- 1Department of Radiation Oncology and Stephenson Cancer Center, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
- 2Institute of Head and Neck Studies and Education, School of Cancer Sciences, University of Birmingham, Birmingham, United Kingdom
- 3Department of Pediatrics and Stephenson Cancer Center, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
- 4Department of Radiation Oncology, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia
- 5Department of Radiation Oncology, Ohio State University Wexner Medical Center, Columbus, OH, United States
- 6Department of Radiation Oncology, Winship Cancer Institute of Emory University, Atlanta, GA, United States
- 7Department of Radiation Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 8Department of Radiation Oncology, Azienda Ospedaliero-Universitaria Careggi, Florence, Italy
- 9Department of Oncology, University of Lausanne and Lausanne University Hospital, Lausanne, Switzerland
- 10Department of Radiation Oncology, Princess Margaret Cancer Centre, University of Toronto, Toronto, ON, Canada
- 11Department of Surgery, Creighton University, and Nebraska Methodist Health System, Omaha, NE, United States
Extranodal extension (ENE) is a pattern of cancer growth from within the lymph node (LN) outward into perinodal tissues, critically defined by disruption and penetration of the tumor through the entire thickness of the LN capsule. The presence of ENE is often associated with an aggressive cancer phenotype in various malignancies including head and neck squamous cell carcinoma (HNSCC). In HNSCC, ENE is associated with increased risk of distant metastasis and lower rates of locoregional control. ENE detected on histopathology (pathologic ENE; pENE) is now incorporated as a risk-stratification factor in human papillomavirus (HPV)-negative HNSCC in the eighth edition of the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) TNM classification. Although ENE was first described almost a century ago, several issues remain unresolved, including lack of consensus on definitions, terminology, and widely accepted assessment criteria and grading systems for both pENE and ENE detected on radiological imaging (imaging-detected ENE; iENE). Moreover, there is conflicting data on the prognostic significance of iENE and pENE, particularly in the context of HPV-associated HNSCC. Herein, we review the existing literature on ENE in HNSCC, highlighting areas of controversy and identifying critical gaps requiring concerted research efforts.
1 Introduction
Extranodal extension (ENE) describes the phenomenon of cancer growth from within the lymph node (LN) outward into the perinodal tissues. The critical event is the disruption and penetration of the tumor through the entire thickness of the LN capsule, which normally acts as a barrier impeding tumor extension and is central to the diagnosis and classification of ENE.
ENE was first reported in 1930 in a retrospective analysis of autopsy material of 20 patients with head and neck cancer. The Australian pathologist Rupert Willis described tumor extension beyond the LN into adjacent structures, including soft tissue and bone (1). Almost three decades later, the negative prognostic impact of ENE was demonstrated in breast cancer, followed by similar findings in head and neck squamous cell carcinoma (HNSCC) ten years later (2).
The underlying pathobiology of ENE remains unclear. However, the presence of ENE is often associated with an aggressive cancer phenotype in HNSCC (3) and other tumour types (4). In HNSCC, ENE is associated with an increased risk of distant metastasis and lower rates of locoregional control (5). Since the publication of the two seminal adjuvant therapy trials in 2004 (6–8), ENE detected on histopathology (pathologic ENE; pENE) has also been considered a high risk feature and an indication for treatment intensification by adding cisplatin to radiotherapy (RT) after surgery, albeit at the cost of higher overall toxicity. Consequently, the presence of pENE has now been incorporated as a risk-stratification factor in Human papillomavirus (HPV) negative HNSCC in the latest (8th) edition of the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) TNM Staging Manual (9).
Although more than ninety years have passed since the first description of ENE, several issues remain unresolved. These include the lack of consensus on definitions, terminology, and widely accepted assessment criteria and classification systems for both pENE and ENE detected on pre-treatment imaging (imaging-detected ENE; iENE). Moreover, there is no agreement on the prognostic significance of iENE and pENE, particularly in the context of HPV-associated HNSCC. Here, we review the existing literature on ENE, highlighting areas of controversy and identifying critical gaps that need further research.
