
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 17 November 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1269971
 Suk Jun Lee1
Suk Jun Lee1 Jieon Go2
Jieon Go2 Byung Soo Ahn3
Byung Soo Ahn3 Jee Hyun Ahn1
Jee Hyun Ahn1 Jee Ye Kim1
Jee Ye Kim1 Hyung Seok Park1
Hyung Seok Park1 Seung Il Kim1
Seung Il Kim1 Byeong-Woo Park1
Byeong-Woo Park1 Seho Park1*
Seho Park1*Purpose: Lymphovascular invasion (LVI) is a well-known poor prognostic factor for early breast cancer. However, the effect of LVI on breast cancer subtype and node status remains unknown. In this study, we aimed to evaluate the clinical significance of LVI on the recurrence and long-term survival of patients with early breast cancer by comparing groups according to the subtype and node status.
Methods: We retrospectively reviewed the medical records of 4554 patients with breast cancer who underwent breast cancer surgery between January 2010 and December 2017. The primary endpoints were disease-free survival (DFS) and overall survival (OS). Univariate and multivariate analyses were performed to identify prognostic factors related to the DFS and OS according to the nodal status and breast cancer subtype.
Results: During a follow-up period of 94 months, the median OS and DFS were 92 and 90 months, respectively. The LVI expression rate was 8.4%. LVI had a negative impact on the DFS and OS, regardless of the lymph node status. LVI was associated with higher recurrence and lower survival in the luminal A, human epidermal growth factor receptor 2-positive, and triple-negative breast cancer subtypes. The Cox proportional hazards model showed that LVI was a significant prognostic factor for both DFS and OS. No correlation has been observed between LVI and the Oncotype Dx results in terms of prognostic value in early breast cancer.
Conclusion: LVI is an independent poor prognostic factor in patients with early breast cancer, regardless of the node status and molecular subtype. Therefore, the LVI status should be considered when making treatment decisions for patients with early stage breast cancer; however, further prospective studies are warranted.
Breast cancer is the most common cancer diagnosed in women worldwide (1). The total number of patients with breast cancer in South Korea has doubled over the last decade (2). Over the years, many studies have identified prognostic factors in breast cancer, such as age, tumor size, axillary lymph node status, histologic grade, estrogen/progesterone receptor (ER/PR) status, human epidermal growth factor receptor 2 (HER2), and Ki-67, which are significant factors that should be considered when deciding on adjuvant treatments (3, 4).
Lymphovascular invasion (LVI) is associated with the recurrence of solid tumors, including early breast cancer (5). However, according to the National Comprehensive Cancer Network (NCCN), St. Gallen, and European Society for Medical Oncology (ESMO) recommendations, sole LVI status has very limited role in deciding adjuvant treatment (6–8). Although genomic assays are widely employed for making decisions regarding adjuvant chemotherapy, LVI can also be an important factor regardless of gene expression status in patients with ER-positive/HER2-negative breast cancer (9). Recent studies have demonstrated that the detection of LVI supplements reliable information to the 21-gene recurrence score (RS) (10, 11).
However, few studies have investigated the correlation between LVI and recurrence and survival according to the molecular subtypes. Therefore, this study aimed to evaluate the prognostic significance of LVI on the recurrence and long-term survival according to the molecular subtype in patients with early breast cancer who underwent breast surgery.
We retrospectively reviewed the data of patients with breast cancer who underwent breast cancer surgery at Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea, between January 2010 and December 2017. Patients who received neoadjuvant chemotherapy, presented with distant metastases at diagnosis, were diagnosed with ductal carcinoma in situ or occult breast cancer, or did not undergo axillary surgery were excluded from the study. Finally, 4,554 patients were included in the analysis (Figure 1).
Survival data were obtained from the medical records of Severance Hospital. This study was approved by the Institutional Review Board of our institution (Approval No. 4-2023-0067), and the requirement for informed consent was waived owing to the retrospective study design.
Basic patient information, such as age and clinicopathological characteristics, including tumor stage, node stage, histologic grade, nuclear grade, ER status, PR status, HER2 status, Ki-67 index value, adjuvant treatments, radiotherapy, breast surgery type, and axillary surgery type, were collected. Tumor stage was evaluated according to the 8th American Joint Committee on Cancer (AJCC) TNM staging system (12).
The surgical specimens were stained with hematoxylin and eosin (H&E) to identify LVI and reported in routine pathology reports. Lymphatic invasion was defined as the presence of tumor emboli within the endothelial line space, whereas vessel invasion was defined as the presence of fibrin clots or erythrocytes and a lack of smooth muscle or elastic fibers in the endothelial line space. Because immunohistochemical staining is not routinely performed, distinguishing between lymphatic and vessel invasion using light microscopy was challenging. Thus, in our study, LVI was defined as the presence of tumor cells within the endothelial line spaces around the tumor (Figure 2).
