- 1Department of Radiation Oncology, National Cancer Center Hospital, Tokyo, Japan
- 2Division of Research and Development for Boron Neutron Capture Therapy, National Cancer Center Exploratory Oncology Research & Clinical Trial Center, Tokyo, Japan
- 3Division of Radiation Safety and Quality Assurance, National Cancer Center Hospital, Tokyo, Japan
- 4Medical Physics Laboratory, Division of Health Science, Graduate School of Medicine, Osaka University, Suita, Osaka, Japan
- 5Department of Dermatologic Oncology, National Cancer Center Hospital, Tokyo, Japan
- 6Department of Radiation Oncology, Jutendo University School of Medicine, Tokyo, Japan
- 7Department of Radiological Science, Graduate School of Human Health Sciences, Tokyo Metropolitan University, Tokyo, Japan
- 8National Cancer Center Hospital, Tokyo, Japan
- 9Shin-Matsudo Accuracy Radiation Therapy Center, Shin-Matsudo Central General Hospital, Chiba, Japan
This study reports the first patient treatment for cutaneous malignant melanoma using a linear accelerator-based boron neutron capture therapy (BNCT) system. A single-center open-label phase I clinical trial had been conducted using the system since November 2019. A patient with a localized node-negative acral malignant melanoma and the largest diameter of the tumor ≤ 15 cm who refused primary surgery and chemotherapy was enrolled. After administering boronophenylalanine (BPA), a single treatment of BNCT with the maximum dose of 18 Gy-Eq delivered to the skin was performed. The safety and efficacy of the accelerator-based BNCT system for treating localized cutaneous malignant melanoma were evaluated. The first patient with cutaneous malignant melanoma in situ on the second finger of the left hand did not develop dose-limiting toxicity in the clinical trial. After BNCT, the treatment efficacy was gradually observed, and the patient achieved PR within 6 months and CR within 12 months. Moreover, during the follow-up period of 12 months after BNCT, the patient did not exhibit a recurrence without any treatment-related grade 2 or higher adverse events. Although grade 1 adverse events of dermatitis, dry skin, skin hyperpigmentation, edema, nausea, and aching pain were noted in the patient, those adverse events were relieved without any treatment. This case report shows that the accelerator-based BNCT may become a promising treatment modality for cutaneous malignant melanoma. We expect further clinical trials to reveal the efficacy and safety of the accelerator-based BNCT for cutaneous malignant melanoma.
Introduction
Boron neutron capture therapy (BNCT) using an accelerator-based neutron source is one of the recently developed radiotherapies. The principle of therapeutic efficacy is primarily based on 10B(n, α)7Li reactions. Those particles exhibit high linear energy transfer, and the relative biological effectiveness of BNCT is greater than that of conventional radiotherapies (1). As the ranges of those particles within the human body are comparable to the size of the target cells, when the concentration of 10B particles is several times higher in the tumor than in the surrounding normal tissues, selective tumor cell killing can be anticipated. Thus, the higher biological effectiveness via boron neutron capture reaction has been experimentally explored (1).
Clinical trials for BNCT have been performed in research reactors, and favorable clinical outcomes have been reported (2, 3). However, the necessity of using a nuclear reactor as a neutron source posed regulatory and safety concerns that prevented the widespread application of BNCT in oncology practice. Recent researches have resulted in implementing accelerator-based neutron sources for BNCT that can be utilized in hospitals (4, 5). Therefore, the linear accelerator-based BNCT system with a lithium target was installed at the National Cancer Center Hospital (NCCH), Tokyo, Japan, and has been in clinical use following the completion of various preclinical studies (5). A phase I clinical trial has been conducted using this system for treating localized cutaneous malignant melanoma and angiosarcoma since November 2019 (6). As neutrons have a limited penetration depth due to the use of epithermal neutron beam, superficial tumors such as cutaneous malignant melanoma are good candidates for BNCT (7).
