- 1Department of Urology, Medical University of Białystok, Bialystok, Poland
- 2Department of Histology and Cytophysiology, Medical University of Białystok, Bialystok, Poland
Introduction: The most common testicular tumors are seminomas. They are characterized by rapid growth and a very high potential for metastasis to other organs. Mutual interactions of tumor cells play an important role in the invasiveness and metastatic capacity, in which complexes of adhesion proteins play a special role. There is a lack of studies on changes in these molecules and their behaviour in testicular cancer. The aim of the study was immunohistochemical identification and evalutaion of adhesive molecules β-catenin, E-cadherin, galectin-3 in testicular cancer – seminoma.
Methods: Tests were performed on sections of testicular cancer – seminoma in comparison with unchanged tissue samples as a control. Material was taken from 30 patients who underwent orchiectomy. Immunohistochemistry and PCR were used to identify β-catenin, E-cadherin and galectin-3 and gene expression.
Results: Immunoreactivity and expression of β-catenin and E-cadherin in seminomas were markedly decreased compared to non-cancerous testicular tissue. Galectin-3 immunoreactivity was found in both control and cancerous tissue, but in different location. In non-cancerous tissue, it was localized in the cytoplasm of the cells of the seminiferous tubules, in seminomas it was localized mainly in the endothelium. The expression of the Lgals3 gene encoding galectin-3 in seminomas was slightl higher in relation to the tissue unchanged by the carcinogenetic process.
Conclusions: The results of the study suggest a significant role of β-catenin, E-cadherin and galectin-3 in the carcinogenesis of seminomas and may indicate new aspects of the patomechanism of seminomas formation, and thus time lead to better understand the biology of these tumors.
1 Introduction
In recent decades, there has been an increase in the incidence of testicular cancer (1–3), which accounts for about 5% of all urological cancers. The most common histopathological form of testicular cancers are seminomas (4–6). They account for about a third of all testicular germ cell tumors. The disease probably has its origin in fetal life as testicular dysgenesis syndrome (TDS). The exact pathomechanism of the development of seminomas is not known. The most likely theory is inhibition of gonocyte maturation, but the exact pathogenesis is still unknown. Therefore, further research on the pathomechanisms of this tumor may prove to be very important in the diagnosis and effective therapy.
Adhesion molecules, which are involved in the organization, differentiation and proliferation of cells, play a very important role in the proper functioning of cells and tissues. Against this background, they seem to play a key role in tumorigenesis. Disturbances in the interaction between cells and the components of the extracellular matrix lead to a loss of cohesion and facilitate cell migration, contributing to the formation of metastases. In addition, adhesion proteins allow cancer cells to penetrate the wall of blood vessels and stimulate angiogenesis, which affects the formation of metastases in sometimes distant organs. Recent studies indicate that adhesion molecules play an important role in various stages of malignant tumor development, stimulate primary tumor growth and, through intracellular signal transduction mechanisms, enable cancer cells to migrate through the blood vessel wall and metastasize (7, 8).
One of the families of adhesion proteins are cadherins, which play a key role in securing intercellular contacts. Test results for several cancers (breast cancer, colorectal cancer, bladder, esophagus) showed poor prognosis and worse course of the disease with reduced expression of E-cadherin and catenins (9–13).
Cadherins are inextricably linked with catenins and form cadherin-catenin complexes which are critically important for cell-to-cell adhesion. However, in the course of carcinogenesis, cadherins are often inactivated or functionally blocked, which enables the development and progression of the tumor (14, 15). Cadherins have been evaluated in various human malignancies, e.g. pancreatic carcinoma, melanoma, hepatocellular carcinoma, glioblastoma, breast tumor and gastric cancer (14, 16). In numerous studies, E-cadherin has been described as a tumor suppressor (17, 18). However, according to more recent studies E-cadherin, particularly in late-stage tumors, can also promote cell migration and invasion, and even cancer progression (19, 20).
The action of catenin is not limited to the E-cadherin-dependent regulation of intercellular adhesion, it is also an important mediator of the Wnt signaling pathway. An increased level of β-catenin expression and its localization in the cell nucleus always induces carcinogenic features and promotes the proliferation and survival of cancer cells (21, 22). Saito et al. showed weak expression of E-cadherin only in 3 out of 16 seminomas in contrast to β-catenin, which was expressed in most cases. Similar results are presented by Guerra et al. (23, 24).
Galectin-3 belongs to the galectin family and has many important biological functions, such as: apoptosis, cell growth, pre-mRNA assembly, angiogenesis, differentiation and transformation. This lectin is mainly found in the cytoplasm, but can also be found in the nucleus, and is also secreted into body fluids such as serum and urine (25). Elevated serum galectin-3 levels have been reported in patients with colorectal and bladder cancer (26, 27). In patients with prostate cancer, serum galectin-3 levels have been shown to positively correlate with prostate-specific antigen, especially in the early clinical stage (28). Studies have shown that galectin-3 can be a diagnostic or prognostic biomarker of many diseases, including cancer. Research by Kayser et al. and Devouassoux-Shisheboran et al. showed that galectin-3 could potentially become a predictor of tumor aggressiveness and survival of patients with testicular cancer (29, 30).
