- 1Department of Gynecology & Obstetrics, Liaocheng People's Hospital, School of Medicine, Liaocheng University, Liaocheng, China
- 2Biomedical Laboratory, School of Medicine, Liaocheng University, Liaocheng, China
- 3Department of Pathology, Liaocheng People's Hospital, Liaocheng, China
Endometrial cancer (EC) is the most common malignant tumor of the female reproductive system, and the majority of ECs are low histological grade and confined to the uterus, resulting in a good prognosis. However, metastasis to the lung from a low-grade and early-stage endometrial endometrioid carcinoma (EEC) is extremely rare. Therefore, it is crucial to accurately differentiate between primary pulmonary malignancy and extra-thoracic malignancy presenting as metastatic disease, and flexible bronchoscopy with tissue acquisition plays a key role in this process. Despite its importance, there is limited literature available on the cytology of metastatic endometrial carcinoma in liquid-based cytology of bronchial brush (BB). In this article, we present two rare cases of lung metastasis from low-grade and early-stage EEC, along with a detailed analysis of the cytologic features observed in BB samples. These cases highlight the significance of cytological and histological pathology, complemented by immunohistochemistry (ICH) analysis, in the diagnosis and management of EEC patients. Pathologists should pay close attention to these aspects, while gynecologists need to be mindful of the follow-up and management of early-stage, low-grade EEC patients. By focusing on these areas, healthcare professionals can effectively contribute to the improved care and outcomes of patients with EEC.
Introduction
Endometrial cancer is the second most common type of gynecologic cancer and the sixth most common cancer in women globally, with 417,000 new diagnoses made in 2020 (1–4), and the incidence of EC is rising (5, 6). The majority of patients are diagnosed with low-grade, uterine-limited disease (7) and have a good prognosis after undergoing surgical treatment to completely remove the lesions (8–10). The reported 5-year survival rate for endometrial adenocarcinoma is over 89%, with stage I tumors having a rate of 94% (11, 12). Furthermore, the 5-year survival rates corresponding to International Federation of Gynecology and Obstetrics (FIGO) grades 1 and 2 stand at 93% and 94%, respectively (13). However, the recurrence rates of early-stage EC range from 2% to 26% in the literature (14), varying widely among histological subtypes, with rates as low as 7% for patients with low-grade endometrioid adenocarcinoma (15). Histological tumor type is an important prognostic predictor in EC (16).
A retrospective study reported that 2.9% of patients with FIGO (2009) grade 1, non-myometrial invasive tumors without lymphovascular space invasion (LVSI) experienced recurrence (17). It is difficult and critical for risk stratification in early-stage, grade 1 EC (18, 19). A small subset of women with low-grade and early-stage EEC may experience recurrence or distant metastasis (20), such as lung and brain metastases, which are rare occurrences (21). However, the incidence of lung metastasis of cancer patients has been increasing due to improvements in therapeutic options (22) and bronchoscopy evaluation is an important tool for the differentiation between primary lung carcinomas and metastases (23). Therefore, it is crucial to accelerate the study of cytological pathology in these cases for pathologists.
In this article, we present two rare cases of stage I and low-grade endometrioid endometrial cancer with pulmonary metastasis. Furthermore, we provide a detailed description of the bronchoscopy brush liquid-based cytological characteristics of lung metastasis from EEC, which has not been reported previously.
Case reports
Case 1
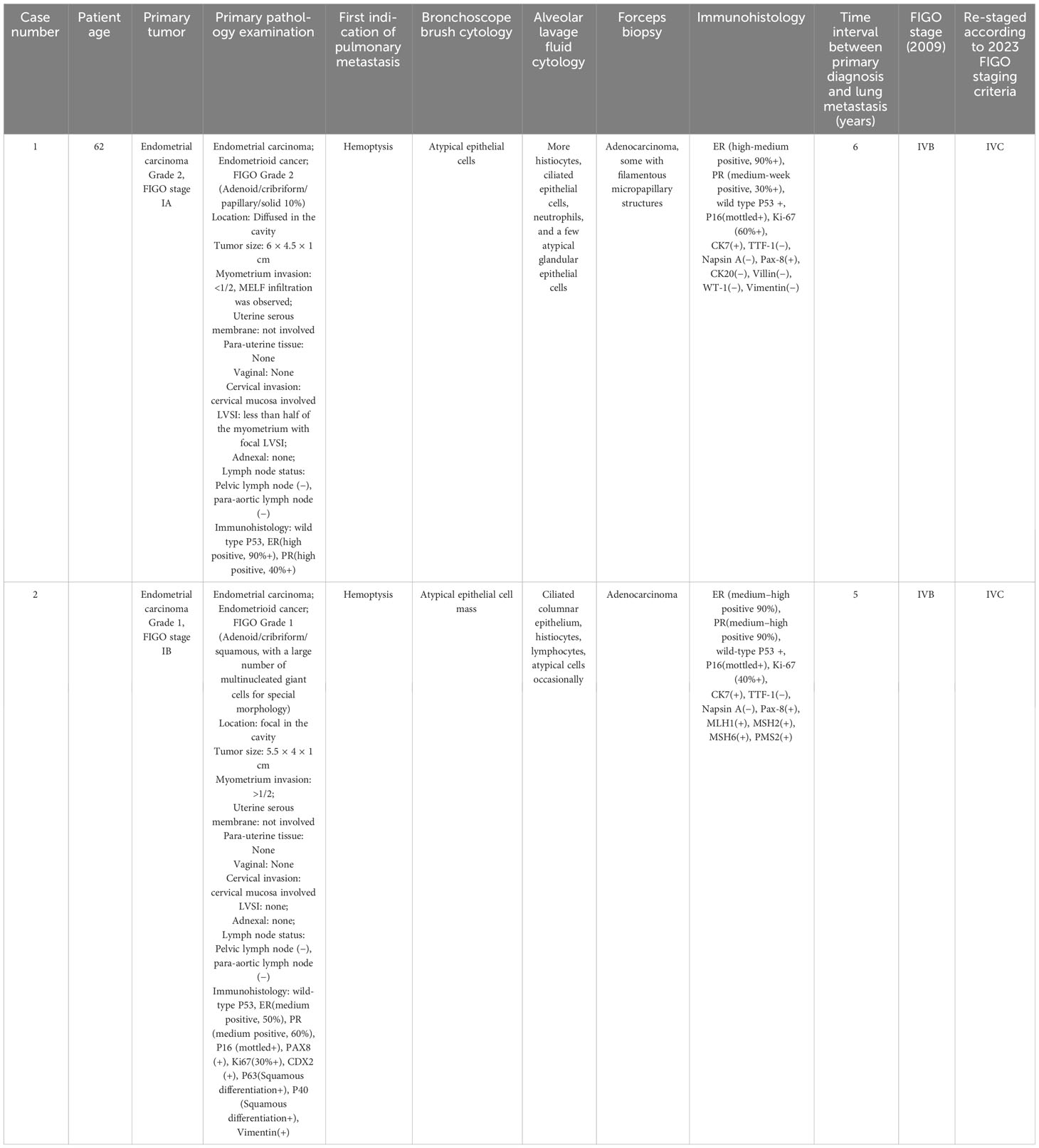
A 70-year-old woman presented with hemoptysis for 6 months and was admitted to our hospital in 2022. Thoracic computed tomography (CT) revealed a mass in the lower lobe of right lung, indicating tumors, as well as multiple small high-density nodules in both lungs.
