
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 10 October 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1265240
 Hyeon-Jong Kim1†
Hyeon-Jong Kim1† Seung Hyuk Lee1†
Seung Hyuk Lee1† Hyun Jeong Shim1
Hyun Jeong Shim1 Hyun Jin Bang1
Hyun Jin Bang1 Sang Hee Cho1
Sang Hee Cho1 Ik-Joo Chung1
Ik-Joo Chung1 Eu Chang Hwang2
Eu Chang Hwang2 Jun Eul Hwang1*†
Jun Eul Hwang1*† Woo Kyun Bae1*†
Woo Kyun Bae1*†Introduction: To investigate the effects of hepatic arterial infusion chemotherapy (HAIC) with or without systemic chemotherapy compared to systemic chemotherapy alone in patients with locally advanced hepatocellular carcinoma (HCC).
Methods: Following a registered protocol (PROSPERO 2023 CRD42023386780 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023386780), a comprehensive search was performed using reputable databases and registries up to December 26, 2022, with no language, publication date, or status restrictions. Only randomized controlled trials (RCTs) investigating the effects of HAIC with or without systemic chemotherapy versus systemic therapy alone were included. The primary outcomes were overall survival (OS), progression-free survival (PFS), and adverse events. The secondary outcomes included the objective response rate (ORR) and disease control rate (DCR). A random-effects model was used, and the certainty of the evidence was rated using GRADE.
Results: Seven RCTs involving 1,010 patients were included. All trials utilized sorafenib as the comparator. Five trials (690 patients) compared HAIC plus sorafenib to sorafenib alone, while two trials (320 patients) compared HAIC to sorafenib. The results indicate that HAIC, with or without sorafenib, may increase OS, PFS, and ORR compared with sorafenib alone. HAIC may enhance DCR, but the evidence is very uncertain. Adverse events were comparable between HAIC plus sorafenib and sorafenib alone. However, adverse events might be decreased in HAIC alone.
Discussion: HAIC with or without systemic chemotherapy may improve survival outcomes and response rates of patients with HCC. Since the current body of evidence is moderate to very low, more robust randomized trials are needed to confirm the efficacy of HAIC.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=386780, identifier CRD42023386780.
The International Agency for Research on Cancer (IARC) reported that liver cancer was the sixth most common cancer and the third leading cause of cancer-related deaths worldwide in 2020 (1). In addition, the incidence of hepatocellular carcinoma (HCC) has increased over the past two decades. In the United States, the incidence of HCC is on the rise, particularly among individuals infected with the hepatitis C virus (HCV) (1, 2).
Current treatment strategies for HCC include surgical resection, transplantation, and locoregional therapies such as ablation, transarterial procedures, and systemic therapies. The treatment goals can vary based on the patient’s cancer stage, underlying liver function, and performance status. Consequently, numerous clinical practice guidelines for HCC recommend a multidisciplinary approach to developing individualized treatment plans (3–5). Usually, patients diagnosed with early-stage HCC are recommended for hepatic resection and liver transplantation. Systemic therapies, including tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors, have achieved remarkable advances and are now recommended for patients with advanced HCC and distant metastasis. Intermediate or locally advanced stages of HCC encompass multifocal, diffuse, and infiltrative HCC. In some cases, patients with limited and well-defined lesions may be candidates for transplantation or transarterial chemoembolization. However, patients with extensive HCC liver involvement have limited therapeutic options (3). The Barcelona Clinic of Liver Cancer (BCLC) group and the National Comprehensive Cancer Network (NCCN) recommend systemic therapies as the primary approach for extensive disease (3, 6). While Asian groups, including groups in Korea, Japan, and China, suggest systemic therapies and hepatic arterial infusion chemotherapy (HAIC) as potential treatment options for these patients (4, 5, 7).
Several studies have demonstrated the effectiveness of cytotoxic chemotherapy on HCC (8, 9). However, accompanied liver cirrhosis affects the absorption and metabolism of chemotherapeutic agents, posing challenges in maintaining therapeutic doses and increasing the risk of toxicity. HAIC is a technique that involves the direct infusion of cytotoxic chemotherapy into the hepatic artery via an implanted catheter port system. This method is designed to expose HCC to high concentrations of chemotherapeutic agents while reducing adverse reactions (4, 10). Previous studies have shown favorable results of HAIC in patients with intermediate or advanced HCC (11–14). As a result, Eastern Asian groups such as the Korean Liver Cancer Association (KLCA) and the Japan Society of Hepatology (JSH) recommend HAIC as an important treatment option. In Korea, HAIC is recommended as a salvage therapy following the failure of first- or second-line systemic therapies, or as a substitute therapy for systemic therapies in advanced HCC patients with preserved liver function and portal vein invasion and without extrahepatic spread. In Japan, HAIC is considered for patients with more than four intrahepatic tumors or vascular invasion and who are not suitable candidates for local treatments, such as radiofrequency ablation (RFA) or transarterial chemoembolization (TACE) (4, 5). However, the effectiveness of HAIC compared to systemic therapy in patients is still controversial. Two randomized controlled trials (RCTs), the SILIUS study and the SCOOP-2 study, were conducted in Japan to compare the effectiveness of HAIC plus sorafenib with the sorafenib monotherapy (10, 15). While these studies did not find a significant difference in the overall survival between the two treatment groups, it is important to note that there were certain limitations in defining the impact of HAIC with these studies. Several meta-analyses and systematic reviews have been published, but most studies analyzed observational studies and RCTs together without distinction (16–18) and did not rate the certainty of evidence. Therefore, this study aimed to summarize the currently available evidence on the effects of HAIC with or without systemic chemotherapy versus systemic chemotherapy in patients with advanced HCC. This study was limited to RCTs, which provided a high certainty of evidence and used rigorous methodological standards for systematic review.
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was based on a registered protocol (PROSPERO: CRD42023386780 from 14/01/2023). Institutional review board (IRB) approval was unnecessary for this type of study. The inclusion criteria for this systematic review and meta-analysis were RCTs involving patients with advanced HCC treated with HAIC, either with or without systemic chemotherapy. Trials in which the patients received HAIC as adjuvant therapy following surgical resection or in combination with RFA or TACE were excluded.
The primary outcomes of this study were overall survival (OS) and progression-free survival (PFS). The secondary outcomes included adverse events (AEs), objective response rate (ORR), and disease control rate (DCR). ORR was defined as the sum of complete responses (CRs) and partial responses (PRs), and DCR was defined as the sum of CRs, PRs, and stable diseases. In all the included RCTs (10–15, 19), the modified Response Evaluation Criteria in Solid Tumors for HCC (mRECIST) was used to assess the treatment responses (20). AEs were assessed with the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE).