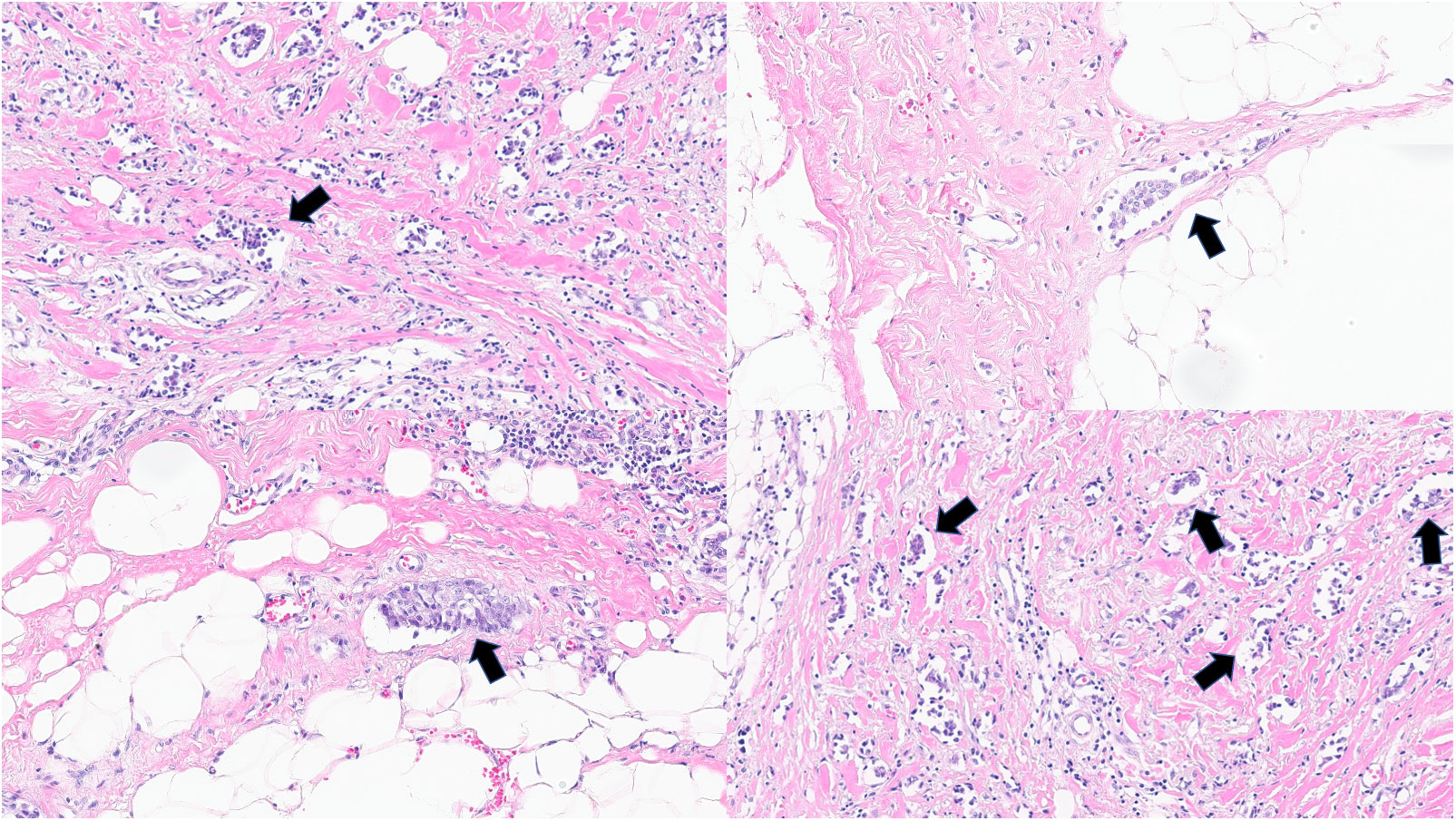
Figure 2 Lymphovascular invasion in invasive breast cancer in hematoxylin and eosin slides. Arrows show lymphovascular invasion. (all magnification, 100×).
Breast cancer molecular subtypes were defined based on the immunohistochemical staining results as follows: a) hormone receptor (HRs)-positive (ER/PR) and HER2 negative tumors, b) HRs positive and HER2 positive tumors, c) HRs negative and HER2 positive tumors, and d) tumors negative for both HRs and HER2, defined as triple-negative breast cancer (TNBC). According to the guidelines of the American Society of Clinical Oncology (ASCO) 2010, the positive status of ER or PR is defined as the presence of at least 1% stained cancer nuclei of ER or PR. HER2 positivity was defined as a score of 3 on immunohistochemical analysis (13, 14). HER2 expression of 0 or 1+ were categorized as HER2 negative, and HER2 expression of 3+ was defined as HER2 positive. If HER2 expression was 2+, the silver in situ hybridization assay was performed. Positive silver in situ hybridization assay results were categorized as HER2 positive, and vice versa. ≥ 20% Ki‐67 index values were classified as highly proliferative tumors.
Recurrence was defined as recurrence in the ipsilateral breast or counterlateral breast, regional or non-regional lymph node areas, or distant organs. Disease-free survival (DFS) was defined as the time from diagnosis to disease recurrence or death, whichever occurred first. Overall survival (OS) was defined as the time from diagnosis to death from any cause.