In 2016, the patient, who had a history of diabetes mellitus (DM) and hypertension, experienced irregular vaginal bleeding for 2 months after menopause. Hysteroscopic biopsy confirmed endometrial cancer, and she subsequently underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy, as well as pelvic and paraaortic lymph node dissection. The diagnosis was endometrioid endometrial carcinoma [FIGO (2009) stage 1A, grade 2]. Adjuvant treatment consisting of six cycles of carboplatin and cyclophosphamide was administered.
Case 2
A 57-year-old woman presented with a persistent cough of unknown cause for nearly 1 year and was admitted to our hospital in 2022. CT scan revealed multiple small high-density nodules in both lungs.
In 2018, the patient with a BMI of 33 kg/m2, was referred to our hospital due to thickened endometrium. Hysteroscopic biopsy confirmed endometrial cancer, and she underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy, as well as pelvic and paraaortic lymph node dissection. The diagnosis was endometrioid endometrial carcinoma [FIGO (2009) stage 1B, grade 1]. Adjuvant treatment included six cycles of carboplatin and cyclophosphamide, as well as pelvic local radiotherapy (DT50Gy/25f).
Both patients underwent flexible bronchoscopy with endobronchial ultrasound (EBUS), which included bronchoalveolar lavage, bronchial brushing, mediastinal lymph node puncture, forceps biopsy, and immunohistochemistry. The results confirmed lung metastasis of endometrial adenocarcinoma.
Pathology
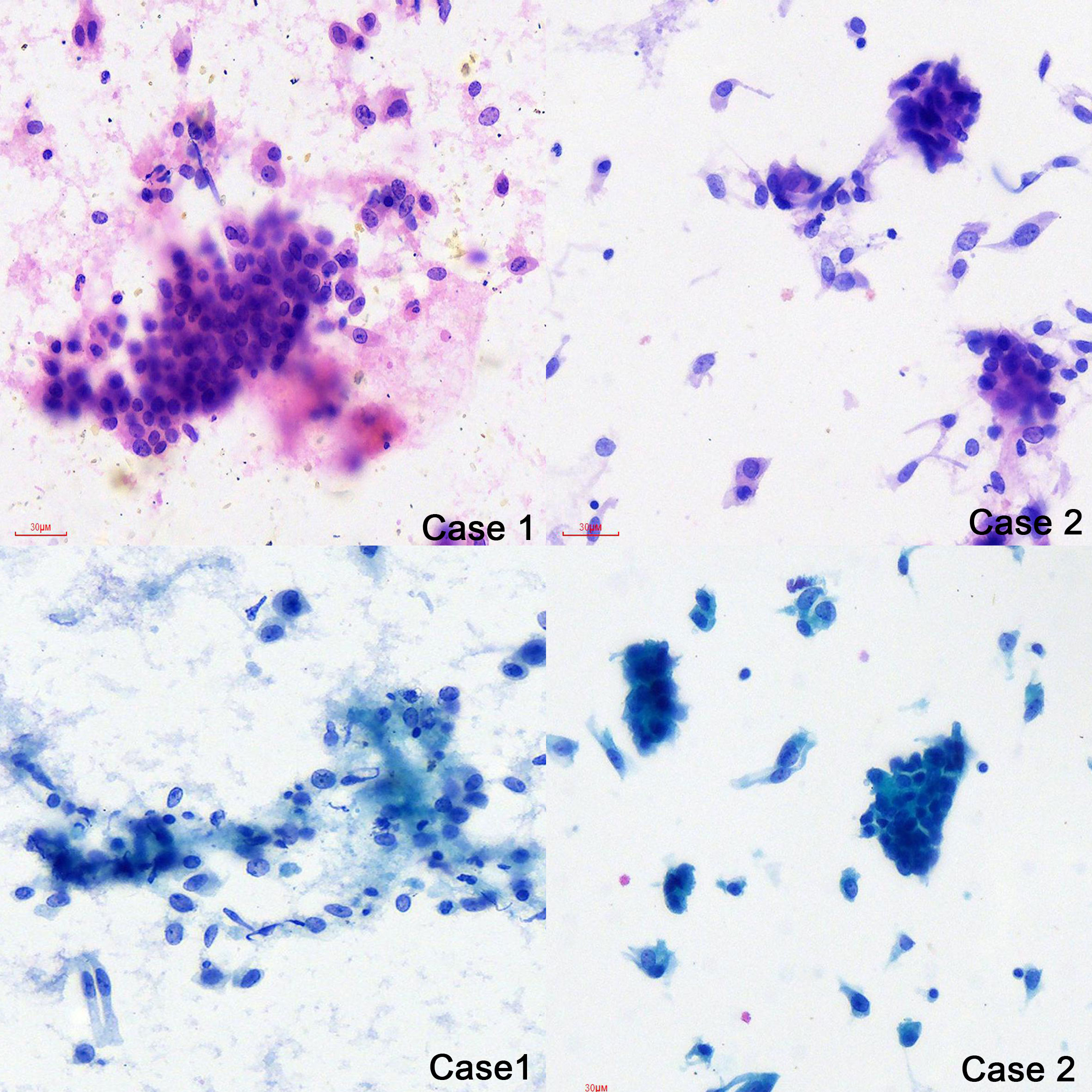
The cytology of bronchial brushing showed cellular morphology that differed from the exfoliated endometrial cancer cells found in cervical fluid-based samples. The morphology was mild, with small atypia, making it difficult to distinguish from bronchial epithelial reactive hyperplasia and carcinoid tumors (Figure 1). Subsequent forceps biopsy pathology revealed adenocarcinoma, with one of the two cases showing papillary configurations. Immunohistochemical staining was positive for estrogen, progesterone, wild-type P53, Pax-8, CK7, Ki-67 (60%+), and mottled positive for P16, while negative for TTF-1 and Napsin A (Figure 2). The expression of the four MMR proteins (MSH2, MLH1, MSH6, and PMS2) was retained in the metastatic tumor tissues, suggesting microsatellite stable carcinoma.
