- 1Department of Medical Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Respiratory and Critical Care Medicine, Fuyang People’s Hospital, Fuyang, China
Background: This study aimed to investigate the efficacy of immunotherapy, as monotherapy or in combination, comparing to chemotherapy with or without anti-angiogenesis for advanced non-small cell lung cancer (NSCLC) patients progressing to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs).
Methods: We retrospectively analyzed patients with advanced NSCLC harboring EGFR mutations who received immune checkpoint inhibitors (ICI) and/or chemotherapy after EGFR-TKIs failure at Shanghai Chest Hospital between Aug 2016 and Oct 2022. According to the subsequent immunotherapy regimen, the patients were assigned to ICI monotherapy (IM), IO plus anti-angiogenesis (IA), ICI plus chemotherapy (IC), ICI plus chemotherapy plus anti-angiogenesis (ICA). Eligible patients undergoing standard chemotherapy were assigned to chemotherapy plus anti-angiogenesis (CA) and chemotherapy alone (CM). Efficacy was evaluated according to the RECIST 1.1version, and calculated the objective response rate (ORR) and disease control rate (DCR). Survival curves were plotted using the Kaplan-Meier method, and the median progression-free survival (PFS) was calculated. Differences among survival curves of the six groups were assessed using the log-rank test.
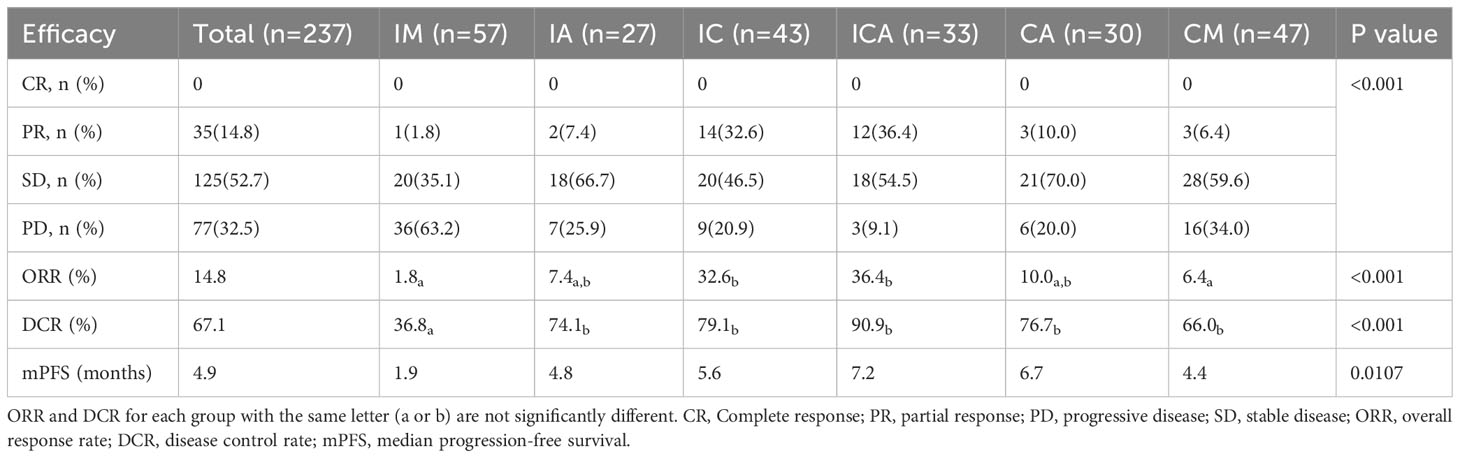
Results: A total of 237 advanced NSCLC patients with EGFR mutations were included in this study. Of the 160 patients who received immunotherapy, 57 received ICI monotherapy, 27 received ICI plus anti-angiogenesis therapy, 43 received ICI plus chemotherapy, and 33 received ICI plus anti-angiogenesis plus chemotherapy. 77 patients received standard chemotherapy, of which 30 received chemotherapy plus anti-angiogenesis and 47 received chemotherapy alone. Patients in ICA group showed significant longer PFS than IM (7.2 vs 1.9 months, P=0.011), IA (7.2 vs 4.8 months, P=0.009) and CM group (7.2 vs 4.4 months, P=0.005). There was no significant difference in PFS between the ICA and IC (7.2 vs 5.6 months, P=0.104) or CA (7.2 vs 6.7 months, P=0.959) group. Meanwhile, the ICA group showed the highest ORR and DCR (36.4% and 90.9%) compared to the other five groups. The IC group had a higher ORR than the IA and CA group (32.6% vs 7.4% vs 10.0%, respectively), but the DCR was comparable (79.1% vs 74.1% vs 76.7%, respectively). The ORR of the CM group was 6.4% and the DCR was 66.0%. IM group showed the lowest ORR and DCR (1.8% and 36.8%). Treatment-related adverse events (TRAEs) of grade 3 or worse occurred in 9 (27.3%) patients in the ICA group, 6 (20.0%) in the CA group, 7 (14.9%) in the CM group, 5 (11.6%) in the IC group, 5 (8.8%) in the IM group, and 2 (7.4%) in the IA group.
Conclusion: NSCLC patients with positive EGFR mutations after EGFR-TKIs failure received subsequent immunotherapy plus anti-angiogenesis and chemotherapy are likely to have more benefits in ORR, DCR and mPFS.
Highlights
Key findings
● Immunotherapy plus anti-angiogenesis and chemotherapy may have survival benefits than other regimens in NSCLC patients after EGFR-TKIs failure.
What is known and what is new?
● Patients with advanced NSCLC harboring EGFR mutations have limited benefit from chemotherapy or ICI monotherapy after TKIs failure, and combination therapy is the trend. But the optimal treatment strategy is currently controversial.
● Our study compared the efficacy of four immunotherapy-based therapies with chemotherapy alone or chemotherapy plus anti-angiogenesis to find the optimal treatment regimen for NSCLC patients after EGFR-TKIs failure.
What is the implication, and what should change now?
● Our results can provide references for clinicians' treatment choice. Immunotherapy plus anti-angiogenesis and chemotherapy may be the optimal immunotherapy-based combination therapy, but more prospective studies are needed.
Introduction
The most common driver gene mutation of non-small cell lung cancer (NSCLC) in East Asians is epidermal growth factor receptor (EGFR) mutation (1). EGFR receptor tyrosine kinase inhibitors (TKIs) are preferred for EGFR-sensitive mutations in clinical setting; however, most patients experience problems with acquired resistance and disease progression after receiving EGFR-TKIs for approximately 10 to 18 months (2, 3). The use of standard platinum-based dual-drug chemotherapy as subsequent therapy had a limited effect; therefore, more effective treatment strategies should be investigated for patients who progress on EGFR-TKIs therapy.
Immune checkpoint inhibitors (ICI), including cytotoxic T lymphocyte-associated antigens-4 (CTLA-4) inhibitors, programmed cell death 1 (PD-1) inhibitors and programmed cell death ligand 1 (PD-L1) inhibitors, counteract the immunosuppressive effect of tumors and reactivate the immune response of T cells to inhibit tumor cell growth (4). Several studies have demonstrated the efficacy of ICI therapy in advanced NSCLC (5). Immune checkpoint inhibitors such as the PD-1 inhibitor Nivolumab, Pembrolizumab, and the PD-L1 inhibitor Atezolizumab, and the CTLA-4 inhibitor Ipilimumab have been approved for the treatment of advanced NSCLC. However, first-line immunotherapy has poor efficacy for NSCLC with EGFR mutations (6). Clinical studies have found that the tumor immune microenvironment is altered after receiving EGFR-TKIs targeted therapy, including an increase in CD8+ tumor-infiltrating lymphocytes (TILs) density, TMB, and PD-L1 expression in tumor cells (7, 8). This finding suggests the possibility of ICI monotherapy or ICI-based combination therapy for this population. Results from the IMpower150 and ORIENT31 studies further support the administration of ICI-based combination therapy in this setting (9, 10). However, the phase 3 clinical trials CheckMate-722 and KEYNOTE-789 comparing immunotherapy combined with chemotherapy with chemotherapy alone did not show significant results (11, 12). There remains controversy regarding subsequent therapeutic strategies for NSCLC patients harboring EGFR mutations previously treated with EGFR-TKIs. Therefore, our study retrospectively reviewed patients with advanced NSCLC after EGFR-TKIs failure in Shanghai Chest Hospital and analyzed clinical outcomes, safety and relevant influential factors of different treatment options.