Categorical factors were analyzed using the chi-square test. The Kaplan–Meier method was used to draw DFS and OS curves, and group differences were calculated using the log-rank test. A multivariate Cox proportional hazards model was used to identify significant independent factors associated with DFS and OS. Statistical significance was defined as p < 0.05. All the statistical analyses were performed using SPSS software (version 26.0; IBM Software, Armonk, NY, USA).
LVI was observed in 381 patients (8.4%). Comparisons of the clinicopathological characteristics of the patients with and without LVI are shown in Table 1. The mean age of the patients in the LVI-negative group was higher than that of the patients in the LVI-positive group. Patients with positive LVI showed higher stages and grades than those with negative LVI. Specifically, a higher N stage was associated with a higher LVI positivity rate. The LVI positivity rates were 15.4%, 19.6%, 31.9%, and 52.5% in the pN1mi, N1, N2, and N3 stages, respectively.
In addition, the LVI-positive group had a higher percentage of patients with high Ki-67 levels than the LVI-negative group. Regarding the molecular subtypes, the LVI-positive group had a lower percentage of luminal type but a higher proportion of HER2 positive and TNBC subtypes. The percentage of patients who received adjuvant chemotherapy and radiotherapy was higher in the LVI-positive group than that in the LVI-negative group (p=0.011). The LVI-positive group underwent more total mastectomies and axillary lymph node dissections than the LVI-negative group.
At a median follow-up of 92 months, the LVI-negative group showed significantly favorable DFS and OS compared to the LVI-positive group (Figure 3). In addition, a significant difference was observed in the DFS between the two groups for both node-negative and node-positive disease (Figure 4). Moreover, the LVI-positive group showed a poorer prognosis than the LVI-negative group, regardless of the breast cancer subtype (Figure 5).
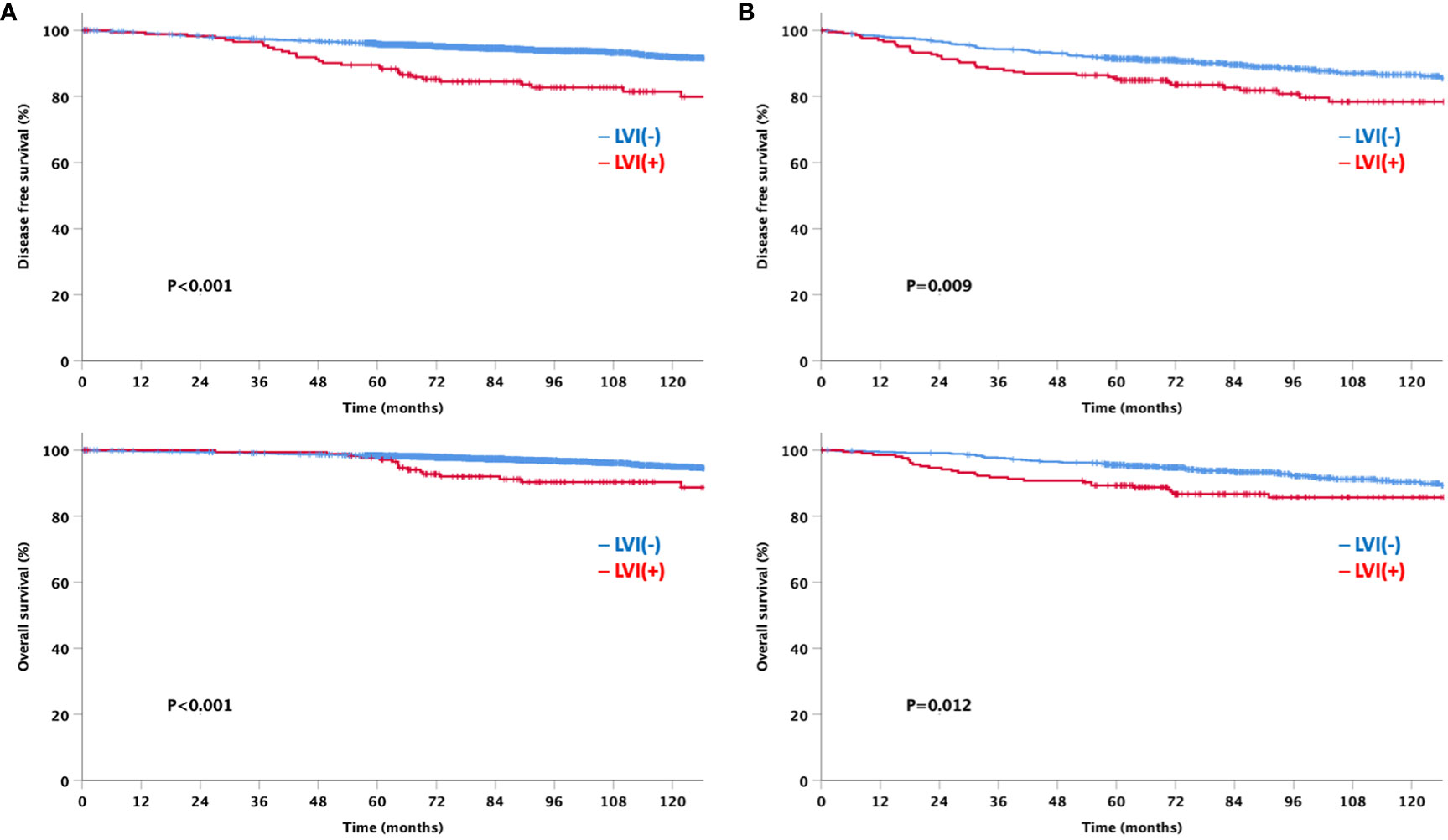
Figure 4 Disease-free and overall survival based on the lymphovascular invasion according to node status (A) Node negative (B) Node positive.