The incidence of seminoma increases from year to year. New factors associated with cancer progression are still being investigated, and the results obtained are inconclusive. E-cadherin, B-catenin and galectin-3 may prove to be important biomarkers that have not yet been jointly evaluated in this type of cancer. The aim of the study is the immunohistochemical identification and evaluation of the expression of E-cadherin, B-catenin and galectin-3 in human testicular seminoma.
2 Materials and methods
2.1 Sample collection
The research was carried out on archival postoperative material collected from thirty patients of the Department of Urology of Medical University of Bialystok, operated on for testicular cancer in the years 2014-2022. The study protocol was approved by the Bioethics Committee of the Medical University of Bialystok (R-I-002/282/2019) and prior written informed consent was obtained from each subject.
The research material consisted of fragments of seminomas obtained during radical orchidectomy. The comparative material consisted of fragments of the surrounding unchanged tissue of the testis. All seminoma lesions were at the same pT1 stage were confined to the testis and epididymis without vascular/lymphatic invasion. Following surgery samples were immediately fixed in Bouin’s solution and routinely embedded in paraffin or placed in RNAlater solution (AM7024 Thermo Fischer) and stored in -80°C.
The paraffin blocks were cut into 4 µm section. Histological examinations were performed on haematoxylin and eosin-stained tissue sections. Immunohistochemical reactions were performed to detect β-catenin, E-cadherin and Gal-3. The material stored in the RNAlater solution was subjected to real-time PCR to evaluate the expression of the genes encoding β-catenin, E-cadherin and Gal-3. The slides were evaluated using an Olympus BX43 optical microscope with a built-in digital camera and connected to a computer. Color microscopic images of β-catenin, E-cadherin and Gal-3 at 200x magnification (at least 5 fields from each slide of a given patient) were archived and saved in jpg format on a computer hard drive.
2.2 Immunohistochemistry
Immunostaining was made by the following protocol (detailed described in Kasacka et al. (2018) (31): paraffin-embedded sections were deparaffined and hydrated in pure alcohols. The sections of testicular tissue were subjected to pretreatment in a pressure chamber and heated using Target Retrieval Solution Citrate pH=6.0 (Agilent Technologies, Inc. Santa Clara, CA, USA). After cooling down to room temperature, the sections were incubated with Dako REAL Peroxidase-Blocking Solution (Agilent Technologies, Inc. Santa Clara, CA, USA). The sections with the primary antibodies: β-catenin (1:2000; ab32572, Abcam, Cambridge, UK), E-cadherin (1:500; ab76055, Abcam, Cambridge, (UK) and galectin-3 (1:1000; MA1-940, Invitrogen) were incubated 24 hours at +4°C in a humidified chamber. Procedure was followed by incubation with secondary antibody (REAL™ EnVision™ Detection System, Peroxidase/DAB, Rabbit/Mouse detection kit (K5007; Agilent Technologies Denmark Ap/S, Produktionsvej 42, 2600 Glostrup, Denmark). The bound antibodies were visualized by incubation with DAB Flex chromogen. Finally the testicular tissue sections were counterstained in hematoxylin QS (H-3404 Vector Laboratories, Burlingame, CA, USA) and observed under a light microscope. Sections with seminoma tissues were dehydrated and the specificity of the antibodies was confirmed using a negative control, where the antibodies were replaced by Antibody Diluent (S3022; Agilent Technologies Denmark Ap/S, Produktionsvej 42, 2600 Glostrup, Denmark). The negative control showed no apparent immunoreactivity in the seminiferous tubules.
2.3 Real-time PCR
Seminoma and normal tissue samples taken from the material after operation were placed in an RNA-later solution. Total RNA was isolated using NucleoSpin® RNA Isolation Kit (Machery-Nagel). Quantification and quality control of total RNA was determined using a spectrophotometer - NanoDrop 2000 (ThermoScientific). An aliquot of 1 µg of total RNA was reverse transcribed into cDNA using iScript™ Advanced cDNA Synthesis Kit for RT-qPCR (BIO-RAD). Synthesis of cDNA was performed in a final volume of 20 μl using an Thermal Cycler (Model SureCycler 8800, Aligent Technologies). For reverse transcription, the mixtures were incubated at 46°C for 20 min, then heated to 95°C for 1 min and finally cooled quickly at 4°C. Quantitative real-time PCR reactions were performed using Stratagene Mx3005P (Agilent Technologies) with the SsoAdvanced™ Universal SYBER® Green Supermix (BIO-RAD). Specific primers for E-cadherin, β-catenin and GAPDH (GAPDH) were designed by BIO-RAD Company. The housekeeping gene GAPDH (GAPDH) was used as a reference gene for quantification. To determine the amounts of levels of test genes expression, standard curves were constructed for each gene separately with serially diluted PCR products. PCR products were obtained by cDNA amplification using specific primers as follows: E-cadherin (qHsaCEP0049339, BIO-RAD), β-catenin (qHsaCID0010363, BIO-RAD), and GAPDH (qHsaCED0038674, BIO-RAD). QRT-PCR was carried out in a doublet in a final volume of 20 μl under the following conditions: 2 min polymerase activation at 95°C, 5 s denaturation at 95°C, 30 s annealing at 60°C for 35 cycles. PCR reactions were checked, including no-RT-controls, omitting of templates, and melting curve to ensure only one product was amplified. The relative quantification of gene expression was determined by comparing Ct values using the ΔΔCt method. All results were normalized to GAPDH.