Materials and methods
Clinical data
This single-center retrospective study was conducted to compare the efficacy of immunotherapy-based regimens with chemotherapy after EGFR-TKIs resistance in EGFR-mutant advanced NSCLC patients. Patients with advanced NSCLC after EGFR-TKIs failure at Shanghai Chest Hospital between September 2016 and May 2020 were identified. Patients who interrupted the treatment or did not have imaging data for efficacy assessment were excluded. Due to the small size of the dual immunotherapy patients, they were ultimately not included in the analysis. The baseline clinical characteristics including age, sex, smoking history, tumor pathology type, EGFR mutation subtype, TNM stage, PD-L1 expression status, Eastern Cooperative Oncology Group (ECOG) performance status (PS) score, and lines of therapy were calculated. This study was conducted in accordance with the tenets of the Declaration of Helsinki and the principles of good clinical practice. The protocol and its amendments were approved by the institutional ethical review board of Shanghai Chest Hospital. The ethical review committee waived the requirement for individual informed consent from the patients in this study because of the use of anonymous medical records.
The inclusion and exclusion criteria
The inclusion criteria were as follows: 1. histologically or cytologically confirmed non-small cell lung cancer; 2. diagnosis of stage IIIB-IV based on the 8th edition of TNM staging system; 3. harboring EGFR-activating mutations confirmed by tumor histology, cytology, or circulating tumor DNA; 4. previously received at least one EGFR-TKI and had evidence of radiological disease progression; 5. the participants had an ECOG PS of 0-1; 6. at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
The exclusion criteria were as follows: 1. Prior systemic immunotherapy before EGFR-TKIs; 2. The clinical data were incomplete; 3. Simultaneous diagnosis of any active autoimmune disease or history of autoimmune diseases; 4. no available imaging data to assess the response to immunotherapy or chemotherapy; 5. discontinuation of treatment for any reason.
Pathology, biomarkers and molecular diagnostics
Tumor samples collected from biopsy or surgical resection were used for immunohistochemical detection and confirmed pathological diagnosis of NSCLC. The PD-L1 status was determined by immunohistochemistry (IHC) staining. PD-L1 tumor proportion score (TPS) was defined as the proportion of tumor cells with partial or complete cell membrane staining. PD-L1 expression <1% was classified as a negative result; PD-L1 expression ≥1% and <50% was classified as a positive result; PD-L1 expression ≥50% was classified as a strong positive result. EGFR gene subtype mutations were evaluated by detecting blood or tumor tissue samples using next-generation sequencing (NGS) or polymerase chain reaction (PCR).
Efficacy and safety assessment
Tumor response was assessed using RECIST v1.1. Complete response (CR), complete disappearance of the target lesion, and the short diameter of the pathological lymph nodes decreased to less than 10 mm; partial response (PR), the sum of the target lesion diameter decreased by at least 30%; progressive disease (PD), the sum of the target lesion diameter increased by at least 20% or the appearance of new lesions; stable disease (SD), the increase in diameter of the target lesion did not achieve PD, and the degree of decrease did not achieve PR. Efficacy evaluation includes the overall response rate (ORR) and disease control rate (DCR), where ORR was defined as the proportion of patients who had a CR or PR, and DCR was defined as the proportion of patients who had CR, PR, or SD. Imaging was performed for at least once per cycle during the treatment phase. Interruption of treatment occurred at the time of radiographically identified disease progression. Long term outcomes were evaluated using progression-free survival (PFS), which was measured from the initiation of treatment to radiographic progression. The cut-off date was March 2023, and patients who remained unprogressive at this time were recorded as censored. Adverse events were reported during study treatment and for 30 days after treatment ended and graded as per the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0).
Statistical analysis
Data were analyzed by the software SPSS version 25.0 and GraphPad Prism version 8.0. Continuous variables (e.g age) were presented as medians and ranges. Categorical variables were presented as numbers and percentiles. Survival curves were plotted and median PFS (mPFS) was calculated using the Kaplan-Meier (KM) method. The Log-Rank test was used to compare survival differences between groups. PFS relevant influential factors were explored by evaluating hazard ratios (HR) and associated 95% CIs using the Cox proportional hazard model. Differences in ORR, DCR or the other categorical variables were evaluated using Chi-square (χ2) test or Fisher exact test, as appropriate. Statistical significance was defined as two-sided P values of less than 0.05.
Results
Patient characteristics
In total, 855 patients were assessed for eligibility. Based on the inclusion and exclusion criteria, 237 patients were finally included in the analysis. According to the subsequent regimen, the patients who received immunotherapy were further subdivided into four groups: 57 received ICI monotherapy (IM), 27 received ICI plus anti-angiogenesis (IA), 43 received ICI plus chemotherapy (IC), and 33 received ICI plus chemotherapy plus anti-angiogenesis (ICA). Patients who received standard chemotherapy were separated into two groups: 30 received chemotherapy plus anti-angiogenesis (CA) and 47 received chemotherapy alone (CM) (Figure 1). The median age of all patients was 61 years (range 35-77 years), and 56.5% of the patients were female (n=134). 98.7% of patients were diagnosed with lung adenocarcinoma (n=234). Two patients who received immunotherapy were diagnosed with poorly differentiated non-small cell lung cancer. One patient who received chemotherapy was diagnosed with lung squamous cell carcinoma. Of the patients, 89% (n=211) harbored common EGFR mutations, with EGFR exon 19 deletion (19del) in 54.8% (n=130), EGFR exon 21 L858R mutation (21L858R) in 34.2% (n=81), and the remaining 11.0% (n=26) harbored EGFR rare mutations. 66.2% (n=157) of patients were diagnosed with stage IVB, 25.3% (n=60) with stage IVA, and 8.4% (n=20) with stage IIIB or IIIC. All patients had good performance status (ECOG=0-1). 30.8% (n=73) of the patients acquired the T790M mutation after receiving the first or second generation of EGFR-TKIs. PD-L1 expression was detected in 45.6% (n=108) of all patients, and 41.4% (n=98) of those patients had received immunotherapy. In total, 61.6% (n=146) of the patients received PD-1 inhibitors, 5.9% (n=14) received PD-L1 inhibitors, and 32.5% (n=77) received chemotherapy. Detailed treatments of immunotherapy populations are showed in Table S1. 36.3% (n=86) of the patients received fourth line or later line of therapy, 29.5% (=70) received third line of therapy, and 34.2% (n=81) received second line of therapy. Except lines of therapy and PD-L1 status, demographic and clinical characteristics were well balanced among the groups at baseline, and the data are listed in Table 1.
