
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 04 October 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1264871
With the widespread use of low-dose chest Computed Tomography (CT), lung nodules are being increasingly detected. Common pulmonary conditions such as lung adenocarcinoma, lung squamous cell carcinoma, and tuberculosis are typically diagnosable through imaging examinations. Nevertheless, when multiple types of lung cancer are combined with other benign tumors, how can an accurate diagnosis be made? In this report, we present a rare case of a patient with the simultaneous occurrence of lung adenocarcinoma, lung squamous cell carcinoma, pulmonary tuberculosis, and pulmonary hamartoma, which has not been previously reported. This patient underwent surgical intervention in the Department of Thoracic Surgery at the Second Hospital of Jilin University and has now fully recovered and been discharged. The patient’s preoperative positron emission tomography-CT(PET-CT)results did not align with the postoperative pathological diagnosis. The imaging findings were atypical, and the pathological diagnosis was exceptionally rare. We share this case report to contribute to the accumulation of clinical experience.
Lung malignancy is one of the most common types of malignant tumors in terms of incidence rate (1, 2). Due to advancements in high-resolution computed tomography (HRCT), an increasing number of patients with multiple pulmonary nodules and multiple lung cancers (MLC) are being diagnosed. On the contrary, the radiological differentiation of lung malignancies can still be challenging at times, especially when accompanied with other benign pulmonary conditions (3). While lung cancer is highly prevalent, the co-occurrence of lung cancer with tuberculosis or pulmonary hamartoma is rare. To the best of our knowledge, cases of lung cancer combined with tuberculosis as well as pulmonary hamartoma have not been reported. Lung cancer combined with tuberculosis not only promotes the deterioration of lung cancer but also leads to the activation of tuberculosis (4, 5). It is reported that pulmonary hamartomas also have the potential for malignancy (6). In this paper, we present a recent case treated at the Second Hospital of Jilin University, involving a patient with primary lung adenocarcinoma combined with lung squamous cell carcinoma, pulmonary hamartoma, and pulmonary tuberculosis. Our aim is to provide a comprehensive analysis of this unique case and enhance the understanding of this particular clinical scenario.