An experienced information specialist conducted the electric searches of multiple databases, including Medline via Ovid, Embase via Elsevier, the Cochrane Central Register of Controlled Trials via Wiley, Web of Science, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP), Koreamed, and Kmbase. The search was performed from the inception of the databases to December 2022 without any language or publication status restrictions.
Two review authors (HJK, SHL) used the Covidence software platform (www.covidence.org) to assess all potentially relevant records and select eligible studies. Any discrepancies in the assessment of eligibility were resolved through discussions with a third member of the review team. After the eligible studies were selected, two review authors independently extracted data from each study in duplicate. The extracted data included study design, duration, setting, country, sample size, patient characteristics, interventions, clinical outcomes such as OS, PFS, DCR, and OCR, and adverse events. A PRISMA flow diagram is presented to illustrate the study selection process. The Cochrane Risk of bias tool was used to evaluate the risk of bias in each study (21). Each bias item was classified as ‘low risk,’ ‘high risk,’ or ‘unclear’.
Dichotomous data such as ORR, DCR, and adverse events are presented as risk ratios (RRs) with 95% confidence intervals (CIs). Time-to-events data, including OS and PFS, are expressed as hazard ratios (HRs) with 95% CIs. Heterogeneity (inconsistency) was identified by the I2 statistics and interpreted according to the Cochrane Handbook (22). A visual inspection with Forest plots was used to assess the overlap of CIs. When any heterogeneities were detected, it was attempted to determine the possible reasons by evaluating the characteristics of each study and subgroup. A random-effects model was employed to summarize the data and interpret the results. The certainty of evidence (CoE) was rated on an outcome-specific basis using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach (23).
To assess the overall quality of evidence, the GRADE framework was used. This framework considers criteria pertaining to validities, such as the risk of bias, imprecision, inconsistency, publication bias, and indirectness of results (23). Each author independently assigned a rating of high, moderate, low, or very low to the quality of evidence for each outcome in every comparison. Any inconsistencies were addressed through a consensus or, if necessary, by engaging other review authors for arbitration using GRADEpro (24). Subsequently, “Summary of findings” tables were generated that presented crucial information about the number of participants and studies, the certainty of the evidence, the estimated relative effects, and the anticipated absolute effects of each treatment strategy for each clinical outcome (25, 26). We used the GRADE guidance to describe the certainty of the evidence and the magnitude of the effect size (27).
We planned to conduct subgroup analyses of the Child–Pugh score, level of tumor markers, and portal vein thrombosis and described them in the protocol. Additionally, subgroup analyses based on chemotherapy regimen were added to compare possible differences in the outcomes of each chemotherapeutic used for HAIC.
Figure 1 is the PRISMA flow diagram that presents the process of selecting eligible studies. A total of 1,076 records from databases and 214 records from registers were identified. After removing duplicates, 916 records were screened by the review authors. One record was excluded by automation tools, and 915 reports were sought for retrieval. Among them, 887 reports were not retrieved because of animal studies or exclusion criteria. Twenty-eight reports were assessed for eligibility. Five reports were excluded because they were ongoing studies and five other reports were excluded due to a wrong comparator (n = 2), wrong study design (n = 2), or wrong measured outcomes (n = 1). Finally, seven RCTs were included in this review (10–15, 19).