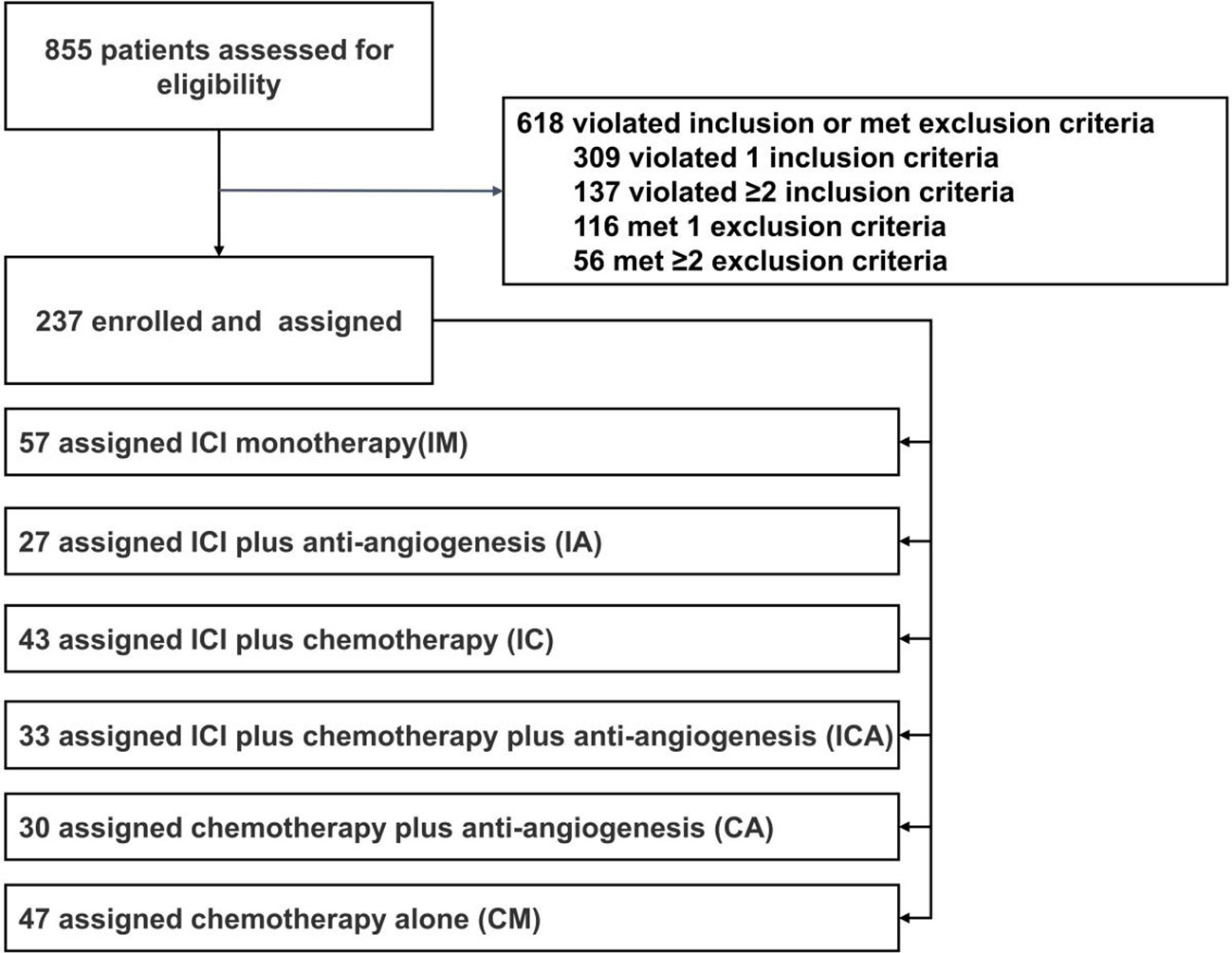
Figure 1 Study flow chart. Between September 2016 and May 2020,855 patients were assessed for eligibility, and 237 patients were finally brought into the analysis, include 57 received ICI monotherapy (IM), 27 received ICI plus anti-angiogenesis (IA), 43 received ICI plus chemotherapy (IC), 33 received ICI plus chemotherapy plus anti-angiogenesis (ICA),30 received chemotherapy plus anti-angiogenesis (CA) and 47 received chemotherapy alone (CM).
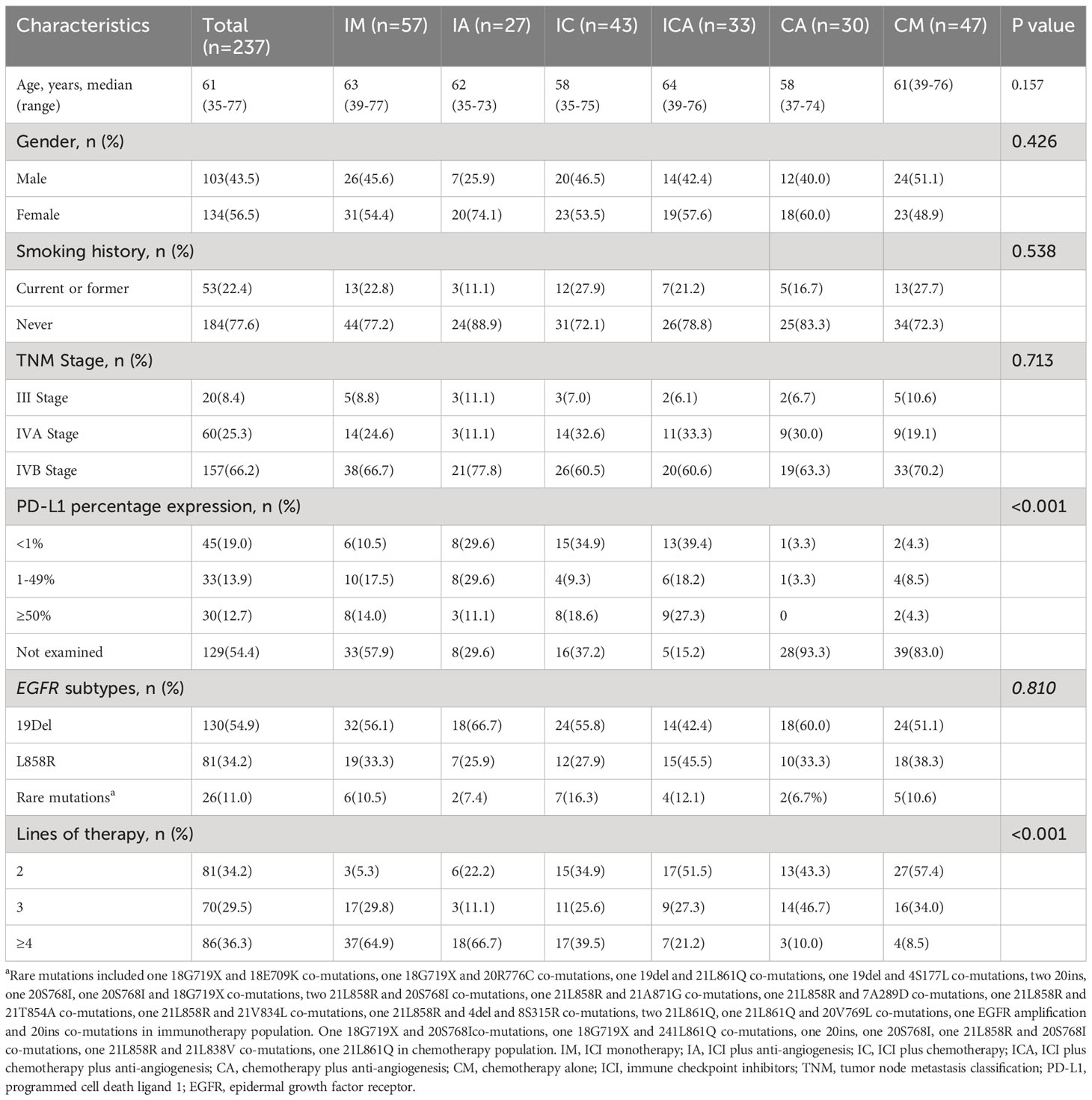
Table 1 Baseline demographic and clinical characteristics of patients stratified by treatment strategies after TKIs failure (n=237).