A 73-year-old male patient was found to have multiple lung nodules during a routine chest Computed Tomography (CT) scan conducted one month ago. Among these, the nodule in the anterior segment of the right upper lobe(RUL) measures 18.3mm, the nodule in the posterior segment of the RUL measures 18.6mm, the nodule in the dorsal segment of the right lower lobe(RLL) measures 20.1mm, the nodule in the posterior apical segment of the left upper lobe(LUL) measures 6mm, and the nodule in the dorsal segment of the left lower lobe (LLL) measures 16.2mm. The patient's Chest CT results are shown in Figure 1. To rule out lung nodules caused by inflammatory conditions, the patient underwent anti-inflammatory treatment. On reevaluation with positron emission tomography-CT(PET-CT)after 14 days, multiple abnormal density shadows were detected in both lungs, accompanied by increased metabolic activity, suggesting malignancy. Among these, the SUVmax values were 13.94 for the nodule in the anterior segment of the RUL, 8.17 for the nodule in the posterior segment of the RUL, 11.39 for the nodule in the RLL, 7.23 for the nodule in the LUL, and 5.3 for the nodule in the LLL. The laboratory test results showed no abnormalities in the blood routine, coagulation function, liver function, and kidney function. The results of the human immunodeficiency virus (HIV) antibody, hepatitis B 5 items, and hepatitis C antibody/syphilis antibody tests were all negative. The physical examination revealed a body temperature of 36.2°C, respiratory rate of 16 breaths per minute, pulse rate of 78 beats per minute, and blood pressure of 144/88 mmHg. The patient predominantly exhibited abdominal breathing with symmetrical respiratory movements. Clear breath sounds were heard in both lungs. The electrocardiogram showed sinus rhythm, indicating a generally normal cardiac activity. Thus, we decided to perform staged video-assisted thoracoscopic wedge resection of the lung lesions in order to achieve complete tumor removal. First, the patient underwent RUL wedge resection and RLL wedge resection in the left lateral decubitus position using video-assisted thoracoscopic surgery (VATS). Preoperatively, the patient’s blood gas analysis showed a pH of 7.41, pCO2 of 40mmHg, pO2 of 76mmHg, HCO3- of 25.4mmol/L, BE of 0.7mmol/L, and