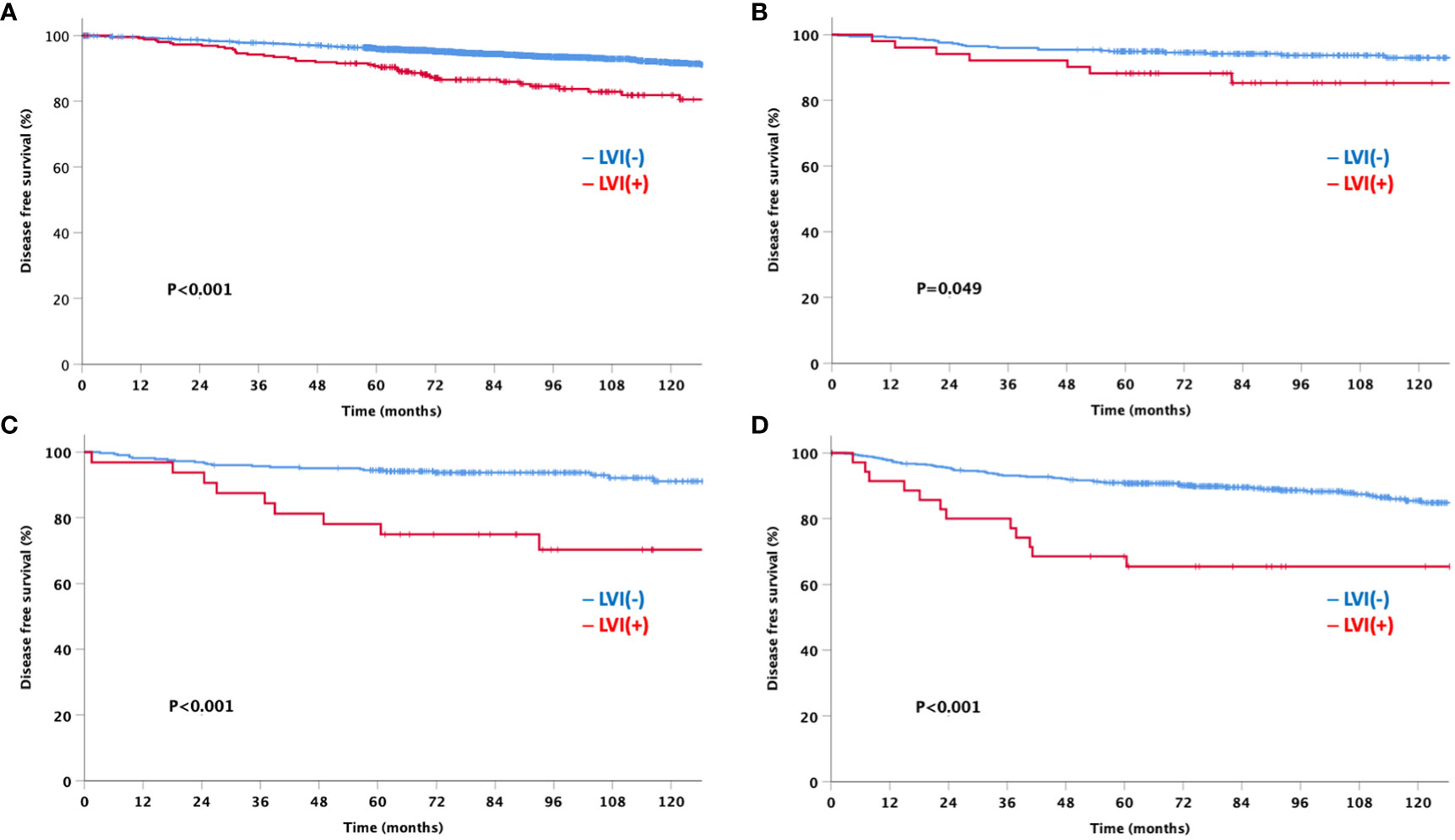
Figure 5 Disease-free survival by lymphovascular invasion according to the breast cancer subtype (A) HRs+HER2- (B) HRs+HER2+ (C) HRs-HER2+ (D) HRs-HER2-HRs: hormone receptor, HER2: human epidermal growth factor receptor 2.
The univariate analyses associated with DFS are presented in Table 2. Older age, higher T stage, higher N stage, higher histological grade, positive LVI, TNBC subtype, adjuvant chemotherapy, endocrine therapy, or radiotherapy, and total mastectomy were significant prognostic factors associated with poor DFS. In the multivariate analysis, higher T stage, higher N stage, higher histologic grade, positive LVI, and radiotherapy were statistically significant.
