- 1Department of Oncology, Huzhou Central Hospital, Affiliated Central Hospital Huzhou University, Huzhou, China
- 2Department of Nephrology, The First People’s Hospital of Huzhou, First Affiliated Hospital of Huzhou University, Huzhou, China
- 3Department of Physical Examination Center, Zhejiang Xinda Hospital, Huzhou, China
Background: Recently, the survival rate of nasopharyngeal carcinoma (NPC) patients has improved greatly due to developments in NPC treatments. But cause-specific mortality in NPC patients remains unclear. This study aims to investigate the common causes of death in NPC patients.
Methods: Eligible patients with NPC were included from the Surveillance, Epidemiology, and End Results (SEER) database. Standardized mortality ratios(SMRs) were calculated to compare death rates in NPC patients with those in the general population.
Results: A total of 3475 patients with NPC were included, of whom 1696 patients died during the follow-up period. 52.83% of deaths were caused by NPC, followed by other cancers (28.13%) and non-cancer causes (18.46%). The proportion of patients who died of NPC decreased over survival time. Moreover, non-cancer causes of death increase from 12.94% to 51.22% over time after 10 years of diagnosis. Heart diseases was the most common non-cancer cause of death in NPC patients.
Conclusions: Although NPC remains the leading cause of death after NPC diagnosis, other non-NPC causes of death represent an increased number of death in NPC patients. These findings support the involvement of multidisciplinary care for follow-up strategy in NPC patients.
1 Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial carcinoma characterized by distinct geographical and ethnic distribution. Multiple risk factors contribute to the occurrence of NPC, including Epstein-Barr virus (EBV) infection, genetic predisposition and environmental factors (1, 2). Recently, there have been rapid evolution of the treatments in NPC patients. With the progress of radiotherapy techniques, optimization of chemotherapy strategies and breakthroughs of immune checkpoint inhibitors, the mortality of NPC patients has been substantially reduced (3–5). In long-term NPC survivors, understanding different causes of death is significant to develop individual follow-up strategies.
Previous studies have well described the causes of death from prostate cancer, breast cancer, small cell lung cancer and other cancers (6–10). But few existing studies have focused on the causes of death from NPC, especially for non-cancer reasons (11). Therefore, risk of death can be underestimated, and early intervention can not be carried out timely. In the current study, we endeavored to concentrate on each cause of death during NPC patients survivorship. We provided the analysis based on demographic-related and tumor-related characteristics and compared the risk of death from each cause with that of the general population.
2 Materials and methods
2.1 Data source
This was a retrospective cohort study. Data was collected from the Surveillance, Epidemiology, and End Results (SEER) 17 registries, November 2021 submission (2000–2019) for SMRs, which includes approximately 26.5% of the U.S. population.
2.2 Patients
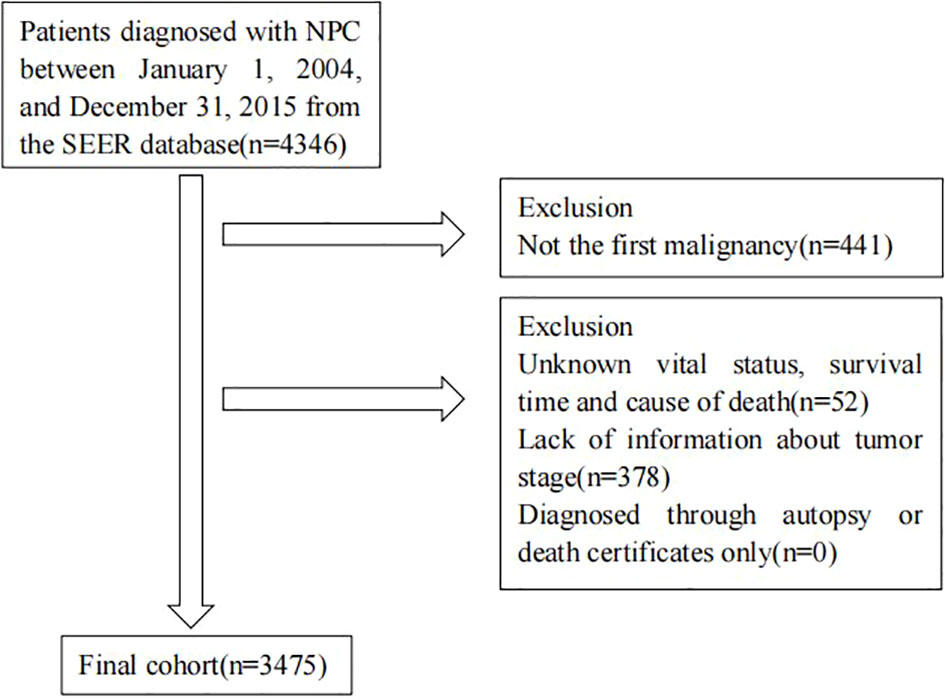
We identified all patients diagnosed with NPC as their first malignancy between 2004 and 2015 from SEER database. Patients diagnosed through autopsy or death certificates only were excluded. We also excluded patients with an unknown vital status, survival time, cause of death or staging information. Figure 1 shows the flowchart of participant selection.
