 4D), TNFSF15/TL1A was only significantly associated with improved survival in liver samples (HR 0.46, p-value = 0.03). Again, we found no immunostimulatory genes that were significantly associated with response to Gem-based therapy in either all samples or the liver subset.
3.5 Quantification of immune cell infiltration using CIBERSORTx
Similar to our immunomodulatory analysis, we observed significant differences using CIBERSORTx in infiltrating immune cell proportions on comparison of the primary lesion pancreatic samples compared with metastatic liver biopsies (Table 2). Metastatic liver samples had both increased macrophage (1.2 times more, p-value < 0.05) and increased eosinophil infiltration (1.4 times more, p-value < 0.05) compared to pancreatic biopsies from the primary lesion. Further analysis of the specific macrophage phenotypes revealed an increased M2 macrophage fraction in the liver samples, indicating a more immunosuppressed metastatic TiME compared to the primary lesion.
For the entire cohort of treated patients, increased infiltration by 8 out of the 9 immune cell aggregate phenotypes except NK cells were significantly associated with response to mFFX treatment. Pre-treatment tumor immune cell populations were not associated with response to Gem-based treatment (Table 3A). On subset analysis of PDAC patients with liver metastasis, only increased pre-treatment CD8 T-cell, dendritic cell, and neutrophil infiltration were only significantly associated with therapy response to mFFX (Table 3B). Conversely, increased total mast cell infiltration was only associated with response to Gem-based therapy in the liver TiME.
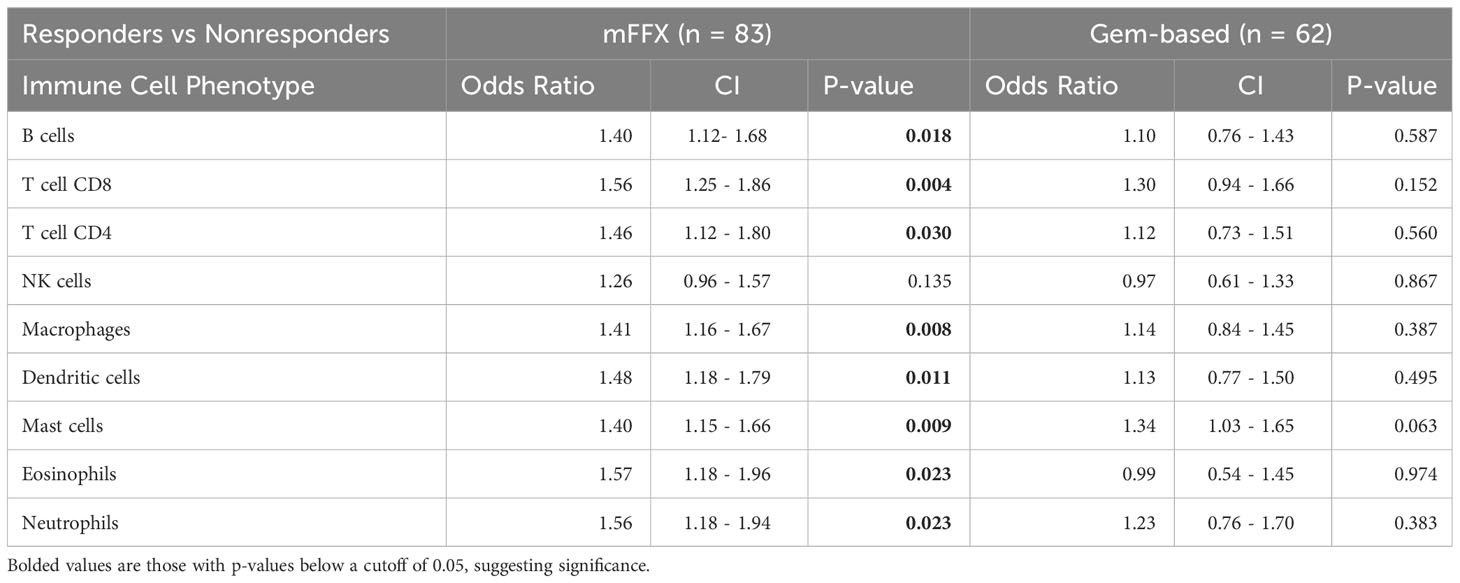
Table 3A Comparison of immune infiltration in responders versus nonresponders for patients treated with mFFX and Gem-based therapy.