Standard treatment for cutaneous malignant melanoma without metastasis is primary surgery with extensive resection, with or without postoperative adjuvant therapy depending on its stage (8). Because malignant melanoma is frequently observed in the extremities and is affected by elderly patients (9), radical resection significantly diminishes the quality of life regarding limb function preservation. Therefore, developing treatment options is expected as an alternative to surgery. Boronophenylalanine (BPA, borofalan(10B)), a commonly utilized 10B-containing drug in BNCT, is taken up by tumor cells via the L-type amino acid transporter 1 (LAT-1) (10), and cutaneous malignant melanoma expresses LAT-1 at high levels (11). Therefore, cutaneous malignant melanoma was chosen as one of the target diseases for the clinical trial.
This report describes the first patient treatment for cutaneous malignant melanoma using an accelerator-based BNCT system.
Case description
Clinical trial overview
The trial utilizes an investigational device (CICS-1, neutron irradiation device manufactured by Cancer Intelligence Care Systems, Inc.) and an investigational drug (SPM-011, borofalan(10B) provided by Stella Pharma Corporation) (6). The trial is a single-center, open-label study. The primary endpoint is to assess the incidence of dose-limiting toxicity at a predetermined radiation dose. The severity of adverse events was evaluated based on CTCAE v 5.0. The dose-limiting toxicity was defined as any treatment-related grade 4 or higher and serious adverse event requiring hospitalization occurring within 90 days after irradiation. Patients diagnosed histopathologically as cutaneous malignant