SaO2 of 95%. One week later, the patient underwent LUL wedge resection and LLL wedge resection in the right lateral decubitus position using VATS. Preoperatively, the patient’s blood gas analysis showed a pH of 7.49, pCO2 of 36mmHg, pO2 of 72mmHg, HCO3- of 27.4mmol/L, BE of 4.1mmol/L, and SaO2 of 96%. The intraoperative pathological diagnosis revealed squamous cell carcinoma for the nodule in the RUL(Nodule 1), chronic granulomatous inflammation suggestive of tuberculosis for the nodules in the RUL(Nodule 2), and LLL, invasive adenocarcinoma for the nodule in the RLL, and hamartoma for the nodule in the LUL. Postoperative pathological examination revealed the following findings: The nodule in the RUL (Nodule 1) is consistent with moderately to poorly differentiated squamous cell carcinoma, supported by immunohistochemical staining and morphological features. The tissue sample from the second nodule in the RUL exhibited extensive necrosis, surrounded by chronic granulomatous inflammation. The nodule in the RLL is diagnosed as invasive adenocarcinoma, primarily exhibiting a follicular pattern, with additional findings of an adherent growth pattern and a small amount of complex glandular structures. The patient's Pathological findings are shown in Figure 2. No definite vascular invasion was observed. The immunohistochemical staining results were as follows: CK (AE1/AE3) (+), TTF-1 (-), NapsinA (-), CK7 (+), CK5/6 (+), P40 (+), Ki67 (80% positivity), CD34 (-), CK8/18 (weak +), vimentin (focal +), INSM1 (-), BRG-1 (+),INI-1(+).Special staining results were as follows: PAS stain(-), antacid stain(-). Postoperatively, the patient underwent genetic testing, which did not detect any gene mutations associated with lung cancer. Furthermore, the patient was staged as pT1b, and no further treatment was deemed necessary. The patient has fully recovered and has been discharged from the hospital.
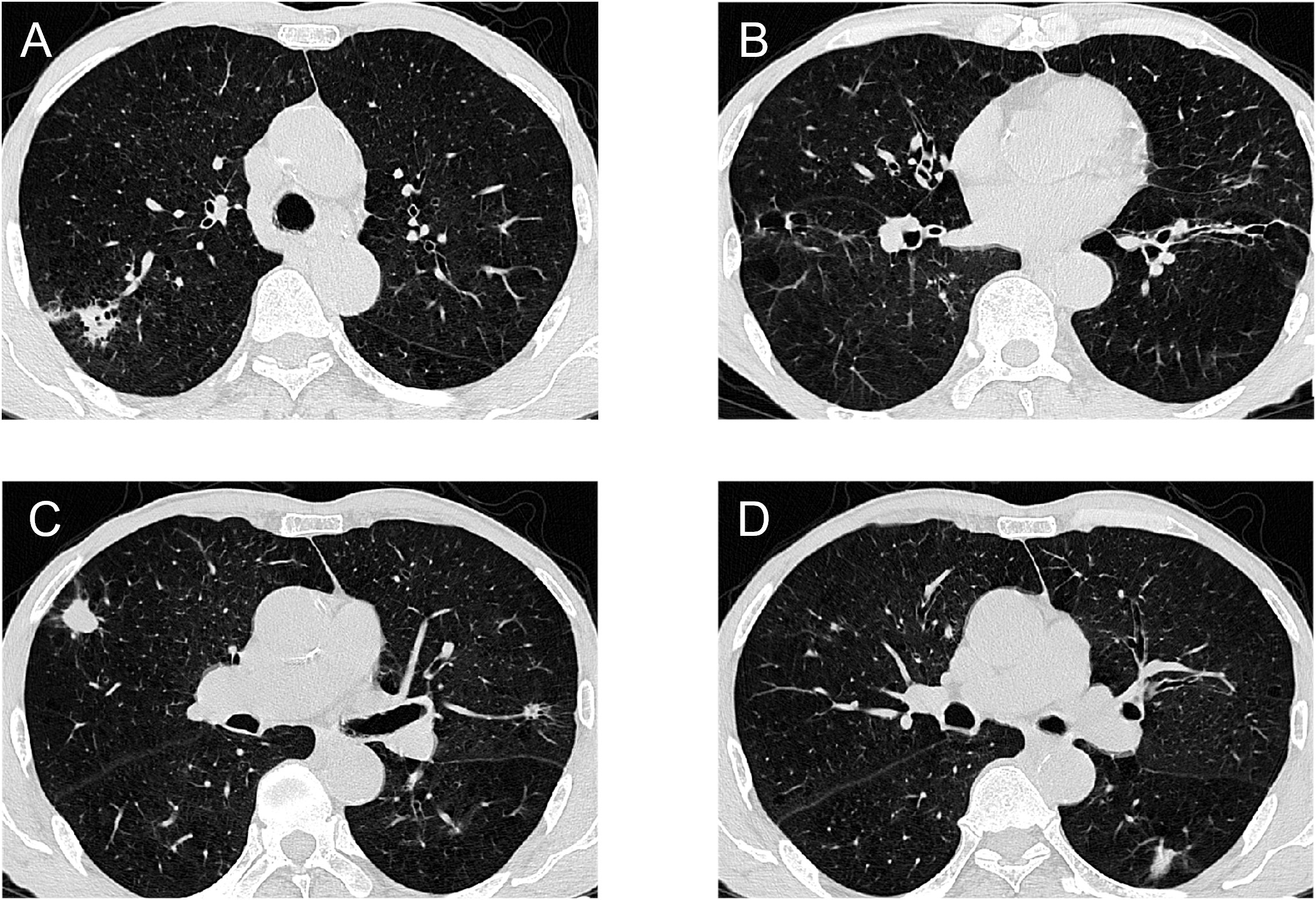
Figure 1 Chest CT results of the patient: (A)Right upper lung mass (B). Right lower lung mass (C)Left upper lung mass and right upper lung mass (D)Left lower lung mass.
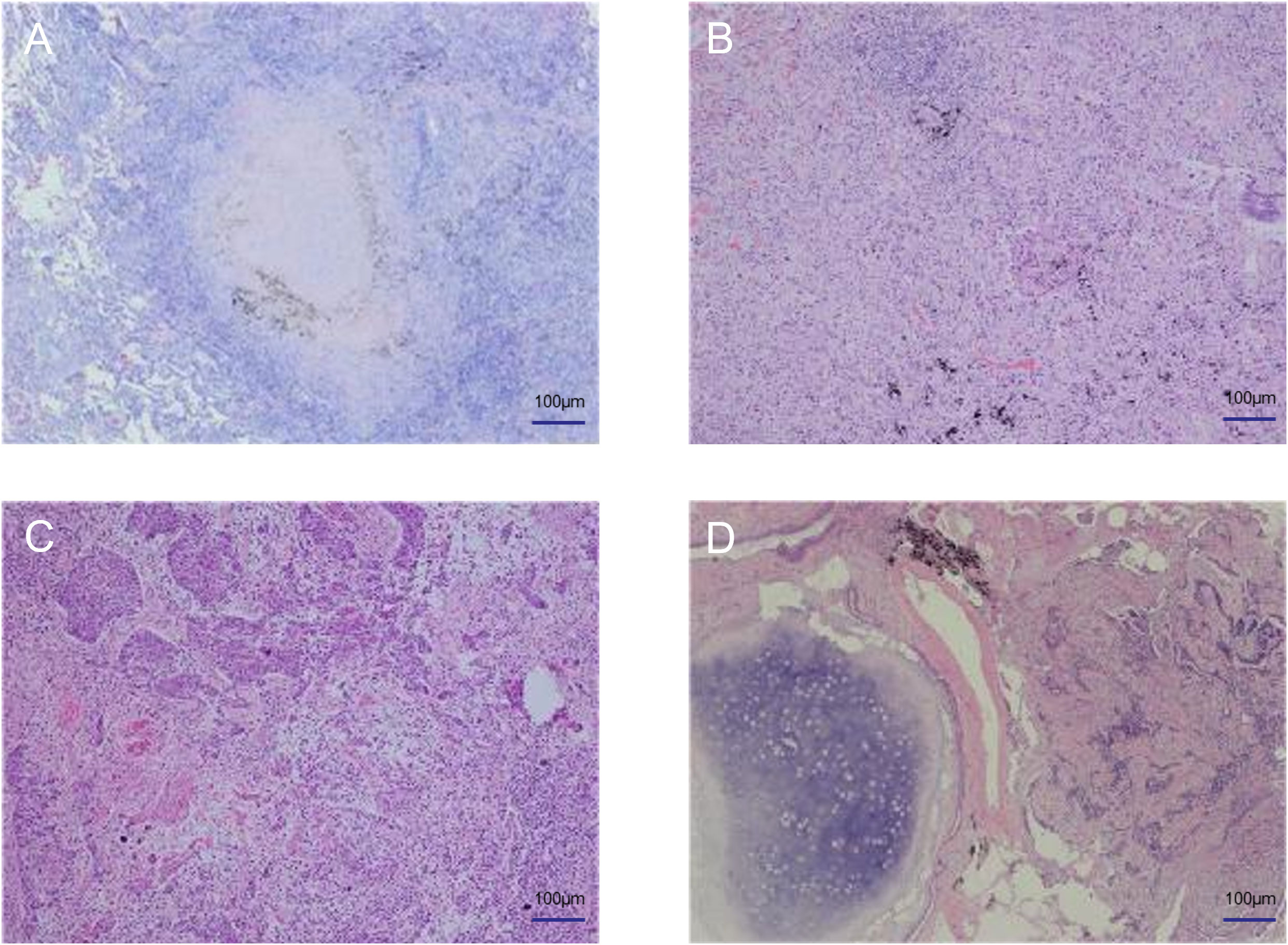
Figure 2 Pathological findings of the patient. (A) Tuberculosis (B) Adenocarcinoma (C) Squamous cell carcinoma (D) Hamartoma.