2.4 Statistical analysis
All data were analyzed for statistical significance using the Statistica version 12.0 computer software package+e. The mean values were computed automatically; significant differences were determined by one-way ANOVA test; p < 0.05 was considered significant.
3 Results
In the present study, sections from thirty seminomas and normal tissues testis were examined. A total of 30 samples were considered for the study. The mean age of the patients at the time of operation was 37 years, with a range 23-63 years. A positive immunohistochemical reaction in the form of a brown stain indicated the presence of the tested antigen.
3.1 Immunohistochemical evaluation
Strong β-catenin immunoreactivity was found in non-cancerous testicular tissue, mainly in the contact areas between epithelial cells of the convoluted tubules of the testis (Figure 1A).
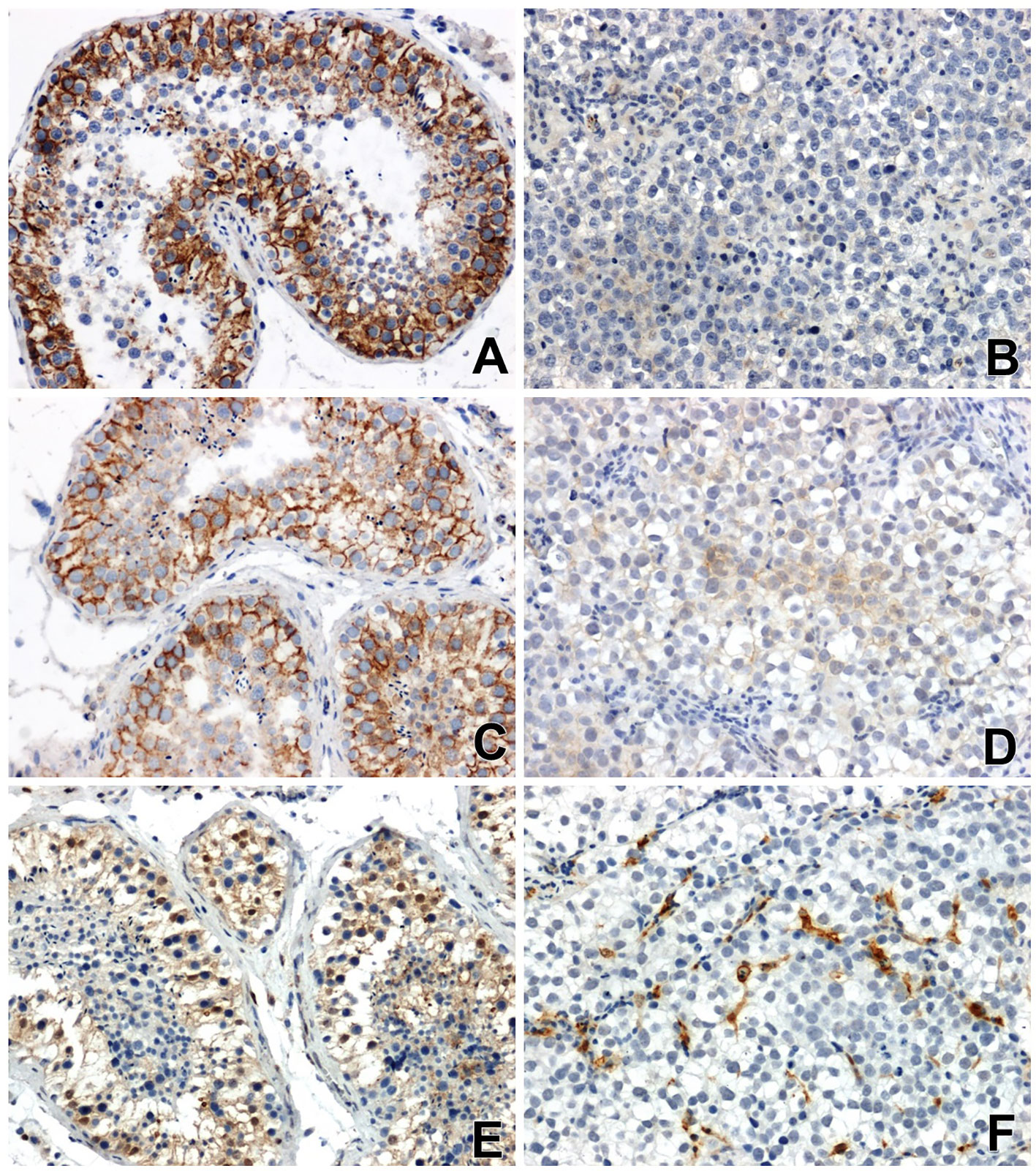
Figure 1 Immunoreactivity and localization in testicular tissues: of β-catenin (A) in normal non-neoplastic tissue, (B) in seminoma (very weak or negative reaction), of E-cadherin (C) in normal tissue, (D) in seminoma (a low specific signal) and Gal-3 (E) in normal tissue, (F) in seminoma, x200.