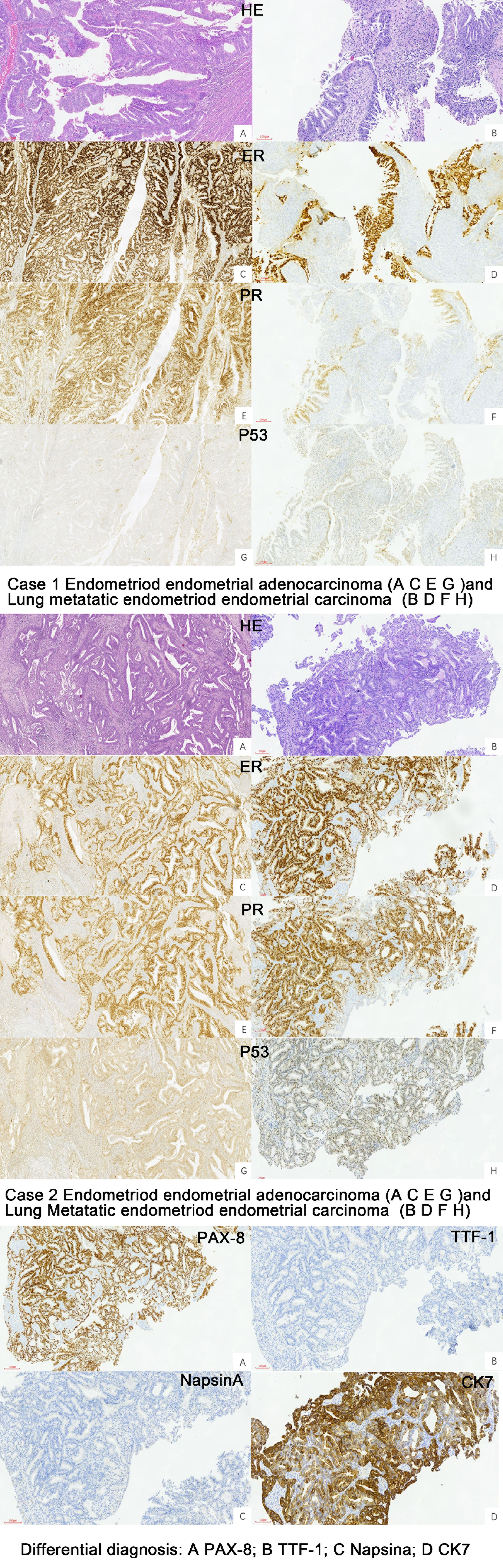
Figure 2 Pathology of the primary EEC and Lung metastatic EEC of the two cases (H&E ×10, IHC stain ×10).
We reviewed the pathology of the primary endometrial malignancy and found that the histological features were consistent with those of the previous primary EC. Therefore, the nodules were considered metastases from the endometrial malignancy rather than primary lung cancer (Table 1).
Treatment and follow-up
Both patients were treated with six cycles of combined paclitaxel and carboplatin chemotherapy. The latter patient also received Bevacizumab as part of her treatment. Regular follow-up examinations revealed a significant decrease in tumor size in the lungs after completing chemotherapy.
Discussion
Prognosis for patients with endometrial adenocarcinoma is generally good. However, disease recurrence, either local or distant, occurs in 7% to 15% of diagnosed patients (15), particularly those with endometrial endometrioid carcinoma (EEC) and those with non-endometrioid histology (24–26). Although 5% to 10% of low-grade EEC patients will experience either local recurrence or distant metastasis (17, 27, 28), the occurrence of distant organ metastasis is still very rare (29) for the stage I with low-grade EEC. In the domain of endometrial carcinoma, the histological classification embodies a seminal prognostic determinant. The two case instances under our scrutiny, which now stand subjected to the nuances of the revised 2023 FIGO staging, find their classification within the ambit of non-aggressive histological phenotypes. This realm encapsulates the realm of low-grade entities, constituting Grades 1 and 2 endometrioid endometrial carcinomas, while the antithetical cadre of aggressive histological counterparts encompasses the expanse of Grade 3 EECs, serous carcinoma (SC), clear cell carcinoma (CCC), mixed carcinoma (MC), undifferentiated carcinoma (UC), carcinosarcoma (CS), and mesonephric-like and gastrointestinal type mucinous carcinomas (16). Molecular features can be used to estimate risk of recurrence and hence survival (30–33). The Cancer Genome Atlas (TCGA) has meticulously categorized endometrial carcinomas into four distinct classifications (16, 34): (1) POLE/Ultramutated: This category is characterized by the presence of somatic inactivating hotspot mutations within the POLE exonuclease domain, resulting in an exceptionally elevated mutational burden. Irrespective of the histological grade, tumors displaying POLE mutations exhibit a remarkably favorable prognosis. (2) Microsatellite Instability-High/Hypermutated: This subset entails endometrioid endometrial carcinomas (EECs) or undifferentiated carcinomas that exhibit a deficiency in mismatch repair (MMRd) leading to microsatellite instability. This category bears an intermediate prognosis. (3) Somatic Copy-Number Alteration High/Serous-Like (SCNA-High): Marked by a low mutation rate, nearly ubiquitous TP53 mutations (95% prevalence), and an exceedingly unfavorable prognosis. While the majority of these malignancies manifest as serous carcinomas, a fraction of up to 25% comprises endometrioid carcinomas (primarily high-grade) and carcinosarcomas. (4) Somatic Copy-Number Alteration Low (SCNA-Low): This class encompasses endometrioid endometrial carcinomas and clear cell carcinomas characterized by scant copy-number alterations and a diminished mutational burden. In this study, we present two cases of low-grade (grades 1 and 2) and early-stage (stage IA and IB) endometrial adenocarcinoma (positive ER/positive PR/wild-type p53) with distant metastasis. If available and feasible, it is recommended to do molecular classification testing (POLEmut, MMRd, non-specific molecular profile [NSMP], and p53 abnormal [p53abn]) in all patients with endometrial cancer to enable the meticulous stratification of patients into discrete prognostic risk groups and to furnish invaluable insights that possess the potential to exert influence over determinations concerning adjuvant and systemic therapeutic strategies (16). The FIGO (2023) staging assign stages I and II based on meticulous surgical–anatomical and histological assessments. Within the realm of molecular classification, the statuses of POLE mutation and p53abn have emerged as significant indicators. p53abn status has been indicative of an unfavorable prognosis; however, in the context of our two cases, it is noteworthy that all instances exhibited wild-type p53 status. The interval from primary diagnosis to metastasis was 6 years and 4 years, respectively. A previous study reported a mean interval time of 4.9 years based on the analysis of 8 endometrial carcinoma patients without subtyping of grade or stage (35).