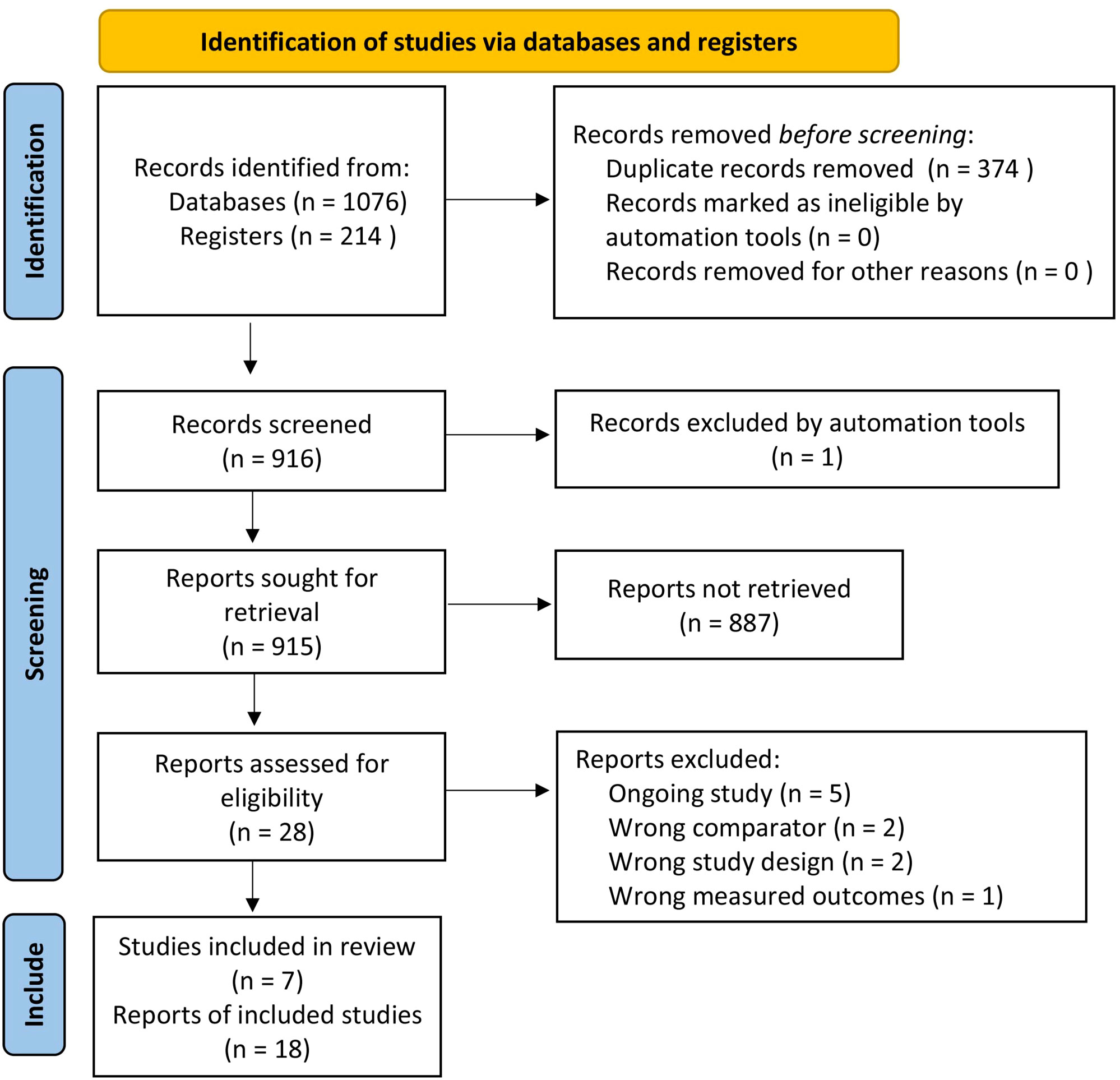
Figure 1 PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Among the seven RCTs included in this study, three studies were conducted in Japan (10, 15, 19), three in China (12–14), and one in South Korea (11). Five trials were multicenter trials (10, 11, 13, 15, 19), and the remaining two were single-center trials (12, 14). The included studies were performed between 2010 and 2020, and their publication dates ranged from 2016 to 2022. The number of eligible participants ranged from 58 to 262 individuals. Among the included RCTs, five trials compared HAIC + sorafenib and sorafenib alone, encompassing a total of 695 patients (10, 13–15, 19). The remaining two trials compared HAIC and sorafenib, with a total of 320 patients included (11, 12). In all the studies, the diagnosis of HCC was confirmed either histologically or clinically, according to the American Association for the Study of Liver disease criteria (2). All eligible patients were adults who were not suitable for surgery or locoregional treatment such as ablation or TACE (10–15, 19). Four trials included patients with ECOG PS 0–1 (10, 11, 15, 19), while the remaining three included patients with ECOG PS 0–2 (12–14). Two trials included patients with Child-Pugh grade A (Child-Pugh score 5 or 6) (13, 14), whereas the other five trials also included Child-Pugh score 7 (10–12, 15, 19). In all the studies, sorafenib was initiated at a dose of 400 mg twice daily (10–15, 19). However, there were variations in the HAIC regimens applied. Ikeda et al. and Kondo et al. utilized a cisplatin monotherapy as a HAIC regimen (15, 19), while Kudo et al. and Choi et al. used a combination of cisplatin and fluorouracil (10, 11). He et al., Zheng et al., and Lyu et al. employed a combination of oxaliplatin, leucovorin, and fluorouracil (12–14). All of the studies allowed for the participation of patients with extrahepatic metastasis as long as it was determined that the metastatic lesions would not have an influence on their prognosis (10–15, 19). One study only enrolled patients with portal vein invasion (13), while two studies exclusively included patients with major portal vein tumor thrombosis (Vp3–4) (11, 14). Although two studies initially used RECIST1.1 and conducted post hoc analyses with mRECIST (13, 14), all the included studies assessed tumor response using mRECIST. Six studies reported funding sources; five studies were supported by government agencies (10, 12–14, 19), and one study was funded by a non-profit organization (15). The other study did not specify its funding source (11). Five studies reported no conflicts of interest (11–14, 19), while two provided details of their conflicts of interest (10, 15). The characteristics of the included studies are summarized in Supplement Table 1.
The risks of bias in the included studies are summarized and presented in Figure 2. Most of the included studies had a low risk of bias across numerous domains; however, all the included studies were considered to have an overall high risk of performance bias and detection bias for the subjective outcomes. In the HAIC groups, patients had to undergo arterial catheterization, while the sorafenib groups did not. As a result, blinding of participants was inevitably impossible.
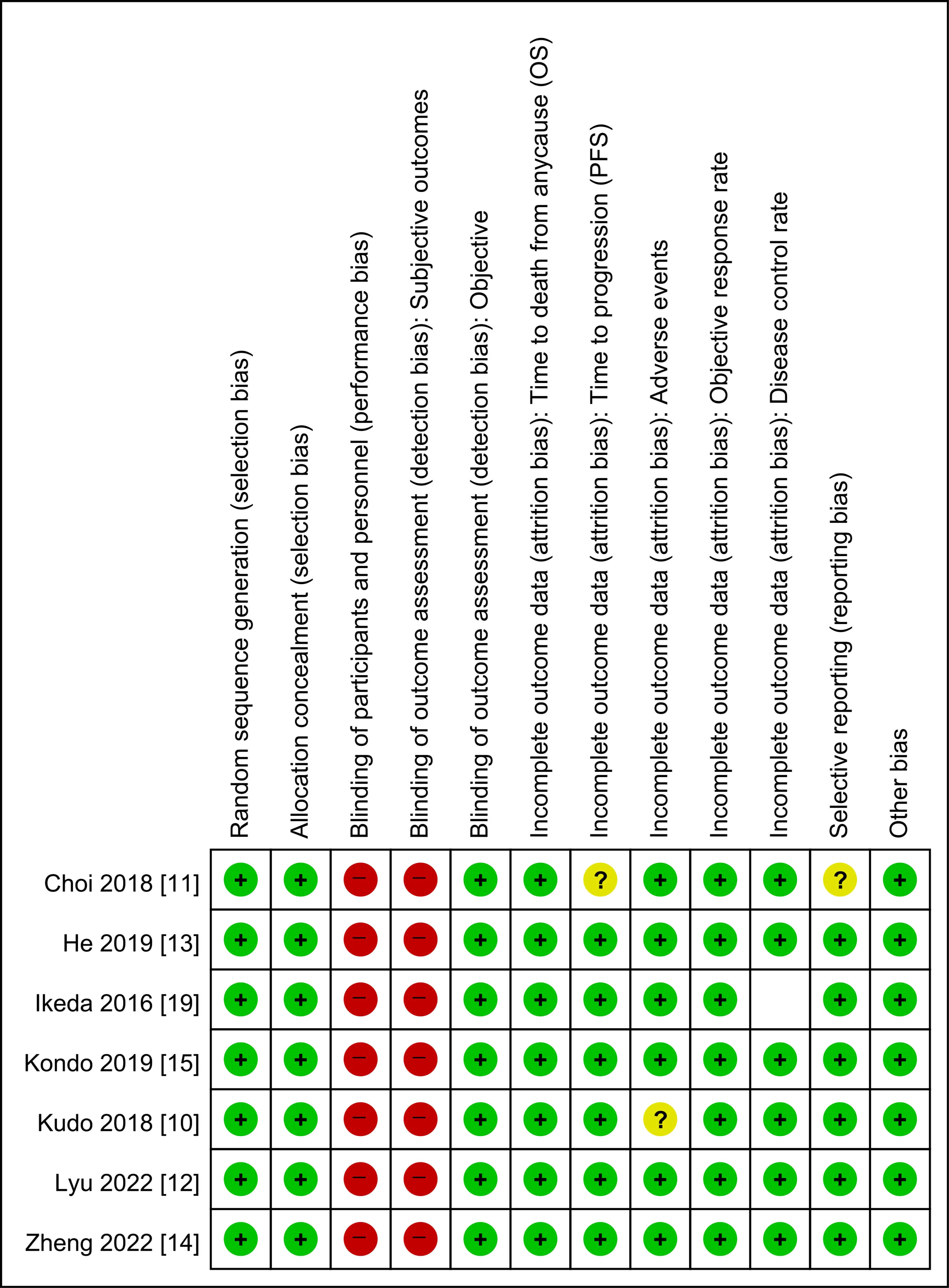
Figure 2 Risk of bias summary: review authors’ judgment on each risk of bias item for each induced study. OS, overall survival; PFS, progression-free survival.