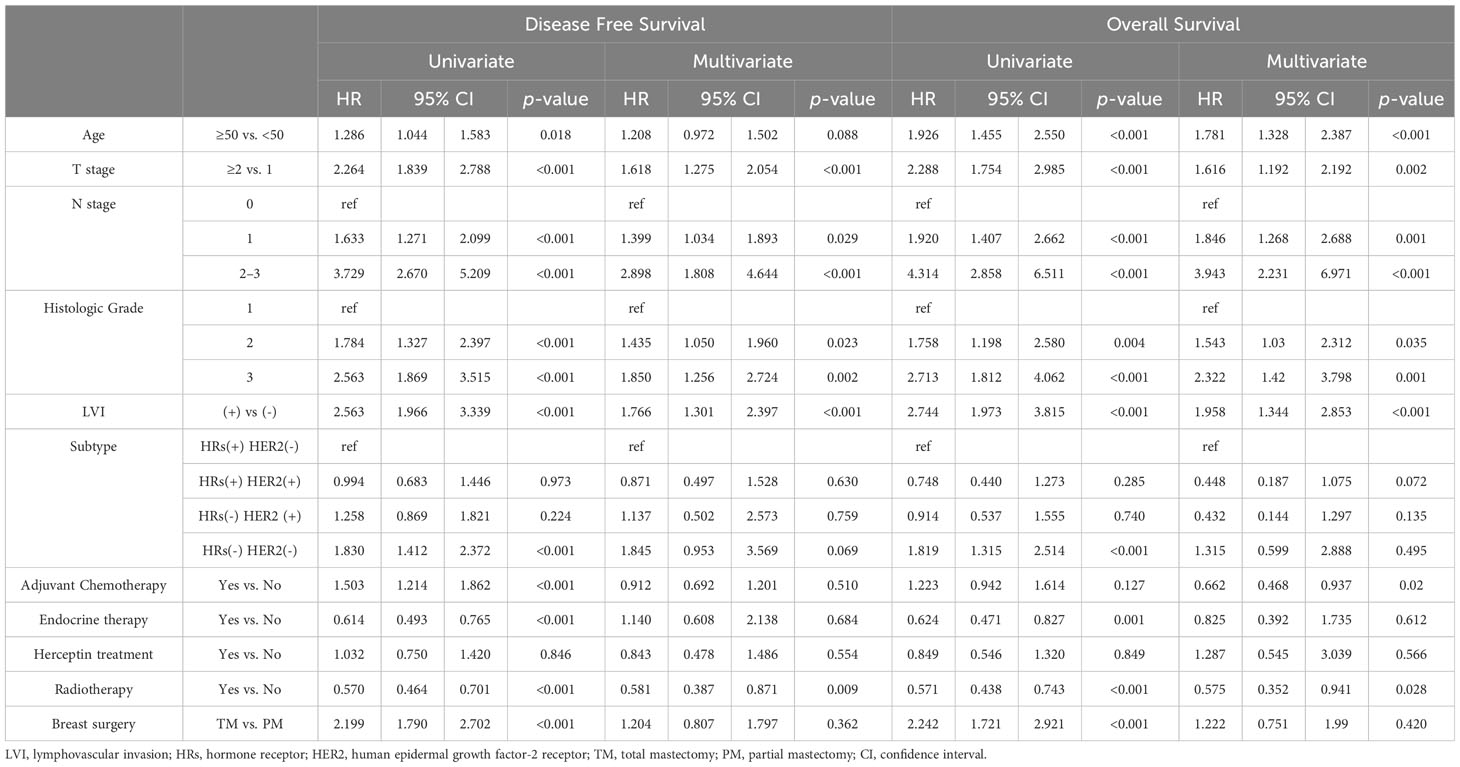
Table 2 Prognostic factors for overall survival and disease-free survival associated with lymphovascular invasion.
Prognostic factors associated with OS are shown in Table 2. In the univariate analysis, age of ≥ 50 years, larger tumor size, advanced nodal stage, grade III tumors, presence of LVI, TNBC subtype, endocrine therapy or radiotherapy, and total mastectomy were worse prognostic factors related to the OS. Multivariate analyses revealed that older age, higher T stage, higher N stage, higher histologic grade, positive LVI, adjuvant chemotherapy, and radiotherapy were significant independent prognostic factors.
