- Department of Thoracic Surgery, Quanzhou First Hospital Affiliated to Fujian Medical University, Quanzhou, Fujian, China
Background: Segmentectomy has been proven to have better survival and perioperative efficacy than lobectomy for non-small cell lung cancer (NSCLC) up to 2 cm. Whether this result is applicable to stage T1cN0M0 NSCLC (2.1 to 3 cm) remains controversial.
Methods: We conducted a comprehensive search across seven databases to identify relevant studies comparing lobectomy and segmentectomy procedures. Our primary focus was on survival indicators (overall survival [OS] and disease-free survival [DFS]), while for secondary outcomes, operative outcomes, hospitalization outcomes, recurrences, and complications were considered.
Results: After screening, the final analysis included 10 studies (involving 22113 patients in the lobectomy group and 1627 patients in the segmentectomy group). The lobectomy procedure achieved better OS (hazard ratio [HR]: 1.19 [1.07~1.33]) and DFS (HR: 1.37 [1.10~1.71]), which were proven in all subgroups. The OS rate at 2-5 years and DFS rate at 4-5 years were higher in the lobectomy group. The advantages of OS and DFS in the lobectomy group increased over the survival time. More lymph node dissections, intraoperative blood loss and total complications were found in the lobectomy group. Similar hospital stays, 90-day mortality and conversion thoracotomy were found between the two groups.
Conclusion: Lobectomy appeared to be the better choice for patients with stage T1cN0M0 NSCLC with better survival (OS and DFS). However, the complications needed to be taken seriously.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identification CRD42023445013.
Introduction
The incidence and mortality of lung cancer has been increasing over the past decades (1, 2). For stage IA non-small cell lung cancer (NSCLC), surgery remains the standard treatment method (2). In traditional concepts, lobectomy is the standard surgical procedure for these patients. However, in recent years, with the introduction of minimally invasive concepts, how to protect lung function as much as possible under the same survival efficacy has received attention from thoracic surgeons around the world (3). For stage IA1-2 (T1a-bN0M0) NSCLC, segmentectomy has been proven to have better perioperative efficacy, lung function protection, and noninferior survival efficacy compared with lobectomy (4–6). However, whether this conclusion is valid in stage IA3 NSCLC remains controversial in clinical practice.
To clarify this debate, several studies have been conducted in the past decade. Ogawa et al.’s and Deng et al.’s studies suggested that lobectomy achieved better overall survival (OS) and disease-free survival (DFS) than segmentectomy (7, 8). However, Forster et al.’s and Wang et al.’s studies did not find a survival advantage in the lobectomy group, and there were more complications in the lobectomy group (9, 10). Kamigaichi et al.’s and Yamashita et al.’s studies did not find any differences in the efficacy of survival and safety between the two groups (11, 12).
To clarify this controversy, we compared the survival and perioperative outcomes between lobectomy and segmentectomy procedures.
Materials and methods
Search strategy
This study adhered to the PRISMA guidelines (meta-analysis) (Table S1) (13). The study was preregistered on PROSPERO (ID: CRD42023445013). Then, we systematically searched seven databases (including Web of Science, EMBASE, etc.) until May 25, 2023. MeSH terms such as “lobectomy,” “segmentectomy,” and “lung cancer” were employed. The retrieval strategies can be found in Table S2. Additionally, we conducted a thorough search of the references in the retrieved literature to identify relevant articles.
Inclusion and exclusion criteria
Studies had to meet the following inclusion criteria:
(1) Population: patients diagnosed with stage IA3 (T1cN0M0) NSCLC based on The Eighth Edition Lung Cancer Stage Classification (14).
(2) Intervention and comparison: lobectomy vs. segmentectomy.
(3) Outcomes: survival, intraoperative outcomes, hospitalization outcomes, recurrences, and complications.
(4) Study design: randomized controlled trial (RCT) or cohort study.
When the same patient populations were involved in 2 or more studies, RCT and propensity score matching study would be prioritized, and if not, study with the largest sample size should be prioritized for inclusion. Animal experiments, meta-analyses, letters, commentaries, and reviews were excluded.
Data extraction
Two independent investigators extracted the following data: baseline characteristic data, survival data (OS and DFS), intraoperative outcomes (operative time, etc.), recurrences (total, locoregional, and distant), hospitalization outcomes (hospital stay, etc.), and complications.
Outcome assessments
At 1-5 years, overall survival rate (OSR) and disease-free survival rate (DFSR) were analyzed. Subgroup analyses of OS and DFS were also conducted based on factors such as publication year, nation, stage, and data sources.
Quality assessment
To evaluate the quality of cohort studies, we employed the Newcastle-Ottawa Scale (NOS), which incorporates three elements: comparability, selection, and outcome. High-quality studies scored 8 or 9 points and medium-quality studies scored 6 or 7 points (15).
Therefore, the highest-quality study would score 9 points. In our analysis, high-quality studies were defined as those that scored 8 or 9 points, and medium-quality studies were those that scored 6 or 7 points.
To assess the evidence level of the results, the Grades of Recommendations Assessment, Development, and Evaluation (GRADE) was utilized, which incorporated five components: publication bias, inconsistency, indirectness, risk of bias, and imprecision. Four levels of evidence existed: high, moderate, low, and very low (16).
Statistical analysis
Review Manager 5.3 and STATA 12.0 were utilized for data analysis. Survival data were evaluated using hazard ratios (HRs), with HR > 1 indicating support for the lobectomy group. Meanwhile, the mean difference (MD) was used to analyze continuous variables, and the risk ratio (RR) was used to analyze dichotomous variables. The corresponding 95% confidence interval (95%CI) was calculated, and statistical significance was set at P<0.05. Heterogeneity was evaluated using the I2 statistic and χ2 test. When heterogeneity was acceptable (I2 < 50%), a fixed-effects model was employed. Conversely, a random-effects model was employed. Funnel plots (17), Egger’s test (18), and Begg’s test (19) were conducted to assess publication bias. Sensitivity analyses were performed by omitting individual studies to determine whether results were dependent on a single study (20).