The widespread use of low-dose chest CT has led to an increased detection rate of early-stage malignant lung tumors. Nonetheless, even with the addition of PET-CT, chest CT still has certain limitations in diagnosing lung malignancies. Firstly, the radiological presentation of the lung malignancy in this case is atypical. The patient’s chest CT showed a relatively low proportion of solid components in the nodule of the right lung’s lower lobe, presenting as ground-glass opacity(GGO). In contrast, the pathology revealed invasive adenocarcinoma. This reminds us that even if CT indicates pure GGO, it may still represent invasive adenocarcinoma. According to the report by Yoon et al., a consolidation tumor ratio (CTR) exceeding 75% is an independent risk factor for lung adenocarcinoma (7). According to the report by Shimomura et al., volume-based consolidation tumor ratio (CTR) can serve as a useful predictive indicator for accurate clinical staging of stage I and II adenocarcinoma patients (8). Based on a retrospective study of 985 cases, Lin et al. concluded that lesions with a lower proportion of solid components, such as pure GGO nodules, had significantly more favorable histological features and prognosis compared to the group with part-solid nodule (PSN) (9). Nevertheless, reliance on such predictions should be cautious, as they may not universally apply, and treatment decisions based solely on radiological measurements of GGO tumors are inappropriate. Research has demonstrated that radiological measurements of lung adenocarcinoma, including consolidation-to-tumor ratio value, consolidation size, and tumor size, cannot accurately predict tumor invasion and prognosis. Accordingly, formulating treatment strategies based solely on radiological measurements of GGO tumors is inappropriate (10). Li et al. acknowledged that the CTR was an independent prognostic factor for the entire non-small cell lung cancer (NSCLC) population, but not for certain subtypes of NSCLC. Tumor size still holds significance for certain solid NSCLC cases (11). Hence, in clinical practice, even for pure ground-glass nodules (GGNs) detected on CT, it is important to consider multiple factors such as tumor diameter, serum tumor markers, maximum standardized uptake value on PET-CT, age, gender, smoking status, current extra-pulmonary cancer, air bronchogram and nodule size (p<0.05),circulating tumor cell,and others (12, 13). After comprehensive evaluation, an appropriate treatment plan can be chosen. Relying solely on imaging findings may introduce certain biases.