Of the 4,554 patients included in the analysis, only 291 were available for the Oncotype Dx 21-gene recurrence score (RS) to explore the association between the multigene assay and the anatomical presence of LVI in patients with HR-positive and HER2-negative tumors. Patients were classified into three groups based on the RS according to the TAILORx trial (15); 163 (56.0%), 76 (26.1%), and 52 (17.9%) patients were in the low risk (RS ≤15), intermediate risk (RS 16–25), and high risk (RS ≥26) groups, respectively (Figure 6). No significant difference was observed in the prevalence of LVI among the three groups (7.4%, 11.8%, and 9.6% in the low-, intermediate-, and high-risk groups, respectively; p=0.518). Although a limited number of patients underwent the 21-gene RS assay and had a favorable luminal subtype, we further analyzed the DFS to explore the impact of the presence of LVI on the survival outcomes according to the Oncotype Dx risk classification. Patients with positive LVI showed a trend towards worse DFS; however, the difference was not statistically significant regardless of the 21-gene RS risk classification (Figure 7). In addition, the OS of LVI-positive patients was significantly worse in the Oncotype Dx low-/intermediate-risk group, whereas no difference in the OS was observed in the Oncotype Dx high-risk group. However, the actual events were too rare for observing any statistical significance in the current analysis.
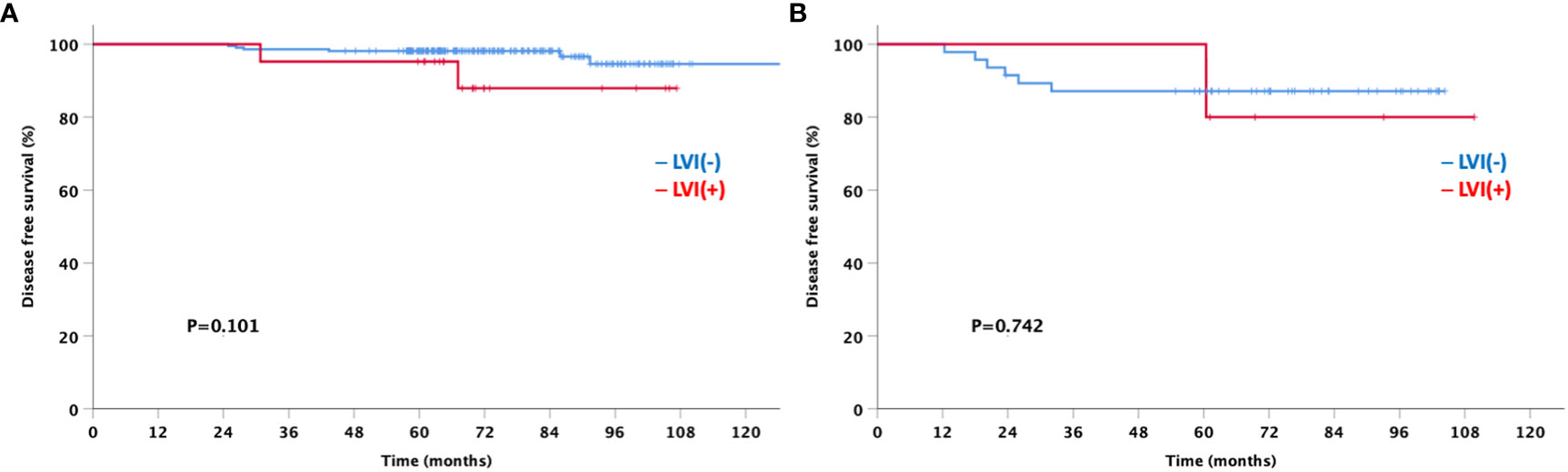
Figure 7 Disease-free survival based on the lymphovascular invasion according to Oncotype Dx group (A) low/intermediate-risk (B) high-risk.
This study demonstrated the clinical significance of LVI in early breast cancer in a large population with a long 10-year follow-up period. The detection rate of LVI was 8.4% in our study, which is consistent with previous studies (10, 16, 17), but lower than some studies reporting up to 30–48% (18, 19). The retrospective design and mere review of routine pathology medical records could be attributed to the difference. The race, limited to the Asian population, may be another reason for this. We assumed that a large proportion of smaller T1 stages in our data may have affected the low percentage of LVI expression.
Our study demonstrated that LVI is an independent poor prognostic factor for survival in patients with early breast cancer. Whether LVI should be considered an independent prognostic factor is debatable. While some studies have suggested that LVI is an independent prognostic variable not affected by the lymph node status or pathological features (20), other studies have stated that LVI is the only dependent factor associated with tumor characteristics, such as histological grade (21, 22). Some studies have even stated that LVI is not related to the treatment outcomes in patients with breast cancer (23, 24). Nevertheless, very few studies have analyzed LVI in the context of node status and molecular subtypes. In this study, we observed that LVI had a negative impact on the DFS and OS, regardless of the node status. Furthermore, LVI is associated with higher recurrence and lower survival rates in all breast cancer subtypes. Moreover, LVI was associated with DFS and OS in both univariate and multivariate analyses. These results suggest that LVI has independent prognostic value in patients with early breast cancer.
According to the findings of this study, while radiation therapy status was not statistically different between LVI negative and positive groups, radiation therapy was also an independent prognostic factor for overall survival. Since postmastectomy radiation therapy is administered for patients with risk factors such as large tumor size, close or positive resection margin, high histologic grade, and hormone receptor negative tumors, such factors might have affected the overall prognosis (6). The St. Gallen International Consensus Guidelines also recommend whole breast irradiation over partial breast irradiation in patients with LVI positive tumors (25).