Pulmonary metastasis is a common occurrence in cases of metastatic carcinoma. In relation to gynecologic tumors, the lungs are reported to be the most frequent site of metastasis from low-grade endometrial stromal sarcoma (36–38), although this is uncommon (35). However, recent reports indicate that lung metastasis is the most common distant organ metastasis in endometrial tumors (39–41), with carcinosarcoma having a significantly higher rate of lung metastasis compared to other histological types, and undifferentiated tumors had the highest rate of lung metastasis when considering tumor grade (29). A study reported that stage IA endometrial adenocarcinoma can exhibit distant metastasis, often spreading hematogenously to the lungs. However, in that study, the metastasis occurred in cases with the papillary serous histological subtype (42). Endometrial mesonephric-like carcinomas (MLCa), constituting an approximate fraction of 1% among endometrial carcinomas (43), have a high incidence of lung metastasis (44); one study has documented that MLCa frequently undergoes recurrence accompanied by distant metastases, with the pulmonary locale predominating (comprising 64% of metastatic cases) (45). MLCa also exhibit a diverse spectrum of morphological presentations, often resulting in their inadvertent under-identification or misclassification as low-grade (grade 1 or 2) endometrioid endometrial carcinomas (46, 47). Immunohistochemistry could help to differentiate the diagnosis, and the MLCa components were characterized by the variable expression of markers supportive of mesonephric differentiation (GATA3, TTF1 and CD10) and lack of hormone receptor (ER and PR) expression, whereas EEC shows the opposite staining pattern (48) when performed. The two patients we reported both had stage I endometrial endometrioid carcinoma (ER and PR positive).
The risk of endometrial cancer increases with age and BMI (34); the first case in our report involved a 70-year-old patient with a history of DM, while the second case involved a 57-year-old patient with a BMI of 33 kg/m2. Evidence from a previous report indicated that DM is a poor prognostic factor in patients with low-grade EEC, specifically those with KRAS mutation (49). BMI and increases with age are the main risks of endometrial cancer; it has the strongest link to obesity among the 20 most common types of tumors. Each 5 kg/m2 increase in BMI is associated with a 54% higher risk of cancer (50, 51). Furthermore, obesity is considered a risk factor for recurrence (52). Obesity creates a proinflammatory environment characterized by high levels of circulating interleukin-6, tumor necrosis factor-α, C-reactive protein, and a relative deficiency of protective immune cell types (53, 54). Obesity also leads to a hyper-estrogenic state due to the peripheral aromatization of adrenal androgens to estrogen by adipose tissue (55). The prevailing theory regarding endometrial carcinogenesis suggests that natural progesterone deficiency contributes to an unopposed estrogen excess driven by obesity in postmenopausal women (56). Therefore, weight management should be included as an essential component of follow-up care for patients with EEC. Optimizing survivorship through weight loss and lifestyle interventions could improve both the survival and quality of life for individuals with endometrial cancer (57).
Imaging techniques play a crucial role in distinguishing between primary and metastatic tumors in the lungs. CT scans are the standard imaging modality for assessing the extent of the disease (58). However, clinical presentation and radiographic findings may exhibit significant overlap between lung metastases and primary lung cancer. To further differentiate these conditions in chest imaging, bronchoscopy is commonly employed as a diagnostic tool for lung cancer (59). Flexible bronchoscopy, which includes procedures like bronchial brushing and forceps biopsy, is particularly useful in evaluating peripheral lung abnormalities. In our report, both patients underwent flexible bronchoscopy with endobronchial ultrasound (EBUS) to obtain accurate diagnoses. This involved techniques such as bronchoalveolar lavage, bronchial brushing, mediastinal lymph node puncture, and forceps biopsy. These procedures allowed for the collection of small histological or cytological samples, enabling tissue biopsies and brushings to contribute to an accurate diagnosis.
Cytological and histological examination, along with immunohistochemistry, are crucial for diagnosing lung metastases. However, there is limited information available regarding the characteristics of liquid-based cytology using bronchial brush samples. In our study, we described two cases involving the cytology of bronchial brushes. The cell morphology observed was distinct from the exfoliated endometrial cancer cells found in cervical fluid-based samples. In these cases, the cellular changes were mild, displaying small atypia, which made it challenging to differentiate them from bronchial epithelial reactive hyperplasia and carcinoid tumors. Following the cytology report, forceps biopsy was performed, and the pathology revealed adenocarcinoma, with one of the cases demonstrating papillary configurations. The utilization of IHC has significantly enhanced the precision of diagnostic categorization in the realm of lung carcinomas, and the immunoprofiles of adenocarcinomas arising from the female genital tract (cervix, endometrium, fallopian tube, and ovary) differ depending on the tumor histotype and primary sites (33). Immunohistochemical analysis in our cases showed positive staining for wild-type p53, positive estrogen receptor (ER), progesterone receptor (PR), Pax-8, CK7, and Ki-67 (60%+). Additionally, there was patchy positive staining for P16 that could help us to distinguish it from SC and EEC, while staining was negative for TTF-1 and Napsin A. The manifestation of wild-type p53 was discerned in our cases, a finding harmoniously aligned with the primary endometrioid endometrial carcinoma immunohistochemical staining. This congruence in p53 status not only bolsters the diagnostic correlation but also plays a pivotal role in guiding the selection of appropriate adjuvant therapeutic modalities. Positive for ER and PR not only helps us to compare the pathology with primary EEC, but also furnishes a decisive means for differentiation from primary lung carcinoma or endometrial MLCa. Pax-8 exhibits a remarkable capacity for recognizing the majority of adenocarcinomas within the female genital tract, a characteristic that starkly contrasts with its applicability to lung adenocarcinomas (60, 61); the positive Pax-8 immunostaining observed in our cases has significantly contributed to the precise elucidation of the diagnosis, firmly establishing the origin of the lung metastasis as stemming from EEC. TTF1 staining is a critical single marker for adenocarcinoma in lung cancer, with Napsin A also showing some diagnostic utility as a secondary marker for adenocarcinoma in lung tumors (62); a combination of TTF1 and Napsin A may yield greater sensitivity for lung adenocarcinoma (63), and the absence of both TTF1 and Napsin A immunoreactivity played a pivotal role in solidifying the diagnosis for the cases. To further validate the findings, we reviewed the pathology results of the primary endometrial malignancy, which exhibited a similar pattern to the lung lesions.