Results
Study characteristics
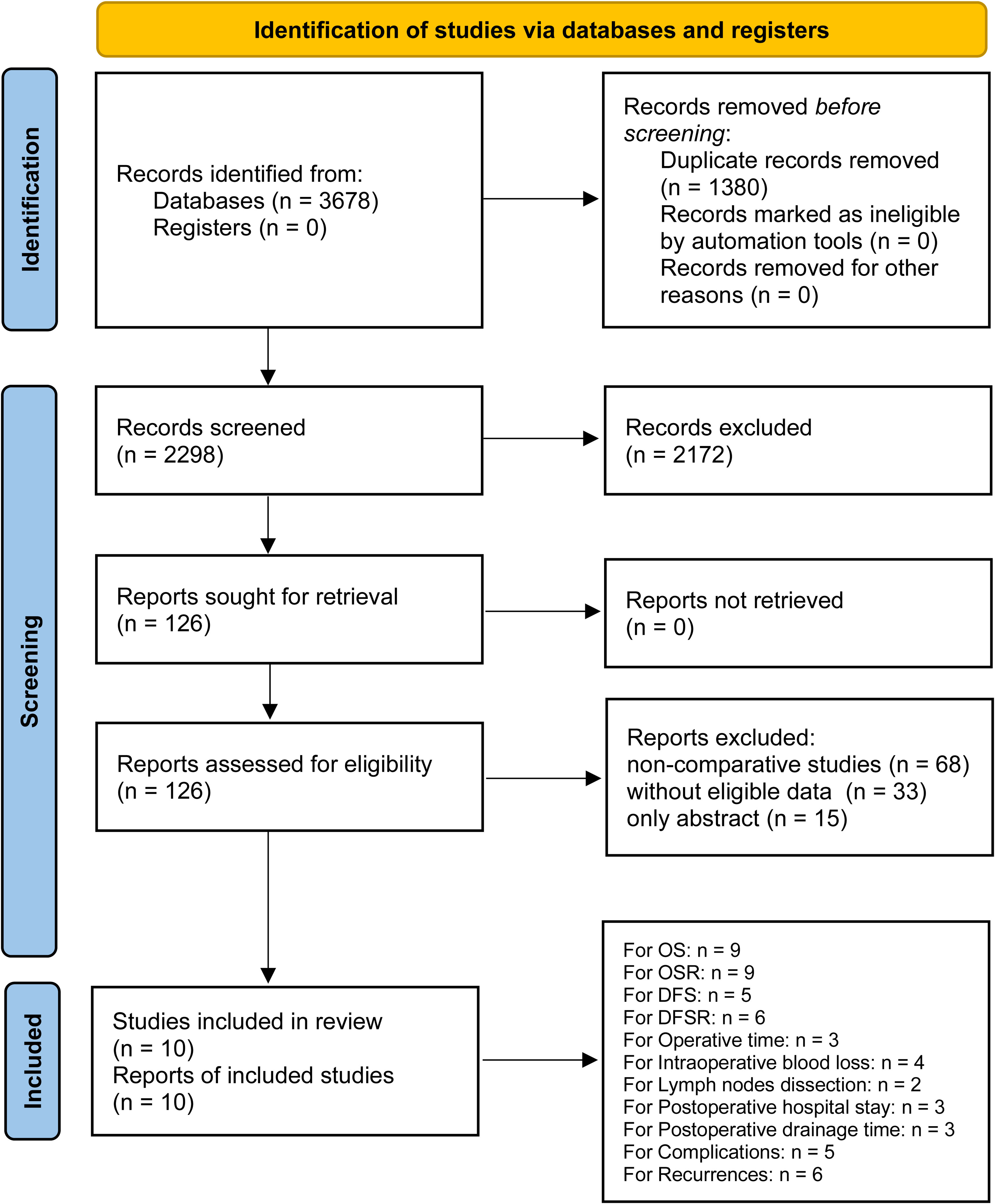
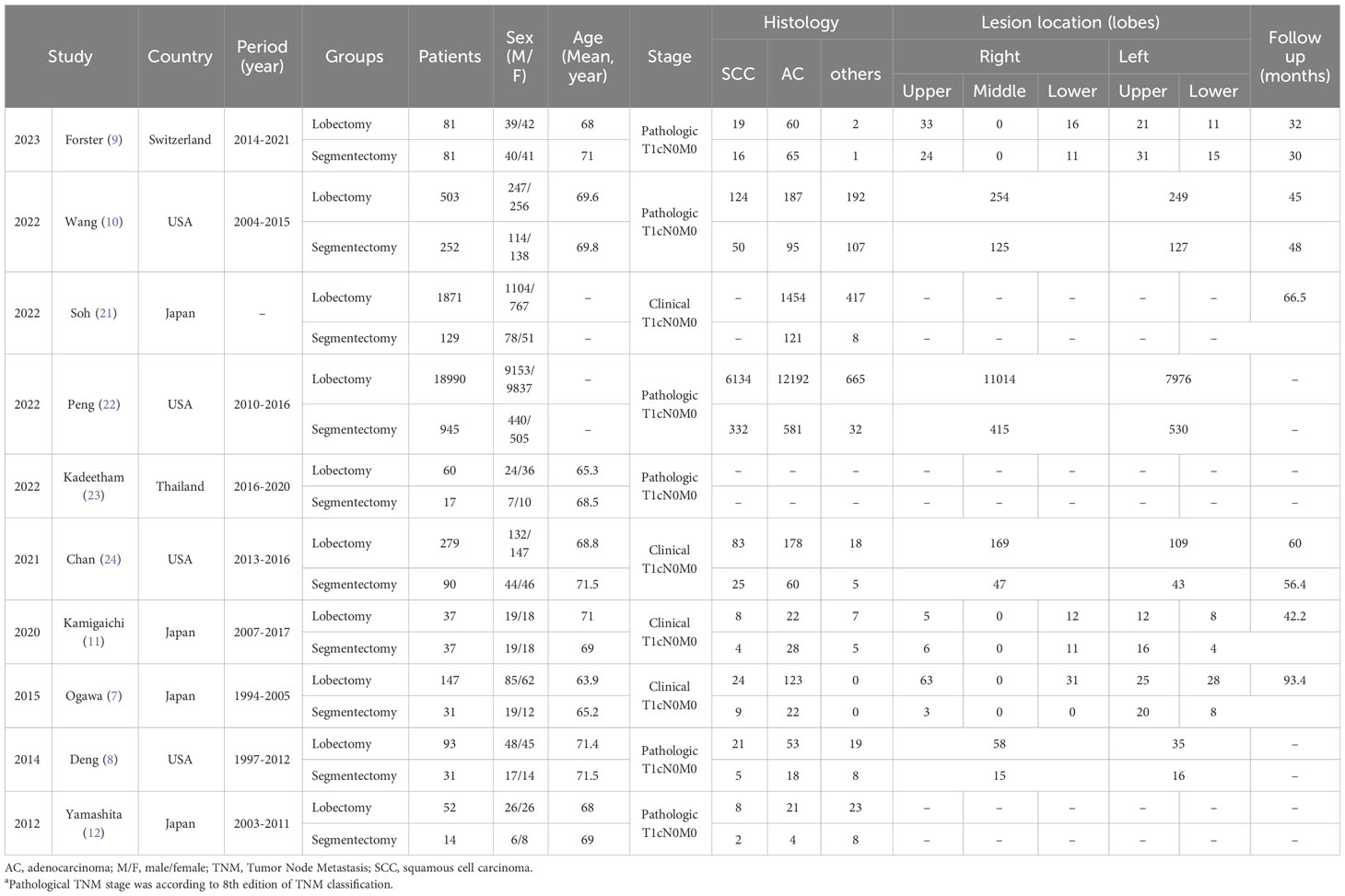
After careful screening of 3678 studies, 10 studies met the inclusion criteria (7–12, 21–24) (Figure 1). The baseline characteristics are summarized in Table 1. Among them, five studies were performed in Asia (7, 11, 12, 21, 23), and five studies were performed in America and Europe (8–10, 22, 24). According to the NOS, 6 studies (9–12, 22, 23) were of high quality, and 4 studies (7, 8, 21, 24) were of medium quality (Table S3). The quality of evidence assessed using the GRADE was found to be low to very low for all outcomes (Table S4).