melanoma or angiosarcoma without lymph node or distant metastasis are eligible for participation in the trial. The trial protocol is registered with ClinicalTrials.gov (NCT04293289) and JapicCTI (JapicCTI-195062). Although the clinical trial duration was up to 180 days, the patients were followed up after the end of the clinical trial period. The clinical trial is being conducted in accordance with the Declaration of Helsinki and Good Clinical Practices and compliance with the registered protocol. Institutional review boards at NCCH approved the clinical trial (T4725). Prior to treatment, all patients provided written informed consent. The detail of the clinical trial overview was also found in the previous report (6).
Case presentation
A patient, a previously healthy 72-year-old female, was referred to us for the evaluation of malignant melanoma in situ on a second finger of the left hand (Figure 1). She was diagnosed with cutaneous malignant melanoma and had no lymph node nor distant metastasis, as determined by standard diagnostic and staging procedures. The tumor size was 20 × 12 mm. Before BNCT, the nail on the left hand’s second finger had been peeled off spontaneously. She declined radical surgery due to its invasiveness and expected aesthetic results, so she was referred for BNCT.
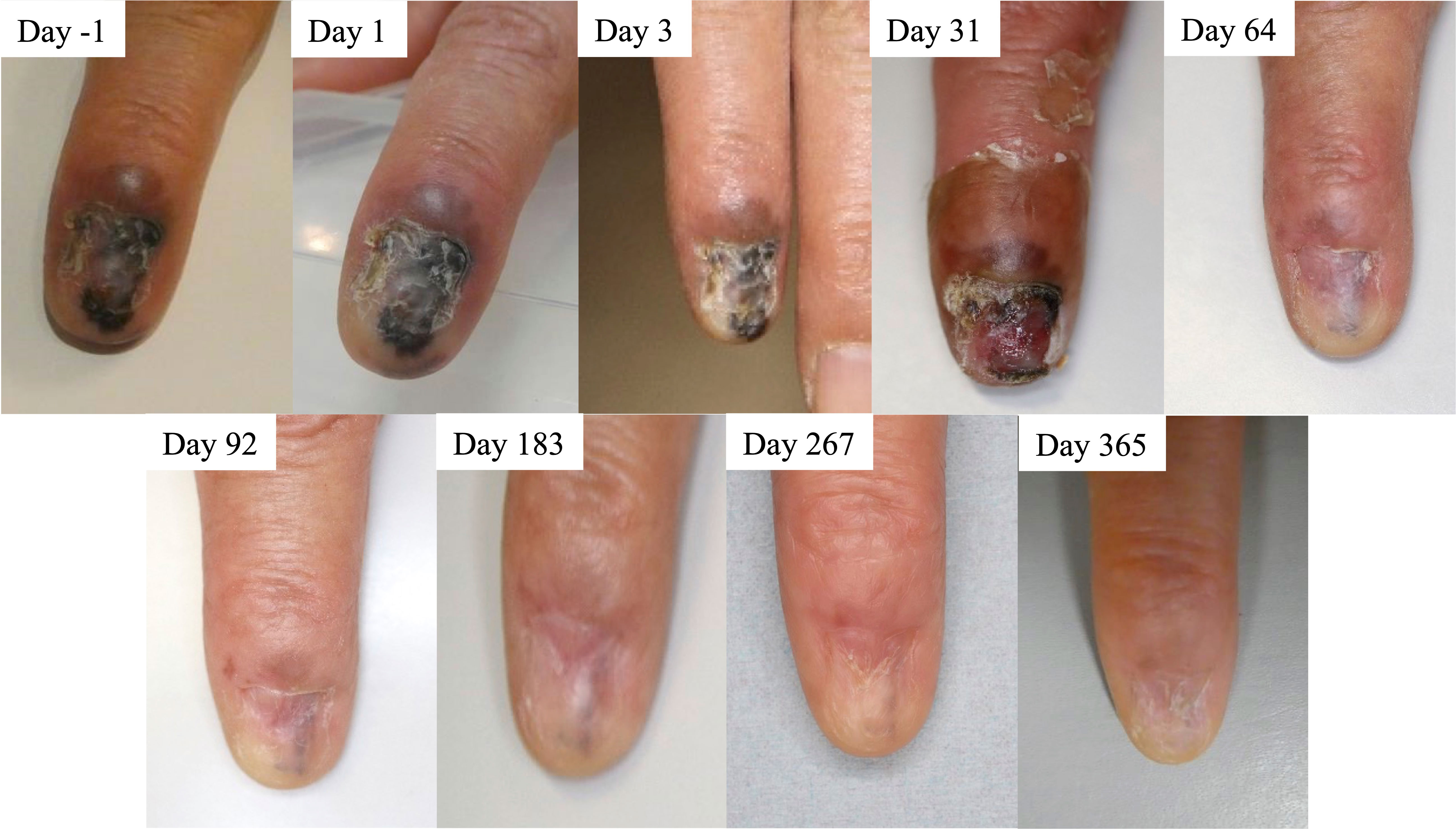
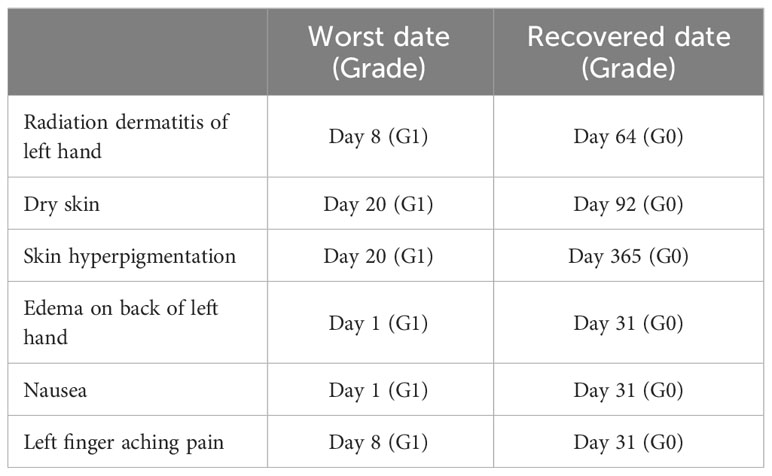
Figure 1 Time course of patient’s tumor before and after boron neutron capture therapy (BNCT). Clinical photos were obtained before treatment start (day -1) and on treatment days 1, 3, 31, 64, 92, 183, 267, and 365. The patient achieved PR day 183 and CR day 365, according to the RECIST 1.1 criteria.