Efficacy evaluation
For the entire study population, the ORR was 14.8% and the DCR was 67.1% (Table 2). Comparative analysis of six groups, the highest ORR was observed in the ICA group, followed by the IC, CA, IA, CM group, and the lowest ORR was observed in the IM group (36.4% vs 32.6% vs 10.0% vs 7.4% vs 6.4% vs 1.8%, Table 2). Similarly, the highest DCR was observed in the ICA group, followed by the IC, CA, IA, CM group, and the lowest DCR was observed in the IM group (90.9% vs 79.1% vs 76.7% vs 74.1% vs 66.0% vs 36.8%, Table 2). The ICA group and IC group showed similar ORR (36.4% and 32.6%, respectively, Table 2), which were higher than those of the other four groups. Meanwhile, the ICA group showed a higher DCR (90.9%) than the other five groups (Table 2). There was no significant difference between the DCR of the IC, IA, and CA group (79.1% vs 74.1% vs 76.7%, respectively) (Table 2). ICI monotherapy resulted in a significant lower DCR (36.8%) than those of other five groups (Table 2).
Long term outcomes
The median PFS of all eligible patients was 4.9 months (Table 2). The mPFS was longest in the ICA group, followed by CA, IC, IA and CM group, and shortest in IM group (7.2 [95%CI: 4.4-10.0 months] vs 6.7 [95%CI: 4.6-8.8 months] vs 5.6 [95%CI: 4.8-6.4 months] vs 4.8 [95%CI: 2.8-6.8 months] vs 4.4 [95%CI: 3.6-5.5 months] vs 1.9 [95%CI: 1.0-2.8 months]) (Figure 2A). Patients in ICA group showed significant longer PFS than IM (7.2 vs 1.9 months, P=0.011), IA (7.2 vs 4.8 months, P=0.009) and CM (7.2 vs 4.4 months, P=0.005) group (Figure 2B). Similarly, patients in CA group showed significant longer PFS than IM (6.7 vs 1.9 months, P=0.012), IA (6.7 vs 4.8 months, P=0.018) and CM (6.7 vs 4.4 months, P=0.008) group (Figure 2B). There was no significant difference in PFS between the ICA and CA (7.2 vs 6.7 months, P=0.959) group (Figure 2B). The mPFS of IC group was longer than IM (5.6 vs 1.9 months, P=0.183), IA (5.6 vs 4.8 months, P=0.083) and CM (5.6 vs 4.4 months, P=0.145) group, but without statistical difference (Figure 2B). In these analyses, both ICA and CA therapy showed superior mPFS than other therapeutic strategies.
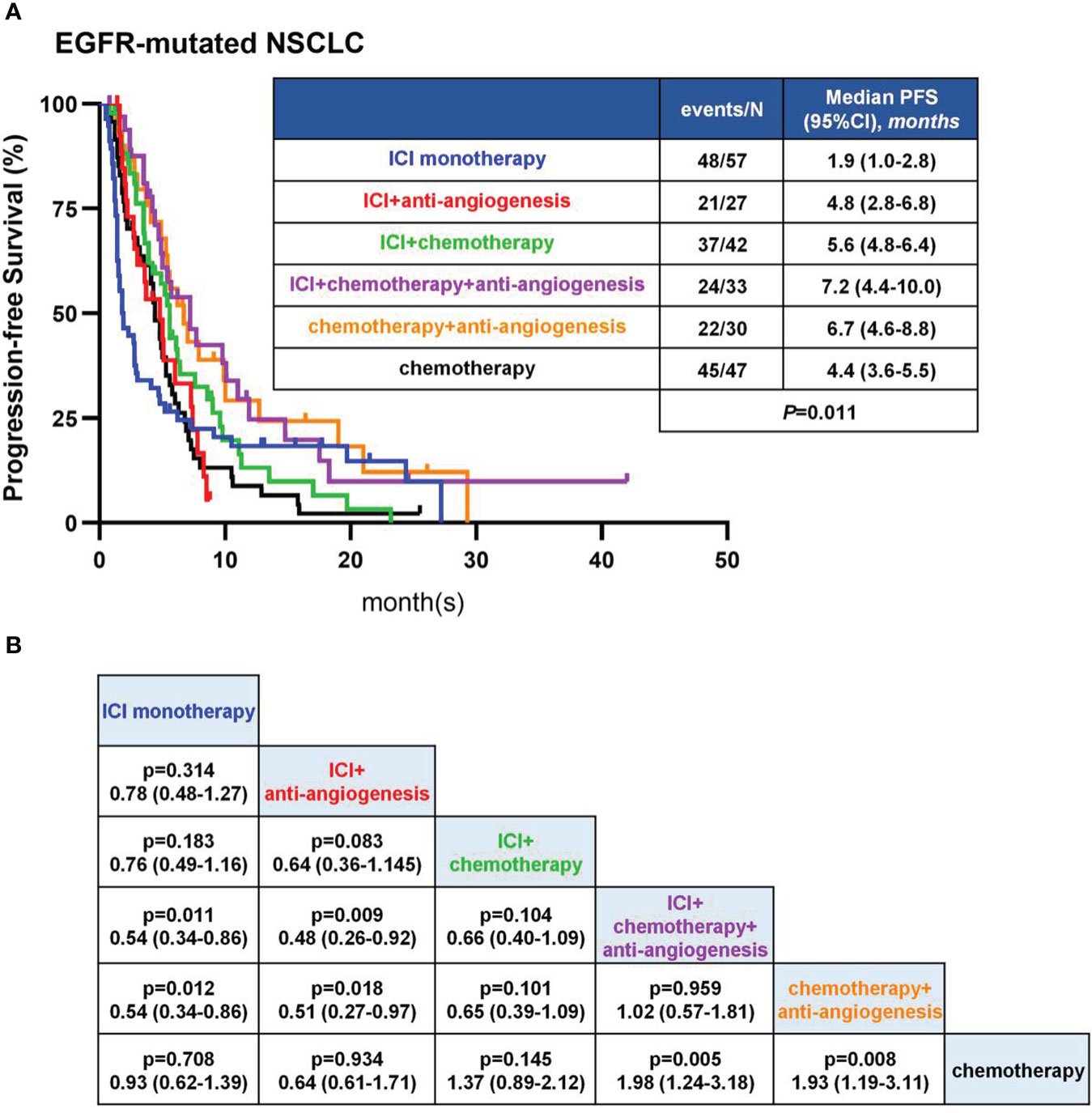
Figure 2 PFS in EGFR-mutant patients. (A) Kaplan-Meier analyses in different therapies. (B) Pairwise comparisons among therapies in log-rank test.
Safety
TRAEs had the highest incidence rate in the ICA group(26 [78.8%]) followed by the CA group(21 [70.0%]).TRAEs of any grade occurred in a similar proportion of patients in IA, IC, CM group (16 [59.3%] vs 25 [58.1%] vs 30 [63.8%]).While the IM group had the lowest incidence of TRAEs(22 [38.6%]).TRAEs of grade 3 or worse occurred in 9 (27.3%) patients in the ICA group, 6 (20.0%) in the CA group, 7 (14.9%) in the CM group, 5 (11.6%) in the IC group, 5 (8.8%) in the IM group, and 2 (7.4%) in the IA group. The most common TRAEs were leukopenia (4 [7.0%] in the IM group vs 0 in the IA group vs 18 [41.9%] in the IC group vs 19 [57.6%] in the ICA group vs 14 [46.7%] in the CA group vs 21 [44.7%] in the CM group). Details of TRAEs were provided in Table S2.