Survival
The lobectomy group showed better OS than the segmentectomy group (HR: 1.19 [1.07~1.33], Figure 2). Subgroup analyses indicated that the lobectomy group had a higher OSR at 2 years (RR: 1.03 [1.00~1.06]), 3 years (RR: 1.04 [1.01~1.08]), 4 years (RR: 1.09 [1.04~1.13]) and 5 years (RR: 1.11 [1.06~1.17]) (Figures S1, 3A). The OSR in the lobectomy group showed an increasing trend over time (Figure 3C).
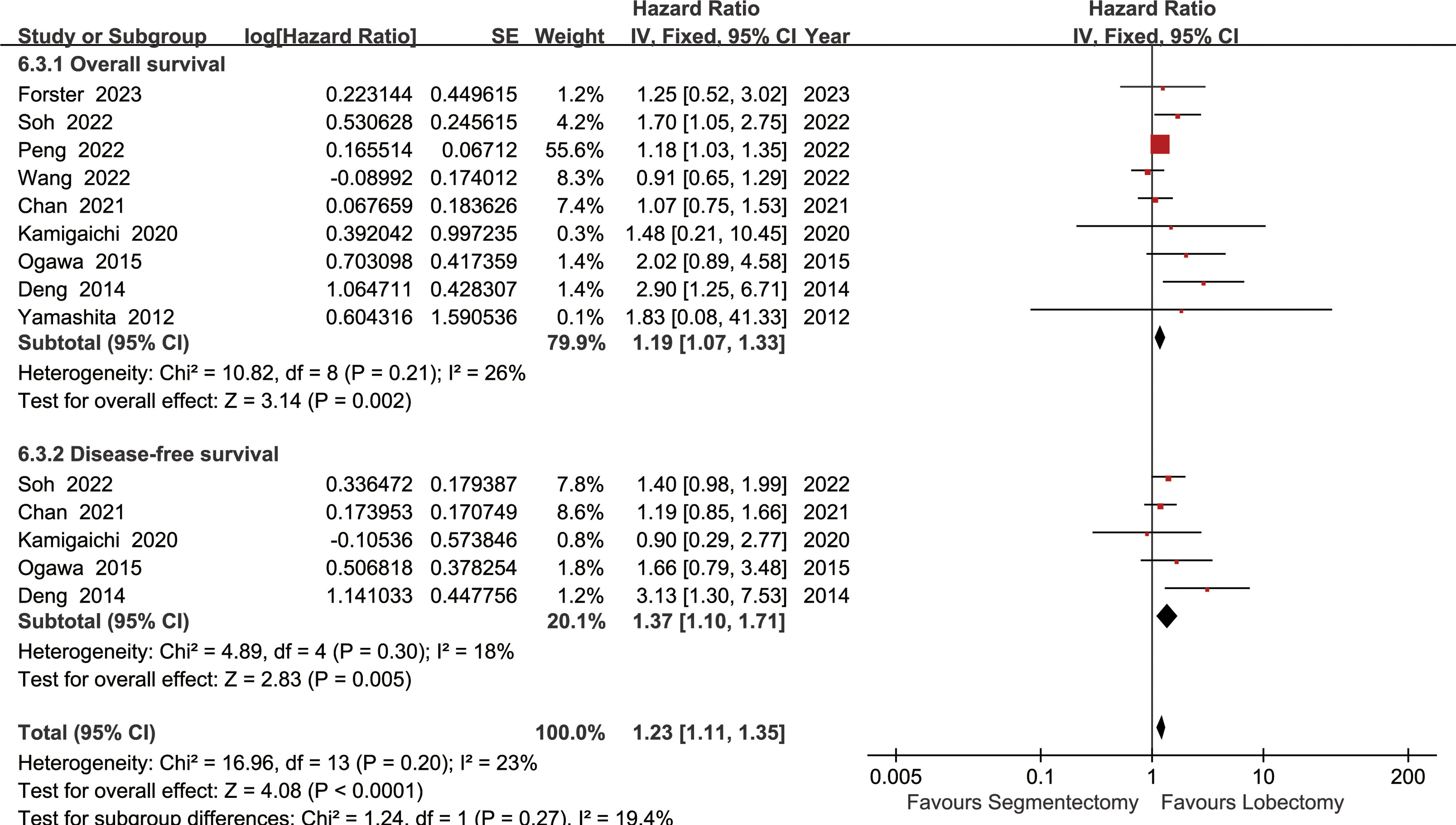
Figure 2 Forest plots of overall survival and disease-free survival associated with lobectomy versus segmentectomy.