Treatment
Two-hundred mg/kg/h of SPM-011 was continuously administered intravenously to the patient for 2 hours before neutron irradiation. After this 2-hour administration, the blood was taken to determine the 10B concentration in blood using an inductivity coupled plasma optical emission spectrometer (SPS3500DD, Hitachi High-Tech Science Corporation, Tokyo, Japan). After them without a pause, the continuous intravenous administration speed of SPM-011 was decreased to 100 mg/kg/h for the next 1 hour (6). Based on the 10B concentration measurement result, the required proton charge was determined to deliver the maximum dose of 18 Gy-Eq to the skin. The neutron irradiation was then performed during the last 1-hour administration. When the neutron irradiation to the patient was complete, the administration of SPM-011 was stopped. At the neutron irradiation, to reduce the delivered dose except for the affected finger, the fingers were flexed except for sticking the affected finger out, and the treatment was then performed. Figure 2 shows the treatment geometry photo. The affected finger was surrounded by the bolus to achieve the optimal dose.
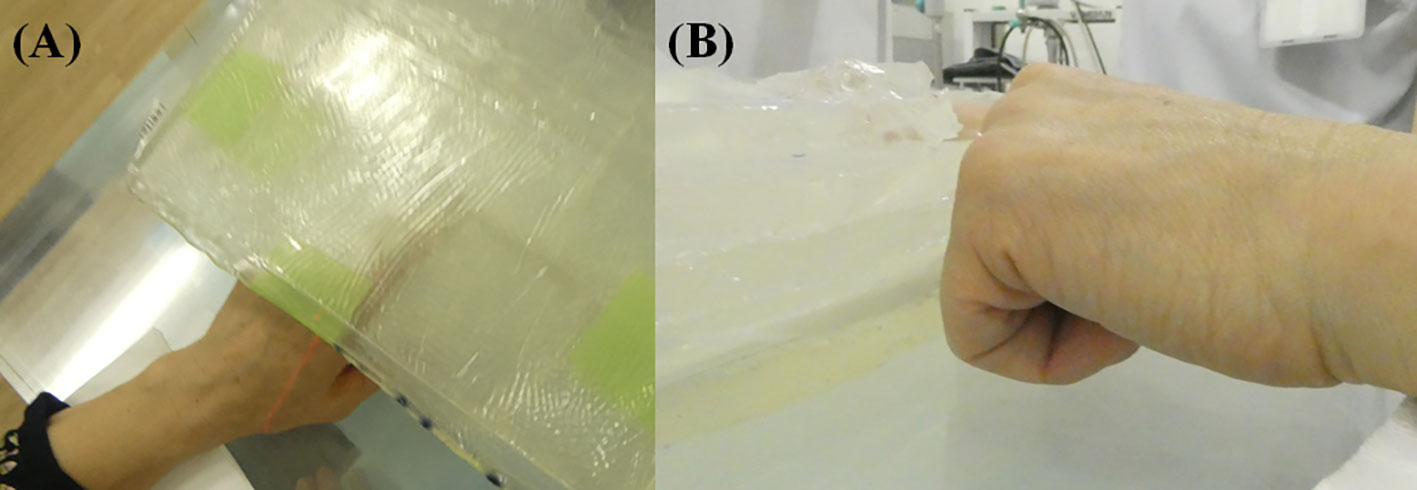
Figure 2 Treatment geometry photos taken at (A) corresponding to the neutron beam axis and (B) perpendicular to the neutron beam axis. The affected finger was surrounded by the bolus and the other fingers were flexed.