2 Definition and classification of ENE
2.1 Pathologic ENE
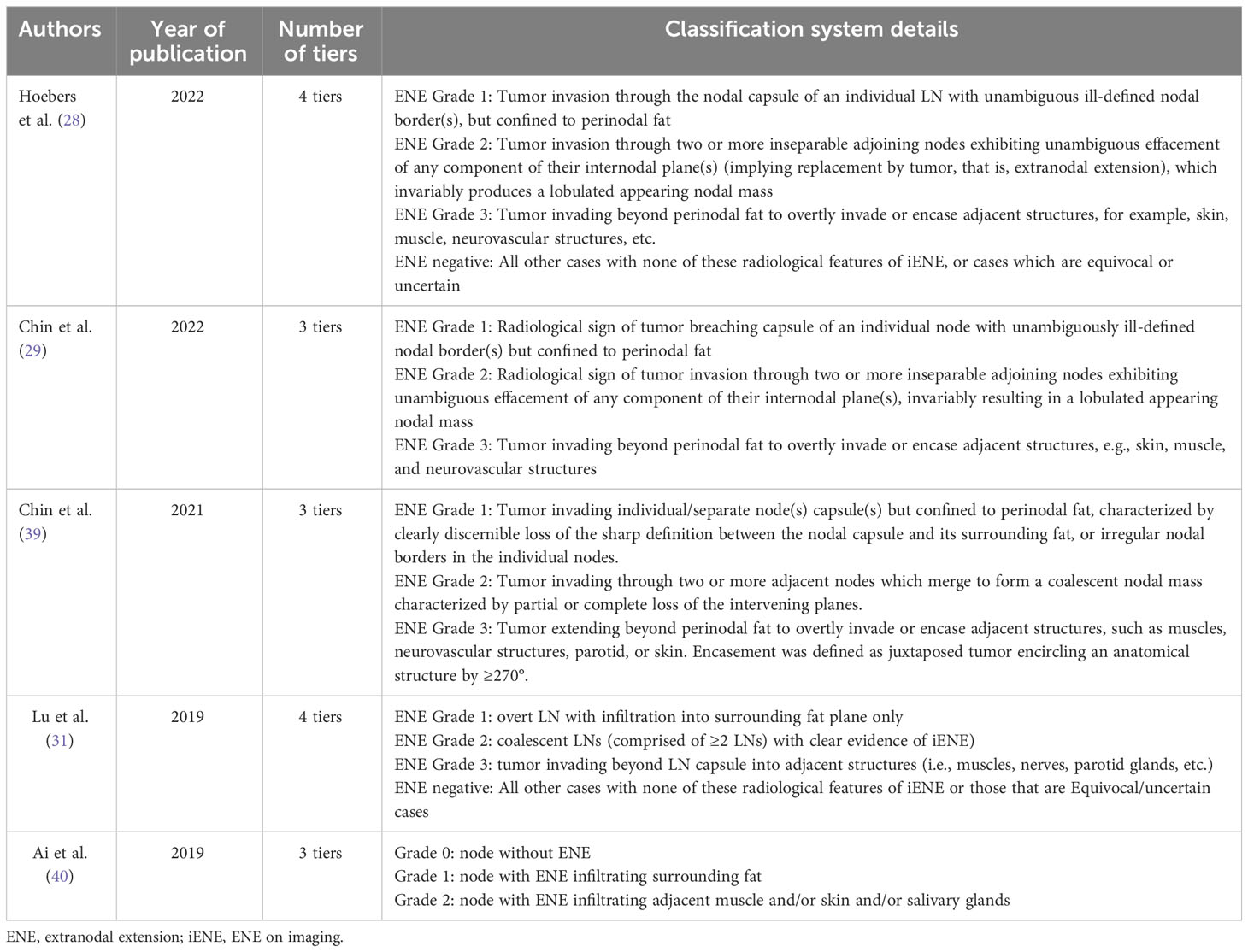
pENE is commonly defined as extension of tumor cells outside the LN capsule into the perinodal soft tissue on histopathologic examination (10). It is often subcategorized as either microscopic/minor (≤2 mm in extent) or macroscopic/major (>2 mm in extent) or as a soft tissue metastasis (STM) (Figure 1), as recommended by the AJCC for documentation purposes (9). The prognostic significance of the 2 mm extension threshold remains contentious, especially in the context of HPV-associated HNSCC (11–16).