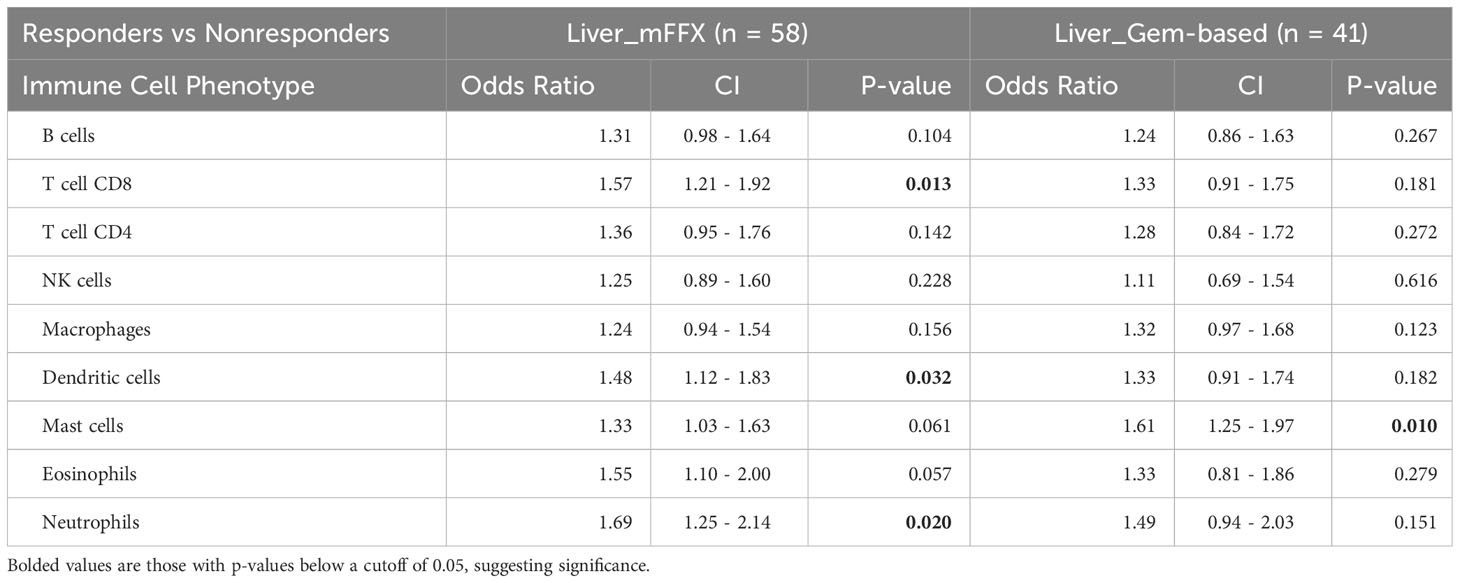
Table 3B Comparison of immune infiltration in responders versus nonresponders for patients treated with mFFX and Gem-based therapy, metastatic liver biopsies only.
Taken together, these data suggest that pre-treatment infiltration by different immune cell phenotypes differs based on site and are associated with therapy response in both a site-specific and therapy-specific fashion. Response to mFFX was more associated with increased infiltration by CD8 T-cells compared to Gem-based regimens, indicating that the pre-treatment TiME may be more impactful for patients receiving mFFX.
4 Discussion
Across cancers, the TiME is a well described mediator of patient survival and can influence treatment response (89). A growing body of evidence supports the ability of FOLFIRINOX to augment tumoral immunity. However, the influence of pre-treatment TiME on FOLFIRINOX response is not well described. Insight into tumor microenvironment features associated with chemotherapy response may help to identify key mediators of efficacy and point to future opportunities in designing next generation chemo-ICI strategies. Utilizing access to a unique dataset of treatment-naïve biopsy samples of advanced or metastatic PDAC patients from the COMPASS trial, we hypothesized that the components of the pre-treatment TiME would differ between metastatic and primary lesions and would be associated with therapy response and survival in a cohort of advanced PDAC patients.
In the present study, we identified key site-specific differences in the cellular and genomic components of the PDAC TiME. Our analysis identified that PDAC metastatic liver biopsies had more variable expression of immunomodulatory genes compared to pancreas biopsies. Multiple immunoinhibitory genes such as CSF1R (84, 85) and components of immune checkpoint signaling pathways such as CD86-CTLA4 (90), PD-1/PD-L1 (91), and PVR-TIGIT (92) were upregulated in liver metastatic samples. Notably, no immunomodulatory genes were identified that were significantly upregulated in the primary lesion TiME relative to the metastatic tumor samples. Compared to the TiME of primary lesion pancreatic biopsies, the metastatic liver biopsies also demonstrated increased infiltration by M0 and M2 macrophages. M0 macrophages are considered undifferentiated macrophages that can be polarized into different functional phenotypes such as M1 and M2 (93). However, a growing body of evidence suggests that M0 macrophages are not a benign member of the TiME. They are associated with worse outcomes in multiple cancers such as breast (94), prostate (95), and lung cancer (96), and possess a transcriptional profile similar to that of M2 macrophages (97, 98). This, combined with an increased infiltration of the immunoinhibitory M2 macrophage (46–52) and the immunomodulatory findings stated above, suggests that the metastatic liver TiME is more dynamically regulated and overall immunosuppressed compared to the TiME of the primary lesion.
An inflamed pre-treatment TiME has been recognized as a predictor of response to neoadjuvant chemotherapy in various cancers (99, 100). One of the earlier markers used to predict response was the systemic immune-inflammatory index (SII) (101), based on peripheral neutrophils, platelets, and lymphocytes that portended survival in NSCLC (102), gastric (103, 104), and colorectal (105) cancer. Similarly, the Immunoscore, which was initially developed in colorectal cancer (106) and is based on CD3+/CD8+ lymphocyte quantification in tumors, predicts disease-free survival and overall survival in colorectal cancer (54, 55) and other cancers (56, 57). Notably, standard chemotherapy regimens for colorectal cancer have significant overlapping antineoplastic agents with FOLFIRINOX, including FOLFOX, XELOX, FOLFIRI, FOLFOXIRI, or CAPIRI (107), implying that a pre-treatment immune contexture can impact therapy response in PDAC as well. This is supported by retrospective studies in PDAC, which have demonstrated associations between survival and various pre-treatment TIL populations. For example, increased CD8 TIL presence correlated with improved survival (108, 109), while M2 macrophage infiltration correlated with worsened survival (110–112). However, limited data exist in PDAC directly addressing the effect of the pre-treatment immune contexture on chemotherapy. In this study, we show that key members of the pre-treatment TiME are also significantly associated with treatment response. Notably, increased infiltration by mutually exclusive immune cell phenotypes were associated with response to different chemotherapeutic regimens. Increased CD8 T-cell infiltration was significantly associated with tumor response to mFFX in the entire cohort and on subset analysis of patients with liver metastasis. Previous reports have demonstrated that mFFX treatment is associated with an increased infiltration of CD8+ T cells and reduced Tregs (41–43, 113) suggesting that mFFX can augment the PDAC TiME. However, our analysis demonstrates that the pre-treatment CD8 T-cell infiltration status of the PDAC TiME may also impact response to FOLFIRINOX. This opens the question of whether the observed increase in CD8+ T cells post-mFFX and the associated favorable response were due to the presence of a high CD8+ T cell population pre-treatment. Our findings implicate the tumor immune status and favorable biology of the treatment-naive tumor may influence response. It is highly likely that both the pre-treatment TiME and chemotherapy-induced CD8+ T cells contribute to a favorable response with mFFX. In contrast, Gem-based regimens were not associated with the presence of any immune cell population across all samples, and only mast cell aggregates in the liver subset. While mast cell infiltration is typically associated with tumor growth (114, 115), higher mast cell infiltration was significantly correlated with overall survival and response to gemcitabine in a cohort of biliary tract cancer patients (116).
