- Department of Medical Oncology, Affiliated Hospital of Hebei University, Baoding, China
Primary colorectal squamous cell carcinoma (CSCC) is a rare pathological subtype. Currently, clinical data with regards to its prognosis and treatment is limited, and there is no optimal treatment method. The case presented involves a proficient mismatch repair (pMMR) and microsatellite-stable (MSS) Colorectal cancer (CRC) patient with squamous cell carcinoma (SCC) located transversely in the colon. Based on the imaging assessment, the tumor infiltration depth is classified as T4. After receiving 4 cycles of neoadjuvant treatment with oxaliplatin and capecitabine (XELOX), the patients were evaluated for partial response (PR) in 2 cycles and stable disease (SD) in 4 cycles. The patient underwent a right hemicolectomy and received postoperative paclitaxel/cisplatin (TC) adjuvant chemotherapy. After 23 months, a systemic examination revealed abdominal metastasis. A needle biopsy was conducted on the detected abdominal metastases, with the resulting pathology indicating the presence of metastatic SCC. The individual exhibited expression of programmed cell death ligand 1 (PD-L1) and a mutation in the TP53 gene. Considering the patient’s disease recurrence based on medical history, a treatment plan was formulated. This involved Sintilimab plus Cetuximab and the combination of leucovorin, fluorouracil, and irinotecan (FOLFIRI) regimen. The patient received four cycles of treatment with an efficacy evaluation of SD- and seven cycles of treatment with an efficacy evaluation of SD+, which resulted in a progression-free survival (PFS) duration of 7 months. This case study presents the conventional XELOX chemotherapy protocol, which has shown limited effectiveness, and highlights the favorable results achieved by implementing the TC adjuvant chemotherapy regimen in individuals diagnosed with primary colonic SCC. Furthermore, combining immune checkpoint blockade (ICB) with other therapies for patients with advanced disease is anticipated to provide an extended duration of survival.
1 Introduction
Primary CSCC is an infrequent form of tumor, representing a mere 0.01-0.025% of the total cases of colorectal cancer (1). The mean age of the patients was 63.5 ± 15.3, with no significant difference in incidence between men and women (2). The most common site of occurrence was the rectum, followed by the right colon (3). The majority of cases were found to be complicated by lymph node and liver metastases (4). The patient’s clinical presentation resembled that of colorectal adenocarcinoma, with nearly a half of patients displaying symptoms of gastrointestinal bleeding or abdominal distress (3, 5). The initial diagnosis is mostly advanced, with a poor prognosis (6). Usually, patients with metastasis in distant organs have a median survival rate of about 8 months (7). The five-year relative survival rate is notably inferior compared to that of colorectal adenocarcinoma (2).
Patients with primary rectal SCC are mainly treated with a combination of surgical intervention and radio chemotherapy (4, 7). Significantly, primary rectal SCC at stage II manifests a high sensitivity to chemoradiotherapy, and the administration of neoadjuvant chemoradiotherapy in patients prior to surgery has demonstrated a positive correlation with improved survival rates (8). Patients diagnosed with stage III or IV rectal SCC are typically treated through a combination of radiotherapy and chemotherapy; adding surgical interventions concurrently does not improve the overall survival (OS) of these patients (1). However, for patients experiencing recurrence or an ineffective response to radiotherapy and chemotherapy, the option of surgical intervention is available (9).
Divergent from rectal SCC, the most important treatment for patients with primary colonic SCC is surgery, and the efficacy of chemotherapy or radiotherapy is still unclear (10). Surgical intervention is the primary therapeutic modality employed for patients diagnosed with colonic SCC in Stage II (11). In cases of stage III colonic SCC, the treatment regimen involves a combination of surgical intervention and chemotherapy. In general, patients diagnosed with this condition commonly receive fluorouracil with or without cisplatin adjuvant chemotherapy (12, 13). Palliative treatment is the main approach for patients with metastatic primary colonic SCC. Hence, it is imperative to engage in further discourse regarding the management of patients diagnosed with colonic SCC.