The total physical BNCT dose, which was comprised of the boron, nitrogen, hydrogen, and gamma-ray dose, was calculated by Monte Carlo Simulation (Particle and Heavy Ion Transport code System (PHITS), ver. 3.020) (12). It was noted that the assumed boron concentration in the blood on treatment planning was 25 ppm. Considering the compound biological effectiveness in the boron dose and the relative biological effectiveness (RBE) in each dose component (Table 1), the maximum and minimum expected RBE-weighted doses to the gross tumor volume in the patient were expected as 61.0 and 53.1 Gy-Eq, respectively. The expected RBE-weighted boron, nitrogen, hydrogen, and gamma-ray doses to the skin were then expected as 14.2, 1.2, 0.2, and 2.4 Gy-Eq, respectively. Furthermore, the some difference on the blood boron concentration may be observed between the treatment planning and the actual treatment. In this case, the boron dose is re-calculated to reflect the actual patient boron concentration, and the final dose is re-prescribed to follow the clinical trial protocol. As a result, the actual required proton charges and the actual irradiation time are derived. A detailed description of the dose calculation method was reported in the previous study (6).
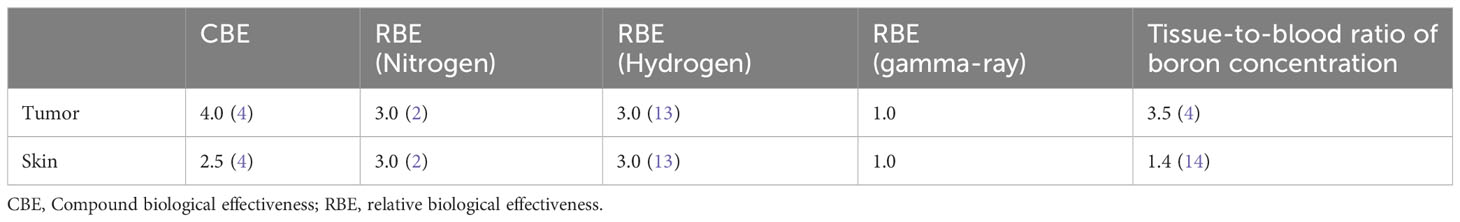
Table 1 Compound biological effectiveness, relative biological effectiveness, and boron concentration parameters in the dose calculation.
Results
All data are current as of January 27, 2023.
Treatment delivered
The treatment was performed on January, 2022. The radiation dose was prescribed so that the maximum dose delivered to the skin was 18 Gy-Eq, which was the last radiation dose level in this trial. The patient received the first patient treatment for cutaneous malignant melanoma using an accelerator-based BNCT system. After the first 2-hour administration of SPM-011, the blood boron concentration measured was 33.7 ppm. Based on the measured boron concentration, the required proton charge and the expected irradiation time were 18230 mC and 30.4 minutes, respectively. The epithermal neutron of 9.7×1011 cm-2 was provided by CICS-1 (5).
Clinical outcomes
The epithelial tumor shrank very slowly and reduced in blackness. The patient reached PR on day 183 and CR on day 365 (Figure 1). No disease recurrence was noted over the follow-up period of 12 months without any treatment-related grade 2 or higher adverse events. Table 2 summarizes treatment-related toxicities. Treatment-related grade 1 adverse events of edema on the back of left hand, nausea, radiation dermatitis of left hand, left finger aching pain, dry skin, and skin hyperpigmentation were noted on day 1, 1, 8, 8, 20, and 20, respectively. The skin hyperpigmentation and the other adverse events were relieved within 12 and 3 months, respectively, without any treatment.
Discussion
This report presents the first patient treatment for cutaneous malignant melanoma treated with an accelerator-based BNCT system. The patient had favorable short-term tumor responses and safe outcomes.
While our follow-up period was relatively short, no progression was observed in the patient. According to the previous report, 28.6% and 47.6% in the recurrent patient group experienced recurrence within 6 and 12 months after surgery although the recurrence pattern of cutaneous malignant melanoma varied depending on the patient’s background (15). Therefore, this case report shows that the accelerator-based BNCT may become a novel treatment option for cutaneous malignant melanoma.