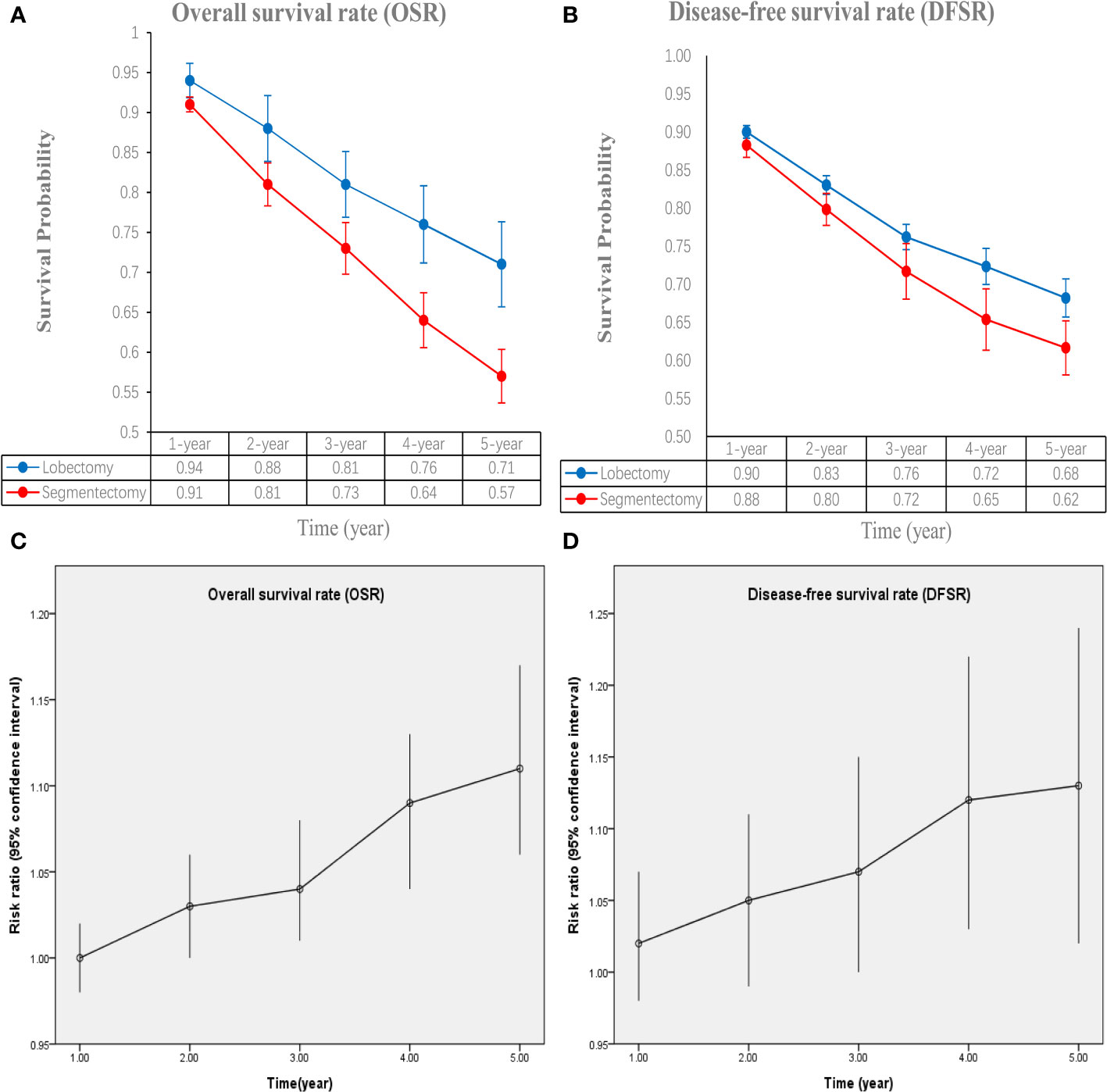
Figure 3 Comparisons of overall survival rate (1-5 years, A: trend of overall survival rate; C: trend of risk ratios) and disease-free survival rate (1-5 years, B: trend of disease-free survival rate; D: trend of risk ratios) associated with lobectomy versus segmentectomy according to survival time.
The lobectomy group showed better DFS than the segmentectomy group (HR: 1.37 [1.10~1.71], Figure 2). Subgroup analyses indicated that the lobectomy group had a higher DFSR at 4 years (RR: 1.12 [1.03~1.22]) and 5 years (RR: 1.13 [1.02~1.24]) (Figures S2, 3B). The DFSR in the lobectomy group showed an increasing trend over time (Figure 3D).
The two groups had similar total recurrences (RR: 1.27 [0.62~2.62]), locoregional recurrences (RR: 1.11 [0.46~2.68]) and distant recurrences (RR: 1.44 [0.60~3.48]) (Figure S3).
Subgroup analysis of survival
Subgroup analyses were performed according to published year, nation, stage, and data sources. The survival advantages of the lobectomy group were achieved in all subgroups (Table 2).