2 Case presentation
The 41-year-old female patient presented to the clinic with abdominal pain and was diagnosed with colon cancer on January 16, 2020 (Figure 1A). Colonoscopy, the pathological results of the biopsy, and immunohistochemistry (IHC) indicated poorly differentiated SCC located in the transverse colon. The patient received a comprehensive examination that ruled out the presence of distant metastases and primary tumors. The imaging assessment results indicate that the tumor infiltration depth was classified as T4, suggesting local progression of the disease. As a result, neoadjuvant treatment was administered. The XELOX regimen was utilized for therapy, which was then followed by 2 cycles of treatment with a PR efficacy evaluation (Figure 1B). Subsequently, 4 cycles of treatment were given with an efficacy evaluation of SD (Figure 1C). The MDT (Multidisciplinary Team) that deliberated on the case after the neoadjuvant treatment concluded that surgical resection of the neoplasia had become feasible. The patient underwent a right hemicolectomy procedure on May 22, 2020. The postoperative pathological examination yielded a poorly differentiated SCC located in the colon (Figure 2A). The tumor’s largest diameter measured 6.5cm. The tumor infiltrated the muscular layer and reached the subserosal fibrous adipose tissue. The malignancy was visible at the circumferential cutting edge, and no clear vascular tumor thrombus or nerve infiltration was found, which was in line with the chemotherapy response (AJCC/TRG grade 3). No metastatic cancer was found in lymph nodes (0/43). The IHC indicated BRAFV600E (-), PMS 2 (+), MLH 1 (+), MSH 2 (+), MSH 6 (+), CD56 (-), Syn (focal weak +), CgA (+), CK20 (partial +), CDX 2 (+), P40 (+), P63 (+), and Ki-67 (70%). Between June 25th, 2020, and November 30th, 2020, the patient commenced treatment with the TC adjuvant chemotherapy protocol. The patients were then regularly monitored, and the medical condition remained stable.
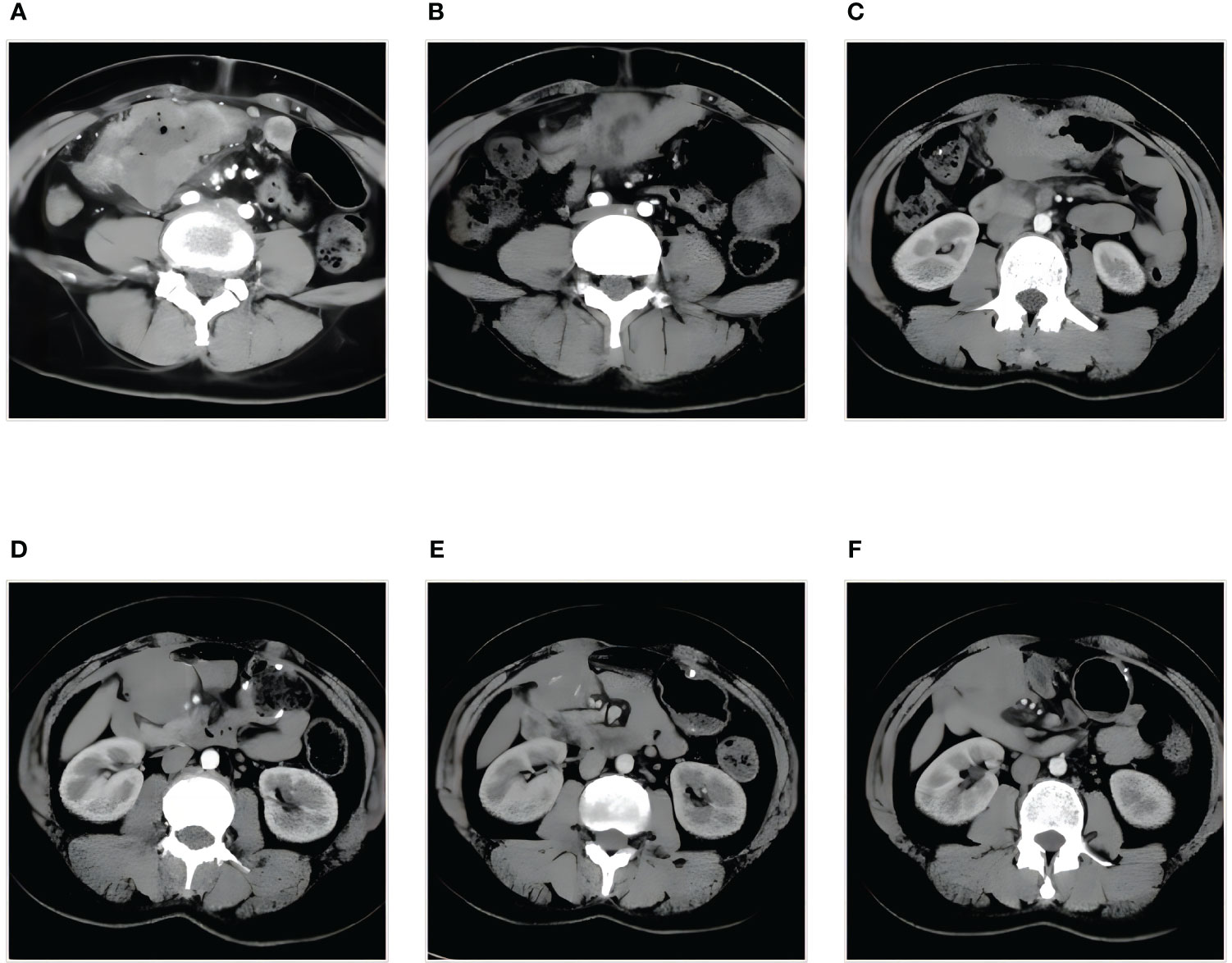
Figure 1 Treatment assessment by abdominal enhanced CT (A-F). (A) Diagnosis, the tumor infiltration depth is classified as T4. (B) PR, after two cycles of XELOX therapy. (C) SD, after four cycles of XELOX therapy. (D) PD, abdominal metastasis. (E) SD-, after four cycles of combination therapy. (F) SD+, after seven cycles of combination therapy. PR, partial response; PD, progressive disease; SD, stable disease.
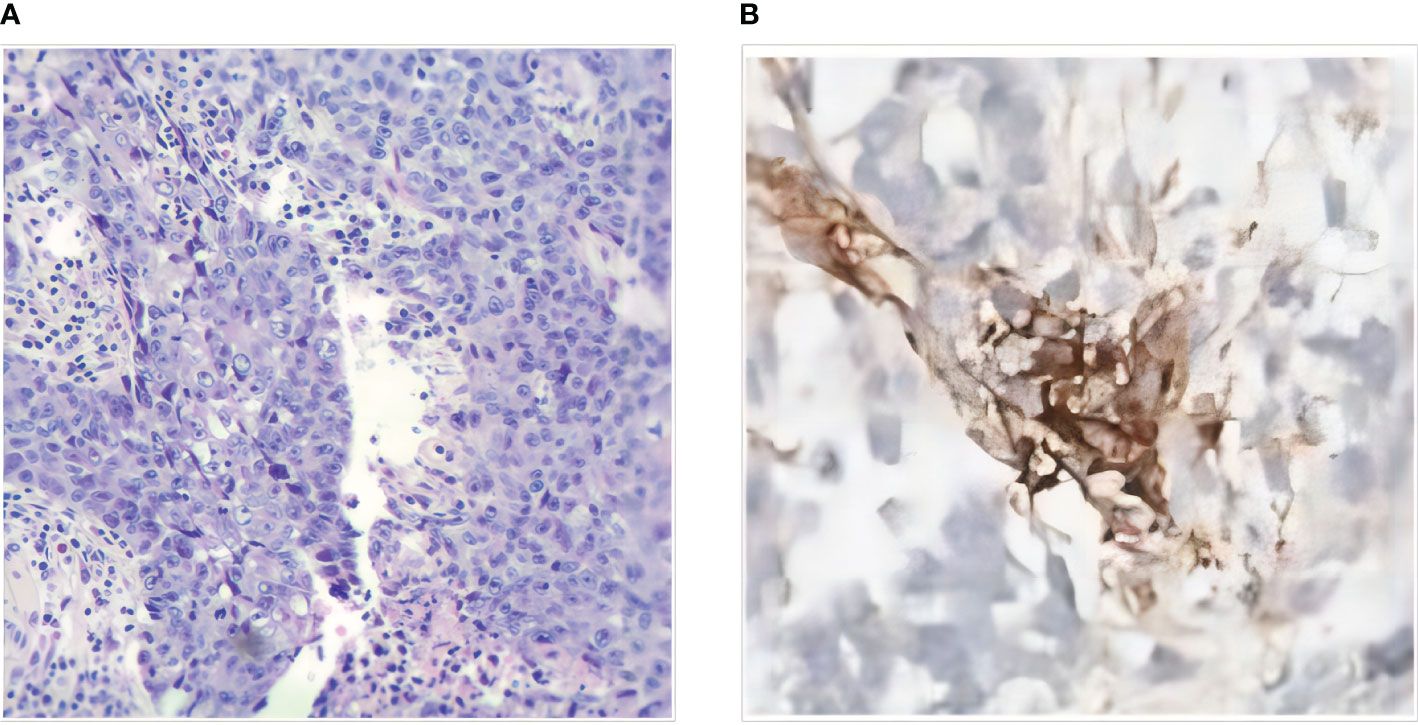
Figure 2 (A) Histopathology of SCC of transverse colon (HE×100). (B) Immunohistochemistry (IHC) of the Abdominal nodule biopsies.