Grade 2 or higher adverse event related to BNCT was not observed in the patient. Those adverse events can also be observed after the conventional radiotherapy, and their severities were similar to milder compared with conventional radiotherapy. While previous studies reported that grade 3 adverse event of a transient asymptomatic hyperamylasemia was frequently observed in patients with head and neck cancer and scalp angiosarcoma after BNCT, it was not observed in this patient (4, 6). It may relate to a tumor location. The previous report investigated hyperamylasemia after the conventional radiotherapy dose to the parotid glands greater than 0.5–1 Gy (16). In this patient, the epithelial tumor was located on the second finger of the left hand. Therefore, radiation exposure to the salivary glands was likely lower than those patients with head and neck cancer and scalp angiosarcoma.
Previous report investigated that the differences between the reactor-based and the accelerator-based BNCT (17). The energy and intensity of neutrons are adjusted to be optimal for BNCT in the accelerator-based BNCT. Thus, previous reports indicated that RBE value of neutron beam in the accelerator-based BNCT was lower than that in the reactor-based BNCT (13, 17, 18). Furthermore, the accelerator-based BNCT also expects shorter irradiation time. However, our clinical trial protocol was developed based on the clinical trials conducted in the reactor-based BNCT, and the delivered dose to a patient was set to be comparable to the clinical study from the reactor-based BNCT (2, 3, 19). As a result, skin reaction from the accelerator-based BNCT was very mild as well as the reactor-based BNCT. Thus, it might be a clinically undetectable difference even if there was a slight different between the reactor-based and the accelerator-based BNCT. Furthermore, previous reports from reactor-based BNCT had manifested the favorable clinical outcome of malignant melanoma (Response rate: 69-95%) (2, 3, 19). Even with this limitation, we expect the accelerator-based BNCT to become a promising option for treating cutaneous malignant melanoma. Furthermore, depending on the progression of the primary lesion, performing sentinel lymph node biopsy and adjuvant therapy may make BNCT a more suitable treatment while this case did not perform. We hope that further cases will reveal optimal pre- and post-treatment strategies for acral cutaneous malignant melanoma treated with the accelerator-based BNCT.
In the conclusion, this case report shows that the accelerator-based BNCT may become a promising treatment modality for cutaneous malignant melanoma. We expects further clinical trials to reveal the efficacy and safety of the accelerator-based BNCT for cutaneous malignant melanoma.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The clinical trial is being conducted in accordance with the Declaration of Helsinki and Good Clinical Practices and compliance with the registered protocol. Institutional review boards at NCCH approved the clinical trial (T4725). Prior to treatment, all patients provided written informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HI: Data curation, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. SN: Formal Analysis, Funding acquisition, Validation, Writing – origBinal draft, Writing – review & editing. NY: Data curation, Resources, Writing – review & editing. ToK: Data curation, Resources, Writing – review & editing. MT: Data curation, Formal Analysis, Resources, Writing – review & editing. TaK: Data curation, Resources, Writing – review & editing. NM: Data curation, Resources, Writing – review & editing. KN: Data curation, Resources, Writing – review & editing. TN: Formal Analysis, Resources, Writing – review & editing. HO: Formal Analysis, Resources, Writing – review & editing. KIi: Formal Analysis, Resources, Writing – review & editing. TC: Formal Analysis, Resources, Writing – review & editing. HirN: Formal Analysis, Resources, Writing – review & editing. AN: Data curation, Resources, Writing – review & editing. MS: Data curation, Resources, Writing – review & editing. KT: Data curation, Resources, Writing – review & editing. KIn: Data curation, Resources, Writing – review & editing. KO: Data curation, Resources, Writing – review & editing. YN: Data ;curation, Resources, Writing – review & editing. KS: Supervision, Writing – review & editing. HitN: Supervision, Writing – review & editing. JI: Supervision, Writing – review & editing, Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a JSPS Grant-in-Aid for Young Scientists (Grant Number 19K17218) and a JSPS Grant-in-Aid for Young Scientists (B) (Grant Number 26860410), and partially supported by a JSPS Grant-in-Aid for Scientific Research (B) and (C) (Grant Number 19H03611 and 16K10410) and by Japanese Agency for Medical Research and Development (23mk0121267h0001).