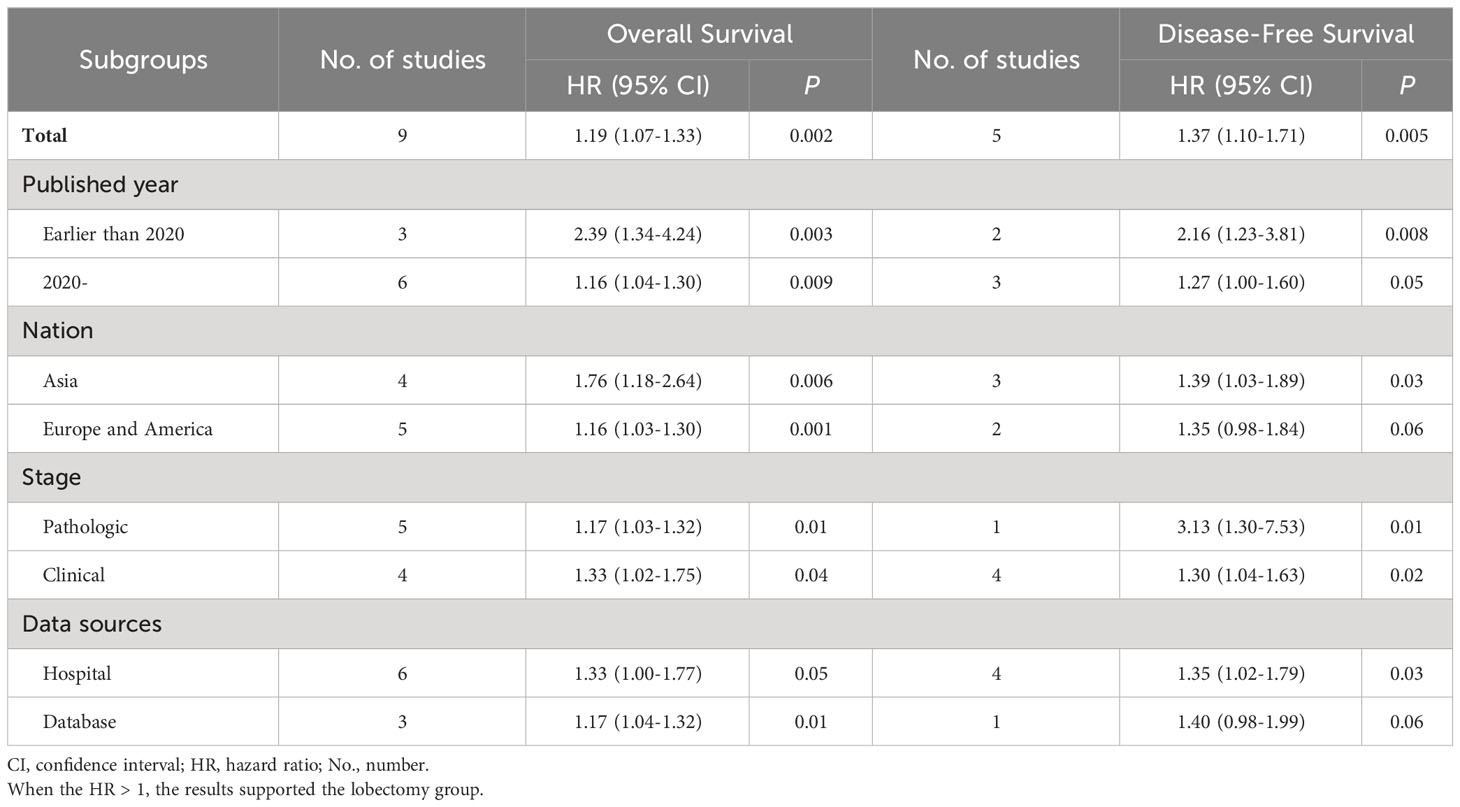
Table 2 Subgroup analysis of overall survival and disease-free survival associated with lobectomy versus segmentectomy.
Intraoperative indicators
More lymph node dissections (MD: 5.27 [0.76~9.79], p = 0.02, Figure 4B) and intraoperative blood loss (MD: 50.32 [31.16~69.48] ml, p < 0.00001, Figure 4C) were found in the lobectomy group. The segmentectomy group tended to have a more favorable operative time (MD: 4.17 [-0.16~8.49] minutes, p = 0.06, Figure 4A), without statistical significance.

Figure 4 Forest plots of operative time (A) lymph nodes dissection (B) and intraoperative blood loss (C) associated with lobectomy versus segmentectomy.
Hospitalization indicators
Similar drainage times (MD: 0.54 [-0.33~1.41] days) and hospital stays (MD: 0.47 [-0.46~1.39] days) were found between the two groups (Figure 5).
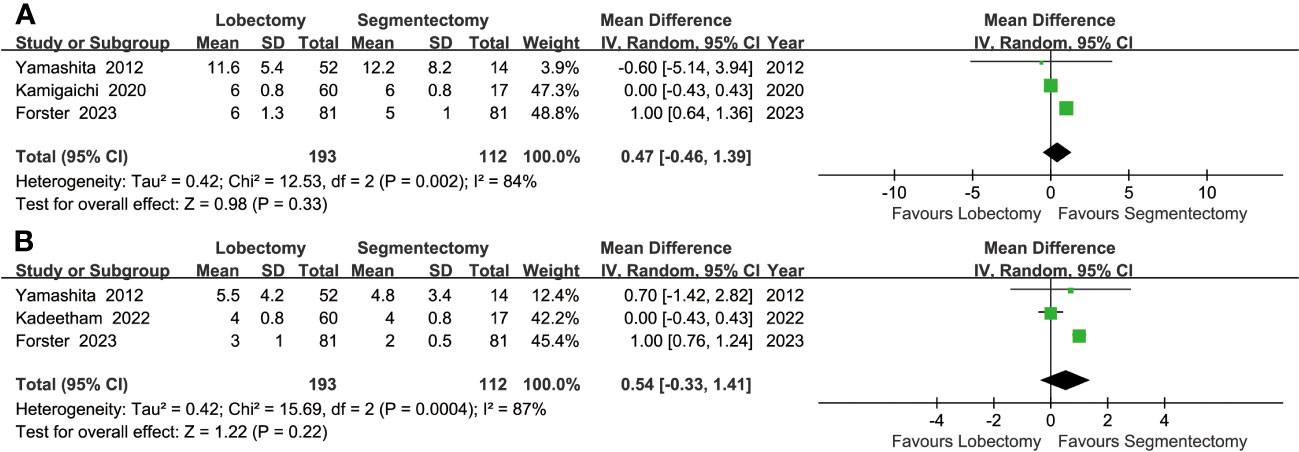
Figure 5 Forest plots of postoperative hospital stay (A) and postoperative drainage time (B) associated with lobectomy versus segmentectomy.