Please refer to Table 1, and Supplement Figures 1-6.
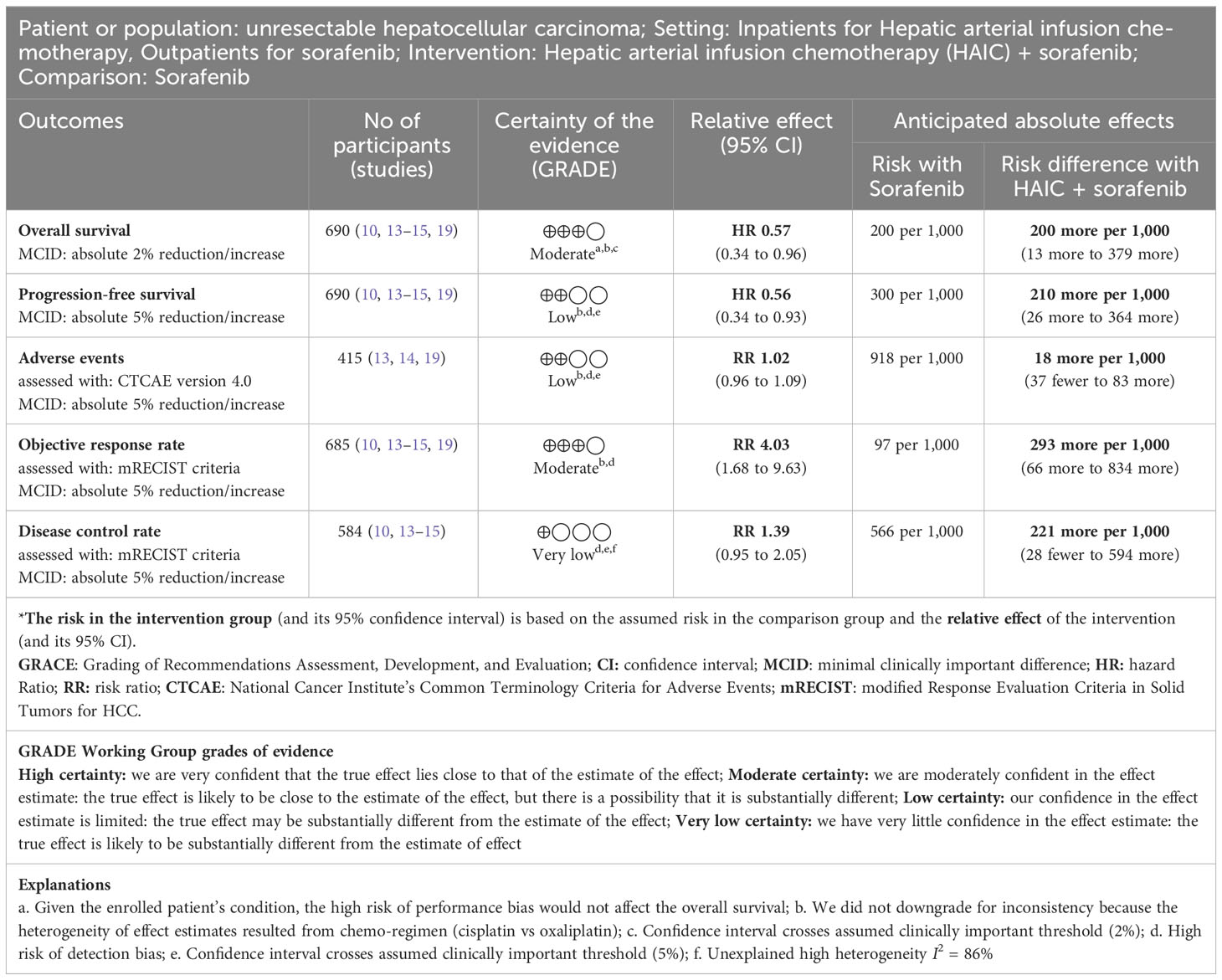
Table 1 Summary of findings table of Hepatic arterial infusion chemotherapy + sorafenib versus sorafenib for advanced hepatocellular carcinoma.
Five RCTs with 690 patients (HAIC + sorafenib n = 359, sorafenib n = 331) reported OS (10, 13–15, 19). HAIC + sorafenib probably increases OS compared to sorafenib (HR 0.57, 95% CI 0.34 to 0.96; I2 = 87%; moderate-certainty evidence). This corresponds to 200 more OS per 1000 patients (95% CI 13 more to 379 more) than sorafenib. We downgraded the certainty of the evidence for serious imprecision (–1). (Table 1; Supplement Figure 1, 2).
Five RCTs with 690 patients (HAIC + sorafenib n = 359, sorafenib n = 331) reported PFS (10, 13–15, 19). HAIC + sorafenib may increase PFS compared to sorafenib (HR 0.56, 95% CI 0.34 to 0.93; I2 = 96.8%; low-certainty evidence). This corresponds to 210 more PFS per 1000 patients (95% CI 26 more to 364 more) than sorafenib. We downgraded the certainty of the evidence for serious study limitations (–1) and serious imprecision (–1) (Table 1; Supplement Figure 3).