The immunoreactivity of β-catenin in all seminomas was very low or negative. Nuclear localization of β-catenin or low-grade perinuclear expression was observed only in single tumor cells (Figure 1B).
Immunohistochemical analysis showed a clear immunoexpression of E-cadherin in non-neoplastic tissues of the testis. A positive result of the reaction, in the form of a colour reaction, was observed mainly in the membrane localization of the epithelial cells of the seminiferous tubules (Figure 1C). Meanwhile, E-cadherin immunoreactivity in seminomas was significantly attenuated and restricted to certain cell groups in membrane and cytoplasmic localization (Figure 1D).
Reaction with anti-antibody Gal-3 showed clearly positive immunoreactivity in the cytoplasm of the cells of the seminiferous tubules, as well as in the nuclei of some cells in the testis without signs of neoplastic transformation (Figure 1E). In seminomas, a clear positive reaction was observed in endothelial cells and single stromal cells (Figure 1F).
3.2 Real-time PCR
QRT-PCR analysis showed a significant decrease in the expression of β-catenin and E-cadherin genes (Figures 2A, B) and a slight increase in the expression of the Lgals3 gene encoding galectin-3 (Figure 2C) in the seminomas compared to healthy tissue.
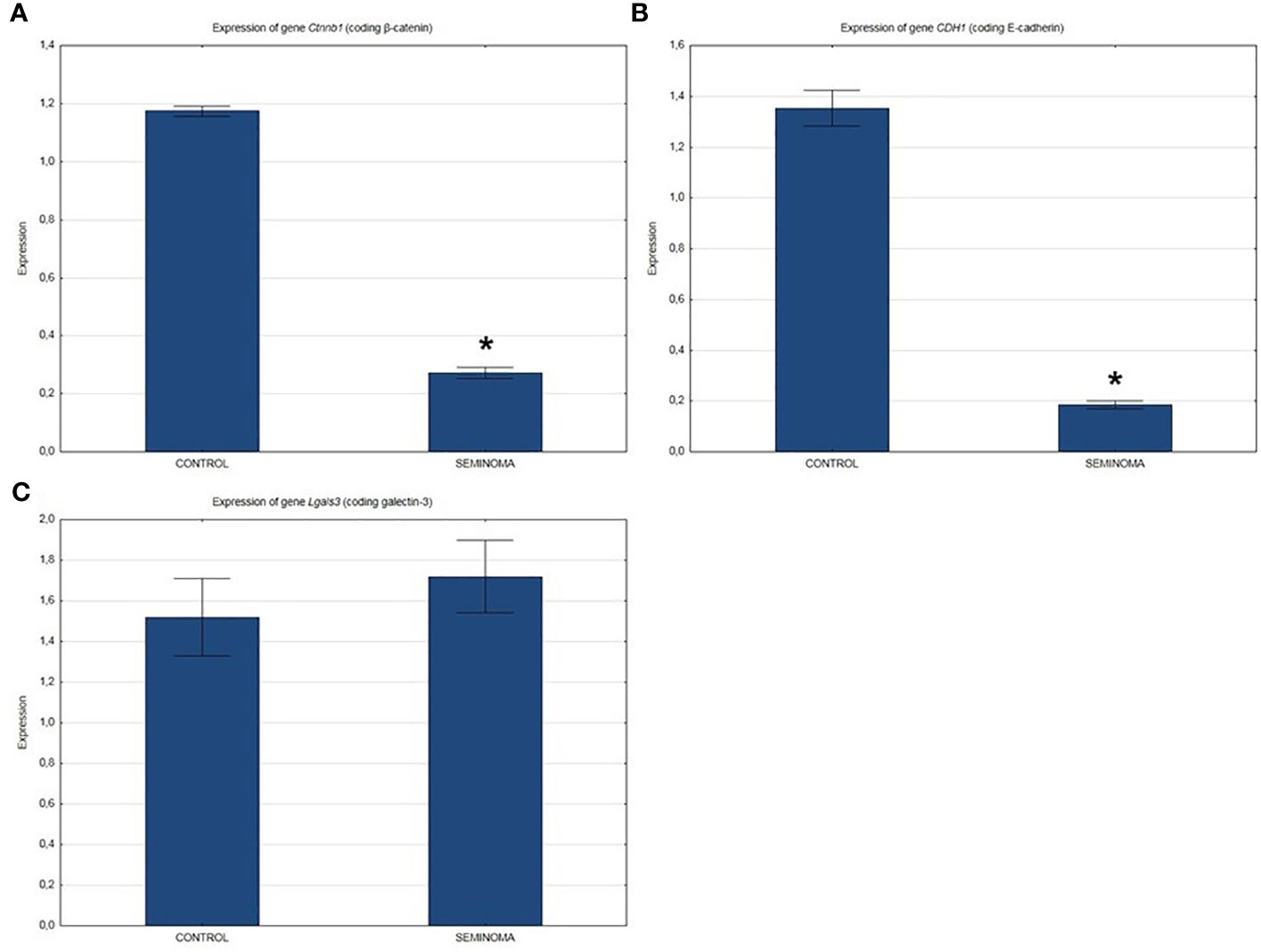
Figure 2 Expression of genes in normal and seminoma tissues encoding: (A) β-catenin, (B) E-cadherin, and (C) galectin-3. *p < 0.05 seminoma vs. control.