2.3 Study variables
Demographic and clinical information was extracted from SEER database, including sex, age, race, marital status, year of diagnosis, the 6th AJCC stage, histology type and treatment(surgery, radiotherapy or chemotherapy). Causes of death were mainly classified into cancer-related death and non-cancer death.
2.4 Outcome assessments
The number of all deaths after NPC diagnosis was calculated in different variables during all follow-up time and at each follow-up period. The cause of death record in SEER database was based on the International Statistical Classification of Diseases and Related Health Problems 10th Revision.
2.5 Statistical analysis
We computed standardized mortality ratios (SMRs), defined as the ratios of observed number of deaths in included NPC patients to expected number of deaths in the general population. Expected number of deaths was adjusted by age, sex, race and calendar year. We calculated the SMRs with 95% confidence intervals by SEER*Stat (version 8.4.0.1). All statistical tests were two-sided and a p value < 0.05 was considered to be statistically significant.
3 Results
3.1 Baseline characteristics
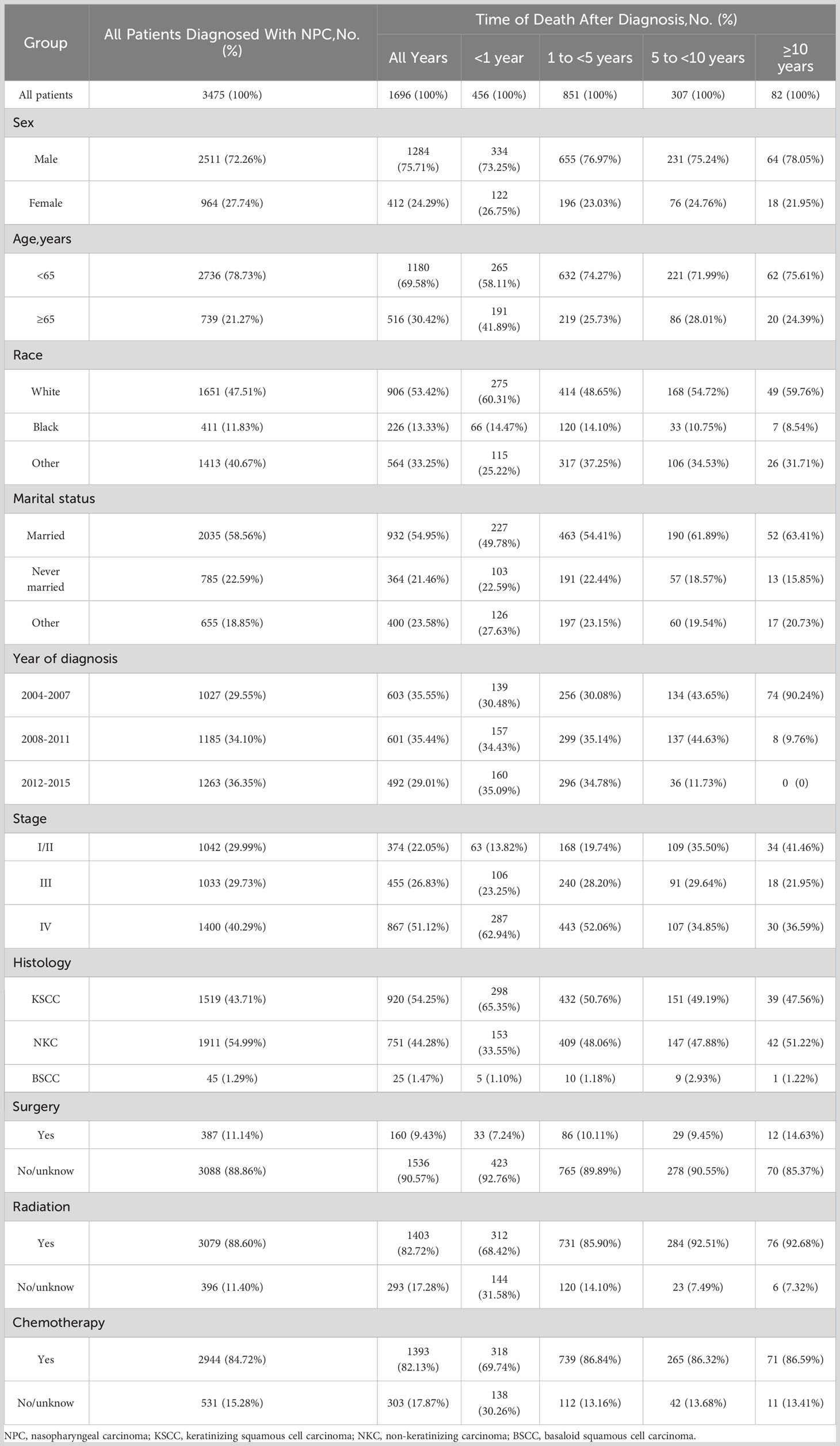
A total of 3475 patients diagnosed with NPC were included in our study. The number of male patients (72.26%) was 2.6 times higher than that of female patients (27.74%). Most patients (78.73%) were < 65 years old. The proportion of stage I-II patients (29.99%) and stage III patients (29.72%) was similar, while stage IV patients (40.29%) outnumbered. The majority of patients had keratinizing squamous cell histology type (43.71%) and non-keratinizing cell histology type (54.99%). Both radiotherapy and chemotherapy were common treatments for NPC patients.