Conflict of interest
This study was supported by research funds Cancer Intelligence Care Systems, Inc. SN. This study was also supported by a commissioned study from Stella Pharma Corporation and Cancer Intelligence Care Systems, Inc. Hiroshi Igaki and Jun Itami. These sponsors were involved in the management of the study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BNCT, Boron Neutron Capture Therapy; BPA, boronophenylalanine; CICS, Cancer Intelligence Care Systems; LAT-1, L-type amino acid transporter 1; NCCH, National Cancer Center Hospital; PHITS, Particle and Heavy Ion Transport code System; RBE, Relative biological effectiveness; PR, Partial response; CR, Complete response.
References
1. Masunaga S, Sakurai Y, Tanaka H, Tano K, Suzuki M, Kondo N, et al. The dependency of compound biological effectiveness factors on the type and the concentration of administered neutron capture agents in boron neutron capture therapy. Springerplus (2014) 3:128. doi: 10.1186/2193-1801-3-128
2. Hiratsuka J, Kamitani N, Tanaka R, Tokiya R, Yoden E, Sakurai Y, et al. Long-term outcome of cutaneous melanoma patients treated with boron neutron capture therapy (BNCT). J Radiat Res (2020) 61(6):945–51. doi: 10.1093/jrr/rraa068
3. Menéndez PR, Roth BM, Pereira MD, Casal MR, González SJ, Feld DB, et al. BNCT for skin melanoma in extremities: updated Argentine clinical results. Appl Radiat Isot (2009) 67(7-8 Suppl):S50–3. doi: 10.1016/j.apradiso.2009.03.020
4. Hirose K, Konno A, Hiratsuka J, Yoshimoto S, Kato T, Ono K, et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan ((10)B) for recurrent or locally advanced head and neck cancer (JHN002): An open-label phase II trial. Radiother Oncol (2021) 155:182–7. doi: 10.1016/j.radonc.2020.11.001
5. Nakamura S, Igaki H, Ito M, Imamichi S, Kashihara T, Okamoto H, et al. Neutron flux evaluation model provided in the accelerator-based boron neutron capture therapy system employing a solid-state lithium target. Sci Rep (2021) 11:8090. doi: 10.1038/s41598-021-87627-8
6. Igaki H, Murakami N, Nakamura S, Yamazaki N, Kashihara T, Takahashi A, et al. Scalp angiosarcoma treated with linear accelerator-based boron neutron capture therapy: A report of two patients. Clin Transl Radiat Oncol (2022) 33:128–33. doi: 10.1016/j.ctro.2022.02.006
7. Nakamura S, Imamichi S, Masumoto K, Ito M, Wakita A, Okamoto H, et al. Evaluation of radioactivity in the bodies of mice induced by neutron exposure from an epi-thermal neutron source of an accelerator-based boron neutron capture therapy system. Proc Jpn Acad Ser B Phys Biol Sci (2017) 93(10):821–31. doi: 10.2183/pjab.93.051
8. National Comprehensive Cancer Network, Inc. NCCN guidelines for Melanoma: Cutaneous V 3 . Available at: www.NCCN.org (Accessed November 17, 2022).