4 Discussion
According to the latest epidemiological data, testicular cancer accounts for 1.6% of all cancers in men and the number of diagnosed cases is increasing every year. The dominant type of cancer is germ cell carcinomas, including seminomas and non-seminomas.
The exact etiology of seminoma is unknown. The last hypothesis is that endocrine-disrupting environmental factors contribute to the abnormal development of gonocytes. Expanding knowledge on the mechanisms of testicular cancer formation is necessary to continuously improve the effectiveness of prevention and treatment of this malignant tumor. Considering the important role of β-catenin, E-cadherin and galectin-3 in the process of carcinogenesis, it seems reasonable to conduct a study aimed at evaluating and comparing gene expression and activity of the above proteins in testicular seminoma compared to healthy tissue. Immunohistochemical tests and real-time PCR were used as research methods (32).
Searching the literature, we were able to find only individual articles on similar topics. Moreover, data describing changes in the biology of seminomas compared to normal testicular tissue are also limited (21, 24, 29, 30, 33–35).
In our study, we showed significant attenuation of immunoreactivity and expression of β-catenin and E-cadherin genes in seminoma compared to strong expression in control tissue. The study of gal-3 showed a change the localization of this protein. In seminomas, quite strong immunoreactivity was demonstrated in endothelial cells and connective tissue stroma, while in normal tissue, the presence of galactin-3 was observed in the cytoplasm of the epithelium of the seminiferous tubules and the intensity of the reaction was moderate.
In the presented study, 30 patients with organ-limited diagnosis of testicular seminoma, without features of distant metastases, were evaluated. The expression of genes encoding β-catenin and E-cadherin was significantly reduced, while in the case of the gene encoding galectin-3, a slight increase in expression was found in comparison to healthy tissue.
Changes in β-catenin immunoreactivity in seminomas compared to control tissue have been demonstrated in several previous studies. An immunohistochemical study by Guerra et al. showed weak expression of β-catenin in investigated seminomas. Low expression of E-cadherin and no activity of beta-catenin in cell nuclei were demonstrated (24). Chovanec et al. evaluated beta-catenin alone in testicular cancer. They showed a marked decrease in its expression in seminomas compared to other types of testicular tumors. They also proved that the intensity of beta-catenin expression may positively correlate with poor prognostic factors (33). Lobo et al. analyzed potential biomarkers for an increased risk of disease recurrence in the first stage. The results obtained by these researchers indicate that patients with elevated beta-catenin expression have a higher risk of recurrence of the disease at this stage (34).
There are few reports relating to E-cadherin in testicular seminoma. Saito et al. evaluated e-cadherin expression in 16 patients diagnosed with seminoma. E-cadherin was detected in only three patients (21). Honecker and his research team also evaluated E-cadherin in testicular cancer. It should be noted that E-cadherin expression was significantly lower in seminomas compared to non-seminomas (36).
There are few reports in the available literature on the importance and function of galectins in testicular seminoma. Kayser et al. analyzed lectins as a prognostic factor in testicular seminoma with lung metastases. Compared to the primary tumor, none of the analyzed features, including galectin-3, was stable and statistically significant in the metastatic lesion. On the basis of the results obtained by the authors of determined galectins, no clear conclusions can be drown as to their significance in the prognosis of testicular seminoma (29). Devouassoux-Shisheboran et al. using immunohistochemistry and RT-PCR, analyzed galectin-3 expression in testicular cancer. Galectin-3 expression, however, was demonstrated in seminomas, with no apparent significant difference compared to control tissue (30). In the available literature, we have not found a study evaluating all three factors related to cell adhesion in seminoma at the same time. It can therefore be assumed that these are the first studies of this type.
The functions of β-catenin as a co-regulator of the transcription process and a protein necessary for intracellular adhesion are well known. Under the control of activation by Wnt, β-catenin accumulates in the cytoplasm and then translocated to the nucleus, where it promotes the transcription of genes encoding mostly oncoproteins. Beta-catenin translocated to the nucleus binds to lymphoid stimulating factor (LEF) and T cell factor (LEF-1/TCF), to form a powerful transcription factor of genes such as c-Myc, c-Jun, CCND1, metalloproteases, cyclin D1, vimentin, etc., all related to cell proliferation, invasion and EMT (epithelial-mesenchymal transition) (Liu et al, 2022). In our study, we demonstrated very weak expression of β-catenin in the nuclei of only single seminoma cells. These results are consistent with several previously cited observations of β-catenin staining in testicular cancer (33, 34, 37).