Clinical factors associated with PFS
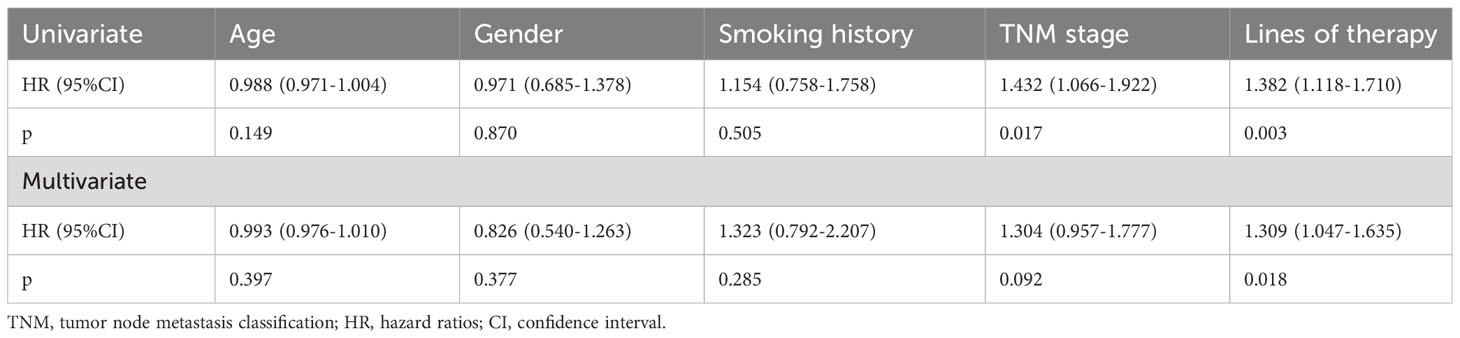
A total of 160 patients who received immunotherapy were included in the univariate and multivariate analyses. Age, gender, smoking history, TNM stage, and line of therapy were included as independent variables. Age was taken as a continuous numerical variable, and its measured value was taken. All of the categorical variables were summarized with frequencies and percentages and were analyzed using the χ2 or Fisher exact test, as appropriate. Univariate COX regression analysis showed that age, sex, and smoking history were not risk factors for disease progression in the patients receiving immunotherapy (Table 3). A more advanced disease stage seems to be related with a shorter PFS (Table 3). Patients receiving an earlier line of immunotherapy showed enhanced survival benefits compared with those who received immunotherapy as a later line (Table 3). Multivariate COX regression analysis showed that gender, age, smoking history, and TNM stage were not risk factors of disease progression in the immunotherapy group (Table 3). Similarly, the earlier immunotherapy was applied, the more it delayed disease progression (Table 3). It should be noted that, owing to the limited case size, the univariate and multivariate analyses did not include the EGFR mutant subtype and PD-L1 expression status as independent variables.
Subgroup analysis of patients with common EGFR mutations
Among 211 patients with common EGFR mutations, 141 patients received immunotherapy, including 88 patients with 19del and 53 patients with 21L858R mutation. There was no significant difference in ORR and DCR between 19del and 21L858R mutations subgroups (P=0.066, P=0.870, respectively) (Table S3). The mPFS was 3.7 months (95%CI: 2.6-4.8 months) in 19del group, and 4.9 months (95%CI: 3.5-6.3 months) in 21L8585R mutations group, with a tendency for longer PFS in 21L858R subgroup, but the difference was not significant (P=0.767) (Figure 3).
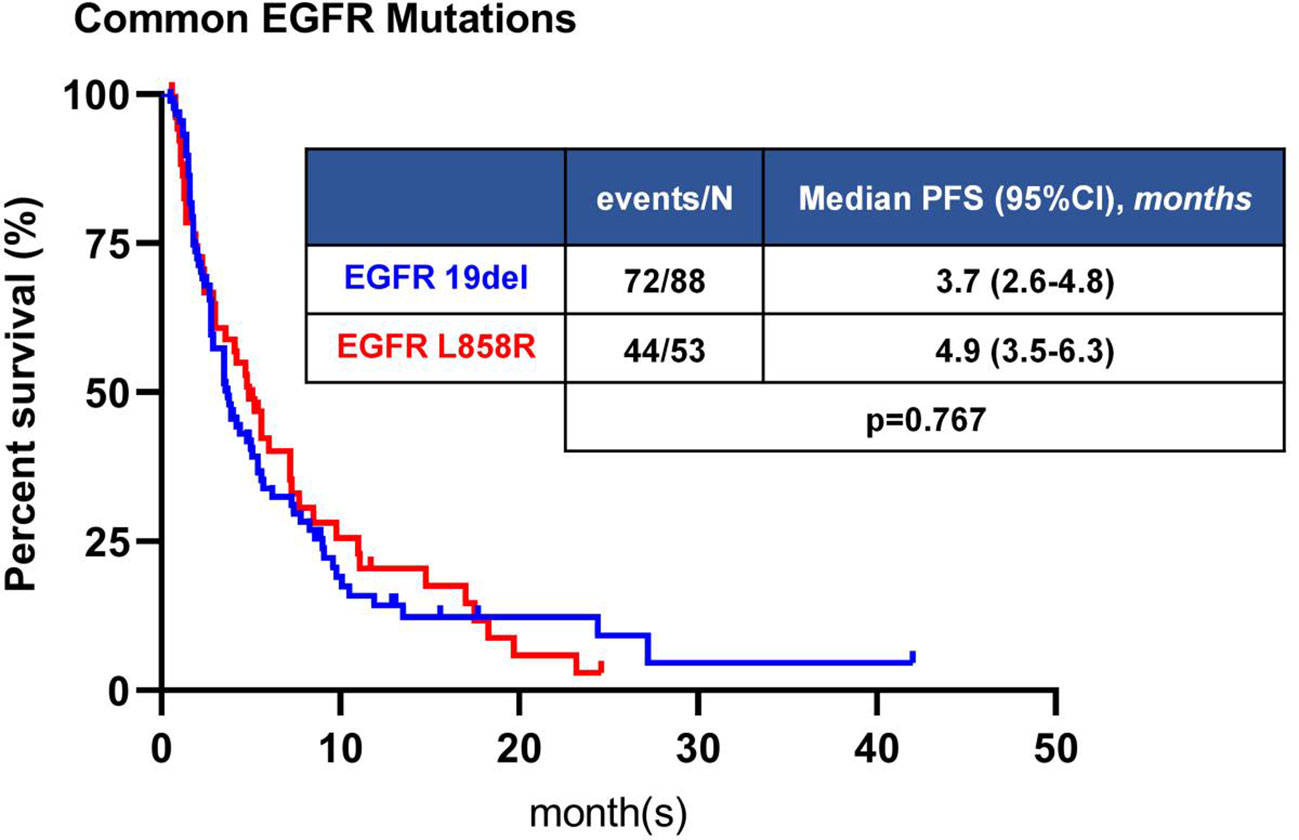
Figure 3 Kaplan-Meier analyses of PFS of patients with common EGFR mutation. The difference in PFS was not statistically significant between 19del and L858R.
Subgroup analysis of patients with PD-L1 expression
Among 160 patients receiving immunotherapy, PD-L1 expression was detected in 98 patients, of which 42 patients were PD-L1 negative (<1%), 28 patients were PD-L1 positive (1% -49%), and 28 patients were PD-L1 strongly positive (≥50%). All patients with PD-L1 expression who received immunotherapy had an ORR of 24.5%, DCR of 72.5%, and median PFS of 5.6 months (95%CI, 4.7-6.5 months) (Table S4). ORR of PD-L1 negative patients was 21.4%, DCR was 71.4%, median PFS was 5.1 months (95%CI, 3.2-7.0 months); for patients with PD-L1 positive, ORR was 14.3%, DCR was 64.3%, and median PFS was 5.6 months (95%CI, 3.6-7.6 months); for patients with PD-L1 strongly positive, ORR was 39.3%, DCR was 82.1%, and median PFS was 5.7 months (95%CI, 4.6-6.8 months) (Table S4). There was no significant difference in the ORR and DCR (P=0.078, P=0.312, respectively) among these three groups for pairwise comparisons (Table S4). The PD-L1 positive and strongly positive groups tended to have a longer PFS than the PD-L1 negative group, but the difference was not significant (P=0.211) (Figure 4).