Our study demonstrated the prognostic power of LVI; however, discordant findings were observed between LVI and node metastasis. Our data showed that 8.7% of the patients were LVI-positive, while 20.4% were node-positive. In the subgroups, node negative disease (N0) was 82.7% in the LVI-negative group and 54.3% in the LVI-positive group. Although the presence of LVI increased as the N stage increased (pN1mi,15.4%; N1,19.6%; N2,31.9%; and N3,52.5%), LVI was not a prerequisite condition for axillary node metastasis. Previous studies have also stated that lymph node metastasis can occur even in the absence of LVI in breast cancer (26). Thus, lymph node metastasis is not completely preventable because LVI does not occur. Taken together, our results are consistent with those of previous studies comparing LVI and node metastasis.
Furthermore, no association was observed between LVI and Oncotype Dx, indicating its prognostic value in early breast cancer. This finding is in agreement with a study by Al-Zawi et al., which stated that LVI did not have a statistically significant impact on the Oncotype Dx RS (9). Recent studies have shown that the detection of LVI adds reliable information to the 21-gene RS (10, 11). LVI provides additional prognostic information for OS in N0 patients with RS of 11-100 (10). In addition, one study investigated the genes used for multi-gene assays in LVI-positive patients. In the study, the authors found out that the Oncotype Dx test generally focuses on genes about proliferation, HER2 status, estrogen receptor and invasion, and only one out of twenty-one ODX gene correlated with LVI (27). Thus, we suggest that LVI should be considered a significant prognostic factor for early stage breast cancer, regardless of the genomic assay results.
Our study had several limitations, mostly owing to its retrospective study design. Second, the rate of LVI expression was slightly lower than that reported in other studies. This is because a smaller tumor size could have affected the results of LVI detection. Third, Oncotype Dx result was only available for small portion of study population. Therefore, we cannot perform powerful analysis about LVI and oncotype Dx result. Finally, the possibility of selection bias cannot be excluded. Despite these limitations, our study had a long follow-up period of up to 140 months and more than 4,000 patients were included in the analysis.
In conclusion, LVI in patients with early breast cancer is an independent risk factor for poor prognosis. Patients with positive LVI showed a higher recurrence rate and poorer survival in all breast cancer subtypes. Therefore, the LVI status should be considered when making treatment decisions for patients with early breast cancer; however, further prospective studies are warranted.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Institutional Review Board of Severance Hospital, Seoul, Republic of Korea (Approval No. 4-2023-0067). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin owing to the retrospective study design.
SL: Writing – original draft, Writing – review & editing. JG: Validation, Writing – review & editing. BA: Data curation, Writing – review & editing. JA: Data curation, Writing – review & editing. JK: Data curation, Writing – review & editing. HP: Data curation, Writing – review & editing. SK: Writing – review & editing. B-WP: Writing – review & editing. SP: Data curation, Formal analysis, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT). (No. 2022R1A2C1011970)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1269971/full#supplementary-material
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in korea: Incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat (2019) 51:417–30. doi: 10.4143/crt.2019.138
3. Schwartz AM, Henson DE, Chen D, Rajamarthandan S. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: A study of 161 708 cases of breast cancer from the seer program. Arch Pathol Lab Med (2014) 138:1048–52. doi: 10.5858/arpa.2013-0435-OA
4. Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P, et al. Estimating the benefits of therapy for early-stage breast cancer: The st. Gallen international consensus guidelines for the primary therapy of early breast cancer 2019. Ann Oncol (2019) 30:1541–57. doi: 10.1093/annonc/mdz235
5. Mohammed RA, Martin SG, Gill MS, Green AR, Paish EC, Ellis IO. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am J Surg Pathol (2007) 31:1825–33. doi: 10.1097/PAS.0b013e31806841f6
6. Gradishar WJ, Anderson BO, Abraham J. Nccn guidelines index table of contents discussion. Breast Cancer (2019) 215:7–10. doi: 10.6004/jnccn.2023.0031
7. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol (2019) 30:1194–220. doi: 10.1093/annonc/mdz173
8. Denduluri N, Chavez-MacGregor M, Telli ML, Eisen A, Graff SL, Hassett MJ, et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: Asco clinical practice guideline focused update. J Clin Oncol (2018) 36:2433–43. doi: 10.1200/JCO.2018.78.8604