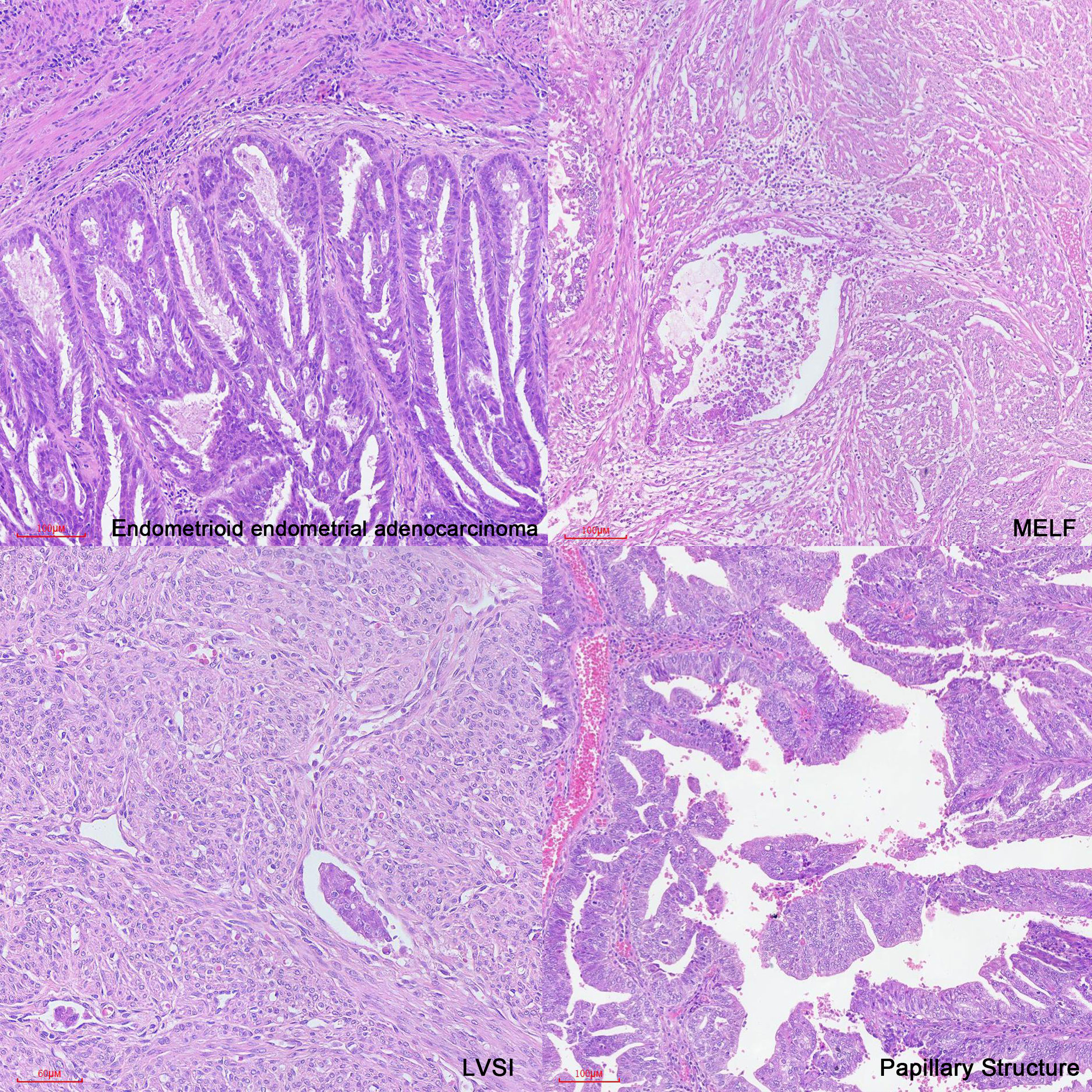
In case 1, the patient had a low prognostic risk (34) as she was stage IA low-grade (grade 2) endometrioid carcinoma and focal LVSI. Classic features associated with distant recurrence included age, stage at presentation, deep myometrial invasion, and LVSI (11). However, we also observed the presence of microcystic, elongated, and fragmented (MELF) pattern of myoinvasion (Figure 3), which is considered a significant feature of recurrence or distant metastasis. MELF patterns are newly described patterns that are typically associated with FIGO grade 1 or 2 endometrioid adenocarcinoma. These patterns could potentially indicate epithelial mesenchymal transition in carcinomas, facilitating infiltration into the surrounding stroma and promoting tumor progression (64, 65). LVSI, characterized by the presence of tumor emboli within lymphatic, capillary, or venous channels (66, 67), is associated with an increased likelihood of metastasis to lymph nodes and other sites (68).
For the patient in case 2, she had intermediate risk as she was stage IB endometrioid carcinoma and low-grade (grade 1) with negative LVSI (34). The immunohistochemistry staining of the primary tumor revealed medium positive expression of ER (50%) and PR (60%). According to a recent study, decreased expression of ER/PR detected by immunohistochemistry can serve as a valuable prognostic biomarker for identifying low-grade EEC that may have distant metastasis (49).
To the best of our knowledge, there have been several reported cases in the current literature of endometrial carcinoma with lung metastases. However, scant literature exists elucidating pulmonary metastases of stage I low-grade (grades 1 and 2) endometrioid endometrial carcinomas, an exceedingly rare phenomenon. In our analysis, we examined the high-risk factors in terms of clinical features and histological pathology, emphasizing the necessity of precise management for early-stage EEC patients, particularly those with a history of DM or obesity. Additionally, we highlighted the importance of paying closer attention to patients exhibiting LVSI, MELF patterns, and a decrease in ER and PR expression.