These treatment-specific patterns in the TiME were also seen when analyzing immunomodulators associated with therapy response and survival. For example, the immunoinhibitory and pro-tumorigenic (117) cytokine TGFB1 was significantly downregulated in responders and associated with worse survival in the mFFX treatment cohort. Other genes such as ADORA2A, CSF1R, and LAG3 were significantly associated with worse survival in patients in the mFFX cohort across all samples and liver-only samples. No immunomodulatory genes were associated with therapy response in the Gem-based therapy cohort when comparing either all biopsies or focusing solely on liver biopsy samples (Supplementary Figures S2A, B). Similarly, no immunomodulatory genes were associated with survival within the Gem-based cohort (Figures 4A–D). In the context of pre-clinical studies which demonstrate that FOLFIRINOX (18–20) and gemcitabine (118, 119) impact tumor immunity, our data suggest that the interactions between the cellular and genomic components of the pre-treatment PDAC TiME with FOLFIRINOX and gemcitabine may be mechanistically different.
As reflected within this patient cohort, overall survival for patients with advanced stage PDAC remains abysmal, with 5-year survival of less than 10% (2) and highlights the need for strategies to improve outcomes for this large subset of PDAC patients. Currently, aside from the patient’s performance status there are no indications for administering one regimen over the other (41, 42). Our data suggest that FOLFIRINOX-based chemotherapy approach may be advantageous in select patients with a favorable pre-existing TiME. In such patients, and in patients of borderline performance status that may sway a clinician against the use of FOLFIRINOX (41, 42), an immune-based indication for chemotherapy may provide a more nuanced approach to cancer therapy and improve patient outcomes. Furthermore, the implication of a dynamically regulated, immunosuppressed metastatic TiME in PDAC suggests potential avenues for targeted therapies and implies the need to incorporate stage of disease into future design of immune-targeted PDAC therapeutic strategies. For example, the presence of multiple upregulated immune checkpoint pathways in the metastatic PDAC TiME may be targeted via existing checkpoint blockade therapies (84, 120–122) and may represent a potential therapy target to improve outcomes for patients with metastatic PDAC. Another consideration would be to capitalize on the impact of a pre-treatment TiME on chemotherapy through immunological priming. For example, preclinical studies show administration of immunomodulatory cytokines such as interferon sensitize PDAC cell lines to gemcitabine (123, 124). Another strategy could combine chemotherapy with the prior use of mRNA vaccines to expand tumor-specific T-cells (45). As multiple immunomodulatory genes were only associated with survival in the mFFX cohort, adjunctive immunotherapeutic strategies could improve mFFX response in either a chemotherapy-only or chemo-ICI regimen. Potential candidates include the use of antibodies or bispecific molecules to target TGFB, which are in clinical testing (125). Agents to block CSF1R, such as surufatinib, are also in testing, and have demonstrated anti-tumoral efficacy in phase III trials (126).
Limitations exist within this study. The COMPASS trial included only patients with advanced or metastatic PDAC, and the results may not be valid in a stage I/II, resectable cohort. CIBERSORTx only provides relative proportions of immune cell infiltration and may not be reflective of absolute infiltration especially for inter-sample comparison (127); as such, we are unable to quantify how potential differences in the absolute immune infiltration may impact chemotherapy response, and will require further study. Additionally, the LM22 signature matrix used in deconvolutional analysis was derived from microarray-derived data from PBMCs (128) and may not be reflective of the totality of immune cell phenotypes within the PDAC TiME. However, this study utilized a large, unique dataset of treatment-naïve PDAC samples to analyze the TiME; future efforts would investigate the in silico analysis in both in vitro and in vivo PDAC models to validate the results and advance our understanding of the PDAC tumor biology.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://ega-archive.org/datasets/EGAD00001004548.
Ethics statement
The studies involving humans were approved by Institutional Review Board approval and written consent for the COMPASS trial was obtained from participating institutions (University Health Network, Toronto, Ontario, Canada; MUHC Centre for Applied Ethics, Montreal, Quebec, Canada; and Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board, Kingston, Ontario, Canada) and a data use agreement was completed by Baylor College of Medicine with the Ontario Institute for Cancer Research for use of the data within this study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. SK: Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. DE: Supervision, Writing – review & editing. JA: Writing – review & editing. GV: Writing – review & editing. WF: Writing – review & editing. ZS: Methodology, Supervision, Writing – review & editing. MR: Writing – review & editing. HL: Data curation, Software, Supervision, Writing – review & editing. EC: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported in part by Merit Review Award # I01 CX001880-01A1 from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Program (ERC) and the National Pancreatic Cancer Foundation. This work was supported by a US Department of Defense Impact Award (Lee: W81XWH-22-1-0657) and a Cancer Prevention and Research Institute of Texas grant (Lee: RP200443). Additional support was provided by a pilot grant from the Dan L Duncan Comprehensive Cancer Center, which is partly funded by the NIH grant P30 CA125123 (Camp & Lee).