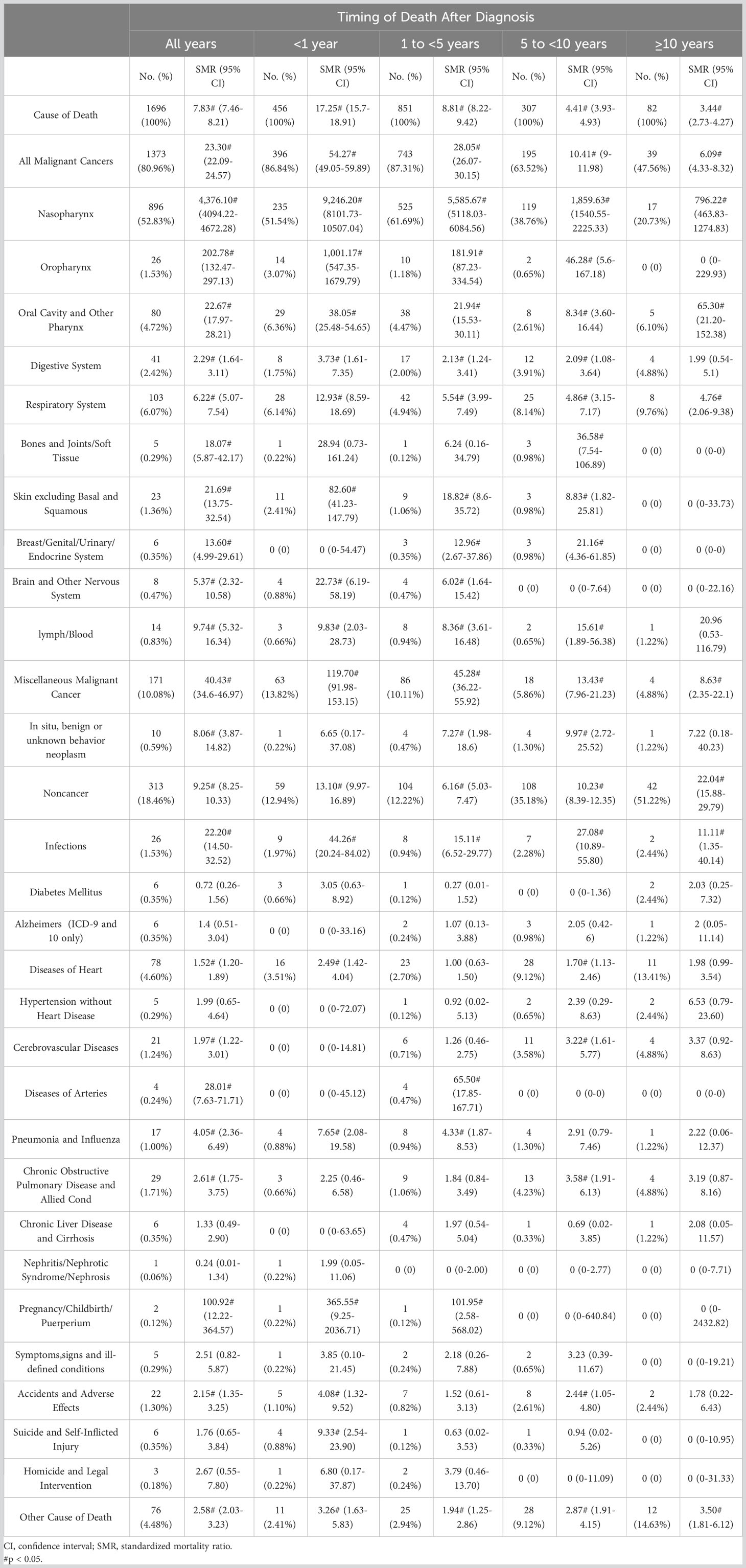
During the follow-up, 1696 (48.81%) patients died. Of total deaths, 26.89% occurred within the first year of diagnosis, 50.18% occurred from 1 to 5 years, 18.10% occurred from 5 to 10 years, and 4.83% individuals survived longer than 10 years. In addition, 52.83% of death were caused by NPC, followed by other cancers (28.13%) and non-cancer causes (18.46%). Besides, NPC patients were at a higher risk dying from most other cancers. As for non-cancer causes, heart disease was the most common one (4.6%).