Conclusion
The occurrence of pulmonary metastases in stage I, low-grade (1 and 2) EEC is extremely rare. Therefore, gynecologists should pay close attention to the management and follow-up of early-stage, low-grade EEC patients. It is crucial for cytological pathologists to recognize the characteristics of bronchoscopy brush liquid-based cytology in cases of lung metastasis from EEC. Early and accurate diagnosis of metastatic EEC is important, as it allows for appropriate treatment to be administered to these patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LW: Writing – review & editing, Project administration, Supervision, Writing – original draft. YL: Writing – review & editing, Methodology. LH: Methodology, Writing – review & editing, Investigation.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
3. Gu B, Shang X, Yan M, Li X, Wang W, Wang Q, et al. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990-2019. Gynecologic Oncol (2021) 161(2):573–80. doi: 10.1016/j.ygyno.2021.01.036
4. Giaquinto AN, Broaddus RR, Jemal A, Siegel RL. The changing landscape of gynecologic cancer mortality in the United States. Obstetrics Gynecology (2022) 139(3):440–2. doi: 10.1097/AOG.0000000000004676
5. Lu KH, Broaddus RR. Endometrial cancer. New Engl J Med (2020) 383(21):2053–64. doi: 10.1056/NEJMra1514010
6. Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, et al. Current recommendations and recent progress in endometrial cancer. CA: Cancer J Clin (2019) 69(4):258–79. doi: 10.3322/caac.21561
7. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
8. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol Off J Am Soc Clin Oncol (2009) 27(32):5331–6. doi: 10.1200/JCO.2009.22.3248
9. Mourits MJ, Bijen CB, Arts HJ, ter Brugge HG, van der Sijde R, Paulsen L, et al. Safety of laparoscopy versus laparotomy in early-stage endometrial cancer: a randomised trial. Lancet Oncol (2010) 11(8):763–71. doi: 10.1016/S1470-2045(10)70143-1
10. Backes FJ, Brudie LA, Farrell MR, Ahmad S, Finkler NJ, Bigsby GE, et al. Short- and long-term morbidity and outcomes after robotic surgery for comprehensive endometrial cancer staging. Gynecologic Oncol (2012) 125(3):546–51. doi: 10.1016/j.ygyno.2012.02.023
11. Kilgore JE, Jackson AL, Ko EM, Soper JT, Van Le L, Gehrig PA, et al. Recurrence-free and 5-year survival following robotic-assisted surgical staging for endometrial carcinoma. Gynecologic Oncol (2013) 129(1):49–53. doi: 10.1016/j.ygyno.2012.12.020
12. Lewin SN, Herzog TJ, Barrena Medel NI, Deutsch I, Burke WM, Sun X, et al. Comparative performance of the 2009 international Federation of gynecology and obstetrics' staging system for uterine corpus cancer. Obstetrics Gynecology (2010) 116(5):1141–9. doi: 10.1097/AOG.0b013e3181f39849
13. Roma AA, Rybicki LA, Barbuto D, Euscher E, Djordjevic B, Frauenhoffer E, et al. Risk factor analysis of recurrence in low-grade endometrial adenocarcinoma. Hum Pathol (2015) 46(10):1529–39. doi: 10.1016/j.humpath.2015.06.015
14. Fujimoto T, Nanjyo H, Fukuda J, Nakamura A, Mizunuma H, Yaegashi N, et al. Endometrioid uterine cancer: histopathological risk factors of local and distant recurrence. Gynecologic Oncol (2009) 112(2):342–7. doi: 10.1016/j.ygyno.2008.10.019
15. Esselen KM, Boruta DM, del Carmen M, Schorge JO, Goodman A, Growdon WB. Defining prognostic variables in recurrent endometrioid endometrial cancer: a 15-year single-institution review. Int J Gynecological Cancer Off J Int Gynecological Cancer Soc (2011) 21(6):1078–83. doi: 10.1097/IGC.0b013e31821872f4
16. Berek JS, Matias-Guiu X, Creutzberg C, Fotopoulou C, Gaffney D, Kehoe S, et al. FIGO staging of endometrial cancer: 2023. Int J gynaecology obstetrics: Off Organ Int Fed Gynaecology Obstetrics (2023) 162(2):383–94. doi: 10.1002/ijgo.14923
17. Stasenko M, Feit N, Lee SSK, Shepherd C, Soslow RA, Cadoo KA, et al. Clinical patterns and genomic profiling of recurrent 'ultra-low risk' endometrial cancer. Int J Gynecological Cancer (2020) 30(6):717–23. doi: 10.1136/ijgc-2020-001241
18. Stelloo E, Nout RA, Osse EM, Jürgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res (2016) 22(16):4215–24. doi: 10.1158/1078-0432.CCR-15-2878
19. Veneris JT, Lee EK, Goebel EA, Nucci MR, Lindeman N, Horowitz NS, et al. Diagnosis and management of a recurrent polymerase-epsilon (POLE)-mutated endometrial cancer. Gynecologic Oncol (2019) 153(3):471–8. doi: 10.1016/j.ygyno.2019.03.247
20. Zhong L, Jiang W, RutieYin, Liu H, Song L. CTNNB1 p.D32A (c.95A > C) somatic mutation in stage I grade 1 endometrioid endometrial carcinoma with lung metastasis: a case report. BMC Med Genomics (2023) 16(1):137. doi: 10.1186/s12920-023-01570-3
21. Gao C, Wang Y, Broaddus R, Sun L, Xue F, Zhang W. Exon 3 mutations of CTNNB1 drive tumorigenesis: a review. Oncotarget (2018) 9(4):5492–508. doi: 10.18632/oncotarget.23695
22. Gerull WD, Puri V, Kozower BD. The epidemiology and biology of pulmonary metastases. J Thorac Dis (2021) 13(4):2585–9. doi: 10.21037/jtd.2020.04.28
23. Mondoni M, Rinaldo RF, Carlucci P, Terraneo S, Saderi L, Centanni S, et al. Bronchoscopic sampling techniques in the era of technological bronchoscopy. Pulmonology (2022) 28(6):461–71. doi: 10.1016/j.pulmoe.2020.06.007
24. Sasada S, Yunokawa M, Takehara Y, Ishikawa M, Ikeda S, Kato T, et al. Baseline risk of recurrence in stage I-II endometrial carcinoma. J Gynecologic Oncol (2018) 29(1):e9. doi: 10.3802/jgo.2018.29.e9