9. Huayllani MT, Restrepo DJ, Boczar D, Avila FR, Bagaria SP, Spaulding AC, et al. National comprehensive analysis of characteristics of acral lentiginous melanoma. Anticancer Res (2020) 40(6):3411–5. doi: 10.21873/anticanres.14325
10. Detta A, Cruickshank GS. L-amino acid transporter-1 and boronophenylalanine-based boron neutron capture therapy of human brain tumors. Cancer Res (2009) 69:2126–32. doi: 10.1158/0008-5472.CAN-08-2345
11. Shimizu A, Kaira K, Kato M, Yasuda M, Takahashi A, Tominaga H, et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) expression in cutaneous melanoma. Melanoma Res (2015) 25(5):399–405. doi: 10.1097/CMR.0000000000000181
12. Sato T, Iwamoto Y, Hashimoto S, Ogawa T, Furuta T, Abe S, et al. Featurs of particle and heavy ion transport code system (PHITS) version 3.02. J Nucl Sci Technol (2018) 55(5-6):684–90. doi: 10.1080/00223131.2017.1419890
13. Kawabata S, Miyatake S-I, Kajimoto Y, Kuroda Y, Kuroiwa T, Imahori Y, et al. The early successful treatment of glioblastoma patients with modified boron neutron capture therapy. Rep two cases J Neurooncol (2003) 65(2):159–65. doi: 10.1023/B:NEON.0000003751.67562.8e
14. Fukuda H, Honda C, Wadabayashi N, Kobayashi T, Yoshino K, Hiratsuka J, et al. Pharmacokinetics of 10B-p-boronophenylalanine in tumors, skin and blood of melanoma patients: a study of boron neutron capture therapy for Malignant melanoma. Melanoma Res (1999) 9(1):75–83. doi: 10.1097/00008390-199902000-00010
15. Fusi S, Ariyan S, Sternlicht A. Data on first recurrence after treatment for Malignant melanoma in a large patient population. Plast Reconstr Surg (1993) 91(1):94–8. doi: 10.1097/00006534-199301000-00014
16. Authors on behalf of I, Stewart FA, Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs–threshold doses for tissue reactions in a radiation protection context. Ann ICRP (2012) 41:1–322. doi: 10.1016/j.icrp.2012.02.001
17. Suzuki M, Tanaka H, Sakurai Y, Kashino G, Yong L, Masunaga S, et al. Impact of accelerator-based boron neutron capture therapy (AB-BNCT) on the treatment of multiple liver tumors and Malignant pleural mesothelioma. Radiother Oncol (2009) 92(1):89–95. doi: 10.1016/j.radonc.2009.01.010
18. Nakamura S, Imamichi S, Shimada K, Takemori M, Kanak Y, Iijima K, et al. Relative bioilogical effectiveness for epithermal neutron beam contamintated with fast neutrons in the linear accelerator-based boron neutron capture therapy system coupled to a solid-state lithium target. J Radiat Res (2023) 64(4):661–7. doi: 10.1093/jrr/rrad037
Keywords: boron neutron capture therapy (BNCT), malignant melanoma, linear accelerator-based BNCT system, solid-state Li target, acral malignant melanoma, first treatment
Citation: Igaki H, Nakamura S, Yamazaki N, Kaneda T, Takemori M, Kashihara T, Murakami N, Namikawa K, Nakaichi T, Okamoto H, Iijima K, Chiba T, Nakayama H, Nagao A, Sakuramachi M, Takahashi K, Inaba K, Okuma K, Nakayama Y, Shimada K, Nakagama H and Itami J (2023) Acral cutaneous malignant melanoma treated with linear accelerator-based boron neutron capture therapy system: a case report of first patient. Front. Oncol. 13:1272507. doi: 10.3389/fonc.2023.1272507
Received: 04 August 2023; Accepted: 26 September 2023;
Published: 13 October 2023.
Edited by:
Minoru Suzuki, Kyoto University, JapanReviewed by:
Koichi Yasuda, Hokkaido University, JapanShintaro Shiba, Shonan Kamakura General Hospital, Japan
Copyright © 2023 Igaki, Nakamura, Yamazaki, Kaneda, Takemori, Kashihara, Murakami, Namikawa, Nakaichi, Okamoto, Iijima, Chiba, Nakayama, Nagao, Sakuramachi, Takahashi, Inaba, Okuma, Nakayama, Shimada, Nakagama and Itami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoshi Nakamura, c2F0b25ha2FAbmNjLmdvLmpw
 Hiroshi Igaki
Hiroshi Igaki Satoshi Nakamura
Satoshi Nakamura Naoya Yamazaki5
Naoya Yamazaki5 Mihiro Takemori
Mihiro Takemori Tairo Kashihara
Tairo Kashihara Kenjiro Namikawa
Kenjiro Namikawa Yuko Nakayama
Yuko Nakayama Jun Itami
Jun Itami