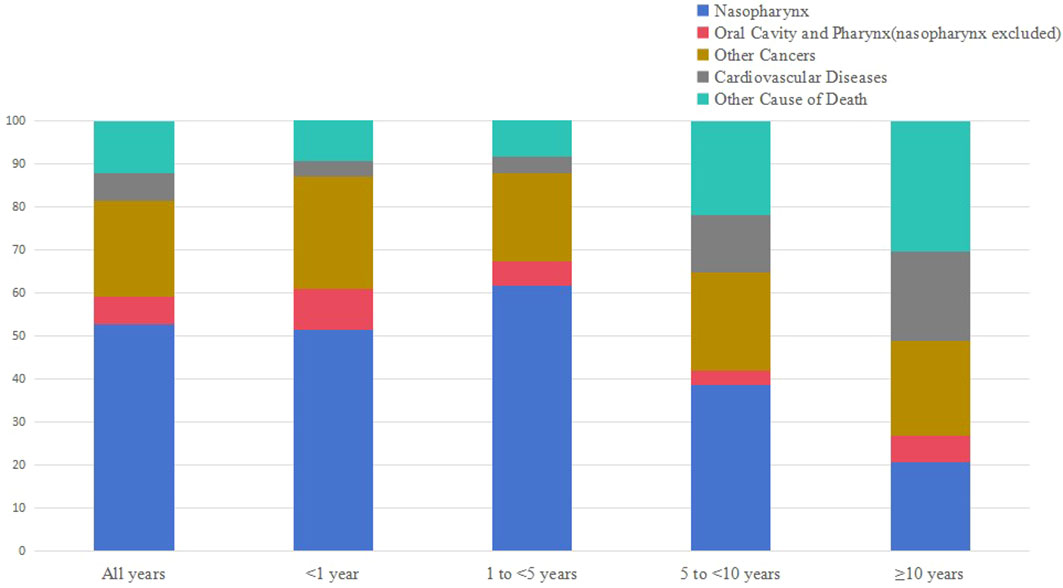
Table 1 shows the baseline characteristics of patients with NPC included in our study. Table 2 shows observed deaths and SMRs for causes of death after diagnosis of NPC. Figure 2 shows causes of death after NPC diagnosis within each follow-up period.
3.2 Cause of death within 1 year following NPC diagnosis
Within 1 year after the diagnosis of NPC, a total of 456 death occurred. 51.54% died of NPC, 35.30% died of other cancers, and 12.94% died of non-cancer causes. Miscellaneous malignant cancer (13.82%) was the most common causes of other cancer deaths, followed by oral cavity and other pharynx cancers (6.36%). For non-cancer causes, the leading causes were heart diseases (3.51%), other cause of death (2.41%), and infections (1.97%), respectively. Besides, the risks of NPC patients dying from pregnancy/childbirth/puerperium (SMR 365.55#; 95%CI 9.25-2036.71), infections (SMR 44.26#; 95%CI 20.24-84.02), suicide and self-inflicted injury (SMR 9.33#; 95%CI 2.54-23.90), and pneumonia and influenza (SMR 7.65#; 95%CI 2.08-19.58) were 5 times higher than what expected in general population.
3.3 Cause of death within 1-5 years following NPC diagnosis
Within 1-5 years following NPC diagnosis, a total of 851 death occurred. NPC was the leading cause of death (61.69%), while other cancers (25.62%) and non-cancer causes (12.22%) accounting for the remaining proportion. Miscellaneous malignant cancer (10.11%) and heart diseases (2.70%) continued to be the most common causes of other cancer deaths and non-cancer cause of death. The SMR elevated to the highest level for pregnancy/childbirth/puerperium (SMR 101.95#; 95%CI 2.58-568.02), diseases of arteries (SMR 65.50#; 95%CI 17.85-167.71), and infections (SMR 15.11#; 95%CI 6.52-29.77) for non-cancer causes.
3.4 Cause of death within 5-10 years following NPC diagnosis
Within 5-10 years following NPC diagnosis, a total of 307 death occurred. 38.76% died of NPC, 24.76% died of other cancers, and 35.18% died of non-cancer causes. The respiratory system cancers (8.14%) turned to be the most common cause of other cancer death. Heart diseases (9.12%) remained to be the leading non-cancer cause of death. The mortality rate of infections was the highest in non-cancer causes of death (SMR 27.08#; 95%CI 10.89-55.80).