On December 9, 2021, a mass located in the right upper quadrant of the anterior abdominal wall was observed during the reexamination of an abdominal enhanced CT (Figure 1D). On December 23, 2021, the abdominal mass was subjected to a CT-guided percutaneous needle biopsy. The pathology report suggested the possibility of metastatic SCC. Genetic testing revealed a mutation in the TP53 gene, but RAS and BRAF were wild-type, and IHC indicated that the patient had PD-L1: CPS (Combined Positive Score) = 20 (Figure 2B). On January 14, 2022, she was treated with Sintilimab plus Cetuximab and the FOLFIRI regimen. The patient received four cycles of treatment with an efficacy evaluation of SD- (Figure 1E) and seven cycles of treatment with an efficacy evaluation of SD+ (Figure 1F). The patient’s review on July 19, 2022, revealed PD in the condition. The patient’s PFS was approximately 7 months. Considering that the patient is progressing with oligolesions, there is presently a lack of a standardized second-line treatment plan with limited effectiveness. Considering that the patient was progressing by developing oligolesions, there did not, at the time, exist a standardized second-line treatment plan with a certain degree of effectiveness. In September of the same year, the patient underwent a surgical procedure to remove the abdominal mass. Postoperative pathological consideration revealed metastatic SCC. Subsequently, the patient did not receive any further anti-tumor treatment. Regrettably, the patient passed away on June 9, 2023. (Figure 3).
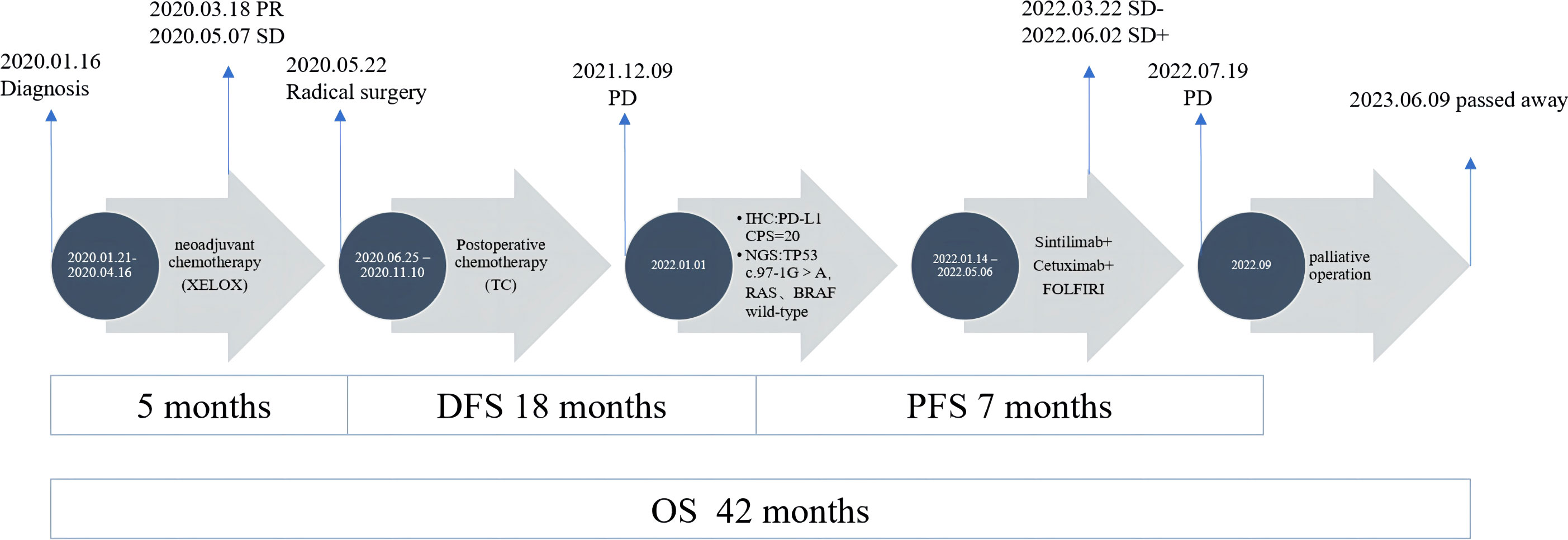
Figure 3 Treatment summary of the patient from diagnosis to last follow-up. PR, partial response; PD, progressive disease; SD, stable disease.