PET-CT has become a widely employed diagnostic tool for malignant lung tumors. Various studies have demonstrated the valuable role of PET-CT in disease identification, lesion localization, differentiation between benign and malignant nodules, and assessment of invasiveness and vascular involvement of lung lesions (14–17). Nonetheless, in this patient, increased uptake of FDG was observed on PET-CT in both the left upper lobe hamartoma and the left lower lobe tuberculosis. The correct diagnosis of hamartoma and lung tuberculosis was only established through surgical resection and pathological examination. This finding is noteworthy as hamartomas and lung tuberculosis can potentially present as false-positive results on PET-CT. As reported, the sensitivity and specificity of CT in distinguishing benign nodules from malignant nodules are approximately 80-90% and 50-60%, respectively (18). However, PET-CT scans may have limitations due to false-positive results in cases of infection or inflammation (19). This report describes a case where lung tuberculosis combined with a hamartoma was initially misdiagnosed as a malignant lung tumor. The presence of lung tuberculosis led to increased uptake of FDG, resulting in a false-positive result (20). Furthermore, there have been studies summarizing 19 cases of false-positive nodules on PET-CT scans, where high standard uptake values (SUV) above the threshold of 2.5 during PET-CT scanning were labeled as malignant tumors. This report indicates that tuberculosis, aspergilloma, bronchiolitis obliterans organizing pneumonia (BOOP), sarcoidosis, sequestration, anthracosis, and hamartoma can all exhibit high uptake on PET scans, leading to false-positive results (21). A study by Sathekge et al. demonstrated that there was no significant difference between the mean %DSUV of benign lesions 17.1%(SD 16.3%) and malignant lesions 19.4%(SD 23.7%). They concluded that PET-CT is unable to differentiate between malignant tumors and tuberculosis (22). Cicco et al. confirmed a series of PET-CT high-uptake pulmonary hamartomas through pathological examination. Their results revealed that approximately 20% of hamartomas exhibited uptake characteristics suggestive of malignant tumors (23). Goo et al. compared the uptake patterns of PET-CT scans in 10 cases of pulmonary tuberculosis nodules. Their findings demonstrated high FDG uptake (range: 9.3-7.21) near the primary lesion in four patients. Whereas, histopathological examination revealed chronic granulomatous inflammation with caseous necrosis or healed tuberculosis with fungal balls. Consequently, they concluded that when interpreting positive PET-CT results for differentiating between benign and malignant pulmonary masses, consideration should be given to the possibility of pulmonary tuberculosis (24). The above research results indicate that both pulmonary tuberculosis and hamartomas can indeed show increased FDG uptake. Accordingly, when encountering a positive PET-CT result, a comprehensive evaluation of the lesion’s size, morphology, and symptoms should be conducted to avoid inappropriate diagnosis and treatment resulting from false-positive findings. Simultaneously, preoperative pathological examination is crucial, as we can determine the benign or malignant nature of the lesion through fine needle aspiration pathology examination.