The important milestone in the pathogenesis of GCNIS (Germ cell neoplasia in situ) is the failure of germ cell differentiation/maturation due to lack of adequate signals from the somatic niche during early fetal development, which should normally decrease the expression of pluripotency factors (38). The atypical gonocyte retains the expression of embryonic markers such as OCT3/4 and AP-2γ, which leads to the dissemination of seminomas. Neoplastic gonocytes advance as the tubular walls lose elasticity, filling and widening the tubules (intratubular neoplasia) until the disappearance of the barrier produces leakage and dispersion of neoplastic cells (solid seminoma). This mechanism is independent of the Wnt/Beta catenin pathway (38).
E-cadherin, due to its important role in maintaining balance in the process of cell adhesion, cell differentiation and growth, is a very important factor in the context of the process of carcinogenesis. Similar to β-catenin, E-cadherin expression follows the same pattern: high in control tissue, low in tumor tissue. Based on the β-catenin/E-cadherin relationship described above, this result fully confirms the relationship between the two proteins and proves that both β-catenin and E-cadherin in seminoma are likely to play an important role in the development of this tumor. A reduced level of elements of the E-cadherin-beta-catenin complex impairs cell differentiation, promotes the development of local advancement and distant metastasis. The obtained results are confirmed in the works of other authors mentioned above (35, 39).
Galactin-3 participates in many processes important from the oncological point of view, such as: cell growth, apoptosis, differentiation and angiogenesis. Galectin-3 in cancer patients induces secretion of IL-6, G-CSF, sICAM-1 and GM-CSF from blood vascular endothelial cells in vitro and in mice. These cytokines interact in an autocrine/paracrine manner with the vascular endothelium to increase the expressions of the endothelial cell surface adhesion molecules E-selectin, ICAM-1 and VCAM-1, resulting in increased endothelial adhesion of cancer cells and increased migration of endothelial cells, with formation of tubules and branch points (angiogenesis) (40). Our results demonstrated galectin-3 expression in both control tissue and seminoma. The difference consisted in a different location, in the healthy tissue it was the cytoplasm of the epithelial cells of the seminiferous tubules and nuclei of some single cells, in the cancerous tissue mainly the endothelium. Due to the small amount of scientific reports on galectin-3 in seminoma, it is not easy for us to refer and draw clear conclusions. Nevertheless, we consider the localization of galectin-3 in the endothelium in the seminoma as an obvious manifestation of the participation of the studied protein in the angiogenesis process in carcinogenesis.
In summary, our study showed significant differences in the immunoreactivity and expression of the assessed factors: β-catenin, E-cadherin and galectin-3 in testicular seminoma compared to control material. While the results concerning β-catenin and E-cadherin were not surprising, the immunoreactivity of galectin-3 and its localization in the seminoma seem to be the most interesting finding in our study. The results obtained in the study indicate a fairly significant role of the above-mentioned proteins in the process of carcinogenesis in testicular seminoma. They may indicate further aspects of the pathomechanism of seminomas formation and at the same time lead to a better understanding of the biology of these tumors. With the intensification of further research on seminomas, they could be a target of therapy. We hope that the information obtained will help in the future in determining new markers of this disease. This would allow earlier detection of the disease, and thus the implementation of appropriate treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by bioethics committee at the Medical University in Białystok. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GM: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. ND: Conceptualization, Data curation, Methodology, Writing – review & editing. IK: Conceptualization, Data curation, Formal Analysis, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Centre of Science (project MINIATURA 2022/06/X/NZ5/00763).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Park JS, Kim J, Elghiaty A, Ham WS. Recent global trends in testicular cancer incidence and mortality. Medicine (2018) 97(37):e12390. doi: 10.1097/MD.0000000000012390