Complications
More total complications were found in the lobectomy group (RR: 1.28 [1.04~1.59], p = 0.02). Similar severe complications, 90-day mortality, conversion thoracotomy, pulmonary complications, cardiac complications, reoperation, readmission, atrial fibrillation, air leak >5 days, postoperative bleeding, acute renal failure, urinary retention, acute myocardial infarction, embolism, chylothorax, empyema and wound infection were found between the groups (Figure S4).
Sensitivity analysis
A sensitivity analysis was performed for comparisons with high heterogeneity (recurrences, lymph node dissection, postoperative hospital stays and drainage time). The RR/HR/MD and 95% CI did not change significantly after removal of any single study, which indicated that the results were stable (Figure S5).
Publication bias
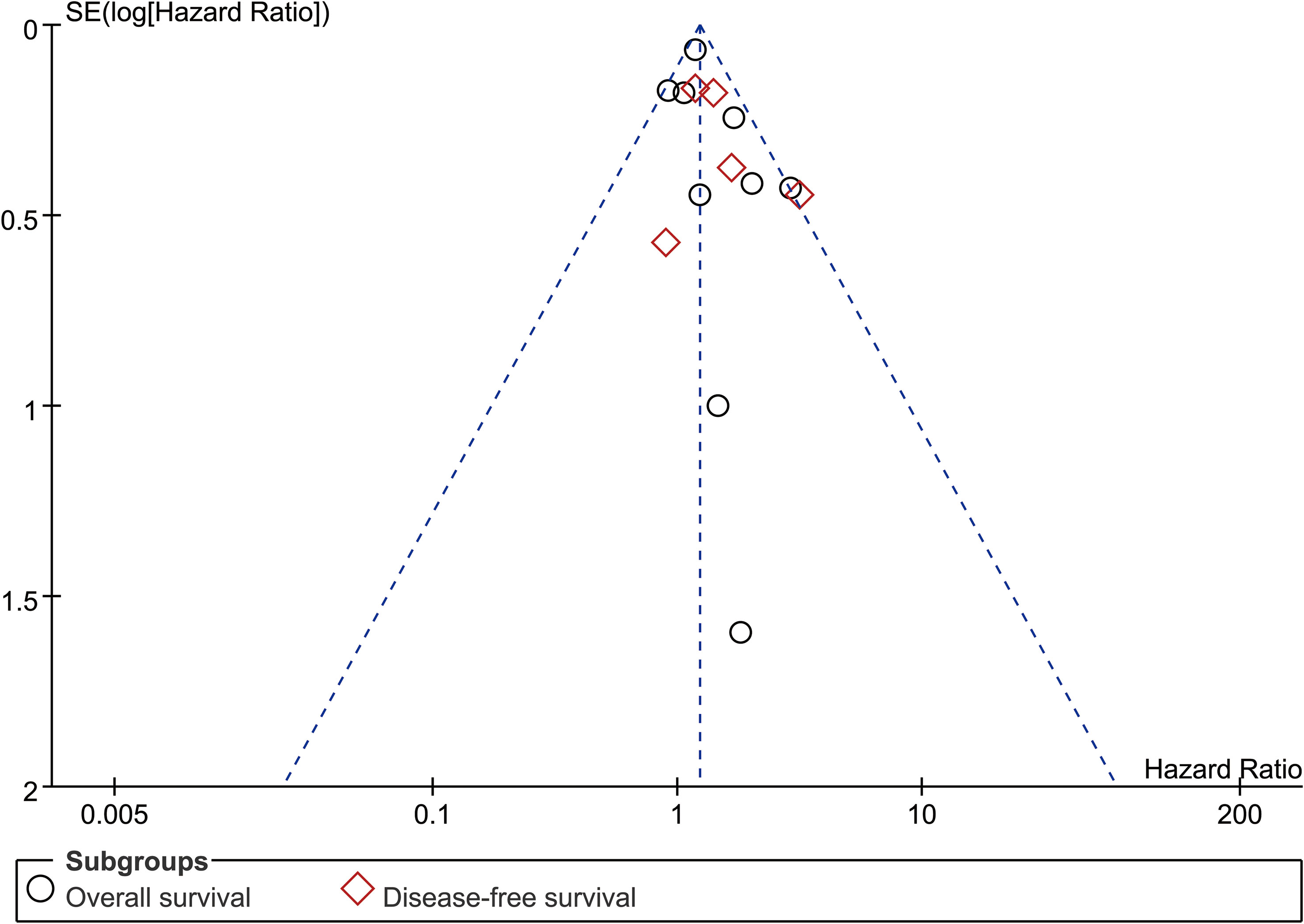
Funnel plots (OS and DFS) showed no significant publication bias, which was also confirmed by Egger’s and Begg’s tests (Figures 6, S6).
Discussion
For a long time, lobectomy is the standard surgical procedure for stage I NSCLC (25). In recent years, some evidence has shown that segmentectomy has better outcomes than lobectomy in stage IA1-2 (T1a-bN0M0) NSCLC (4–6). However, whether this conclusion is valid in stage IA3 (T1cN0M0) NSCLC remains controversial in clinical practice. This meta-analysis compared the two surgical procedures in IA3 (T1cN0M0) NSCLC. The results suggested that the lobectomy group achieved better OS and DFS. The survival advantages in the lobectomy group increased over the survival time. More lymph nodes dissection, intraoperative blood loss and complications were found in lobectomy group.