3.5 Cause of death after 10 years following NPC diagnosis
After 10 years of survival after NPC diagnosis, 82 patients died. 20.73% died of NPC, 26.83% died of other cancers, and 51.22% died of non-cancer causes. The proportion of respiratory system cancers(9.76%) was the highest one for patients died of other cancer causes. The frequency of NPC patients dying of heart diseases reached 13.41% among patients who survived more than 10 years. Patients with NPC also had a higher risk of infections related death (SMR 11.11#; 95%CI 1.35-40.14).
3.6 Subgroup analysis
Male and female NPC patients had a similar risk of cancer related death and non-cancer causes of death, but female patients (SMR 10434.51#; 95%CI 9150.85-11847.82) had a higher risk of death from NPC than male patients (SMR 3616.59#; 95%CI 3345.50-3903.80) (Tables S1, 2). Patients aged < 65 years old had a higher risk of death than that of patients ≥ 65 years, no matter it was a cancer or non-cancer factor (Tables S3, 4). The risk of all cause death in patients of other races (SMR 10.09#; 95%CI 9.28-10.96) was higher than white(SMR 6.95#; 95%CI 6.51-7.42) and black (SMR 7.45#; 95%CI 6.51-8.48) patients (Tables S5, 7). Compared with patients who are married or in other marital statues, patients who have never been married had a higher risk of death, regardless of cancer or non-cancer causes (Tables S8-10). Patients diagnosed in recent years showed an increase in non-cancer causes of death (Tables S11-13). Patients with stage IV NPC had a higher risk of death from caner or non-cancer causes than patients with I-III NPC (Tables S14-16). The prognosis of patients with nasopharyngeal basaloid squamous cell carcinoma (BSCC) was the worst, while the prognosis of patients with nasopharyngeal non-keratinizing carcinoma (NKC) was the best (Tables S17-19). Patients who underwent surgery (SMR 14.77#; 95%CI 12.27-17.62) had a lower risk of cancer related death than those without surgery (SMR 24.71#; 95%CI 23.36-26.12) (Tables S20-21). All causes of death were lower in patients who received radiotherapy (SMR 7.06#; 95%CI 6.69-7.43) than those who did not (SMR 16.56#; 95%CI 14.72-18.57) (Tables S22-23). There was no significant difference in risk of death among patients given chemotherapy or not (Tables S24, 25).
4 Discussion
The survival time of NPC patients is extended because of improved anti-tumor treatments. Several studies have explored the malignant causes of NPC-related mortality, but information on non-cancer causes of death remains limited in NPC survivors (12, 13). Using population-based data from the united states, our study detailed the causes of death in NPC patients. These results provide vital guidance for the health maintenance of NPC patients.
Our findings shown that the proportion of NPC-related death decreased gradually with the extension of survival time, while death due to non-cancer causes increased. Among NPC patients who survived more than 10 years, the incidence of non-cancer related death reached 51.22%.
The most common non-cancer related cause of death from NPC patients was heart disease during the whole follow-up periods after NPC diagnosis. Previous studies have indicated that cancer patients confronted a higher risk of cardiovascular death throughout their lives (14–16). This may be attributable to the adverse effects from anti-tumor treatments (17). Concurrent chemoradiation therapy is the mainstay treatment for NPC patients, leading to a progressive and dynamic cardiovascular autonomic dysfunction (18). Besides, immune checkpoint inhibitors (ICIs) have greatly improved the survival rate of NPC patients in recent years. Meanwhile, cardiac toxicities caused by ICIs should not be ignored (19, 20). Therefore, early interventions with cardiologists in these patients is suggested to provide individualized comprehensive care.
The frequency of other non-cancer related causes of death changed over time, with cerebrovascular diseases, infectious diseases, COPD and allied conditions becoming more frequent as time passed after NPC diagnosis. Radiotherapy for NPC patients may cause cerebrovascular diseases including transient ischemic attack and ischemic stroke, which can lead to severe disability (21). Besides, intracranial aneurysms are a rare complication of radiotherapy, but irradiated NPC patients had higher morbidity and mortality rates after aneurysm rupture and a higher angiographic recurrence rate after treatment (22). In addition, NPC patients had a higher risk of death from infectious diseases. This may be due to the use of chemotherapy, which is associated with the dysfunction of immune system. In our analysis, COPD was another common non-cancer cause of death. Smoking is a risk factor affecting the occurrence of NPC and related to higher NPC mortality (23–25). Tight association between NPC and COPD may be partly owing to shared risk factor of tobacco use.