25. Francis SR, Ager BJ, Do OA, Huang YJ, Soisson AP, Dodson MK, et al. Recurrent early stage endometrial cancer: Patterns of recurrence and results of salvage therapy. Gynecologic Oncol (2019) 154(1):38–44. doi: 10.1016/j.ygyno.2019.04.676
26. Takahashi A, Matsuura M, Matoda M, Nomura H, Okamoto S, Kanao H, et al. Clinicopathological features of early and late recurrence of endometrial carcinoma after surgical resection. Int J Gynecological Cancer (2017) 27(5):967–72. doi: 10.1097/IGC.0000000000000984
27. Iavazzo C, Gkegkes ID, Vrachnis N. Early recurrence of early stage endometrioid endometrial carcinoma: possible etiologic pathways and management options. Maturitas (2014) 78(3):155–9. doi: 10.1016/j.maturitas.2014.04.009
28. Laban M, El-Swaify ST, Ali SH, Refaat MA, Sabbour M, Farrag N, et al. The prediction of recurrence in low-risk endometrial cancer: is it time for a paradigm shift in adjuvant therapy? Reprod Sci (Thousand Oaks Calif) (2022) 29(4):1068–85. doi: 10.1007/s43032-021-00565-8
29. Mao W, Wei S, Yang H, Yu Q, Xu M, Guo J, et al. Clinicopathological study of organ metastasis in endometrial cancer. Future Oncol (London England) (2020) 16(10):525–40. doi: 10.2217/fon-2020-0017
30. León-Castillo A, de Boer SM, Powell ME, Mileshkin LR, Mackay HJ, Leary A, et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy. J Clin Oncol (2020) 38(29):3388–97. doi: 10.1200/JCO.20.00549
31. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature (2013) 497(7447):67–73. doi: 10.1038/nature12113
32. Piulats JM, Guerra E, Gil-Martín M, Roman-Canal B, Gatius S, Sanz-Pamplona R, et al. Molecular approaches for classifying endometrial carcinoma. Gynecologic Oncol (2017) 145(1):200–7. doi: 10.1016/j.ygyno.2016.12.015
33. Talhouk A, McConechy MK, Leung S, Li-Chang HH, Kwon JS, Melnyk N, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer (2015) 113(2):299–310. doi: 10.1038/bjc.2015.190
34. Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet (London England) (2022) 399(10333):1412–28. doi: 10.1016/S0140-6736(22)00323-3
35. Vella JE, Ganesan R, Hirschowitz L. Review of lung and pleural biopsies received in a gynecologic pathology department over a 14-yr period. Int J Gynecological Pathol (2017) 36(2):154–64. doi: 10.1097/PGP.0000000000000296
36. Binesh F, Zahir ST, Akhavan A, Bovanlu TR. Endometrial stromal sarcoma of the uterus presenting as pulmonary metastasis. BMJ Case Rep (2013) 2013:bcr2013008565. doi: 10.1136/bcr-2013-008565
37. Kang DO, Choi SI, Oh JY, Sim JK, Choi JH, Choo JY, et al. Endometrial stromal sarcoma presented as an incidental lung mass with multiple pulmonary nodules. Tuberculosis Respir Dis (2014) 76(3):131–5. doi: 10.4046/trd.2014.76.3.131
38. Takizawa M, Tanaka N, Tsunezuka Y, Katayanagi K, Kurumaya H. [Solitary pulmonary metastasis of low-grade uterine endometrial stromal sarcoma resected 31 years before]. Kyobu geka Japanese J Thorac Surg (2014) 67(4):333–6.
39. Weinberger V, Bednarikova M, Hausnerova J, Ovesna P, Vinklerova P, Minar L, et al. A novel approach to preoperative risk stratification in endometrial cancer: the added value of immunohistochemical markers. Front Oncol (2019) 9:265. doi: 10.3389/fonc.2019.00265
40. van Weelden WJ, Reijnen C, Küsters-Vandevelde HVN, Bulten J, Bult P, Leung S, et al. The cutoff for estrogen and progesterone receptor expression in endometrial cancer revisited: a European Network for Individualized Treatment of Endometrial Cancer collaboration study. Hum Pathol (2021) 109:80–91. doi: 10.1016/j.humpath.2020.12.003
41. Köbel M, Ronnett BM, Singh N, Soslow RA, Gilks CB, McCluggage WG. Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecological Pathol (2019) 38 Suppl 1(Iss 1 Suppl 1):S123–s31. doi: 10.1097/PGP.0000000000000488
42. Khaja M, Yapor L, Haider A, Anwar MY, Ronderos DM, Shin D. A case of Malignant pleural effusion secondary to endometrial cancer after one year of hysterectomy. Cureus (2022) 14(9):e28907. doi: 10.7759/cureus.28907
43. Kolin DL, Costigan DC, Dong F, Nucci MR, Howitt BE. A combined morphologic and molecular approach to retrospectively identify KRAS-mutated mesonephric-like adenocarcinomas of the endometrium. Am J Surg Pathol (2019) 43(3):389–98. doi: 10.1097/PAS.0000000000001193
44. Hardy NL, Staats PN. Metastatic mesonephric-like endometrial adenocarcinoma diagnosed on transbronchial needle aspirate cytology. Diagn Cytopathology (2022) 50(2):86–90. doi: 10.1002/dc.24917
45. Pors J, Segura S, Chiu DS, Almadani N, Ren H, Fix DJ, et al. Clinicopathologic characteristics of mesonephric adenocarcinomas and mesonephric-like adenocarcinomas in the gynecologic tract: a multi-institutional study. Am J Surg Pathol (2021) 45(4):498–506. doi: 10.1097/PAS.0000000000001612
46. Clement PB, Young RH, Keh P, Ostör AG, Scully RE. Malignant mesonephric neoplasms of the uterine cervix. A report of eight cases, including four with a Malignant spindle cell component. Am J Surg Pathol (1995) 19(10):1158–71. doi: 10.1097/00000478-199510000-00006
47. Na K, Kim HS. Clinicopathologic and molecular characteristics of mesonephric adenocarcinoma arising from the uterine body. Am J Surg Pathol (2019) 43(1):12–25. doi: 10.1097/PAS.0000000000000991
48. Pors J, Cheng A, Leo JM, Kinloch MA, Gilks B, Hoang L. A comparison of GATA3, TTF1, CD10, and calretinin in identifying mesonephric and mesonephric-like carcinomas of the gynecologic tract. Am J Surg Pathol (2018) 42(12):1596–606. doi: 10.1097/PAS.0000000000001142