Better survival is the main advantage with lobectomy, and this advantage will increase with the prolongation of survival. The OSR-5y is 71% in the lobectomy group and 57% in the segmentectomy group. Tendency for survival advantage were supported by six included studies (7–9, 11, 12, 21). Yu et al.’s study based on 9580 patients also suggested that lobectomy and complete lymph node dissection should be the recommended standard of care for patients with stage IA3 NSCLC (26). Three reasons may explain this result: (1) Farther tumor margins reduce the possibility of local tumor recurrence in the lobectomy group (8); (2) More lymph node dissection numbers reduce the lymph node recurrence in the lobectomy group (12, 24); (3) Some N1 lymph nodes were not removed in the segmentectomy group, which may affect the staging judgment. Higher actual pathologic staging may affect the prognosis of patients in the segmentectomy group (7). Meanwhile, the survival advantage of lobectomy was proved in all subgroups according to the published year, nation, stage, and data sources. Therefore, we believe that lobectomy should be performed for stage IA3 NSCLC, which not only meets the requirements of the guidelines but also meets the survival needs of patients
Surgical safety is another important indicator for evaluating surgery. Lower intraoperative and postoperative complications often indicate better quality of life and lower costs (27). In this study, more operative time and intraoperative blood loss were found during lobectomy procedure, which is consistent with the actual situation in our surgeries. From the specific data, an average bleeding increment of 50.22 ml and a 4.17 minutes surgical time increment per surgery are acceptable by most patients and surgeons. Meanwhile, more total complications were found in the lobectomy group (RR: 1.28 [1.04~1.59]), which is also in line with our actual postoperative situation. Similar results were also reported in the Ichinose et al.’s study based on 59663 patients (28). However, no significant difference was found in the comparison of all single complications. Pulmonary complications and atrial fibrillation are the two most common complications after lung surgery, with incidence rates of approximately 20% in each group, respectively. Meanwhile, age, operation time and number of lymph node dissected during operation are independent risk factors affecting postoperative complications (29). Thus, although lobectomy may result in better survival outcomes, its more frequent complications need to be taken seriously.
The main consideration for choosing segmentectomy is to protect lung function as much as possible. For NSCLC up to 2 cm, Bao et al. (30) and Ji et al. (29) reported that segmentectomy shows less loss of lung function than lobectomy. Xu et al. (31) suggested that segmentectomy is helpful to minimize the loss of FVC, but not FEV1 or DLCO. However, for tumors of this size (2-3 cm), in order to ensure sufficient distance between the tumor margins, segmental resection of lung tissue is often larger than single lung segment, and even requires combined segmental resection. In these patients, it is controversial whether lung function could be protected, as compared to lobectomy (32). In the current study, due to database limitations, no analysis results related to lung function protection have been obtained, which is important in the future research.
There are still some limitations in the study. First, all retrieved databases are in English, which might result in some non-English published papers that meet the standards not being included. Second, the evidence level of all results is low or very low, which may reduce the credibility of the conclusion. Third, data of lung function was lacking in all the included studies, and it is important to compare the two surgery procedures. Fourth, there are two forms of staging: clinical staging and pathological staging, which might increase the heterogeneity. Fifth, the data sources are different because some of the data were from large databases and some were from single centers, which might increase the heterogeneity.
Conclusion
In summary, lobectomy appeared to be the better choice for patients with stage IA3 NSCLC with better survival (OS and DFS). The survival advantages in the lobectomy group increased over the survival time. Meanwhile, more attention should be given to the control of postoperative complications. However, due to insufficient evidence of the results, large sample RCTs need to be conducted to confirm the conclusion.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
WZ: Conceptualization, Data curation, Formal Analysis, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. SC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. XL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. HC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. RH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
This study was supported by Natural Science Foundation of Fujian Province (grant no: 2020J011291). Role of the Funding: The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
The authors thank professor Wenxiong Zhang, MD (The second affiliated hospital of Nanchang University) for his statistical advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1270030/full#supplementary-material
Supplementary Figure 1 | Comparisons of overall survival rate (1-5 years) associated with lobectomy versus segmentectomy.
Supplementary Figure 2 | Comparisons of disease-free survival rate (1-5 years) associated with lobectomy versus segmentectomy.
Supplementary Figure 3 | Comparisons of recurrences associated with lobectomy versus segmentectomy.
Supplementary Figure 4 | Forest plots of complications associated with lobectomy versus segmentectomy.
Supplementary Figure 5 | Sensitivity analysis of recurrences (A), lymph nodes dissection (B), postoperative hospital stay (C) and postoperative drainage time (D).
Supplementary Figure 6 | Egger’s and Begg’s tests of overall survival (A) and disease-free survival (B).
Abbreviations
AC, adenocarcinoma; CI, confidence interval; CT, cohort study; DFS, disease-free survival; DFSR, disease-free survival rate; DLCO, diffusion capacity of lung to carbon monoxide; FEV1, Forced expiratory volume in one minute; FVC, forced Vital capacity; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HR, hazard ratio; MD, mean difference; No., number; NOS, Newcastle-Ottawa Scale; NSCLC, Non-small cell lung cancer; OS, overall survival; OSR, overall survival rate; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized clinical trial; RR, risk ratio; SCC, squamous cell carcinoma; TNM, Tumor Node Metastasis.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
2. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines® Insights: non-small cell lung cancer, version 2.2023. J Natl Compr Canc Netw (2023) 21(4):340–50. doi: 10.6004/jnccn.2023.0020
3. Li WWL, van der Heide SM, Verhagen AFTM. Extent of surgery for stage IA non-small-cell lung cancer. N Engl J Med (2023) 388(17):1629. doi: 10.1056/NEJMc2302856
4. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet (2022) 399(10335):1607–17. doi: 10.1016/S0140-6736(21)02333-3
5. Altorki N, Wang X, Kozono D, Watt C, Landrenau R, Wigle D, et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med (2023) 388(6):489–98. doi: 10.1056/NEJMoa2212083
6. Potter AL, Kim J, McCarthy ML, Senthil P, Mathey-Andrews C, Kumar A, et al. Segmentectomy versus lobectomy in the United States: Outcomes after resection for first primary lung cancer and treatment patterns for second primary lung cancers. J Thorac Cardiovasc Surg (2023) S0022-5223(23)00613-X.