Furthermore, in the present study, we found that NPC patients are more likely to develop second primary cancers such as respiratory, digestive and miscellaneous malignant cancers. Previous studies also have shown an increased risk for second primary cancers in NPC patients (26). Immune suppression, shared genetic factors and shared environmental risk factors might account for the associations (27). Besides, second cancer risk after definitive intensity-modulated radiotherapy (IMRT) was substantial in NPC patients (28). So it is advocated that NPC survivors should follow proper screening measures for other cancers.
The anatomical location and high sensitivity of NPC to radiotherapy make it the main treatment modality. Radical radiotherapy is the preferred treatment for early-stage NPC patients and the cornerstone of multidisciplinary treatment for locally advanced NPC patients. Lower risk of all causes of death was also found in NPC patients who received radiotherapy in our study. With the developments of irradiation techniques, the way of radiotherapy in NPC is constantly changing. Compared to conventional radiotherapy, IMRT has improved local control rate and been widespread adopted in clinical practice (29, 30). Besides, previous studies have suggested that IMRT was associated with decreased risk of radiation-induced injury (31, 32). To manage the errors in the positioning of patients or compensate for movements of organs, various methods of image guided radiotherapy (IGRT) are being developed. Meanwhile, intensity-modulated proton therapy (IMPT) has shown excellent locoregional control rate and significantly reduce complications in comparison with IMRT, and prospective studies are warranted (33).
Chemotherapy is associated with multiple adverse events depending on anti-tumor drugs. Cisplatin is commonly used to treat locally advanced and metastasis NPC patients. Cisplatin-based concurrent chemoradiotherapy has been identified as standard treatment for locally advanced NPC patients. But cisplatin-based chemotherapy is known to increase the adverse effects of radiotherapy (34). The main adverse events of cisplatin is nephrotoxicity, which limits the use of cisplatin. Apart from hydration regimens, several possible therapeutic targets preventing nephrotoxicity have been identified (35). Other multiple adverse effects such as gastrointestinal reactions, hematotoxicity, neurotoxicity may also influence the quality of life in second-line or higher treatment settings of NPC patients.
Reirradiation in recurrent NPC patients presents unique challenges with significant treatment-related toxicities, such as subcutaneous necrosis, large-vessel integrity, dysphagia and middle ear dysfunction. Hyperfractionated IMRT could improve overall survival and decrease the risk of severe late complications (36). In addition, with recent progress of endoscopic techniques, endoscopic resection of locally recurrent NPC have been reported in recent years. Local endoscopic resection achieved higher survival rates with fewer adverse events (37, 38). In our study, NPC patients who underwent surgery had a lower risk of cancer related death than those without surgery. We hypothesize that surgery could be recommended for recurrent NPC patients with operable tumors.
Several limitations should be acknowledged in our study. First, the study was retrospective and we tried our best to reduce bias. We designed strict screening criteria to reduce selection bias and used SMRs to control differences in age, sex and race to reduce confounding bias. Second, information about treatments and complications is incomplete in SEER database, which may influence survival durations and death patterns. Finally, NPC is particular prevalent in East and Southeast Asia, but most participants in our study were white. Whether our findings can be expended into other races needs to be further investigated. Despite these limitations, our study provides the most comprehensive assessments of causes of death in NPC patients.
5 Conclusions
In summary, deaths from non-NPC causes account for approximately 1/2 during follow-up after NPC diagnosis. Moreover, as survival time prolonged, the incidence of death from non-NPC causes increased. Heart diseases, infections diseases, COPD and allied conditions were the most common non-cancer causes of death in NPC patients. Therefore, we should not only pay attention to anti-tumor therapy, but also take notice of the occurrence of other risks to achieve better long-term outcomes in NPC patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
JZ: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. ZJ: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. YL: Data curation, Methodology, Writing – original draft, Writing – review & editing. XS: Data curation, Methodology, Writing – original draft, Writing – review & editing. HL: Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors are thankful for all the individuals at the National Cancer Institute(USA) for their contribution to the SEER database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1269118/full#supplementary-material
References
1. Perri F, Sabbatino F, Ottaiano A, Fusco R, Caraglia M, Cascella M, et al. Impact of epstein barr virus infection on treatment opportunities in patients with nasopharyngeal cancer. Cancers (Basel). (2023) 15(5):1626. doi: 10.3390/cancers15051626
2. Jicman Stan D, Niculet E, Lungu M, Onisor C, Rebegea L, Vesa D, et al. Nasopharyngeal carcinoma: A new synthesis of literature data (Review). Exp Ther Med (2022) 23(2):136. doi: 10.3892/etm.2021.11059
3. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet (2016) 387(10022):1012–24. doi: 10.1016/S0140-6736(15)00055-0
4. Chen P, Liu B, Xia X, Huang P, Zhao J. Current progress in immunotherapy of nasopharyngeal carcinoma. Am J Cancer Res (2023) 15(4):1140–7.