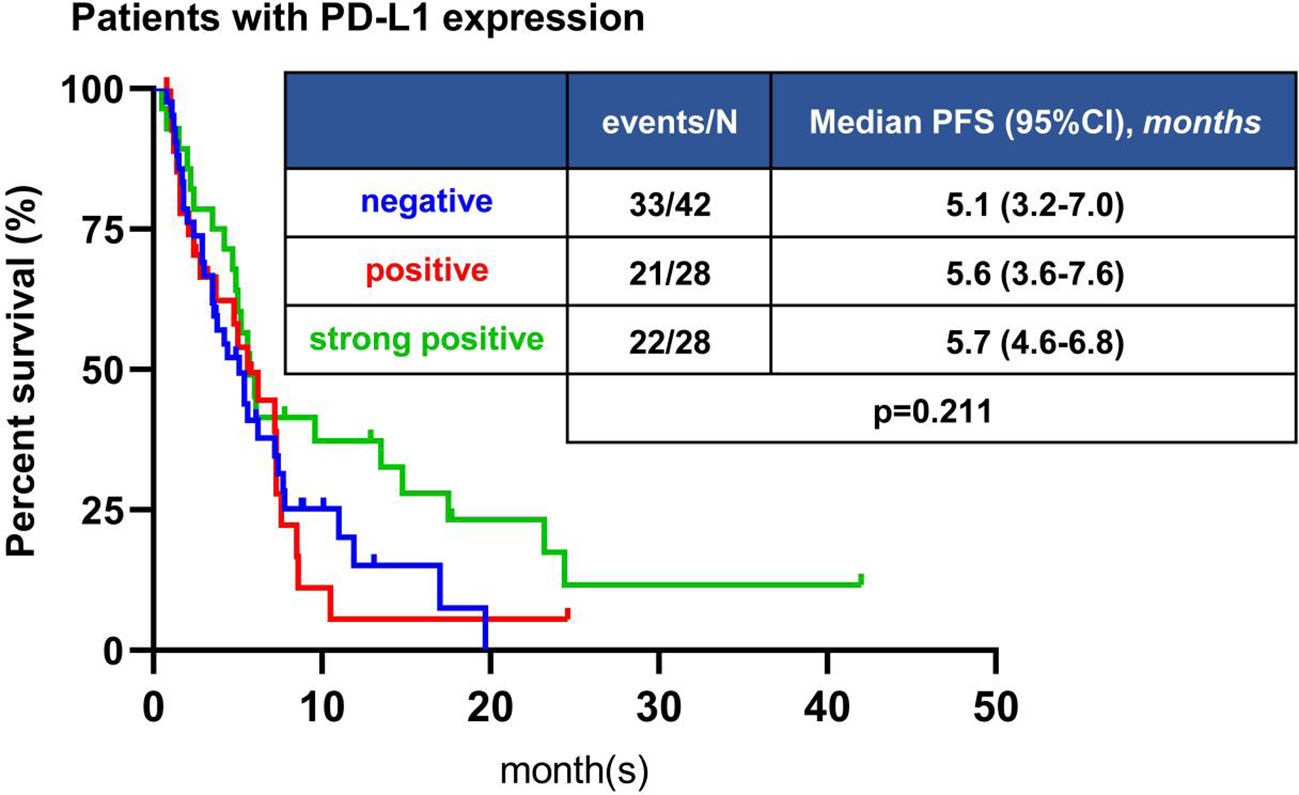
Figure 4 Kaplan-Meier analyses of PFS of patients with different PD-L1 expression. The difference in PFS was not statistically significant among different PD-L1 status.
Discussion
The standard first-line treatment for patients with advanced EGFR-mutated NSCLC are EGFR-TKIs. There are limited treatment options for patients who are refractory to third-generation EGFR-TKIs and for T790M-negative patients who have received first- or second-generation EGFR-TKIs. The most commonly recommended treatment is platinum-containing doublet chemotherapy with or without bevacizumab. In vitro experiments confirmed that the co-culture system of EGFR-mutant tumor cells with immune cells was able to reduce the viability of tumor cells after treatment with PD-1 inhibitors (13). The anti-tumor effect of PD-1 inhibitors has also been demonstrated in preclinical models with EGFR mutations (14). These preclinical studies suggested the possibility of treating EGFR-mutant NSCLC with ICI. However, the initial clinical findings did not support this. Several prospective studies have shown no survival benefit from the use of immunosuppressive agents versus chemotherapy in NSCLC patients with EGFR mutations (15–18). Moreover, most clinical trials of immune checkpoint suppression have also excluded NSCLC patients with driver gene mutations. Therefore, there is an urgent need to develop immune-based combination therapies for NSCLC patients with EGFR mutations.
Our study basically included a variety of mainstream and non-mainstream treatment regimens after TKIs resistance. Analysis of these regimens revealed that the mPFS was longest in the ICA group, followed by the CA, IC, IA, and CM group, and the shortest in the IM group. The ICA group had the highest ORR and DCR, followed by the IC, CA, IA, and CM group, and the lowest was in the IM group. Further differential analysis showed that in terms of mPFS, there was no significant difference between the ICA, CA, and IC group for pairwise comparisons. The same results were obtained from the IC, IA, CM, and IM group for pairwise comparisons. There were many interesting conclusions to draw from these analyses.
Combination regimens were superior to monotherapy. In terms of PFS, the ICA group was longer than the CM or IM group in our study. In terms of the ORR and DCR, the ICA group was better than the IM group. The prospective study ORIENT31 included 444 patients with EGFR mutations after EGFR-TKIs failure, randomly assigned to sintilimab plus IBI305 plus pemetrexed and cisplatin (ICA), sintilimab plus pemetrexed and cisplatin (IC), or pemetrexed and cisplatin groups (CM). The mPFS of the ICA group was 6.9 months and the mPFS for the CM group was 4.3 months in ORIENT31. The difference in PFS between the two groups was statistically significant (P<0.0001) (9). The mPFS in both groups was consistent with the results of our study. Otherwise, our study analysis showed a longer mPFS in the CA group than in the CM or IM group. According to a prospective study, the mPFS of patients with advanced NSCLC received bevacizumab plus chemotherapy was 6.2 months and chemotherapy alone was 4.5 months, and the difference was statistically significant (19). The results were also consistent with the results of our analysis. However, one study showed that chemotherapy combined with anti-angiogenesis was not superior to chemotherapy alone in patients with advanced non-small cell lung cancer (20). Many studies have confirmed the poor efficacy of ICI monotherapy in patients with EGFR-mutant NSCLC. A Japanese randomized controlled study included 102 patients with EGFR-mutant NSCLC after TKIs resistance, with a mPFS of 1.7 months in the nivolumab group and 5.6 months in the carboplatin-pemetrexed group and differences were significant (21). Therefore, CA efficacy was better than IM, which was in line with our expectations.
A subsequent question has to be raised: which regimen has better efficacy comparing ICA with CA? The prospective study Impower150 included 124 chemotherapy-naive NSCLC patients with EGFR mutations, of which 91 patients with EGFR sensitive mutations were assigned to three different regimens: atezolizumab plus bevacizumab plus carboplatin plus paclitaxel (ABCP), bevacizumab plus carboplatin plus paclitaxel (BCP), and atezolizumab plus carboplatin plus paclitaxel (ACP). The results showed that in the 124 EGFR mutation subgroup, the HR for ABCP versus BCP was 0.61 (95% CI 0.36-1.03). The mPFS was 10.2 months for the ABCP group, 6.9 months for the BCP group. With respect to the mOS, the ABCP group also improved compared to the BCP group (10). In conclusion, ICA tended to be better than CA, which was consistent with our results. Given that IMpower150 is a small-scale subgroup analysis, it cannot be concluded that ICA was superior to the CA regimen, and the results need to be confirmed by more prospective studies. The mPFS from each treatment group obtained from the IMpower150 study had a gap to compare with our results, and it needed to be considered that we were based on real-world research; most patients received overline therapy, immunotherapy for these patients was a very posterior treatment, and our study showed that the line of therapy on the efficacy of immunotherapy is very obvious. In addition, IMpower150 enrolled patients before osimertinib was approved as first-line treatment.