9. Al-Zawi ASA, Yin SL, Aladili Z. Lymphovascular invasion in hormone-positive, human epidermal growth factor-negative, low-burden axillary disease in early breast cancer patients tested for oncotype dx recurrence score. Contemp Oncol (Pozn) (2022) 26:139–43. doi: 10.5114/wo.2022.118220
10. Makower D, Lin J, Xue X, Sparano JA. Lymphovascular invasion, race, and the 21-gene recurrence score in early estrogen receptor-positive breast cancer. NPJ Breast Cancer (2021) 7:20. doi: 10.1038/s41523-021-00231-x
11. Mutai R, Goldvaser H, Shochat T, Peretz I, Sulkes A, Yerushalmi R. Prognostic value of the detection of lymphovascular invasion in hormone receptor-positive early breast cancer in the era of molecular profiling. Oncology (2019) 96:14–24. doi: 10.1159/000492429
12. Teichgraeber DC, Guirguis MS, Whitman GJ. Breast cancer staging: Updates in the ajcc cancer staging manual, 8th edition, and current challenges for radiologists, from the ajr special series on cancer staging. AJR Am J Roentgenol (2021) 217:278–90. doi: 10.2214/AJR.20.25223
13. Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol (2011) 5:5–23. doi: 10.1016/j.molonc.2010.11.003
14. Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, et al. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast (2012) 21:50–7. doi: 10.1016/j.breast.2011.07.008
15. Sparano JA, Gray RJ, Makower DF, Albain KS, Saphner TJ, Badve SS, et al. Clinical outcomes in early breast cancer with a high 21-gene recurrence score of 26 to 100 assigned to adjuvant chemotherapy plus endocrine therapy: A secondary analysis of the tailorx randomized clinical trial. JAMA Oncol (2020) 6:367–74. doi: 10.1001/jamaoncol.2019.4794
16. Kang YJ, Kim HS, Jang WS, Kwon JK, Yoon CY, Lee JY, et al. Impact of lymphovascular invasion on lymph node metastasis for patients undergoing radical prostatectomy with negative resection margin. BMC Cancer (2017) 17:321. doi: 10.1186/s12885-017-3307-4
17. Yoon KH, Park S, Kim JY, Park HS, Kim SI, Cho YU, et al. Is the frozen section examination for sentinel lymph node necessary in early breast cancer patients? astr (2019) 97:49–57. doi: 10.4174/astr.2019.97.2.49
18. Nishimura R, Osako T, Okumura Y, Nakano M, Ohtsuka H, Fujisue M, et al. An evaluation of lymphovascular invasion in relation to biology and prognosis according to subtypes in invasive breast cancer. Oncol Lett (2022) 24:245. doi: 10.3892/ol.2022.13366
19. Houvenaeghel G, Cohen M, Classe JM, Reyal F, Mazouni C, Chopin N, et al. Lymphovascular invasion has a significant prognostic impact in patients with early breast cancer, results from a large, national, multicenter, retrospective cohort study. ESMO Open (2021) 6:100316. doi: 10.1016/j.esmoop.2021.100316
20. Rakha EA, Martin S, Lee AH, Morgan D, Pharoah PD, Hodi Z, et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer (2012) 118:3670–80. doi: 10.1002/cncr.26711
21. Freedman GM, Li T, Polli LV, Anderson PR, Bleicher RJ, Sigurdson E, et al. Lymphatic space invasion is not an independent predictor of outcomes in early stage breast cancer treated by breast-conserving surgery and radiation. Breast J (2012) 18:415–9. doi: 10.1111/j.1524-4741.2012.01271.x
22. Ovcaricek T, Frkovic SG, Matos E, Mozina B, Borstnar S. Triple negative breast cancer - prognostic factors and survival. Radiol Oncol (2011) 45:46–52. doi: 10.2478/v10019-010-0054-4
23. Kim SH, Simkovich-Heerdt A, Tran KN, Maclean B, Borgen PI. Women 35 years of age or younger have higher locoregional relapse rates after undergoing breast conservation therapy. J Am Coll Surg (1998) 187:1–8. doi: 10.1016/S1072-7515(98)00114-8
24. Camp RL, Rimm EB, Rimm DL. A high number of tumor free axillary lymph nodes from patients with lymph node negative breast carcinoma is associated with poor outcome. Cancer (2000) 88:108–13. doi: 10.1002/(SICI)1097-0142(20000101)88:1<108::AID-CNCR15>3.0.CO;2-B
25. Burstein H, Curigliano G, Thürlimann B, Weber W, Poortmans P, Regan M, et al. Panelists of the st gallen consensus conference. Customizing local and systemic therapies for women with early breast cancer: The st. Gallen international consensus guidelines for treatment of early breast cancer 2021. Ann Oncol (2021) 32:1216–35. doi: 10.1016/j.annonc.2021.06.023
26. Kuhn E, Gambini D, Despini L, Asnaghi D, Runza L, Ferrero S. Updates on lymphovascular invasion in breast cancer. Biomedicines (2023) 11:968. doi: 10.3390/biomedicines11030968
Keywords: lymphovascular invasion, breast cancer subtypes, node metastasis, oncotype Dx, breast cancer
Citation: Lee SJ, Go J, Ahn BS, Ahn JH, Kim JY, Park HS, Kim SI, Park B-W and Park S (2023) Lymphovascular invasion is an independent prognostic factor in breast cancer irrespective of axillary node metastasis and molecular subtypes. Front. Oncol. 13:1269971. doi: 10.3389/fonc.2023.1269971
Received: 31 July 2023; Accepted: 31 October 2023;
Published: 17 November 2023.
Edited by:
Sulma Mohammed, Purdue University, United StatesReviewed by:
Miguel J. Gil Gil, Catalan Institute of Oncology, SpainCopyright © 2023 Lee, Go, Ahn, Ahn, Kim, Park, Kim, Park and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seho Park, UFNIMTAyNUB5dWhzLmFj
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.