49. Chibbar R, Foerstner S, Suresh J, Chibbar R, Piche A, Kundapur D, et al. Estrogen/progesterone receptor loss, CTNNB1 and KRAS mutations are associated with local recurrence or distant metastasis in low-grade endometrial endometrioid carcinoma. Appl immunohistochemistry Mol Morphology AIMM (2023) 31(3):181–8. doi: 10.1097/PAI.0000000000001102
50. Aune D, Navarro Rosenblatt DA, Chan DS, Vingeliene S, Abar L, Vieira AR, et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Ann Oncol (2015) 26(8):1635–48. doi: 10.1093/annonc/mdv142
51. Renehan AG, Soerjomataram I, Tyson M, Egger M, Zwahlen M, Coebergh JW, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer (2010) 126(3):692–702. doi: 10.1002/ijc.24803
52. Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer epidemiology Biomarkers Prev Publ Am Assoc Cancer Research cosponsored by Am Soc Prev Oncol (2010) 19(12):3119–30. doi: 10.1158/1055-9965.EPI-10-0832
53. Dashti SG, Chau R, Ouakrim DA, Buchanan DD, Clendenning M, Young JP, et al. Female hormonal factors and the risk of endometrial cancer in lynch syndrome. Jama (2015) 314(1):61–71. doi: 10.1001/jama.2015.6789
54. Naqvi A, MacKintosh ML, Derbyshire AE, Tsakiroglou AM, Walker TDJ, McVey RJ, et al. The impact of obesity and bariatric surgery on the immune microenvironment of the endometrium. Int J Obes (2005) (2022) 46(3):605–12. doi: 10.1038/s41366-021-01027-6
55. Agnew HJ, Kitson SJ, Crosbie EJ. Gynecological Malignancies and Obesity. Best Pract Res Clin Obstetrics Gynaecology (2023) 88:102337. doi: 10.1016/j.bpobgyn.2023.102337
56. Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiology Biomarkers Prev Publ Am Assoc Cancer Research cosponsored by Am Soc Prev Oncol (2002) 11(12):1531–43.
57. Agnew H, Kitson S, Crosbie EJ. Interventions for weight reduction in obesity to improve survival in women with endometrial cancer. Cochrane Database systematic Rev (2023) 3(3):Cd012513. doi: 10.1002/14651858.CD012513.pub3
58. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford Engl 1990) (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
59. Sakr L, Roll P, Payan MJ, Liprandi A, Dutau H, Astoul P, et al. Cytology-based treatment decision in primary lung cancer: is it accurate enough? Lung Cancer (Amsterdam Netherlands) (2012) 75(3):293–9. doi: 10.1016/j.lungcan.2011.09.001
60. Toriyama A, Mori T, Sekine S, Yoshida A, Hino O, Tsuta K. Utility of PAX8 mouse monoclonal antibody in the diagnosis of thyroid, thymic, pleural and lung tumours: a comparison with polyclonal PAX8 antibody. Histopathology (2014) 65(4):465–72. doi: 10.1111/his.12405
61. Laury AR, Perets R, Piao H, Krane JF, Barletta JA, French C, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol (2011) 35(6):816–26. doi: 10.1097/PAS.0b013e318216c112
62. Yatabe Y, Dacic S, Borczuk AC, Warth A, Russell PA, Lantuejoul S, et al. Best practices recommendations for diagnostic immunohistochemistry in lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2019) 14(3):377–407. doi: 10.1016/j.jtho.2018.12.005
63. Tran L, Mattsson JS, Nodin B, Jönsson P, Planck M, Jirström K, et al. Various Antibody Clones of Napsin A, Thyroid Transcription Factor 1, and p40 and Comparisons With Cytokeratin 5 and p63 in Histopathologic Diagnostics of Non-Small Cell Lung Carcinoma. Appl immunohistochemistry Mol Morphology AIMM. (2016) 24(9):648–59. doi: 10.1097/PAI.0000000000000235
64. Stewart CJ, Brennan BA, Leung YC, Little L. MELF pattern invasion in endometrial carcinoma: association with low grade, myoinvasive endometrioid tumours, focal mucinous differentiation and vascular invasion. Pathology (2009) 41(5):454–9. doi: 10.1080/00313020903041135
65. Stewart CJ, Little L. Immunophenotypic features of MELF pattern invasion in endometrial adenocarcinoma: evidence for epithelial-mesenchymal transition. Histopathology (2009) 55(1):91–101. doi: 10.1111/j.1365-2559.2009.03327.x
66. Singh N, Hirschowitz L, Zaino R, Alvarado-Cabrero I, Duggan MA, Ali-Fehmi R, et al. Pathologic prognostic factors in endometrial carcinoma (Other than tumor type and grade). Int J Gynecological Pathol (2019) 38 Suppl 1(Iss 1 Suppl 1):S93–s113. doi: 10.1097/PGP.0000000000000524
67. Concin N, Creutzberg CL, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP Guidelines for the management of patients with endometrial carcinoma. Virchows Archiv an Int J Pathol (2021) 478(2):153–90. doi: 10.1007/s00428-020-03007-z
68. Bosse T, Peters EE, Creutzberg CL, Jürgenliemk-Schulz IM, Jobsen JJ, Mens JW, et al. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer–A pooled analysis of PORTEC 1 and 2 trials. Eur J Cancer (Oxford Engl 1990) (2015) 51(13):1742–50. doi: 10.1016/j.ejca.2015.05.015
Keywords: pulmonary metastasis, endometrioid carcinoma, early-stage, low-grade, bronchoscope brush liquid-based cytology
Citation: Wang L, Li Y and Han L (2023) Pulmonary metastasis of stage I, low-grade endometrioid carcinoma: two case reports and the literature review. Front. Oncol. 13:1266485. doi: 10.3389/fonc.2023.1266485
Received: 25 July 2023; Accepted: 20 September 2023;
Published: 12 October 2023.
Edited by:
Alberto Farolfi, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), ItalyReviewed by:
Giuseppe Angelico, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), ItalyMatteo Pavone, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), Italy
Komsun Suwannarurk, Thammasat University, Thailand
Copyright © 2023 Wang, Li and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Han, bGluaGFuMjAyM0AxMjYuY29t
 Li Wang
Li Wang Yingxue Li3
Yingxue Li3 Lin Han
Lin Han