2. Nigam M, Aschebrook-Kilfoy B, Shikanov S, Eggener S. Increasing incidence of testicular cancer in the United States and Europe between 1992 and 2009. World J Urol (2015) 33(5):623–31. doi: 10.1007/s00345-014-1361-y
3. Gurney JK, Florio AA, Znaor A, Ferlay J, Laversanne M, Sarfati D, et al. International trends in the incidence of testicular cancer: lessons from 35 years and 41 countries. Eur Urol (2019) 76(5):615–23. doi: 10.1016/j.eururo.2019.07.002
4. Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer (2005) 5(3):210–22. doi: 10.1038/nrc1568
5. Looijenga LHJ, van der Kwast TH, Grignon D, Egevad L, Kristiansen G, Kao CS, et al. Report from the international society of urological pathology (ISUP) consultation conference on molecular pathology of urogenital cancers: IV: current and future utilization of molecular-genetic tests for testicular germ cell tumors. Am J Surg Pathol (2020) 44(7):e66–79. doi: 10.1097/PAS.0000000000001465
6. Moch H, Amin MB, Berney DM, Compérat EM, Gill AJ, Hartmann A, et al. The 2022 world health organization classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol (2022) 82(5):458–68. doi: 10.1016/j.eururo.2022.06.016
7. Harjunpää H, Llort Asens M, Guenther C, Fagerholm SC. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front Immunol (2019) 10:1078. doi: 10.3389/fimmu.2019.01078
8. Janiszewska M, Primi MC, Izard T. Cell adhesion in cancer: Beyond the migration of single cells. J Biol Chem (2020) 295(8):2495–505. doi: 10.1074/jbc.REV119.007759
9. Chu YQ, Ye ZY, Tao HQ, Wang YY, Zhao ZS. Relationship between cell adhesion molecules expression and the biological behavior of gastric carcinoma. World J Gastroenterol (2008) 14(13):1990–6. doi: 10.3748/wjg.14.1990
10. Nair KS, Naidoo R, Chetty R. Expression of cell adhesion molecules in oesophageal carcinoma and its prognostic value. J Clin Pathol (2005) 58:343–51. doi: 10.1136/jcp.2004.018036
11. Popov Z, Gil-Diez de Medina S, Lefrere-Belda MA, Hoznek A, Bastuji-Garin S, Abbou CC, et al. Low E-cadherin expression in bladder cancer at the transcriptional and protein level provides prognostic information. Br J Cancer (2000) 83(2):209–14. doi: 10.1054/bjoc.2000.1233
12. Ślubowski T, Ślubowska M. Biomarkery w raku piersi. Część II: markery białkowe, DNA, adhezji komórkowej i oporności lekowej. Współczesna Onkologia (2007) 11(5):240–6.
13. Wideł MS, Wideł M. Mechanizmy przerzutowania i molekularne markery progresji nowotworów złośliwych. I. Rak jelita grubego. Postępy Hig. Med Dosw (2006) 60:453–70.
14. Kaszak I, Witkowska-Piłaszewicz O, Niewiadomska Z, Dworecka-Kaszak B, Ngosa Toka F, Jurka P. Role of cadherins in cancer-A review. Int J Mol Sci (2020) 21(20):7624. doi: 10.3390/ijms21207624
15. Oda H, Takeichi M. Evolution: Structural and functional diversity of cadherin at the adherens junction. J Cell Biol (2011) 193:1137–46. doi: 10.1083/jcb.201008173
16. Rossi T, Tedaldi G, Petracci E, Khouzam RA, Ranzani GN, Morgagni P, et al. E-cadherin downregulation and microRNAs in sporadic intestinal-type gastric cancer. Int J Mol Sci (2019) 20:4452. doi: 10.3390/ijms20184452
17. Kourtidis A, Lu R, Pence LJ, Anastasiadis PZ. A central role for cadherin signaling in cancer. Exp Cell Res (2017) 358:78–85. doi: 10.1016/j.yexcr.2017.04.006
18. Ceresa D, Alessandrini F, Bosio L, Marubbi D, Reverberi D, Malatesta P, et al. Cdh4 down-regulation impairs in vivo infiltration and Malignancy in patients derived glioblastoma cells. Int J Mol Sci (2019) 20:4028. doi: 10.3390/ijms20164028
19. Labernadie A, Kato T, Brugues A, Serra-Picamal X, Derzsi S, Arwert E, et al. A mechanically active heterotypic E-cadherin- N-cadherin adhesion enables fibroblast to drive cancer cell invasion. Nat Cell Biol (2017) 19:224–37. doi: 10.1038/ncb3478
20. Daulagala AC, Bridges MC, Kourtidis A. E-cadherin Beyond Structure: A signaling hub in colon homeostasis and disease. Int J Mol Sci (2019) 20:2756. doi: 10.3390/ijms20112756
21. Shang S, Hua F, Hu ZW. The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget (2017) 8(20):33972–89. doi: 10.18632/oncotarget.15687
22. Prakash S, Swaminathan U. β catenin in health: A review. J Oral Maxillofac Pathol (2015) 19(2):230–8. doi: 10.4103/0973-029X.164537
23. Saito T, Katagiri A, Watanabe R, Tanikawa T, Kawasaki T, Tomita Y, et al. Expression of E-cadherin and catenins on testis tumor. Urol Int (2000) 65(3):140–3. doi: 10.1159/000064859