Acknowledgments
The authors would like to acknowledge the members of the PanCuRx Translational Research Initiative and the Ontario Institute for Cancer Research for providing access to the data from the COMPASS trial.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1274783/full#supplementary-material
Supplementary Figure 1 | Kaplan-Meier estimate of overall survival of patients who received mFFX versus Gem-based therapy.
Supplementary Figure 2 | Volcano plots of differential expression of immunomodulatory genes from TISIDB for (A) patients who received Gem-based therapy, and (B) patients who received Gem-based therapy and had metastatic liver biopsies.
Supplementary Table 1 | Comparison of immune infiltration of mFFX versus Gem-based therapy.
References
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res (2014) 74(11):2913–21. doi: 10.1158/0008-5472.CAN-14-0155
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
3. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul J-L, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med (2018) 379(25):2395–406. doi: 10.1056/NEJMoa1809775
4. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med (2013) 369(18):1691–703. doi: 10.1056/NEJMoa1304369
5. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. New Engl J Med (2019) 381(16):1535–46. doi: 10.1056/NEJMoa1910836
6. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn M-J, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non–small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol (2020) 6(5):661–74. doi: 10.1001/jamaoncol.2020.0237
7. Oh D-Y, He AR, Qin S, Chen L-T, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid (2022) 1(8):EVIDoa2200015. doi: 10.1056/EVIDoa2200015
8. O’Reilly EM, Oh D-Y, Dhani N, Renouf DJ, Lee MA, Sun W, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol (2019) 5(10):1431–8. doi: 10.1001/jamaoncol.2019.1588
9. Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol (2020) 17(12):725–41. doi: 10.1038/s41571-020-0413-z
10. Guan Y, Kraus SG, Quaney MJ, Daniels MA, Mitchem JB, Teixeiro E. FOLFOX chemotherapy ameliorates CD8 T lymphocyte exhaustion and enhances checkpoint blockade efficacy in colorectal cancer. Front Oncol (2020) 10:586. doi: 10.3389/fonc.2020.00586
11. Liu P, Chen J, Zhao L, Hollebecque A, Kepp O, Zitvogel L, et al. PD-1 blockade synergizes with oxaliplatin-based, but not cisplatin-based, chemotherapy of gastric cancer. Oncoimmunology (2022) 11(1):2093518. doi: 10.1080/2162402X.2022.2093518
12. Song W, Shen L, Wang Y, Liu Q, Goodwin TJ, Li J, et al. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat Commun (2018) 9(1):2237. doi: 10.1038/s41467-018-04605-x
13. Dosset M, Vargas TR, Lagrange A, Boidot R, Vegran F, Roussey A, et al. PD-1/PD-L1 pathway: an adaptive immune resistance mechanism to immunogenic chemotherapy in colorectal cancer. Oncoimmunology (2018) 7(6):e1433981. doi: 10.1080/2162402X.2018.1433981
14. Maharjan R, Choi JU, Kweon S, Pangeni R, Lee NK, Park SJ, et al. A novel oral metronomic chemotherapy provokes tumor specific immunity resulting in colon cancer eradication in combination with anti-PD-1 therapy. Biomaterials (2022) 281:121334. doi: 10.1016/j.biomaterials.2021.121334
15. Cubas R, Moskalenko M, Cheung J, Yang M, McNamara E, Xiong H, et al. Chemotherapy combines effectively with anti-PD-L1 treatment and can augment antitumor responses. J Immunol (2018) 201(8):2273–86. doi: 10.4049/jimmunol.1800275
16. Wu Y, Deng Z, Wang H, Ma W, Zhou C, Zhang S. Repeated cycles of 5-fluorouracil chemotherapy impaired anti-tumor functions of cytotoxic T cells in a CT26 tumor-bearing mouse model. BMC Immunol (2016) 17(1):29. doi: 10.1186/s12865-016-0167-7
17. Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discovery (2005) 4(4):307–20. doi: 10.1038/nrd1691
18. Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene (2010) 29(4):482–91. doi: 10.1038/onc.2009.356
19. Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity (2016) 44(2):343–54. doi: 10.1016/j.immuni.2015.11.024
20. Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res (2010) 70(8):3052–61. doi: 10.1158/0008-5472.CAN-09-3690
21. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
22. Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med (2020) 382(9):810–21. doi: 10.1056/NEJMoa1910549
23. Gadgeel S, Rodriguez-Abreu D, Speranza G, Esteban E, Felip E, Domine M, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol (2020) JCO1903136. doi: 10.1200/JCO.19.03136
24. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (2021) 398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2
25. Powles T, Csoszi T, Ozguroglu M, Matsubara N, Geczi L, Cheng SY, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(7):931–45. doi: 10.1016/S1470-2045(21)00152-2
26. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0
27. Gogishvili M, Melkadze T, Makharadze T, Giorgadze D, Dvorkin M, Penkov K, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med (2022). doi: 10.1038/s41591-022-01977-y
28. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol (2020) 15(8):1351–60. doi: 10.1016/j.jtho.2020.03.028
29. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol (2021) 16(4):653–64. doi: 10.1016/j.jtho.2020.11.025
30. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA (2021) 326(10):916–25. doi: 10.1001/jama.2021.12836
31. Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol (2022) 23(2):220–33. doi: 10.1016/S1470-2045(21)00650-1
32. Galsky MD, Arija JAA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395(10236):1547–57. doi: 10.1016/S0140-6736(20)30230-0
33. Rexer H, Ohlmann CH, Retz M. [First-line therapy for locally advanced or metastatic urothelial carcinoma : A randomized, controlled phase III trial comparing pembrolizumab with or without platinum-based combination chemotherapy and chemotherapy only in patients with advanced or metastatic urothelial carcinoma (keynote 361-AB 54/16 of the AUO)]. Urologe A (2017) 56(5):659–61. doi: 10.1007/s00120-017-0380-x
34. Wang Y, Han H, Zhang F, Lv T, Zhan P, Ye M, et al. Immune checkpoint inhibitors alone vs immune checkpoint inhibitors-combined chemotherapy for NSCLC patients with high PD-L1 expression: a network meta-analysis. Br J Cancer (2022) 127(5):948–56. doi: 10.1038/s41416-022-01832-4
35. Emens LA, Molinero L, Loi S, Rugo HS, Schneeweiss A, Dieras V, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. J Natl Cancer Inst (2021) 113(8):1005–16. doi: 10.1093/jnci/djab004
36. Burris H 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol (1997) 15(6):2403–13. doi: 10.1200/JCO.1997.15.6.2403
37. Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg (2015) 261(1):12–7. doi: 10.1097/SLA.0000000000000867
38. Cloyd JM, Katz MH, Prakash L, Varadhachary GR, Wolff RA, Shroff RT, et al. Preoperative therapy and pancreatoduodenectomy for pancreatic ductal adenocarcinoma: a 25-year single-institution experience. J Gastrointest Surg (2017) 21(1):164–74. doi: 10.1007/s11605-016-3265-1
39. Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol (2020) 38(27):3217–30. doi: 10.1200/JCO.20.01364
40. Network NCC. Pancreatic Cancer (Version 2.2023) (2023). Available at: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
41. Peng H, James CA, Cullinan DR, Hogg GD, Mudd JL, Zuo C, et al. Neoadjuvant FOLFIRINOX therapy is associated with increased effector T cells and reduced suppressor cells in patients with pancreatic cancer. Clin Cancer Res (2021) 27(24):6761–71. doi: 10.1158/1078-0432.CCR-21-0998
42. Farren MR, Sayegh L, Ware MB, Chen HR, Gong J, Liang Y, et al. Immunologic alterations in the pancreatic cancer microenvironment of patients treated with neoadjuvant chemotherapy and radiotherapy. JCI Insight (2020) 5(1). doi: 10.1172/jci.insight.130362
43. Van Der Kraak L, Goel G, Ramanan K, Kaltenmeier C, Zhang L, Normolle DP, et al. 5-Fluorouracil upregulates cell surface B7-H1 (PD-L1) expression in gastrointestinal cancers. J Immunother Cancer (2016) 4:65. doi: 10.1186/s40425-016-0163-8
44. Ma J, Sun D, Wang J, Han C, Qian Y, Chen G, et al. Immune checkpoint inhibitors combined with chemotherapy for the treatment of advanced pancreatic cancer patients. Cancer Immunol Immunother (2020) 69(3):365–72. doi: 10.1007/s00262-019-02452-3
45. Rojas LA, Sethna Z, Soares KC, Olcese C, Pang N, Patterson E, et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature (2023) 618(7963):144–50. doi: 10.1038/s41586-023-06063-y
46. Balachandran VP, Beatty GL, Dougan SK. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology (2019) 156(7):2056–72. doi: 10.1053/j.gastro.2018.12.038
47. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell (2017) 168(4):707–23. doi: 10.1016/j.cell.2017.01.017
48. Anderson KG, Stromnes IM, Greenberg PD. Obstacles posed by the tumor microenvironment to T cell activity: A case for synergistic therapies. Cancer Cell (2017) 31(3):311–25. doi: 10.1016/j.ccell.2017.02.008
49. Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol (2020) 17(9):527–40. doi: 10.1038/s41571-020-0363-5
50. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med (2018) 24(5):541–50. doi: 10.1038/s41591-018-0014-x
51. Beatty GL, Eghbali S, Kim R. Deploying immunotherapy in pancreatic cancer: defining mechanisms of response and resistance. Am Soc Clin Oncol Educ Book (2017) 37):267–78. doi: 10.1200/EDBK_175232
52. Hu H, Hang J-J, Han T, Zhuo M, Jiao F, Wang L-W. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumor Biol (2016) 37:8657–64. doi: 10.1007/s13277-015-4741-z
53. Wörmann SM, Diakopoulos KN, Lesina M, Algül H. The immune network in pancreatic cancer development and progression. Oncogene (2014) 33(23):2956–67. doi: 10.1038/onc.2013.257