7. Ogawa H, Uchino K, Tanaka Y, Shimizu N, Okuda Y, Tane K, et al. Outcomes of segmentectomy for cT1bN0M0 lung adenocarcinoma and squamous cell carcinoma: a possible association with pathological invasion. Eur J Cardiothorac Surg (2015) 48(1):77–82. doi: 10.1093/ejcts/ezu429
8. Deng B, Cassivi SD, de Andrade M, Nichols FC, Trastek VF, Wang Y, et al. Clinical outcomes and changes in lung function after segmentectomy versus lobectomy for lung cancer cases. J Thorac Cardiovasc Surg (2014) 148(4):1186–1192.e3. doi: 10.1016/j.jtcvs.2014.03.019
9. Forster C, Abdelnour-Berchtold E, Bédat B, Perentes JY, Zellweger M, Sauvain MO, et al. Local control and short-term outcomes after video-assisted thoracoscopic surgery segmentectomy versus lobectomy for pT1c pN0 non-small-cell lung cancer. Interdiscip Cardiovasc Thorac Surg (2023) 36(2):ivad037. doi: 10.1093/icvts/ivad037
10. Wang L, Ge L, You S, Liu Y, Ren Y. Lobectomy versus segmentectomy in patients with stage T (> 2 cm and ≤ 3 cm) N0M0 non-small cell lung cancer: a propensity score matching study. J Cardiothorac Surg (2022) 17(1):110. doi: 10.1186/s13019-022-01867-x
11. Kamigaichi A, Tsutani Y, Kagimoto A, Fujiwara M, Mimae T, Miyata Y, et al. Comparing segmentectomy and lobectomy for clinical stage IA solid-dominant lung cancer measuring 2.1 to 3 cm. Clin Lung Cancer (2020) 21(6):e528–38. doi: 10.1016/j.cllc.2020.04.015
12. Yamashita S, Tokuishi K, Anami K, Moroga T, Miyawaki M, Chujo M, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg (2012) 42(1):83–8. doi: 10.1093/ejcts/ezr254
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71.
14. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest (2017) 151(1):193–203. doi: 10.1016/j.chest.2016.10.010
15. Wells GA, Shea BJ, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
16. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol (2011) 64(4):380–2. doi: 10.1016/j.jclinepi.2010.09.011
17. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (2011) 343:d4002. doi: 10.1136/bmj.d4002
18. Langhorne P. Bias in meta-analysis detected by a simple, graphical test. Prospectively identified trials could be used for comparison with meta-analyses. BMJ (1998) 316(7129):471.
19. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446
20. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev (2019) 10(10):ED000142. doi: 10.1002/14651858.ED000142
21. Soh J, Toyooka S, Shintani Y, Okami J, Ito H, Ohtsuka T, et al. Limited resection for stage IA radiologically invasive lung cancer: a real-world nationwide database study. Eur J Cardiothorac Surg (2022) 62(1):ezac342. doi: 10.1093/ejcts/ezac342
22. Peng T, Wightman SC, Ding L, Lieu DK, Atay SM, David EA, et al. Lobectomy offers improved survival outcomes relative to segmentectomy for >2 but ≤4 cm non-small cell lung cancer tumors. JTCVS Open (2022) 10:356–67. doi: 10.1016/j.xjon.2022.03.002
23. Kadeetham K, Ngodngamthaweesuk M, Kantathut N, Samankatiwat P, Cherntanomwong P, Leelayana P, et al. Overall 5-year survival rate and disease-free survival after segmentectomy versus lobectomy in patients with non-small cell lung cancer. SAGE Open Med (2022) 10:20503121221142171. doi: 10.1177/20503121221142171
24. Chan EG, Chan PG, Mazur SN, Normolle DP, Luketich JD, Landreneau RJ, et al. Outcomes with segmentectomy versus lobectomy in patients with clinical T1cN0M0 non-small cell lung cancer. J Thorac Cardiovasc Surg (2021) 161(5):1639–1648.e2. doi: 10.1016/j.jtcvs.2020.03.041
25. Doerr F, Stange S, Michel M, Schlachtenberger G, Menghesha H, Wahlers T, et al. Redefining the role of surgery in early small-cell lung cancer. Langenbecks Arch Surg (2022) 407(7):2663–71. doi: 10.1007/s00423-022-02631-4
26. Yu X, Zhang R, Zhang M, Lin Y, Zhang X, Wen Y, et al. Segmental resection is associated with decreased survival in patients with stage IA non-small cell lung cancer with a tumor size of 21-30 mm. Transl Lung Cancer Res (2021) 10(2):900–13. doi: 10.21037/tlcr-20-1217
27. Yamamichi T, Ichinose J, Omura K, Hashimoto K, Matsuura Y, Nakao M, et al. Impact of postoperative complications on the long-term outcome in lung cancer surgery. Surg Today (2022) 52(9):1254–61. doi: 10.1007/s00595-022-02452-4
28. Ichinose J, Yamamoto H, Aokage K, Kondo H, Sato Y, Suzuki K, et al. Real-world perioperative outcomes of segmentectomy versus lobectomy for early-stage lung cancer: a propensity score-matched analysis. Eur J Cardiothorac Surg (2022) 63(1):ezac529. doi: 10.1093/ejcts/ezac529
29. Ji D, Sun R, Wu Z. Effects of uniportal thoracoscopic pulmonary segmentectomy and lobectomy on patients with early-stage non-small-cell lung cancer and risk factors of postoperative complications. Am J Transl Res (2023) 15(6):4369–79.
30. Bao M, Lang Z, Wang Z, Zhang X, Zhao L. Changes in pulmonary function in lung cancer patients after segmentectomy or lobectomy: a retrospective, non-intervention, observation study. Eur J Cardiothorac Surg (2023) 2023:ezad256. doi: 10.1093/ejcts/ezad256
31. Xu Y, Qin Y, Ma D, Liu H. The impact of segmentectomy versus lobectomy on pulmonary function in patients with non-small-cell lung cancer: a meta-analysis. J Cardiothorac Surg (2022) 17(1):107. doi: 10.1186/s13019-022-01853-3
Keywords: lobectomy, segmentectomy, survival, non-small cell lung cancer, meta-analysis
Citation: Zhang W, Chen S, Lin X, Chen H and He R (2023) Lobectomy versus segmentectomy for stage IA3 (T1cN0M0) non-small cell lung cancer: a meta-analysis and systematic review. Front. Oncol. 13:1270030. doi: 10.3389/fonc.2023.1270030
Received: 31 July 2023; Accepted: 06 September 2023;
Published: 02 October 2023.
Edited by:
Marco Anile, Sapienza University of Rome, ItalyReviewed by:
Massimo Baudo, Spedali Civili Brescia, ItalyLuigi Ventura, Barts Health NHS Trust, United Kingdom
Copyright © 2023 Zhang, Chen, Lin, Chen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongqi He, hrqfj@126.com
 Wanfei Zhang
Wanfei Zhang