24. Guerra F, Fisch P, Jufe L, Avagnina MA, Mendeluk G, Palaoro LA. Is the Wnt/β catenin signalling pathway activated in Seminoma?: An immunohistochemical study. J Cancer Res Ther (2016) 12(2):1075–9. doi: 10.4103/0973-1482.147392
25. Dong R, Zhang M, Hu Q, Zheng S, Soh A, Zheng Y, et al. F as a novel biomarker for disease diagnosis and a target for therapy (Review). Int J Mol Med (2018) 41(2):599–614. doi: 10.3892/ijmm.2017.3311
26. El Gendy H, Madkour B, Abdelaty S, Essawy F, Khattab D, Hammam O, et al. Galectin 3 for the diagnosis of bladder cancer. Arab J Urol (2014) 12:178−181. doi: 10.1016/j.aju.2013.10.004
27. Gendy HE, Madkour B, Abdelaty S, Essawy F, Khattab D, Hammam O, et al. Diagnostic and prognostic significance of serum and tissue Galectin 3 expression in patients with carcinoma of the bladder. Curr Urol (2014) 7:185−190. doi: 10.1159/000365673
28. Nakajima K, Heilbrun LK, Hogan V, Smith D, Heath E, Raz A. Positive associations between galectin−3 and PSA levels in prostate cancer patients: A prospective clinical study−I. Oncotarget (2016) 7:82266−82272. doi: 10.18632/oncotarget.12619
29. Kayser K, Hoeft D, Hufnagi P, Caselitz J, Zick Y, André S, et al. Combined analysis of tumor growth pattern and expression of endogenous lectins as a prognostic tool in primary testicular cancer and its lung metastases. Histol Histopathol (2003) 18(3):771–9. doi: 10.14670/HH-18.771
30. Devouassoux-Shisheboran M, Deschildre C, Mauduit C, Berger G, Mejean-Lebreton F, Bouvier R, et al. Expression of galectin-3 in gonads and gonadal sex cord stromal and germ cell tumors. Oncol Rep (2006) 16(2):335–40. doi: 10.3892/or.16.2.335
31. Kasacka I, Piotrowska Ż, Filipek A, Lebkowski W. Comparative evaluation of cannabinoid receptors, apelin and S100A6 protein in the heart of women of different age groups. BMC Cardiovasc Disord (2018) 18(1):190. doi: 10.1186/s12872-018-0923-0
32. Yazici S, Del Biondo D, Napodano G, Grillo M, Calace FP, Prezioso D, et al. Risk factors for testicular cancer: environment, genes and infections-is it all? Medicina (Kaunas) (2023) 59(4):724. doi: 10.3390/medicina59040724
33. Chovanec M, Cierna Z, Miskovska V, Machalekova K, Kalavska K, Rejlekova K, et al. βcatenin is a marker of poor clinical characteristics and suppressed immune infiltration in testicular germ cell tumors. BMC Cancer (2018) 18(1):1062. doi: 10.1186/s12885-018-4929-x
34. Lobo J, Gillis AJM, van den Berg A, Looijenga LHJ. Prediction of relapse in stage I testicular germ cell tumor patients on surveillance: investigation of biomarkers. BMC Cancer (2020) 20(1):728. doi: 10.1186/s12885-020-07220-6
35. Borcherding N, Cole K, Kluz P, Jorgensen M, Kolb R, Bellizzi A, et al. Re-evaluating E-cadherin and β-catenin: A pan-cancer proteomic approach with an emphasis on breast cancer. Am J Pathol (2018) 188(8):1910–20. doi: 10.1016/j.ajpath.2018.05.003
36. Honecker F, Kersemaekers AM, Molier M, Van Weeren PC, Stoop H, De Krijger RR, et al. Involvement of E-cadherin and beta-catenin in germ cell tumours and in normal male fetal germ cell development. J Pathol (2004) 204(2):167–74. doi: 10.1002/path.1614
37. Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther (2022) 7(1):3. doi: 10.1038/s41392-021-00762-6
38. Jørgensen A, Lindhardt Johansen M, Juul A, Skakkebaek NE, Main KM, Rajpert-De Meyts E. Pathogenesis of germ cell neoplasia in testicular dysgenesis and disorders of sex development. Semin Cell Dev Biol (2015) 45:124–37. doi: 10.1016/j.semcdb.2015.09.013
39. Tian X, Liu Z, Niu B, Zhang J, Tan TK, Lee SR, et al. E-cadherin/β-catenin complex and the epithelial barrier. J BioMed Biotechnol (2011) 2011:567305. doi: 10.1155/2011/567305
Keywords: β-catenin, E-cadherin, galectin-3, testicular cancer, seminoma
Citation: Młynarczyk G, Domian N and Kasacka I (2023) Changes in adhesion molecules: β-catenin, E-cadherin and Galectin-3 in cells of testicular seminoma. Front. Oncol. 13:1269637. doi: 10.3389/fonc.2023.1269637
Received: 30 July 2023; Accepted: 23 November 2023;
Published: 08 December 2023.
Edited by:
Pinuccia Faviana, University of Pisa, ItalyReviewed by:
Luis Palaoro, University of Buenos Aires, ArgentinaFrancisco Sáez, University of the Basque Country, Spain
Copyright © 2023 Młynarczyk, Domian and Kasacka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Irena Kasacka, a2FzYWNrYUB1bWIuZWR1LnBs; aGlzdG9sb2dpYS5jeXRvZml6am9sb2dpYUB1bWIuZWR1LnBs
 Grzegorz Młynarczyk
Grzegorz Młynarczyk Natalia Domian
Natalia Domian Irena Kasacka
Irena Kasacka