Three RCTs with 415 patients (HAIC + sorafenib n = 221, sorafenib n = 194) reported adverse events (13, 14, 19). HAIC + sorafenib may result in little to no difference in adverse events compared to sorafenib (RR 1.02, 95% CI 0.96 to 1.09; I2 = 46%; low-certainty evidence). This corresponds to 18 more adverse events per 1000 patients (95% CI 37 fewer to 83 more) than sorafenib. We downgraded the certainty of the evidence for serious study limitations (–1) and serious imprecision (–1). (Table 1; Supplement Figure 4).
Five RCTs with 685 patients (HAIC + sorafenib n = 354, sorafenib n = 331) reported ORR (10, 13–15, 19). HAIC + sorafenib probably increases ORR compared to sorafenib (RR 4.03, 95% CI 1.68 to 9.63; I2 = 75%; moderate-certainty evidence). This corresponds to 293 more ORR per 1000 patients (95% CI 66 more to 834 more) than sorafenib. We downgraded the certainty of the evidence for serious study limitations (–1) (Table 1; Supplement Figure 5).
Four RCTs with 584 patients (HAIC + sorafenib n = 294, sorafenib n = 290) reported DCR (10, 13–15). HAIC + sorafenib may increase the disease control rate, but the evidence is very uncertain. (RR 1.39, 95% CI 0.95 to 2.05; I2 = 86%; very low-certainty evidence). We downgraded the certainty of the evidence for serious study limitations (–1), serious inconsistency (–1), and serious imprecision (–1) (Table 1; Supplement Figure 6).
Subgroup analyses (stratified by chemo-regimen and portal vein thrombosis) on the primary outcomes were performed. Pre-planned subgroup analyses stratified by Child-Pugh scores and level of tumor markers could not be performed because there were no available data.
In terms of OS and PFS, of the 690 patients, 379 were using cisplatin (HAIC + sorafenib n = 202; sorafenib n = 177), and 331 were using oxaliplatin (HAIC + sorafenib n = 157; sorafenib n = 154).
For patients using cisplatin, the HR was 0.88 (95% CI 0.69 to 1.13), and for those using oxaliplatin, the HR was 0.34 (95% CI 0.26 to 0.44). The test for interaction was significant (P = 0.001, I2 = 96.3%) (Supplement Figure 1).
For patients using cisplatin, the HR was 0.81 (95% CI 0.65 to 1.00), and for those using oxaliplatin, the HR was 0.31 (95% CI 0.25 to 0.40). The test for interaction was significant (P = 0.001, I2 = 96.8%) (Supplement Figure 3).
Of the 415 patients; 106 were using cisplatin (HAIC + sorafenib n = 65; sorafenib n = 41), and 309 were using oxaliplatin (HAIC + sorafenib n = 156; sorafenib n = 153). For the patients using cisplatin, the RR was 1.00 (95% CI 0.96 to 1.04), and for those using oxaliplatin, the RR was 1.05 (95% CI 0.99 to 1.12). The test for interaction was not significant (P = 0.18, I2 = 43.3%) (Supplement Figure 4).
Of the 690 patients, 559 had portal vein thrombosis (HAIC + sorafenib n = 291; sorafenib n = 268), and 131 had no portal vein thrombosis (HAIC + sorafenib n = 68; sorafenib n = 63). For the patients with portal vein thrombosis, the HR was 0.58 (95% CI 0.32 to 1.05), and for those without portal vein thrombosis, the HR was 0.80 (95% CI 0.55 to 1.15). The test for interaction was not significant (P = 0.37, I2 = 0%) (Supplement figure 2).
Please refer to Table 2, and Supplement Figures 7-11.
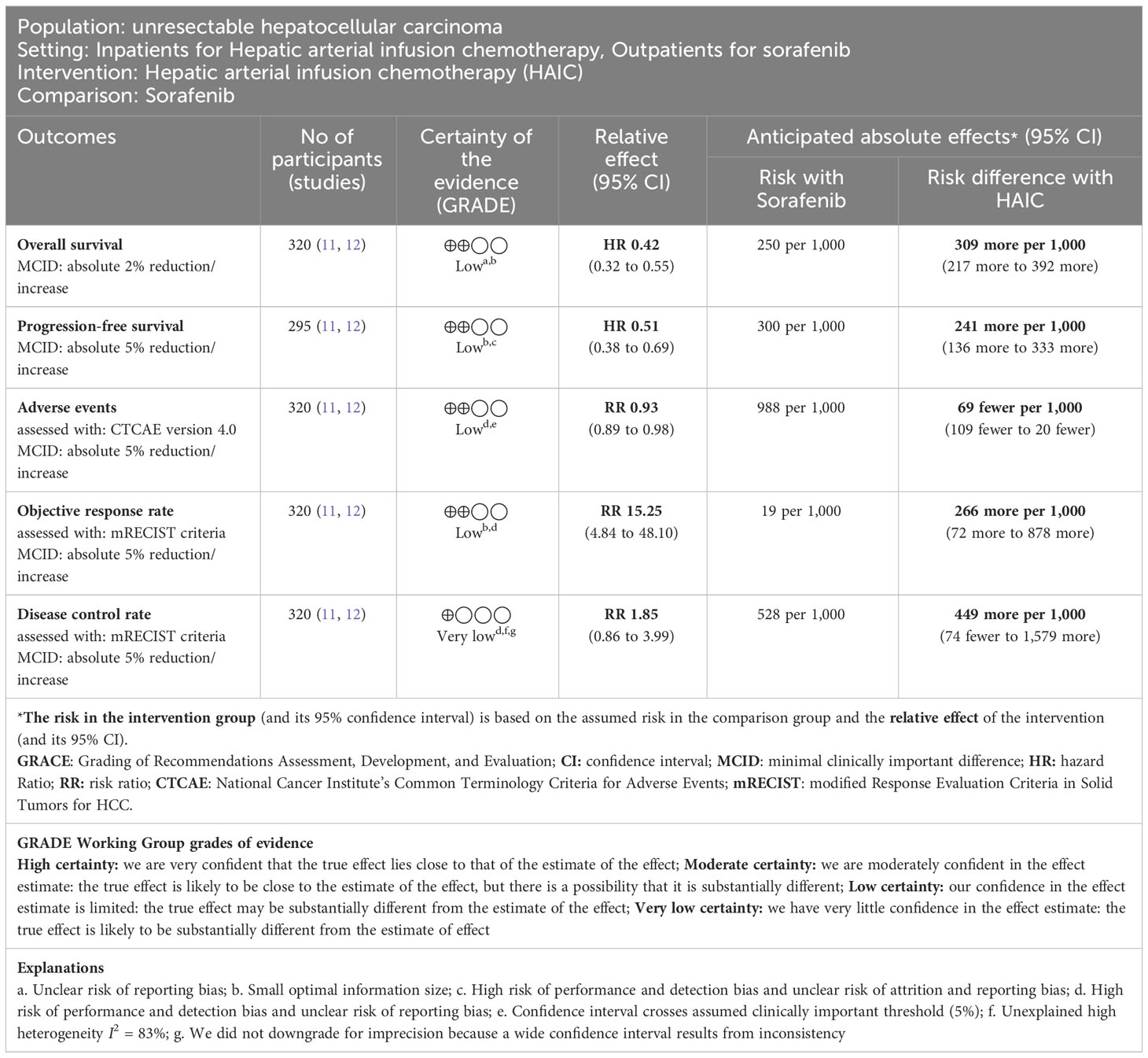
Table 2 Summary of findings table of Hepatic arterial infusion chemotherapy versus sorafenib for advanced hepatocellular carcinoma.