54. Pagès F, Mlecnik B, Marliot F, Bindea G, Ou F-S, Bifulco C, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet (2018) 391(10135):2128–39. doi: 10.1016/S0140-6736(18)30789-X
55. Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol (2011) 29(6):610–8. doi: 10.1200/JCO.2010.30.5425
56. Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, et al. ImmunoScore signature: a prognostic and predictive tool in gastric cancer. Ann Surg (2018) 267(3):504–13. doi: 10.1097/SLA.0000000000002116
57. Donnem T, Kilvaer TK, Andersen S, Richardsen E, Paulsen EE, Hald SM, et al. Strategies for clinical implementation of TNM-Immunoscore in resected nonsmall-cell lung cancer. Ann Oncol (2016) 27(2):225–32. doi: 10.1093/annonc/mdv560
58. Son S-M, Woo CG, Kim DH, Yun HY, Kim H, Kim HK, et al. Distinct tumor immune microenvironments in primary and metastatic lesions in gastric cancer patients. Sci Rep (2020) 10(1):1–12. doi: 10.1038/s41598-020-71340-z
59. Dötzer K, Schlüter F, Schoenberg MB, Bazhin AV, Edler von Koch F, Schnelzer A, et al. Immune heterogeneity between primary tumors and corresponding metastatic lesions and response to platinum therapy in primary ovarian cancer. Cancers (2019) 11(9):1250. doi: 10.3390/cancers11091250
60. Lee H, Na KJ, Choi H. Differences in tumor immune microenvironment in metastatic sites of breast cancer. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.649004
61. Rozenblit M, Huang R, Danziger N, Hegde P, Alexander B, Ramkissoon S, et al. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J Immunother Cancer (2020) 8(2). doi: 10.1136/jitc-2020-001558
62. Kim R, Keam B, Kim S, Kim M, Kim SH, Kim JW, et al. Differences in tumor microenvironments between primary lung tumors and brain metastases in lung cancer patients: therapeutic implications for immune checkpoint inhibitors. BMC Cancer (2019) 19(1):19. doi: 10.1186/s12885-018-5214-8
63. Aung KL, Fischer SE, Denroche RE, Jang GH, Dodd A, Creighton S, et al. Genomics-driven precision medicine for advanced pancreatic cancer: early results from the COMPASS trial. Clin Cancer Res (2018) 24(6):1344–54. doi: 10.1158/1078-0432.CCR-17-2994
64. O’Kane GM, Grünwald BT, Jang G-H, Masoomian M, Picardo S, Grant RC, et al. GATA6 expression distinguishes classical and basal-like subtypes in advanced pancreatic cancer. Clin Cancer Res (2020) 26(18):4901–10. doi: 10.1158/1078-0432.CCR-19-3724
65. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
66. Vera Alvarez R, Pongor LS, Mariño-Ramírez L, Landsman D. TPMCalculator: one-step software to quantify mRNA abundance of genomic features. Bioinformatics (2018) 35(11):1960–2. doi: 10.1093/bioinformatics/bty896
67. Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol (2019) 37(7):773–82. doi: 10.1038/s41587-019-0114-2
68. van der Woude LL, Gorris MAJ, Halilovic A, Figdor CG, de Vries IJM. Migrating into the tumor: a roadmap for T cells. Trends Cancer (2017) 3(11):797–808. doi: 10.1016/j.trecan.2017.09.006
69. Timothy MN, Brian AB, Darren RC, Roheena ZP, Booyeon JH, Dominic ES, et al. Targeting both tumour-associated CXCR2<sup<+</sup< neutrophils and CCR2<sup<+</sup< macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut (2018) 67(6):1112. doi: 10.1136/gutjnl-2017-313738
70. Singh S, Srivastava S, Bhardwaj A, Owen L, Singh A. CXCL12–CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br J Cancer (2010) 103(11):1671–9. doi: 10.1038/sj.bjc.6605968
71. Reyes ME, de La Fuente M, Hermoso M, Ili CG, Brebi P. Role of CC chemokines subfamily in the platinum drugs resistance promotion in cancer. Front Immunol (2020) 11. doi: 10.3389/fimmu.2020.00901
72. Ru B, Wong CN, Tong Y, Zhong JY, Zhong SSW, Wu WC, et al. TISIDB: an integrated repository portal for tumor–immune system interactions. Bioinformatics (2019) 35(20):4200–2. doi: 10.1093/bioinformatics/btz210
73. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics (2010) 26(1):139–40. doi: 10.1093/bioinformatics/btp616
74. Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol (2010) 11(3):R25. doi: 10.1186/gb-2010-11-3-r25
75. Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity (2018) 48(4):812–830.e14. doi: 10.1016/j.immuni.2018.03.023
76. Maier M. DirichletReg: Dirichlet regression for compositional data in R. Res Rep Ser / Department Stat Mathematics (2014) 125.
77. Yoo J, Sun Z, Greenacre M, Ma Q, Chung D, Kim YM. A guideline for the statistical analysis of compositional data in immunology. Commun Stat Appl Methods (2022) 29(4):453–69. doi: 10.29220/CSAM.2022.29.4.453
78. Team RDC. a language and environment for statistical computing (2009). Available at: http://www.R-project.org.
80. Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol (2011) 29(34):4548.