Thirdly, we should pay attention to the relationship between pulmonary tuberculosis and lung cancer. In the case of this patient, both pulmonary tuberculosis and lung cancer coexisted, prompting an exploration of the potential mutual influence between the two conditions. Firstly, pulmonary tuberculosis has been found to have a promoting effect on lung cancer. Sanchez et al. concluded that the potential risk of lung cancer was higher after an episode of tuberculosis (25). Preda et al. mentioned in their article that genomic modifications, chronic inflammation, and fibrosis resulting from tuberculosis may contribute to carcinogenesis. Therapies used for lung cancer, such as checkpoint inhibitors, may reactivate latent tuberculosis or exacerbate its progression. As a consequence, it is important to prioritize the treatment of concurrent pulmonary tuberculosis and lung cancer (26). Through meta-analysis, Abdeahad et al. concluded that active pulmonary tuberculosis increases the risk of lung cancer. Their findings indicate an association between active pulmonary tuberculosis and an elevated risk of adenocarcinoma, squamous cell carcinoma, and small cell lung cancer. Additionally, they concluded that the risk of developing lung cancer remains elevated even after recovery from pulmonary tuberculosis (4). On the other hand, Heuvers et al. argued that a history of pulmonary tuberculosis may serve as an important prognostic factor for lung cancer survival (27). The coexistence of pulmonary tuberculosis and lung cancer is relatively rare. The mechanism underlying this phenomenon may be attributed to several hypotheses, as follows. Firstly, old pulmonary tuberculosis lesions can cause persistent inflammation, leading to fibrosis and scar formation, which enhances the accumulation of carcinogenic substances in that area. The formation of pulmonary scars after repeated tuberculosis treatment favors tumor growth. This type of cancer is known as scar cancer (28). Additionally, the cell wall components of Mycobacterium tuberculosis induce the production of nitric oxide and reactive oxygen species, both of which contribute to the carcinogenic process (29). Conversely, lung cancer also plays a promoting role in the development of pulmonary tuberculosis. Research has shown that within one year of the diagnosis of respiratory or digestive tract cancer, tobacco-related cancer, or hematological malignancies, there is a significantly increased risk of developing tuberculosis. They believe that this may be related to reduced infection barriers, immune suppression, and shared risk factors, and they consider cancer as a clinical predictive indicator for increased active pulmonary tuberculosis cases (5). Hence, we can consider that there is a bidirectional relationship between lung cancer and pulmonary tuberculosis. When faced with cases involving the coexistence of lung cancer and pulmonary tuberculosis, apart from conventional surgical treatment, the management of tuberculosis and long-term postoperative follow-up are critical.
In addition, pulmonary hamartomas also have the potential to transform into cancer. Karasik et al., through a clinical study of 1976 patients with chondromatous hamartoma, concluded that the risk of chondromatous hamartoma developing into lung cancer is significantly higher compared to the general population (30). Lee et al. reported a case of pulmonary squamous cell carcinoma originating from a hamartoma. The pathological results of the patient revealed that the pulmonary hamartoma and squamous cell carcinoma were present within the same nodule. They concluded that pulmonary hamartomas can develop into squamous cell carcinoma (31). Ribet et al. also suggested that pulmonary hamartomas can develop into lung cancer, and they believe that smoking and environmental carcinogens play a crucial role in this transformation process (32). In summary, we have found that the coexistence of pulmonary hamartoma and malignant lung tumors is rare in clinical practice. Nonetheless, there is a possibility of malignant transformation in pulmonary hamartomas. Accordingly, even in histopathologically confirmed cases of pulmonary hamartoma, follow-up examinations should be conducted to prevent potential malignant transformation.