3 Discussion
As a rare pathological type, primary CSCC currently has no established standard treatment available. Most of the studies were focused on rectal SCC, with limited research available on colonic SCC. There was no comprehensive and systematic evidence-based study on its treatment regimen and survival prognosis. Most of the information comes from individual case reports. The present article presents the case of a patient who initially received a diagnosis of locally advanced SCC of the transverse colon. However, the surgical evaluation did not result in an R0 resection. Consequently, the patient underwent four cycles of neoadjuvant chemotherapy, followed by a right hemicolectomy procedure. Postoperative pathology suggested an AJCC/TRG grading of 3. The patient was administered adjuvant chemotherapy with TC with a PFS of 18 months. According to reports, the TC systemic chemotherapy regimen has shown superior treatment outcomes when compared to the 5-fluorouracil and cisplatin (FP) regimen for the management of esophageal squamous cell carcinoma (ESCC) in patients who receive adjuvant chemotherapy after surgery (14). In the postoperative treatment of head and neck squamous cell carcinoma (HNSCC), TC combined with radiotherapy improves the disease control rate (DCR) (15). Meanwhile, the utilization of TC in postoperative settings has been found to extend the period of disease-free survival (DFS) among patients diagnosed with cervical SCC (16). The TC regimen for individuals diagnosed with SCC has some clinical benefits. In the case of colonic SCC, it may be worth comparing the effectiveness of TC and FP.
Hence, given its unique pathological characteristics, CSCC appears to require a distinct approach to treatment when compared to colon adenocarcinoma. Currently, the accepted adjuvant therapy protocol for colon adenocarcinoma consists of the XELOX, mFOLFOX6 (oxaliplatin, fluorouracil, and leucovorin), etc. As per the standard treatment protocol, patients diagnosed with colon adenocarcinoma have shown a 3-year PFS rate of 76% (17). The primary approach for treating patients with advanced colon adenocarcinoma continues to be chemotherapy, plus Bevacizumab or Cetuximab is a feasible treatment option (18, 19). Immunotherapy has shown limited efficacy in treating gastrointestinal tumors, particularly in patients with colon cancer. However, it has been observed that Pembrolizumab is effective as a first-line treatment for metastatic colon cancer patients who have MSI-H or dMMR (20, 21). In recent times, it has been put forth the notion that the fusion of ICB and other treatment methods could emerge as a novel therapeutic choice for pMMR or MSS CRC (22). Research has demonstrated that Avelumab and Cetuximab possess complementary modes of operation that can effectively collaborate to counter the negative feedback of immunosuppression through synergistic action (23). The joint utilization of Pembrolizumab and Cetuximab results in a synergistic antitumor outcome by promoting a more advantageous anti-tumor microenvironment via the amplification of intracellular cytotoxic T lymphocytes and NK cells (24). The phase II CAVE clinical trial findings indicate that the combination of Cetuximab and Avelumab effectively targets patients with MSS metastatic CRC, exhibiting significant rechallenge therapy (25). Incorporating Avelumab into the treatment regimen consisting of Cetuximab and chemotherapy resulted in a noteworthy increase in the objective response rate (ORR) to 83% among patients with MSS CRC (26).