Two RCTs with 320 patients (HAIC n = 159, sorafenib n = 161) reported OS (11, 12). HAIC may increase OS compared to sorafenib (HR 0.42, 95% CI 0.32 to 0.55; I2 = 0%; low-certainty evidence). This corresponds to 309 more OS per 1000 patients (95% CI 217 more to 392 more) than sorafenib. We downgraded the certainty of the evidence for serious study limitations (–1) and serious imprecision (–1) (Table 2; Supplement Figure 7).
Two RCTs with 295 patients (HAIC n = 144, sorafenib n = 151) reported PFS (11, 12). HAIC may increase PFS compared to sorafenib (HR 0.51, 95% CI 0.38 to 0.69; I2 = 39%; low-certainty evidence). This corresponds to 241 more PFS per 1000 patients (95% CI 136 more to 333 more) than sorafenib. We downgraded the certainty of the evidence for serious study limitations (–1) and serious imprecision (–1). (Table 2; Supplement Figure 8).
Two RCTs with 320 patients (HAIC n = 159, sorafenib n = 161) reported adverse events (11, 12). HAIC may reduce adverse events slightly compared to sorafenib (RR 0.93, 95% CI 0.89 to 0.98; I2 = 0%; low-certainty evidence). This corresponds to 69 fewer adverse events per 1000 patients (95% CI 109 fewer to 20 fewer) than sorafenib. We downgraded the certainty of the evidence for serious study limitations (–1) and serious imprecision (–1) (Table 2; Supplement Figure 9).
Two RCTs with 320 patients (HAIC n = 159, sorafenib n = 161) reported ORR (11, 12). HAIC may increase ORR compared to sorafenib (RR 15.25, 95% CI 4.84 to 48.10; I2 = 0%; low-certainty evidence). This corresponds to 266 more ORR per 1000 patients (95% CI 72 more to 878 more) than sorafenib. We downgraded the certainty of the evidence for serious study limitations (–1) and serious imprecision (–1) (Table 2; Supplement Figure 10).
Two RCTs with 320 patients (HAIC n = 159, sorafenib n = 161) reported DCR (11, 12). HAIC may increase the disease control rate, but the evidence is very uncertain (RR 1.85, 95% CI 0.86 to 3.99; I2 = 83%; very low-certainty evidence). We downgraded the certainty of the evidence for serious study limitations (–1), serious inconsistency (–1), and serious imprecision (–1) (Table 2; Supplement Figure 11).
Subgroup analyses (stratified by chemo-regimen) on the primary outcomes were performed. Pre-planned subgroup analyses stratified by portal vein thrombosis, Child-Pugh scores, and level of tumor markers could not be performed because there were no available data.
Regarding OS and adverse events, of the 320 patients, 58 were using cisplatin (HAIC n = 29; sorafenib n = 29), and 162 were using oxaliplatin (HAIC n = 130; sorafenib n = 132).
For the patients using cisplatin, the HR was 0.48 (95% CI 0.27 to 0.85), and for those using oxaliplatin, the HR was 0.41 (95% CI 0.30 to 0.55). The test for interaction was not significant (P = 0.62, I2 = 0%) (Supplement Figure 7).
Of the 295 patients; 33 were using cisplatin (HAIC n = 14; sorafenib n = 19), and 162 were using oxaliplatin (HAIC n = 130; sorafenib n = 132). For the patients using cisplatin, the HR was 0.61 (95% CI 0.42 to 0.90), and for those using oxaliplatin, the HR was 0.45 (95% CI 0.34 to 0.60). The test for interaction was not significant (P = 0.20, I2 = 38.8%) (Supplement Figure 8).
For the patients using cisplatin, the RR was 0.93 (95% CI 0.78 to 1.10), and for those using oxaliplatin, the RR was 0.93 (95% CI 0.89 to 0.98). The test for interaction was not significant (P = 0.95, I2 = 0%) (Supplement Figure 9).
Seven RCTs comprising 1,010 patients that compared sorafenib monotherapy with either HAIC + sorafenib or HAIC alone were identified (10–15, 19). The findings of this meta-analysis study suggest that HAIC, with or without sorafenib, may improve OS, PFS, and response rates in advanced HCC patients without significant differences in adverse events.
Most of the included RCTs demonstrated the survival benefits of HAIC treatments, while the studies conducted by Kondo et al. and Kudo et al. did not show such benefits (10, 15). However, it is imperative to approach the interpretation of their results with caution. Both trials utilized cisplatin monotherapy or low-dose cisplatin with fluorouracil. In the study by Kondo et al., the concept of “Clinical PD” was introduced based on the levels of alpha-fetoprotein (AFP) and des-gamma carboxyprothrombin (DCP). However, the authors interpreted that clinical PD is unlikely to be disadvantageous. Furthermore, in the sorafenib group, more than half of the patients who received subsequent treatments underwent HAIC, suggesting a potential crossover effect that should not be overlooked (15). In the study by Kudo et al., the calculated sample size for each group was 95 patients, and 102 patients were allocated to the HAIC + sorafenib group. However, 14 patients did not receive treatment, resulting in an underpowered sample size (10).