We present a case involving the simultaneous occurrence of pulmonary adenocarcinoma, squamous cell carcinoma, hamartoma, and pulmonary tuberculosis. The patient’s PET-CT images of the lesions showed increased FDG uptake. Nevertheless, as previously discussed, PET-CT has limited capability in distinguishing between lung cancer, hamartoma, and pulmonary tuberculosis. As a consequence, these nodules were identified with a higher likelihood of malignancy. Remarkably, tuberculosis lesions were observed in both lungs of the patient. We hypothesize that the chronic inflammation and fibrosis resulting from recurrent pulmonary tuberculosis infections may have created an environment favorable for the development of cancer, ultimately leading to its occurrence.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics Committee of the Second Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JK: Conceptualization, Writing – original draft. MW: Investigation, Writing – original draft. PH: Validation, Writing – review & editing. BW: Supervision, Validation, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Medical Foundation of China (project number: zgyxjjh-wcwk-2023062001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J (2016) 48(3):889–902. doi: 10.1183/13993003.00359-2016
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Tian S, Li F, Pu J, Zheng Y, Shi H, Dong Y, et al. Differential diagnostic value of histology in mplc and ipm: A systematic review and meta-analysis. Front Oncol (2022) 12:871827. doi: 10.3389/fonc.2022.871827
4. Abdeahad H, Salehi M, Yaghoubi A, Aalami AH, Aalami F, Soleimanpour S. Previous pulmonary tuberculosis enhances the risk of lung cancer: systematic reviews and meta-analysis. Infect Dis (Lond) (2022) 54(4):255–68. doi: 10.1080/23744235.2021.2006772
5. Simonsen DF, Farkas DK, Horsburgh CR, Thomsen RW, Sørensen HT. Increased risk of active tuberculosis after cancer diagnosis. J Infect (2017) 74(6):590–8. doi: 10.1016/j.jinf.2017.03.012
6. Gurumurthy S, Hezel AF, Sahin E, Berger JH, Bosenberg MW, Bardeesy N. Lkb1 deficiency sensitizes mice to carcinogen-induced tumorigenesis. Cancer Res (2008) 68(1):55–63. doi: 10.1158/0008-5472.Can-07-3225
7. Yoon DW, Kim CH, Hwang S, Choi YL, Cho JH, Kim HK, et al. Reappraising the clinical usability of consolidation-to-tumor ratio on ct in clinical stage ia lung cancer. Insights Imaging (2022) 13(1):103. doi: 10.1186/s13244-022-01235-2
8. Shimomura M, Iwasaki M, Ishihara S, Inoue M. Volume-based consolidation-to-tumor ratio is a useful predictor for postoperative upstaging in stage I and ii lung adenocarcinomas. Thorac Cardiovasc Surg (2022) 70(3):265–72. doi: 10.1055/s-0039-1694061
9. Lin B, Wang R, Chen L, Gu Z, Ji C, Fang W. Should resection extent be decided by total lesion size or solid component size in ground glass opacity-containing lung adenocarcinomas? Transl Lung Cancer Res (2021) 10(6):2487–99. doi: 10.21037/tlcr-21-132
10. Ye T, Deng L, Xiang J, Zhang Y, Hu H, Sun Y, et al. Predictors of pathologic tumor invasion and prognosis for ground glass opacity featured lung adenocarcinoma. Ann Thorac Surg (2018) 106(6):1682–90. doi: 10.1016/j.athoracsur.2018.06.058
11. Li Z, Xu W, Gu T, Cao X, Wu W, Chen L. Tumor size, but not consolidation-to-tumor ratio, is an independent prognostic factor for part-solid clinical T1 non-small cell lung cancer. Thorac Cancer (2023) 14(6):602–11. doi: 10.1111/1759-7714.14788
12. Jin F, Zhu L, Shao J, Yakoub M, Schmitt L, Reißfelder C, et al. Circulating tumour cells in patients with lung cancer universally indicate poor prognosis. Eur Respir Rev (2022) 31(166):220151. doi: 10.1183/16000617.0151-2022
13. Jacob M, Romano J, Ara Jo D, Pereira JM, Ramos I, Hespanhol V. Predicting lung nodules Malignancy. Pulmonology (2022) 28(6):454–60. doi: 10.1016/j.pulmoe.2020.06.011
14. Orlacchio A, Schillaci O, Antonelli L, D'Urso S, Sergiacomi G, Nicolì P, et al. Solitary pulmonary nodules: morphological and metabolic characterisation by fdg-pet-mdct. Radiol Med (2007) 112(2):157–73. doi: 10.1007/s11547-007-0132-x
15. Khiewvan B, Ziai P, Houshmand S, Salavati A, Ziai P, Alavi A. The role of pet/ct as a prognosticator and outcome predictor in lung cancer. Expert Rev Respir Med (2016) 10(3):317–30. doi: 10.1586/17476348.2016.1147959
16. Hu Q, Li K, Yang C, Wang Y, Huang R, Gu M, et al. The role of artificial intelligence based on pet/ct radiomics in nsclc: disease management, opportunities, and challenges. Front Oncol (2023) 13:1133164. doi: 10.3389/fonc.2023.1133164
17. Huber RM, Kauffmann-Guerrero D, Hoffmann H, Flentje M. New developments in locally advanced nonsmall cell lung cancer. Eur Respir Rev (2021) 30(160):200227. doi: 10.1183/16000617.0227-2020
18. Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: accp evidence-based clinical practice guidelines (2nd edition). Chest (2007) 132(3 Suppl):94s–107s. doi: 10.1378/chest.07-1352
19. Jeong YJ, Yi CA, Lee KS. Solitary pulmonary nodules: detection, characterization, and guidance for further diagnostic workup and treatment. AJR Am J Roentgenol (2007) 188(1):57–68. doi: 10.2214/ajr.05.2131
20. Jindal T, Sharma N, Kumar A, Iyer VK. Pulmonary hamartoma with tuberculosis masquerading as metastasis. Ann Thorac Med (2011) 6(3):152–3. doi: 10.4103/1817-1737.82452