The current case report showcases a patient diagnosed with colonic SCC and exhibiting PD-L1 expression. After experiencing disease relapse, the patient underwent treatment with a regimen consisting of Sintelimab, Cetuximab, and chemotherapy. As a result, the patient achieved a PFS of 7 months. In the palliative treatment of colonic SCC, there are individual case reports indicating that immunotherapy can be used for these patients with PD-L1 expression. A case of a patient diagnosed with pMMR/MSS primary rectosigmoid-junction SCC and presented with high PD-L1 (CPS = 60) expression and tumor mutational burden (TMB-High, 18.99 mutations/mb), received Sintilimab combined with chemotherapy, and obtained a disease-stabilizing period of one year (27). A case of a patient suffering from pMMR/MSS primary colonic SCC with high PD-L1 (CPS = 20) expression underwent treatment involving Sintilimab combined with mFOLFOX6 and achieved a PFS of 8.5 months (28). According to previous data, the median OS for patients with advanced colonic SCC who only received chemotherapy was approximately 8 months (7). It is evident that immunotherapy treatment for colon SCC offers a survival advantage, possibly linked to the expression of PD-L1.
Based on preclinical experiments, it has been demonstrated that SCC with PD-L1 expression can be effectively suppressed through the use of ICB (29). The expression of PD-L1 on tumors reflects an immunocompetent microenvironment and is considered a major factor in anti-PD-1 therapy (30). And research has shown that the expression of PD-L1 on tumor cells is directly proportional to the response to ICB (31, 32). According to previous reports, the expression of PD-L1 is found in diverse solid tumors, encompassing lung, esophageal, and head and neck squamous cell carcinomas (33). However, there is limited available data on the expression of PD-L1 in colonic SCC.
In addition, immune response as a potential target for the treatment of SCC is associated with distinct gene expression profiles (34). The different mRNA expression patterns suggest that each SCC possesses unique immune signatures (35). Song et al. analyzed the proteome of SCC cancers from 17 organs and identified six distinct immune subtypes of pan SCC, each exhibiting unique tumor microenvironment (TME) characteristics and varying prognostic outcomes. However, it is worth noting that these samples contain common and rare sites of SCC, but do not involve the colon (36). Therefore, there is still no good description of the molecular mechanism of CSCC.
According to this report, it is suggested that the use of TC as an adjuvant chemotherapy regimen may exhibit favorable anti-tumor effects when treating colonic SCC. Additionally, the report recommends exploring the potential of ICB when used in conjunction with other therapies for treating patients with progressive colonic SCC. And the expression level of PD-L1 could be used as a biomarker for the application of ICB therapy in patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Affiliated Hospital of Hebei University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XW: Writing – original draft. SS: Writing – review & editing. YW: Writing – review & editing. DH: Writing – review & editing. ZW: Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dyson T, Draganov PV. Squamous cell cancer of the rectum. World J Gastroenterol (2009) 15:4380–6. doi: 10.3748/wjg.15.4380
2. Kang H, O'Connell JB, Leonardi MJ, Maggard MA, McGory ML, Ko CY. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis (2007) 22:183–9. doi: 10.1007/s00384-006-0145-2
3. Schizas D, Katsaros I, Mastoraki A, Karela NR, Zampetaki D, Lazaridis II, et al. Primary squamous cell carcinoma of colon and rectum: A systematic review of the literature. J Invest Surg (2022) 35:151–6. doi: 10.1080/08941939.2020.1824044