The initial expectation was that the combination of sorafenib and HAIC would yield better results compared to HAIC alone. Overall, HAIC plus sorafenib showed favorable OS, PFS, and ORRs compared with sorafenib alone; however, those of the HAIC alone groups were numerically better (Tables 1, 2). Due to significant heterogeneities observed in the analyses of HAIC plus sorafenib, conducting subgroup analyses based on the HAIC regimen provided valuable insights into the underlying reasons (Supplement Figures 1, 3). Among the five RCTs that used HAIC plus sorafenib, three utilized cisplatin-based chemotherapy (10, 15, 19), while the other two employed oxaliplatin-based chemotherapy (13, 14). As depicted in Supplement Figures 1 and 3, the subgroup receiving the oxaliplatin-based treatment demonstrated significantly better survival outcomes compared with the subgroup receiving the cisplatin-based treatment. The two RCTs investigating HAIC alone employed a higher dose of cisplatin (60 mg/m2/cycle) or oxaliplatin in combination with fluorouracil (11, 12). Additionally, notable differences were observed in the intervals between each HAIC cycle. In the subgroup receiving the cisplatin-based chemotherapy, the intervals ranged from 4 to 6 weeks (10, 15, 19), which were longer than the intervals in the other studies, which ranged from 3 to 4 weeks (11–14).
In all the included RCTs, patients treated with HAIC demonstrated higher response rates than patients receiving sorafenib (range, HAIC with or without sorafenib vs. sorafenib, 17.1–54.8% vs. 3.1–18.0%). Particularly, He et al. (13). reported that a significantly higher number of patients in the HAIC plus sorafenib group proceeded to curative surgery compared with the sorafenib group (HAIC + sorafenib vs. sorafenib, 16 [12.8%] vs. 1 [0.8%], P < 0.001). Similar results were reported by Lyu et al. (12). These findings suggest that HAIC may contribute to downstaging and enabling a switch to curative surgery, thereby improving survival outcomes. In the phase III trial of sorafenib conducted in Western countries, the ORR of sorafenib was 2% (7 of 299 patients), and in the Asia-Pacific region, the ORR was 3.3% (5 of 150 patients) (28, 29). In the updated report of the IMbrave150 study, the ORR based on mRECIST for atezolizumab plus bevacizumab was 30% (97 of 326 patients), while the ORR for sorafenib was 11% (18 of 159 patients) (30). Considering the relatively high ORR observed with HAIC and the possibility of crossover to curative resection or locoregional therapy, HAIC may have a crucial role in the treatment of HCC, even in the era of immune checkpoint inhibitors.
Major vascular invasion or portal vein thrombosis are significant adverse prognostic factors for HCC (3). In the subgroup analyses according to the portal vein invasion and thrombosis (PVTT), HAIC plus sorafenib showed a trend toward improved OS and PFS compared to sorafenib alone, especially in patients with PVTT. However, it did not reach statistical significance.
There have been several published systematic reviews on this topic that have presented the positive effect of HAIC on HCC treatments. Long et al. recently reported that sorafenib plus HAIC showed significantly better OS (HR 0.56 [95% CI 0.37–0.83]; P < 0.01), PFS (HR 0.44 [95% CI 0.27–0.72]; P < 0.01), and ORR (RR 3.77 [95% CI 1.87–7.58]; P < 0.01) than sorafenib alone (31). Zhang et al. also conducted a recent systematic review, comparing HAIC to sorafenib in advanced HCC with PVTT, and reported significant improvements in OS (HR 0.50, 95% CI 0.40–0.63, P < 0.05), PFS (HR = 0.49, 95% CI 0.35–0.67, P < 0.05), and ORR (RR 4.21, 95% CI 2.44–7.28, P < 0.000001) (32). Additionally, this study demonstrated the benefits of HAIC, whether with or without sorafenib.
However, prior reported systematic reviews have exhibited some methodologic errors. Two systematic reviews were published in 2019 and 2022. Although the authors assessed the quality of the studies, they did not evaluate the risk of bias in the included studies (16, 33). Zhang et al. evaluated the quality of the cohort studies using the Newcastle Ottawa scale and conducted a risk assessment according to the Cochrane Collaboration Network recommendations (32). However, they also neglected to include the risk of bias assessment. Long et al. used the GRADE method and presented the risk of bias but did not incorporate this into the interpretation of the review results (31). However, the most considerable error is that all the aforementioned systematic reviews conducted meta-analyses by combining retrospective or observational studies with prospective RCTs. These methodologic errors can introduce flaws in the results.
The rigorous methodology is a strength of this review. This systematic review was conducted based on a prospectively registered protocol, and an experienced information specialist performed the comprehensive literature search. Unlike most of the previous reviews, this study exclusively included RCTs for the meta-analysis. Furthermore, this review is unique in that the GRADE method was adopted, incorporating a certainty of evidence rating and presenting the absolute effect sizes within a clinical context.
However, this study also has some limitations. The combination of atezolizumab with bevacizumab is currently the first choice for advanced HCC, and other targeted agents, such as Lenvatinib, are also an acceptable alternative treatment for advanced HCC. However, since there were no RCTs comparing HAIC to these systemic therapies, we were unable to investigate the relative impacts of HAIC compared to the recently updated treatment for HCC. All of the included RCTs were performed in Asian countries, where the main cause of HCC is hepatitis B virus (HBV) infections. In contrast, patients with hepatitis C virus (HCV) infections, which are the primary cause of HCC in Western countries, comprised a small portion of this study. Therefore, it is necessary to conduct more RCTs in Western countries to obtain a comprehensive understanding of the effectiveness of HAIC for HCC treatment across different populations and etiologies. The small sample size of RCTs was another limitation of this study. Since the current body of evidence is moderate to very low, more robust randomized trials are needed to confirm the efficacy of HAIC.
This study provides evidence supporting the use of HAIC to treat advanced HCC. Furthermore, this study suggests that HAIC with an oxaliplatin-based regimen may contribute to a higher survival rate than HAIC with a cisplatin-based regimen.