21. Akgul AG, Liman ST, Topcu S, Yuksel M. False positive pet scan deserves attention. J Buon (2014) 19(3):836–41.
22. Sathekge MM, Maes A, Pottel H, Stoltz A, van de Wiele C. Dual time-point fdg pet-ct for differentiating benign from Malignant solitary pulmonary nodules in a tb endemic area. S Afr Med J (2010) 100(9):598–601. doi: 10.7196/samj.4082
23. De Cicco C, Bellomi M, Bartolomei M, Carbone G, Pelosi G, Veronesi G, et al. Imaging of lung hamartomas by multidetector computed tomography and positron emission tomography. Ann Thorac Surg (2008) 86(6):1769–72. doi: 10.1016/j.athoracsur.2008.08.033
24. Goo JM, Im JG, Do KH, Yeo JS, Seo JB, Kim HY, et al. Pulmonary tuberculoma evaluated by means of fdg pet: findings in 10 cases. Radiology (2000) 216(1):117–21. doi: 10.1148/radiology.216.1.r00jl19117
25. Cabrera-Sanchez J, Cuba V, Vega V, van der Stuyft P, Otero L. Lung cancer occurrence after an episode of tuberculosis: A systematic review and meta-analysis. Eur Respir Rev (2022) 31(165):220025. doi: 10.1183/16000617.0025-2022
26. Preda M, Tănase BC, Zob DL, Gheorghe AS, Lungulescu CV, Dumitrescu EA, et al. The bidirectional relationship between pulmonary tuberculosis and lung cancer. Int J Environ Res Public Health (2023) 20(2):1282. doi: 10.3390/ijerph20021282
27. Heuvers ME, Aerts JG, Hegmans JP, Veltman JD, Uitterlinden AG, Ruiter R, et al. History of tuberculosis as an independent prognostic factor for lung cancer survival. Lung Cancer (2012) 76(3):452–6. doi: 10.1016/j.lungcan.2011.12.008
28. Sun W, Liu Y, Zhao L, Wang H, Ye L, Liu X, et al. New progress of tuberculosis scar carcinoma. Cancer Metastasis Rev (2023). doi: 10.1007/s10555-023-10128-9
29. Luo YH, Wu CH, Wu WS, Huang CY, Su WJ, Tsai CM, et al. Association between tumor epidermal growth factor receptor mutation and pulmonary tuberculosis in patients with adenocarcinoma of the lungs. J Thorac Oncol (2012) 7(2):299–305. doi: 10.1097/JTO.0b013e31823c588d
30. Karasik A, Modan M, Jacob CO, Lieberman Y. Increased risk of lung cancer in patients with chondromatous hamartoma. J Thorac Cardiovasc Surg (1980) 80(2):217–20. doi: 10.1016/S0022-5223(19)37792-X
31. Lee BJ, Kim HR, Cheon GJ, Koh JS, Kim CH, Lee JC. Squamous cell carcinoma arising from pulmonary hamartoma. Clin Nucl Med (2011) 36(2):130–1. doi: 10.1097/RLU.0b013e318203bc27
Keywords: lung adenocarcinoma, lung squamous cell carcinoma, pulmonary tuberculosis, pulmonary hamartoma, case report
Citation: Kang J, Wang M, Hua P and Wang B (2023) A rare case report: co-occurrence of two types of lung cancer with hamartoma and pulmonary tuberculosis. Front. Oncol. 13:1264871. doi: 10.3389/fonc.2023.1264871
Received: 21 July 2023; Accepted: 18 September 2023;
Published: 04 October 2023.
Edited by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesReviewed by:
Sen Tian, The First Affiliated Hospital of Naval Medical University, ChinaCopyright © 2023 Kang, Wang, Hua and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Wang, YndhbmdAamx1LmVkdS5jbg==; Peiyan Hua, aHVhcGVpeWFuQGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.