4. Frizelle FA, Hobday KS, Batts KP, Nelson H. Adenosquamous and squamous carcinoma of the colon and upper rectum: a clinical and histopathologic study. Dis Colon Rectum (2001) 44:341–6. doi: 10.1007/BF02234730
5. Linardoutsos D, Frountzas M, Feakins RM, Patel NH, Simanskaite V, Patel H. Primary colonic squamous cell carcinoma: a case report and review of the literature. Ann R Coll Surg Engl (2020) 102:e1–7. doi: 10.1308/rcsann.2020.0149
6. Ozuner G, Aytac E, Gorgun E, Bennett A. Colorectal squamous cell carcinoma: a rare tumor with poor prognosis. Int J Colorectal Dis (2015) 30:127–30. doi: 10.1007/s00384-014-2058-9
7. Yang Y, Yu J, Hu J, Zhou C, Niu J, Ma H, et al. A systematic and comprehensive analysis of colorectal squamous cell carcinoma: Implication for diagnosis and treatment. Cancer Med (2022) 11:2492–502. doi: 10.1002/cam4.4616
8. Wang ML, Heriot A, Leong T, Ngan SY. Chemoradiotherapy in the management of primary squamous-cell carcinoma of the rectum. Colorectal Dis (2011) 13:296–301. doi: 10.1111/j.1463-1318.2009.02154
9. Clark J, Cleator S, Goldin R, Lowdell C, Darzi A, Ziprin P. Treatment of primary rectal squamous cell carcinoma by primary chemoradiotherapy: should surgery still be considered a standard of care? Eur J Cancer (2008) 44:2340–3. doi: 10.1016/j.ejca.2008.07.004
10. Nassar H, Ataya K, Hafez B, El Bsat A, Geagea L, Faraj W. Primary squamous cell carcinoma of the colon: A rare case report. Int J Surg Case Rep (2022) 9:107383. doi: 10.1016/j.ijscr.2022.107383
11. Abdelqader A, Jabaji R, Albugeaey M, Palese C. Squamous cell carcinoma of the ascending colon: two cases. J Community Hosp Intern Med Perspect (2017) 7:53–5. doi: 10.1080/20009666.2017.1309339
12. Mondal SK. Primary squamous cell carcinoma of the cecum. J Cancer Res Ther (2009) 5:328–30. doi: 10.4103/0973-1482.59900
13. Yokoyama Y, Wada R, Yamada T, Uchida E, Naito Z. A case of ulcerative colitis with squamous cell carcinomas and multiple foci of squamous dysplasia. Pathol Int (2017) 67:414–8. doi: 10.1111/pin.12539
14. Zhang L, Li W, Lyu X, Song Y, Mao Y, Wang S, et al. Adjuvant chemotherapy with paclitaxel and cisplatin in lymph node-positive thoracic esophageal squamous cell carcinoma. Chin J Cancer Res (2017) 29:149–55. doi: 10.21147/j.issn.1000-9604.2017.02.08
15. Rosenthal DI, Harris J, Forastiere AA, Weber RS, Ridge JA, Myers JN, et al. Early postoperative paclitaxel followed by concurrent paclitaxel and cisplatin with radiation therapy for patients with resected high-risk head and neck squamous cell carcinoma: report of the phase II trial RTOG 0024. J Clin Oncol (2009) 27:4727–32. doi: 10.1200/JCO.2008.21.4197
16. Qin T, Zhen J, Zhou M, Wu H, Ren R, Qu B, et al. Efficacy of neoadjuvant chemotherapy plus radical surgery in patients with bulky stage II cervical squamous cell carcinoma: A retrospective cohort study. Int J Surg (2016) 30:121–5. doi: 10.1016/j.ijsu.2016.04.038
17. André T, Vernerey D, Mineur L, Bennouna J, Desrame J, Faroux R, et al. Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized, open-label, international duration evaluation of adjuvant (IDEA) France, phase III trial. J Clin Oncol (2018) 36(15):1469–77. doi: 10.1200/JCO.2017.76.0355
18. Cervantes A, Prager GW. FOLFOXIRI plus bevacizumab as standard of care for first-line treatment in patients with advanced colon cancer. ESMO Open (2023) 8(2):100883. doi: 10.1016/j.esmoop.2023.100883
19. Qin S, Li J, Wang L, Xu J, Cheng Y, Bai Y, et al. Efficacy and tolerability of first-line cetuximab plus leucovorin, fluorouracil, and oxaliplatin (FOLFOX-4) versus FOLFOX-4 in patients with RAS wild-type metastatic colorectal cancer: the open-label, randomized, phase III TAILOR trial. J Clin Oncol (2018) 36(30):3031–9. doi: 10.1200/JCO.2018.78.3183
20. Casak SJ, Marcus L, Fashoyin-Aje L, Mushti SL, Cheng J, Shen YL, et al. FDA approval summary: pembrolizumab for the first-line treatment of patients with MSI-H/dMMR advanced unresectable or metastatic colorectal carcinoma. Clin Cancer Res (2021) 27(17):4680–4. doi: 10.1158/1078-0432.CCR-21-0557
21. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
22. Wang H, Zhou Y, Zhang Y, Fang S, Zhang M, Li H, et al. Subtyping of microsatellite stability colorectal cancer reveals guanylate binding protein 2 (GBP2) as a potential immunotherapeutic target. J Immunother Cancer (2022) 10:e004302. doi: 10.1136/jitc-2021-004302
23. Bourhis J, Stein A, Paul de Boer J, Van Den Eynde M, Gold KA, Stintzing S, et al. Avelumab and cetuximab as a therapeutic combination: An overview of scientific rationale and current clinical trials in cancer. Cancer Treat Rev (2021) 97:102172. doi: 10.1016/j.ctrv.2021.102172
24. Fountzilas C, Bajor DL, Mukherjee S, Saltzman J, Witkiewicz AK, Maguire O, et al. Phase ib/II study of cetuximab plus pembrolizumab in patients with advanced RAS wild-type colorectal cancer. Clin Cancer Res (2021) 27:6726–36. doi: 10.1158/1078-0432.CCR-21-1650
25. Martinelli E, Martini G, Famiglietti V, Troiani T, Napolitano S, Pietrantonio F, et al. Cetuximab rechallenge plus avelumab in pretreated patients with RAS wild-type metastatic colorectal cancer: the phase 2 single-arm clinical CAVE trial. JAMA Oncol (2021) 7:1529–35. doi: 10.1001/jamaoncol.2021.2915
26. Stein A, Simnica D, Schultheiß C, Scholz R, Tintelnot J, Gökkurt E, et al. PD-L1 targeting and subclonal immune escape mediated by PD-L1 mutations in metastatic colorectal cancer. J Immunother Cancer (2021) 9:e002844. doi: 10.1136/jitc-2021-002844
27. He Y, Wang L, Li X, Zhang T, Song T, Zhang J, et al. Rectosigmoid-junction squamous cell carcinoma with pMMR/MSS achieved a partial response following PD-1 blockade combined with chemotherapy: A case report. Front Oncol (2021) 11:596342. doi: 10.3389/fonc.2021.596342
28. Liu Y, Du J, Zhang P, Xiao H. Squamous cell carcinoma of ascending colon with pMMR/MSS showed a partial response to PD-1 blockade combined with chemotherapy: A case report. Front Oncol (2023) 13:1051786. doi: 10.3389/fonc.2023.1051786
29. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res (2014) 20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271
30. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer (2016) 16:275–87. doi: 10.1038/nrc.2016.36
31. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4
32. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. (2022) 377:e068714. doi: 10.1136/bmj-2021-068714
33. Ritprajak P, Azuma M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol (2015) 51:221–8. doi: 10.1016/j.oraloncology.2014.11.014
34. Campbell JD, Yau C, Bowlby R, Liu Y, Brennan K, Fan H, et al. Genomic, pathway network, and immunologic features distinguishing squamous carcinomas. Cell Rep (2018) 23(1):194–212.e6. doi: 10.1016/j.celrep.2018.03.063
35. Li B, Cui Y, Nambiar DK, Sunwoo JB, Li R. The immune subtypes and landscape of squamous cell carcinoma. Clin Cancer Res (2019) 25(12):3528–37. doi: 10.1158/1078-0432.CCR-18-4085
Keywords: colorectal cancer, squamous cell carcinoma, adjuvant chemotherapy, immune checkpoint inhibitors, microsatellite instability, case report
Citation: Wu X, Su S, Wei Y, Hong D and Wang Z (2023) Case Report: A management strategy and clinical analysis of primary squamous cell carcinoma of the colon. Front. Oncol. 13:1265421. doi: 10.3389/fonc.2023.1265421
Received: 22 August 2023; Accepted: 18 September 2023;
Published: 11 October 2023.
Edited by:
Francesk Mulita, General University Hospital of Patras, GreeceReviewed by:
Georgios-Ioannis Verras, Epsom and St Helier University Hospitals NHS Trust, United KingdomFotios Iliopoulos, General University Hospital of Patras, Greece
David Dimitris Chlorogiannis, University of Patras, Greece
Christos Pitros, General University Hospital of Patras, Greece
Copyright © 2023 Wu, Su, Wei, Hong and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Hong, MjUzNzI0Mzk3OUBxcS5jb20=; Zhiyu Wang, MTg5MzEyMDA4MjZAMTg5LmNu
 Xiang Wu
Xiang Wu Shenyong Su
Shenyong Su