81. Kunzmann V, Ramanathan RK, Goldstein D, Liu H, Ferrara S, Lu B, et al. Tumor reduction in primary and metastatic pancreatic cancer lesions with nab-paclitaxel and gemcitabine: an exploratory analysis from a phase 3 study. Pancreas (2017) 46(2):203. doi: 10.1097/MPA.0000000000000742
82. Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-γ production in murine CD4+ T cells. J Immunol (2005) 174(2):1073–80. doi: 10.4049/jimmunol.174.2.1073
83. Stagg J, Smyth M. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene (2010) 29(39):5346–58. doi: 10.1038/onc.2010.292
84. Cannarile MA, Weisser M, Jacob W, Jegg A-M, Ries CH, Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J ImmunoTher Cancer (2017) 5(1):53. doi: 10.1186/s40425-017-0257-y
85. Li J, Chen K, Zhu L, Pollard JW. Conditional deletion of the colony stimulating factor-1 receptor (c-fms proto-oncogene) in mice. Genesis (2006) 44(7):328–35. doi: 10.1002/dvg.20219
86. Steinberg MW, Cheung TC, Ware CF. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol Rev (2011) 244(1):169–87. doi: 10.1111/j.1600-065X.2011.01064.x
87. Shui JW, Steinberg MW, Kronenberg M. Regulation of inflammation, autoimmunity, and infection immunity by HVEM-BTLA signaling. J Leukoc Biol (2011) 89(4):517–23. doi: 10.1189/jlb.0910528
88. Van De Ven K, Borst J. Targeting the T-cell co-stimulatory CD27/CD70 pathway in cancer immunotherapy: rationale and potential. Immunotherapy (2015) 7(6):655–67. doi: 10.2217/imt.15.32
89. Jia Q, Wang A, Yuan Y, Zhu B, Long H. Heterogeneity of the tumor immune microenvironment and its clinical relevance. Exp Hematol Oncol (2022) 11(1):24. doi: 10.1186/s40164-022-00277-y
90. Seliger B, Marincola FM, Ferrone S, Abken H. The complex role of B7 molecules in tumor immunology. Trends Mol Med (2008) 14(12):550–9. doi: 10.1016/j.molmed.2008.09.010
91. Hirano F, Ophir E, Kotturi MF, Levy O, Ganguly S, Leung L, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res (2005) 65(3):1089–96. doi: 10.1158/0008-5472.1089.65.3
92. Whelan S, Ophir E, Kotturi MF, Levy O, Ganguly S, Leung L, et al. PVRIG and PVRL2 are induced in cancer and inhibit CD8+ T-cell function. Cancer Immunol Res (2019) 7(2):257–68. doi: 10.1158/2326-6066.CIR-18-0442
93. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. Functional differentiation. Front Immunol (2014) 5. doi: 10.3389/fimmu.2014.00514
94. Ali HR, Chlon L, Pharoah PD, Markowetz F, Caldas C. Patterns of immune infiltration in breast cancer and their clinical implications: a gene-expression-based retrospective study. PloS Med (2016) 13(12):e1002194. doi: 10.1371/journal.pmed.1002194
95. Jairath NK, Farha MW, Srinivasan S, Jairath R, Green MD, Dess RT, et al. Tumor immune microenvironment clusters in localized prostate adenocarcinoma: prognostic impact of macrophage enriched/plasma cell non-enriched subtypes. J Clin Med (2020) 9(6):1973. doi: 10.3390/jcm9061973
96. Liu X, Wu S, Yang Y, Zhao M, Zhu G, Hou Z. The prognostic landscape of tumor-infiltrating immune cell and immunomodulators in lung cancer. Biomed Pharmacother (2017) 95:55–61. doi: 10.1016/j.biopha.2017.08.003
97. Zhang Q, Li H, Mao Y, Wang X, Zhang X, Yu X, et al. Apoptotic SKOV3 cells stimulate M0 macrophages to differentiate into M2 macrophages and promote the proliferation and migration of ovarian cancer cells by activating the ERK signaling pathway. Int J Mol Med (2020) 45(1):10–22.
98. Gabrusiewicz K, Rodriguez B, Wei J, Hashimoto Y, Healy LM, Maiti SN, et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight (2016) 1(2). doi: 10.1172/jci.insight.85841
99. Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med (2014) 20(11):1301–9. doi: 10.1038/nm.3708
100. Castaneda CA, Mittendorf E, Casavilca S, Wu Y, Castillo M, Arboleda P, et al. Tumor infiltrating lymphocytes in triple negative breast cancer receiving neoadjuvant chemotherapy. World J Clin Oncol (2016) 7(5):387. doi: 10.5306/wjco.v7.i5.387
101. Hu B, Yang X-R, Xu Y, Sun Y-F, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res (2014) 20(23):6212–22. doi: 10.1158/1078-0432.CCR-14-0442
102. Guo W, Cai S, Zhang F, Shao F, Zhang G, Zhou Y, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non-small cell lung cancer. Thorac Cancer (2019) 10(4):761–8. doi: 10.1111/1759-7714.12995
103. Wang Q, Zhu D. The prognostic value of systemic immune-inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. J Gastrointest Oncol (2019) 10(5):965–78. doi: 10.21037/jgo.2019.05.03
104. Ding PA, Guo H, Sun C, Yang P, Kim NH, Tian Y, et al. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: a prospective study. BMC Gastroenterol (2022) 22(1):121. doi: 10.1186/s12876-022-02199-9
105. Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol (2017) 23(34):6261–72. doi: 10.3748/wjg.v23.i34.6261
106. Angell HK, Bruni D, Barrett JC, Herbst R, Galon J. The immunoscore: colon cancer and beyond. Clin Cancer Res (2020) 26(2):332–9. doi: 10.1158/1078-0432.CCR-18-1851
107. Network NCC. Colorectal Cancer (Version 3.2023) (2023). Available at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
108. Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, et al. CD8+ Tumor-infiltrating lymphocytes together with CD4+ Tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas (2004) 28(1):e26–31. doi: 10.1097/00006676-200401000-00023