HAIC represents a potential alternative for advanced HCC treatment, offering advantages over systemic therapies. Combinations of cytotoxic chemotherapy and targeted agents have emerged as a major trend in anti-cancer treatments, but their application in HCC has been limited due to the low efficacy of systemic administration of cytotoxic chemotherapy. HAIC offers the possibility of combining therapies in HCC while minimizing systemic adverse events. Additionally, HAIC may serve as a bridging therapy or induction therapy for curative resection or locoregional treatments.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
HK: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. SL: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. HS: Investigation, Methodology, Supervision, Validation, Writing – review & editing. HB: Formal Analysis, Investigation, Methodology, Writing – original draft. SC: Investigation, Methodology, Supervision, Validation, Writing – review & editing. IC: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – review & editing. EH: Formal Analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing – review & editing. JH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing, Writing – original draft. WB: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Bio & Medical Technology Development Program of NRF (NRF-2020M3A9G3080281) and NRF grant (NRF-2020R1A5A2031185) funded by MSIT to WB.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1265240/full#supplementary-material
1. International Agency for Research on Cancer, GLOBOCAN 2020. Available at: http://gco.iarc.fr/.
2. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology (2018) 68(2):723–50. doi: 10.1002/hep.29913
3. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol (2022) 76(3):681–93. doi: 10.1016/j.jhep.2021.11.018
4. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol (2022) 28(4):583–705. doi: 10.3350/cmh.2022.0294
5. Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer (2021) 10(3):181–223. doi: 10.1159/000514174
6. National Comprehensive Cancer Network. Hepatocellular Carcinoma (Version 1.2023. Available at: https://www.nccn.org/professionals/physician_gls/pdf/hcc.pdf.
7. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr (2020) 9(4):452–63. doi: 10.21037/hbsn-20-480
8. Mir O, Coriat R, Boudou-Rouquette P, Ropert S, Durand JP, Cessot A, et al. Gemcitabine and oxaliplatin as second-line treatment in patients with hepatocellular carcinoma pre-treated with sorafenib. Med Oncol (2012) 29(4):2793–9. doi: 10.1007/s12032-012-0208-x
9. Lee JE, Bae SH, Choi JY, Yoon SK, You YK, Lee MA. Epirubicin, cisplatin, 5-FU combination chemotherapy in sorafenib-refractory metastatic hepatocellular carcinoma. World J Gastroenterol (2014) 20(1):235–41. doi: 10.3748/wjg.v20.i1.235
10. Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol (2018) 3(6):424–32. doi: 10.1016/S2468-1253(18)30078-5
11. Choi JH, Chung WJ, Bae SH, Song DS, Song MJ, Kim YS, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol (2018) 82(3):469–78. doi: 10.1007/s00280-018-3638-0
12. Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li SL, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: A biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol (2022) 40(5):468–80. doi: 10.1200/JCO.21.01963
13. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial. JAMA Oncol (2019) 5(7):953–60. doi: 10.1001/jamaoncol.2019.0250
14. Zheng K, Zhu X, Fu S, Cao G, Li WQ, Xu L, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: A randomized trial. Radiology (2022) 303(2):455–64. doi: 10.1148/radiol.211545
15. Kondo M, Morimoto M, Kobayashi S, Ohkawa S, Hidaka H, Nakazawa T, et al. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer (2019) 19(1):954. doi: 10.1186/s12885-019-6198-8
16. Zhuang BW, Li W, Xie XH, Hu HT, Lu MD, Xie XY. Sorafenib versus hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: a systematic review and meta-analysis. Jpn J Clin Oncol (2019) 49(9):845–55. doi: 10.1093/jjco/hyz069
17. Long GB, Xiao CW, Zhao XY, Zhang J, Li X. Effects of hepatic arterial infusion chemotherapy in the treatment of hepatocellular carcinoma: A meta-analysis. Med (Baltimore) (2020) 99(26):e20745. doi: 10.1097/MD.0000000000020745
18. Liu M, Shi J, Mou T, Wang Y, Wu Z, Shen A. Systematic review of hepatic arterial infusion chemotherapy versus sorafenib in patients with hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol Hepatol (2020) 35(8):1277–87. doi: 10.1111/jgh.15010
19. Ikeda M, Shimizu S, Sato T, Morimoto M, Kojima Y, Inaba Y, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol (2016) 27(11):2090–6. doi: 10.1093/annonc/mdw323
20. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis (2010) 30(1):52–60. doi: 10.1055/s-0030-1247132
21. Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane (2022). Available at: www.training.cochrane.org/handbook.
22. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane (2022). Available at: www.training.cochrane.org/handbook.
23. Kirmayr M, Quilodrán C, Valente B, Loezar C, Garegnani L, Franco JVA. The GRADE approach, Part 1: how to assess the certainty of the evidence. Medwave (2021) 21(2):e8109. doi: 10.5867/medwave.2021.02.8109
24. GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University and Evidence Prime (2022). Available from: https://gradepro.org/
25. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol (2011) 64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026
26. Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane (2022). Available at: www.training.cochrane.org/handbook.
27. Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol (2020) 119:126–35. doi: 10.1016/j.jclinepi.2019.10.014
28. Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol (2009) 10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7
29. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, et al. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med (2008) 359(4):378–90. doi: 10.1056/NEJMoa0708857
30. Cheng A-L, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol (2022) 76(4):862–73. doi: 10.1016/j.jhep.2021.11.030
31. Long Y, Song X, Guan Y, Lan R, Huang Z, Li S, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib alone for advanced hepatocellular carcinoma: A systematic review and meta-analysis. J Gastroenterol Hepatol (2023) 38(4):486–95. doi: 10.1111/jgh.16088
32. Zhang W, Ouyang D, Huang Z, Che X. Hepatic arterial infusion chemotherapy versus sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombus: An updated meta-analysis and systematic review. Front Oncol (2023) 13. doi: 10.3389/fonc.2023.1085166
Keywords: intra-arterial infusions, carcinoma, hepatocellular carcinoma, drug therapy, survival, GRADE approach (MeSH)
Citation: Kim HJ, Lee SH, Shim HJ, Bang HJ, Cho SH, Chung IJ, Hwang EC, Hwang JE and Bae WK (2023) Hepatic arterial infusion chemotherapy versus systemic therapy for advanced hepatocellular carcinoma: a systematic review and meta-analysis. Front. Oncol. 13:1265240. doi: 10.3389/fonc.2023.1265240
Received: 22 July 2023; Accepted: 18 September 2023;
Published: 10 October 2023.
Edited by:
Alberto Brolese, Department of General Surgery and HPB Unit - APSS, ItalyReviewed by:
Robert Damm, University Hospital Magdeburg, GermanyCopyright © 2023 Kim, Lee, Shim, Bang, Cho, Chung, Hwang, Hwang and Bae. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Woo Kyun Bae, ZHJ3b29reXVuQGNob25uYW0uYWMua3I=; Jun Eul Hwang, aGp1bnlsQG5hdmVyLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.