5. Ng WT, Chow JCH, Beitler JJ, Corry J, Mendenhall W, Lee AWM, et al. Current radiotherapy considerations for nasopharyngeal carcinoma. Cancers (Basel). (2022) 14(23):5773. doi: 10.3390/cancers14235773
6. Weiner AB, Li EV, Desai AS, Press DJ, Schaeffer EM. Cause of death during prostate cancer survivorship: A contemporary, US population-based analysis. Cancer (2021) 127(16):2895–904. doi: 10.1002/cncr.33584
7. Afifi AM, Saad AM, Al-Husseini MJ, Elmehrath AO, Northfelt DW, Sonbol MB. Causes of death after breast cancer diagnosis: A US population-based analysis. Cancer (2020) 126(7):1559–67. doi: 10.1002/cncr.32648
8. Wu XQ, Li JY, Du WJ. Causes of death following small cell lung cancer diagnosis: a population-based analysis. BMC Pulm Med (2022) 22(1):262. doi: 10.1186/s12890-022-02053-4
9. Zhan X, Chen L, Jiang M, Fu B. Trends in the cause of death among patients with bladder cancer in the US SEER population. 1992-2018 (2022) 40(6):1497–503. doi: 10.1007/s00345-022-03971-y
10. Gao J, Chen Y, Wu P, Wang F, Tao H, Shen Q, et al. Causes of death and effect of non-cancer-specific death on rates of overall survival in adult classic Hodgkin lymphoma: a populated-based competing risk analysis. BMC Cancer. (2021) 21(1):955. doi: 10.1186/s12885-021-08683-x
11. Yang SP, Rao MY, Chen QS, Zhou P, Lian CL, Wu SG. Causes of death in long-term nasopharyngeal carcinoma survivors. Front Public Health (2022) 10:912843. doi: 10.3389/fpubh.2022.912843
12. Long Z, Wang W, Liu W, Wang F, Meng S, Liu J, et al. Trend of nasopharyngeal carcinoma mortality and years of life lost in China and its provinces from 2005 to 2020. Int J Cancer. (2022) 151(5):684–91. doi: 10.1002/ijc.33998
13. Ji MF, Sheng W, Cheng WM, Ng MH, Wu BH, Yu X, et al. Incidence and mortality of nasopharyngeal carcinoma: interim analysis of a cluster randomized controlled screening trial (PRO-NPC-001) in southern China. Ann Oncol (2019) 30(10):1630–7. doi: 10.1093/annonc/mdz231
14. Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J (2019) 40(48):3889–97. doi: 10.1093/eurheartj/ehz766
15. Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol (2019) 280:163–75. doi: 10.1016/j.ijcard.2019.01.038
16. Kobo O, Moledina SM, Raisi-Estabragh Z, Shanmuganathan JWD, Chieffo A, Al Ayoubi F, et al. Emergency department cardiovascular disease encounters and associated mortality in patients with cancer: A study of 20.6 million records from the USA. Int J Cardiol (2022) 363:210–7. doi: 10.1016/j.ijcard.2022.06.053
17. Nonaka M, Hosoda H, Uezono Y. Cancer treatment-related cardiovascular disease: Current status and future research priorities. Biochem Pharmacol (2021) 190:114599. doi: 10.1016/j.bcp.2021.114599
18. Huang CC, Lai YR, Chiu WC, Fang FM, Hsieh DY, Lien CY, et al. Concurrent chemoradiation therapy is associated with an accelerated risk of cardiovascular autonomic dysfunction in patients with nasopharyngeal carcinoma: A 9-year prospective follow-up study. Radiother Oncol (2022) 170:129–35. doi: 10.1016/j.radonc.2022.03.004
19. Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol (2018) 19(9):e447–e58. doi: 10.1016/S1470-2045(18)30457-1
20. Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res (2019) 115(5):854–68. doi: 10.1093/cvr/cvz026
21. Yeh CF, Chin YC, Hung W, Huang PI, Lan MY. Vertebral artery stenosis predicts cerebrovascular diseases following radiotherapy for nasopharyngeal carcinoma. Support Care Cancer. (2022) 30(7):5821–30. doi: 10.1007/s00520-022-07011-8
22. Chan SHV, Woo YMP, Wong KSA, Chan KY, Leung KM. The angiographic and clinical outcomes of intracranial aneurysms following irradiation in patients with nasopharyngeal carcinoma: A 13-year experience and literature review. J Neuroradiol (2018) 45(4):224–9. doi: 10.1016/j.neurad.2018.01.056
23. Chang ET, Ye W, Zeng YX, Adami HO. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev (2021) 30(6):1035–47. doi: 10.1158/1055-9965.EPI-20-1702
24. Long M, Fu Z, Li P, Nie Z. Cigarette smoking and the risk of nasopharyngeal carcinoma: a meta-analysis of epidemiological studies. BMJ Open (2017) 7(10):e016582. doi: 10.1136/bmjopen-2017-016582