109. Diana A, Wang LM, D'Costa Z, Allen P, Azad A, Silva MA, et al. Prognostic value, localization and correlation of PD-1/PD-L1, CD8 and FOXP3 with the desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget (2016) 7(27):40992–1004. doi: 10.18632/oncotarget.10038
110. Sugimoto M, Mitsunaga S, Yoshikawa K, Kato Y, Gotohda N, Takahashi S, et al. Prognostic impact of M2 macrophages at neural invasion in patients with invasive ductal carcinoma of the pancreas. Eur J Cancer (2014) 50(11):1900–8. doi: 10.1016/j.ejca.2014.04.010
111. Zeng L, Guo Y, Liang J, Chen S, Peng P, Zhang Q, et al. Perineural invasion and TAMs in pancreatic ductal adenocarcinomas: review of the original pathology reports using immunohistochemical enhancement and relationships with clinicopathological features. J Cancer (2014) 5(9):754–60. doi: 10.7150/jca.10238
112. Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res (2011) 167(2):e211–9. doi: 10.1016/j.jss.2009.05.026
113. Michelakos T, Cai L, Villani V, Sabbatino F, Kontos F, Fernández-del Castillo C, et al. Tumor microenvironment immune response in pancreatic ductal adenocarcinoma patients treated with neoadjuvant therapy. JNCI: J Natl Cancer Inst (2020) 113(2):182–91. doi: 10.1093/jnci/djaa073
114. Chang DZ, Ma Y, Ji B, Wang H, Deng D, Liu Y, et al. Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal AdenocarcinomaMast cells promote PDAC growth. Clin Cancer Res (2011) 17(22):7015–23. doi: 10.1158/1078-0432.CCR-11-0607
115. Strouch MJ, Cheon EC, Salabat MR, Krantz SB, Gounaris E, Melstrom LG, et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res (2010) 16(8):2257–65. doi: 10.1158/1078-0432.CCR-09-1230
116. Bo X, Wang J, Suo T, Ni X, Liu H, Shen S, Li M, et al, et al. Tumor-infiltrating mast cells predict prognosis and gemcitabine-based adjuvant chemotherapeutic benefit in biliary tract cancer patients. BMC Cancer (2018) 18(1):313. doi: 10.1186/s12885-018-4220-1
117. Liang C, Xu J, Meng Q, Zhang B, Liu J, Hua J, et al. TGFB1-induced autophagy affects the pattern of pancreatic cancer progression in distinct ways depending on SMAD4 status. Autophagy (2020) 16(3):486–500. doi: 10.1080/15548627.2019.1628540
118. Plate JMD, Plate AE, Shott S, Bograd S, Harris JE. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother (2005) 54(9):915–25. doi: 10.1007/s00262-004-0638-1
119. Bauer C, Sterzik A, Bauernfeind F, Duewell P, Conrad C, Kiefl R, et al. Concomitant gemcitabine therapy negatively affects DC vaccine-induced CD8+ T-cell and B-cell responses but improves clinical efficacy in a murine pancreatic carcinoma model. Cancer Immunol Immunother (2014) 63(4):321–33. doi: 10.1007/s00262-013-1510-y
120. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. New Engl J Med (2015) 372(21):2018–28. doi: 10.1056/NEJMoa1501824
121. Hoos A, Ibrahim R, Korman A, Abdallah K, Berman D, Shahabi V, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol (2010). doi: 10.1053/j.seminoncol.2010.09.015
122. Rodriguez-Abreu D, Johnson ML, Hussein MA, Cobo M, Patel AJ, Secen NM, et al. Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). Am Soc Clin Oncol (2020). doi: 10.1200/JCO.2020.38.15_suppl.9503
123. Tomimaru Y, Eguchi H, Wada H, Tomokuni A, Kobayashi S, Marubashi S, et al. Synergistic antitumor effect of interferon-ß with gemcitabine in interferon-α-non-responsive pancreatic cancer cells. Int J Oncol (2011) 38(5):1237–43. doi: 10.3892/ijo.2011.954
124. Blaauboer A, Booy S, van Koetsveld PM, Karels B, Dogan F, van Zwienen S, et al. Interferon-beta enhances sensitivity to gemcitabine in pancreatic cancer. BMC Cancer (2020) 20(1):913. doi: 10.1186/s12885-020-07420-0
125. Terabe M, Robertson FC, Clark K, De Ravin E, Bloom A, Venzon DJ, et al. Blockade of only TGF-β 1 and 2 is sufficient to enhance the efficacy of vaccine and PD-1 checkpoint blockade immunotherapy. Oncoimmunology (2017) 6(5):e1308616. doi: 10.1080/2162402X.2017.1308616
126. Xu J, Shen L, Zhou Z, Li J, Bai C, Chi Y, et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol (2020) 21(11):1500–12. doi: 10.1016/S1470-2045(20)30496-4
127. Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol (2016) 17(1):218. doi: 10.1186/s13059-016-1070-5
Keywords: PDACpancreatic ductal adenocarcinoma1, CIBERSORT2, FOLFIRINOX3, immunomodulator(s)4, RNA sequencing5
Citation: Gao Z, Kang SW, Erstad D, Azar J, Van Buren G, Fisher W, Sun Z, Rubinstein MP, Lee HS and Camp ER (2023) Pre-treatment inflamed tumor immune microenvironment is associated with FOLFIRINOX response in pancreatic cancer. Front. Oncol. 13:1274783. doi: 10.3389/fonc.2023.1274783
Received: 08 August 2023; Accepted: 31 October 2023;
Published: 23 November 2023.
Edited by:
Dong Wu, City of Hope, United StatesReviewed by:
Amanda Rae Muñoz, Texas A&M International University, United StatesZiv Radisavljevic, Harvard Medical School, United States
Copyright © 2023 Gao, Kang, Erstad, Azar, Van Buren, Fisher, Sun, Rubinstein, Lee and Camp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: E. Ramsay Camp, cmFtc2F5LmNhbXBAYmNtLmVkdQ==
 Zachary Gao
Zachary Gao Sung Wook Kang1,2,3
Sung Wook Kang1,2,3 Derek Erstad
Derek Erstad Joseph Azar
Joseph Azar Zequn Sun
Zequn Sun Hyun-Sung Lee
Hyun-Sung Lee E. Ramsay Camp
E. Ramsay Camp