In addition, pairwise combination regimens of anti-angiogenesis, immunotherapy, and chemotherapy included the CA, IC, and IA group. For the CA or IA group, anti-angiogenesis combination chemotherapy or immunotherapy was feasible. According to previous studies, there was a synergistic effect between chemotherapeutic agents and antiangiogenic agents. Elevated VEGF levels could lead to tumor vascular disorders, increased permeability and interstitial pressure, and affect the delivery of chemotherapeutic agents into the tumor (22). Bevacizumab could promote the delivery of chemotherapeutic agents into the tumor (23). On the other hand, there was also synergy between antiangiogenic agents and immune checkpoint inhibitors, and antiangiogenic agents showed immunomodulatory effects (24), could improve the tumor microenvironment for immunosuppression in patients with EGFR mutations. Our results indicate that the CA group had a significant longer mPFS than the IA group. However, the ORR and DCR were similar between the two groups. For the CA and IC group, combining immunotherapy or anti-angiogenesis based on chemotherapy, the former mPFS was longer than the latter, but there was no significant difference. In the IMpower150 study, in the EGFR mutation subgroup, the HR for PFS with ACP versus BCP was 1.14 (95% CI 0.73-1.18), mPFS 6.9 months in the ACP group, and mPFS 6.9 months in the BCP group. However, in the 56 patients who had previously received EGFR-TKIs, the mPFS of the BCP group was 6.1 months, which was slightly longer than that of the ACP group (mPFS=5.7 months) (10). The results of the subgroup analysis of IMpower150 were similar to our conclusions. Another real-world retrospective study from Shanghai Pulmonary Hospital achieved similar conclusions: the mPFS was 6.90 months in the CA group and 7.59 months in the IC group, and there was no significant difference in PFS (25). However, another retrospective study showed that OS was worse in the IC group comparing with the CA group (HR 2.37, 95%CI 1.09-5.65, P=0.030) (26). Similarly, comparing IC with IA regimens, based on immunotherapy combined with chemotherapy or anti-angiogenesis, the former mPFS was longer than the latter, no significant difference was observed in PFS.
Although combination therapy was superior to monotherapy, some exceptions existed. For example, the IC group had a longer mPFS than the CM and IM group, but there was no significant difference in PFS. CheckMate-722 and KEYNOTE-789 study also showed no survival benefit between the IC and CM group (11, 12). However, the results of the latest ORIENT31 second interim analysis showed that mPFS was 5.5 months in the IC group and 4.3 months in the CM group, which was similar with our results. But the difference between these two groups in ORIENT31 trail was statistically significant (P=0.016) (27). In addition, the mPFS of the IA group was longer than the CM and IM group, and no significant difference was observed. For IA and CM regimen, the prospective study of Runbo Zhong’s team showed that anlotinib combined with PD-1 inhibitors exhibited better mPFS than chemotherapy group (4.33 vs 3.6 months), a significant increase of nearly 1 month (P=0.005). The mOS was 14.17 months in the combination therapy group and 9.00 months in the chemotherapy group, with a significant extension of 5.17 months (P=0.029). The DCR of anlotinib combined with PD-1 inhibitor group was 92.1% (28). Compared with our analysis results, we found that the study IA regimen mPFS was longer, the DCR was higher, and the CM regimen mPFS was shorter.
In terms of safety, despite higher rates of grade ≥3 TRAEs, our results show that the ICA group was generally well tolerated with no new safety signals. This is similar to previous studies of such regimens in other patient populations (9, 10).
Multivariate analysis showed that for NSCLC patients with EGFR mutations who failed EGFR-TKIs treatment, the earlier they received immunotherapy, the superior benefit in PFS. A clinical study reported that patients who received EGFR-TKIs had alteration in their tumor microenvironment that contributed to immunotherapy. If other treatments were performed during this period, it might lead to the interference of the favorable tumor microenvironment, which could affect the subsequent treatment of ICI (29). Therefore, the timing of immunotherapy administration was equally important.
Subgroups with common EGFR mutations receiving immunotherapy were analyzed in our study. The results indicated that patients with the 21L858R mutation tended to have longer mPFS compared with the 19del-mutant population, but the difference was not statistically significant. However, a clinical study showed that patients with the L858R mutation receiving immunotherapy had a better response and an OS benefit compared with 19del population (30).
Analysis of the population detecting PD-L1 status showed that there was no significant difference in the median PFS, ORR and DCR in the PD-L1 high expression group compared with the low expression and negative groups. However, clinically relevant studies were controversial. A single-arm study of toripalimab combined with chemotherapy at the Shanghai Pulmonary Hospital confirmed that the PD-L1 expression levels were not associated with ICI efficacy (31). The study by Shunli Peng et al. found that the ORR in the high PD-L1 expression group was higher than that in the low PD-L1 expression group in EGFR mutant patients receiving ICI (32). Another study showed that TMB might be a more suitable biomarker for predicting ICI efficacy than PD-L1 (33). Although high PD-L1 expression suggested a better response to PD-1/PD-L1 inhibitors, it was not absolute, and some patients with high PD-L1 expression still could not benefit from ICI. The reasons included a range of factors, such as tumor mutation burden (TMB), coexisting other gene mutations, microsatellite instability (MSI), “cold” or “hot” tumor microenvironment can affect the efficacy of ICI (34–37).
Our study had several limitations. It was retrospective in nature, leading to the possibility of bias in clinical data. No additional patients receiving dual immunotherapy were included because only eight patients were identified when we reviewed the medical records. However, our study is the first to compare most treatment regimens after EGFR-TKIs failure. Moreover, because driver gene-positive patients did not respond to ICI, most of the current studies did not set an IM group for patients after receiving EGFR-TKIs, and our study precisely included these population. In contrast, our study might able to draw more comprehensive conclusions. To some extent, our analytical results provided a reference for subsequent immunotherapy strategies for similar patients. ICI-based combination therapy, especially immunotherapy combination chemotherapy and anti-angiogenesis might be the preferred treatment regimen for advanced NSCLC patients with EGFR mutations after EGFR- TKIs failure.
Conclusions
In conclusion, our results suggest that immunotherapy combination chemotherapy and anti-angiogenesis are more likely to prolong PFS and improve ORR and DCR than other strategies. However, more prospective clinical studies are still needed to further confirm the efficacy of this strategy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by institutional ethical review board of Shanghai Chest Hospital (ethical approval number:KS22021). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because individual consent for this retrospective analysis was waived.
Author contributions
YY: Conceptualization, Formal Analysis, Methodology, Validation, Writing – review & editing. XL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. HH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YS: Conceptualization, Validation, Writing – review & editing. QJ: Conceptualization, Data curation, Validation, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Beijing Xisike Clinical Oncology Research Foundation (grant number: Y-HR2020MS-0982) and the National Natural Science Foundation of China (grant numbers: 82172633).
Acknowledgments
We thank all investigators in our center for supporting this study.