25. Lin JH, Jiang CQ, Ho SY, Zhang WS, Mai ZM, Xu L, et al. Smoking and nasopharyngeal carcinoma mortality: a cohort study of 101,823 adults in Guangzhou, China. BMC Cancer. (2015) 15:906. doi: 10.1186/s12885-015-1902-9
26. Chen MC, Feng IJ, Lu CH, Chen CC, Lin JT, Huang SH, et al. The incidence and risk of second primary cancers in patients with nasopharyngeal carcinoma: a population-based study in Taiwan over a 25-year period (1979-2003). Ann Oncol (2008) 19(6):1180–6. doi: 10.1093/annonc/mdn003
27. Scelo G, Boffetta P, Corbex M, Chia KS, Hemminki K, Friis S, et al. Second primary cancers in patients with nasopharyngeal carcinoma: a pooled analysis of 13 cancer registries. Cancer Causes Control. (2007) 18(3):269–78. doi: 10.1007/s10552-006-0101-z
28. Chow JCH, Tam AHP, Cheung KM, Lee VHF, Chiang CL, Tong M, et al. Second primary cancer after intensity-modulated radiotherapy for nasopharyngeal carcinoma: A territory-wide study by HKNPCSG. Oral Oncol (2020) 111:105012. doi: 10.1016/j.oraloncology.2020.105012
29. Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol (2009) 27(22):3684–90. doi: 10.1200/JCO.2008.19.9109
30. Co J, Mejia MB, Dizon JM, Eisele DW. Evidence on effectiveness of intensity-modulated radiotherapy versus 2-dimensional radiotherapy in the treatment of nasopharyngeal carcinoma: Meta-analysis and a systematic review of the literature. Head Neck. (2016) 38(S1):E2130–E42. doi: 10.1002/hed.23977
31. Peng G, Wang T, Yang K-y, Zhang S, Zhang T, Li Q, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol (2012) 104(3):286–93. doi: 10.1016/j.radonc.2012.08.013
32. Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol (2007) 25(31):4873–9. doi: 10.1200/JCO.2007.11.5501
33. Li X, Kitpanit S, Lee A, Mah D, Sine K, Sherman EJ, et al. Toxicity profiles and survival outcomes among patients with nonmetastatic nasopharyngeal carcinoma treated with intensity-modulated proton therapy vs intensity-modulated radiation therapy. JAMA Netw Open (2021) 4(6):e2113205. doi: 10.1001/jamanetworkopen.2021.13205
34. Tang LQ, Chen DP, Guo L, Mo HY, Huang Y, Guo SS, et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II–IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol (2018) 19(4):461–73. doi: 10.1016/S1470-2045(18)30104-9
35. Tang C, Livingston MJ, Safirstein R,ZD. Cisplatin nephrotoxicity: new insights and therapeutic implications. Nat Rev Nephrol. (2023) 19(1):53–72. doi: 10.1038/s41581-022-00631-7
36. You R, Liu YP, Xie YL, Lin C, Duan CY, Chen DP, et al. Hyperfractionation compared with standard fractionation in intensity-modulated radiotherapy for patients with locally advanced recurrent nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet (2023) 401(10380):917–27. doi: 10.1016/S0140-6736(23)00269-6
37. You R, Zou X, Hua YJ, Han F, Li L, Zhao C, et al. Salvage endoscopic nasopharyngectomy is superior to intensity-modulated radiation therapy for local recurrence of selected T1-T3 nasopharyngeal carcinoma – A case-matched comparison. Radiother Oncol (2015) 115(3):399–406. doi: 10.1016/j.radonc.2015.04.024
38. Liu Y-P, Wen Y-H, Tang J, Wei Y, You R, Zhu X-L, et al. Endoscopic surgery compared with intensity-modulated radiotherapy in resectable locally recurrent nasopharyngeal carcinoma: a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol (2021) 22(3):381–90. doi: 10.1016/S1470-2045(20)30673-2
Keywords: nasopharyngeal carcinoma, cause of death, SEER, survival, standardized mortality ratios (SMRs)
Citation: Zhou J, Jiang Z, Li Y, Shao X and Liao H (2023) Cause of death during nasopharyngeal carcinoma survivorship: a population-based analysis. Front. Oncol. 13:1269118. doi: 10.3389/fonc.2023.1269118
Received: 29 July 2023; Accepted: 03 October 2023;
Published: 18 October 2023.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Ciprian Camil Mirestean, University of Medicine and Pharmacy of Craiova, RomaniaYuri Ueda, Tokyo Medical University Hospital, Japan
Copyright © 2023 Zhou, Jiang, Li, Shao and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haihong Liao, haihonglll@126.com
†These authors have contributed equally to this work
 Jie Zhou
Jie Zhou