Conflict of interest
YY reported that he received fundings of the Beijing Xisike Clinical Oncology Research Foundation grant number: Y-HR2020MS-0982, the National Natural Science Foundation of China 82172633.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1265236/full#supplementary-material
References
1. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer (2007) 7(3):169–81. doi: 10.1038/nrc2088
2. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet (2021) 398(10299):535–54. doi: 10.1016/S0140-6736(21)00312-3
3. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med (2018) 378(2):113–25. doi: 10.1056/NEJMoa1713137
4. Zarour HM. Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res (2016) 22(8):1856–64. doi: 10.1158/1078-0432.CCR-15-1849
5. Dafni U, Tsourti Z, Vervita K, Peters S. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A Systematic Rev Netw Meta-Analysis Lung Cancer (2019) 134:127–40. doi: 10.1016/j.lungcan.2019.05.029
6. Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol (2018) 13(8):1138–45. doi: 10.1016/j.jtho.2018.03.035
7. Sugiyama E, Togashi Y, Takeuchi Y, Shinya S, Tada Y, Kataoka K, et al. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR-mutated non-small cell lung cancer. Sci Immunol (2020) 5(43):eaav3937. doi: 10.1126/sciimmunol.aav3937
8. Isomoto K, Haratani K, Hayashi H, Shimizu S, Tomida S, Niwa T, et al. Impact of EGFR-TKI treatment on the tumor immune microenvironment in EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res (2020) 26(8):2037–46. doi: 10.1158/1078-0432.CCR-19-2027
9. Lu S, Wu L, Jian H, Chen Y, Wang Q, Fang J, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol (2022) 23(9):1167–79. doi: 10.1016/S1470-2045(22)00382-5
10. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med (2019) 7(5):387–401. doi: 10.1016/S2213-2600(19)30084-0
11. Mok TSK, Nakagawa K, Park K, Ohe Y, Girard N, Kim HR, et al. LBA8 Nivolumab (NIVO) + chemotherapy (chemo) vs chemo in patients (pts) with EGFR-mutated metastatic non-small cell lung cancer (mNSCLC) with disease progression after EGFR tyrosine kinase inhibitors (TKIs) in CheckMate 722. Ann Oncol (2022) 33:S1561–S2. doi: 10.1016/j.annonc.2022.10.350
12. Yang JC-H, Lee DH, Lee J-S, Fan Y, de Marinis F, Okamoto I, et al. Pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor (TKI)-resistant, EGFR-mutant, metastatic nonsquamous NSCLC: Phase 3 KEYNOTE-789 study. Am Soc Clin Oncol (2023) 41(17_suppl):LBA9000-LBA9000. doi: 10.1200/JCO.2023.41.17_suppl.LBA9000
13. Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol (2015) 10(6):910–23. doi: 10.1097/JTO.0000000000000500
14. Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discovery (2013) 3(12):1355–63. doi: 10.1158/2159-8290.CD-13-0310
15. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
16. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
17. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet (2016) 387(10030):1837–46. doi: 10.1016/S0140-6736(16)00587-0
18. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
19. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med (2006) 355(24):2542–50. doi: 10.1056/NEJMoa061884
20. Zinner RG, Obasaju CK, Spigel DR, Weaver RW, Beck JT, Waterhouse DM, et al. PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol (2015) 10(1):134–42. doi: 10.1097/JTO.0000000000000366
21. Hayashi H, Sugawara S, Fukuda Y, Fujimoto D, Miura S, Ota K, et al. A randomized phase II study comparing nivolumab with carboplatin-pemetrexed for EGFR-mutated NSCLC with resistance to EGFR tyrosine kinase inhibitors (WJOG8515L). Clin Cancer Res (2022) 28(5):893–902. doi: 10.1158/1078-0432.CCR-21-3194
22. Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med (2001) 7(9):987–9. doi: 10.1038/nm0901-987
23. Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med (2004) 10(2):145–7. doi: 10.1038/nm988
24. Manegold C, Dingemans AC, Gray JE, Nakagawa K, Nicolson M, Peters S, et al. The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol (2017) 12(2):194–207. doi: 10.1016/j.jtho.2016.10.003
25. Yu X, Li J, Ye L, Zhao J, Xie M, Zhou J, et al. Real-world outcomes of chemo-antiangiogenesis versus chemo-immunotherapy combinations in EGFR-mutant advanced non-small cell lung cancer patients after failure of EGFR-TKI therapy. Transl Lung Cancer Res (2021) 10(9):3782–92. doi: 10.21037/tlcr-21-681
26. White MN, Piper-Vallillo AJ, Gardner RM, Cunanan K, Neal JW, Das M, et al. Chemotherapy plus immunotherapy versus chemotherapy plus bevacizumab versus chemotherapy alone in EGFR-mutant NSCLC after progression on osimertinib. Clin Lung Cancer (2022) 23(3):e210–e21. doi: 10.1016/j.cllc.2021.11.001
27. Lu S, Wu L, Jian H, Cheng Y, Wang Q, Fang J, et al. Sintilimab plus chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer with disease progression after EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): second interim analysis from a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med (2023) 11(7):624–36. doi: 10.1016/S2213-2600(23)00135-2
28. Yu L, Hu Y, Xu J, Qiao R, Zhong H, Han B, et al. Multi-target angiogenesis inhibitor combined with PD-1 inhibitors may benefit advanced non-small cell lung cancer patients in late line after failure of EGFR-TKI therapy. Int J Cancer (2023) 153(3):635–43. doi: 10.1002/ijc.34536
29. Tian T, Yu M, Li J, Jiang M, Ma D, Tang S, et al. Front-line ICI-based combination therapy post-TKI resistance may improve survival in NSCLC patients with EGFR mutation. Front Oncol (2021) 11:739090. doi: 10.3389/fonc.2021.739090
30. Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J, et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol (2019) 30(8):1311–20. doi: 10.1093/annonc/mdz141
31. Jiang T, Wang P, Zhang J, Zhao Y, Zhou J, Fan Y, et al. Toripalimab plus chemotherapy as second-line treatment in previously EGFR-TKI treated patients with EGFR-mutant-advanced NSCLC: a multicenter phase-II trial. Signal Transduct Target Ther (2021) 6(1):355. doi: 10.1038/s41392-021-00751-9
32. Peng S, Wang R, Zhang X, Ma Y, Zhong L, Li K, et al. EGFR-TKI resistance promotes immune escape in lung cancer via increased PD-L1 expression. Mol Cancer (2019) 18(1):165. doi: 10.1186/s12943-019-1073-4
33. Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology (2017) 6(11):e1356145. doi: 10.1080/2162402X.2017.1356145
34. Ribas A, Hu-Lieskovan S. What does PD-L1 positive or negative mean? J Exp Med (2016) 213(13):2835–40. doi: 10.1084/jem.20161462
35. Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell (2015) 160(1-2):48–61. doi: 10.1016/j.cell.2014.12.033
36. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med (2012) 366(26):2455–65. doi: 10.1056/NEJMoa1200694
Keywords: non-small cell lung cancer, immunotherapy, EGFR mutation, progression, EGFR-TKIs
Citation: Li X, Huang H, Sun Y, Jiang Q and Yu Y (2023) Immunotherapy strategies for EGFR-mutated advanced NSCLC after EGFR tyrosine-kinase inhibitors failure. Front. Oncol. 13:1265236. doi: 10.3389/fonc.2023.1265236
Received: 22 July 2023; Accepted: 11 September 2023;
Published: 05 October 2023.
Edited by:
Jie Mei, Wuxi People’s Hospital Affiliated to Nanjing Medical University, ChinaReviewed by:
Wenying Peng, Yunnan Cancer Hospital, ChinaAlberto Pavan, Azienda ULSS 3 Serenissima, Italy
Copyright © 2023 Li, Huang, Sun, Jiang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongfeng Yu, eXV5b25nZmVuZzIxMkAxMjYuY29t
†These authors share first authorship
 Xingyuan Li
Xingyuan Li Huayan Huang1†
Huayan Huang1† Yingjia Sun
Yingjia Sun Qing Jiang
Qing Jiang Yongfeng Yu
Yongfeng Yu