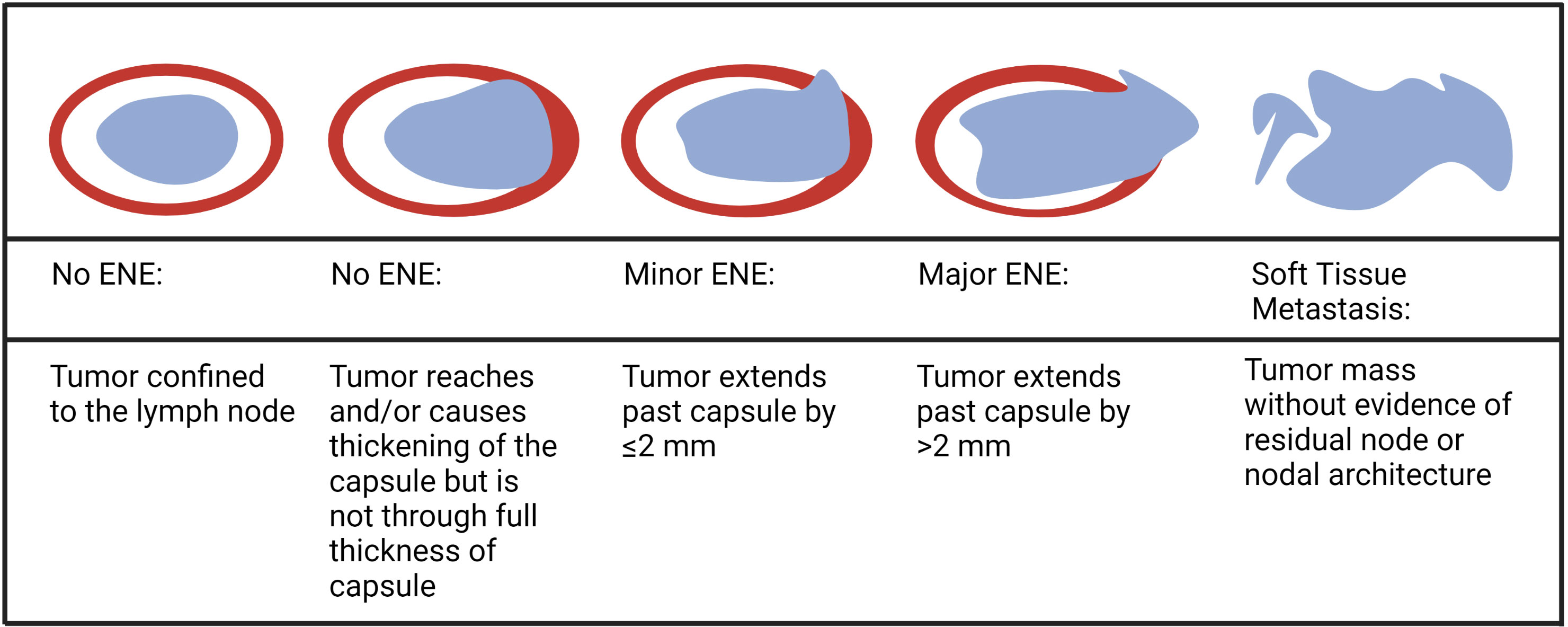
Figure 1 Depiction of various extents of lymph node involvement with tumor (Created in Biorender.com).
Determining pENE can be complicated by the presence of an incomplete nodal capsule. This can occur as a result of sample preparation, or because the capsule has thinned and is difficult to identify, which is especially common at the hilum of the lymph node (17). When the capsule is deficient, pathologists sometimes elect to reconstruct it virtually based on the remaining evident portions, which can introduce heterogeneity and impact the reproducibility of results (12). Another clinical conundrum may occur where there is continuity of a primary tumor and an adjacent lymph node. Some pathologists may elect to diagnose ENE in this case, as it cannot be definitely excluded, while other pathologists may restrict the diagnosis of pENE to those cases where there is evidence of remnant capsule that is discontinuous with the primary tumor (10). A further challenge arises when grossly confluent LNs (also known as matted or coalescent nodes) are present. In this case, additional sections are recommended to exclude ENE, as confluent LNs may simply represent closely aggregated LNs with thickened capsules without actual microscopic evidence of ENE (10). Despite the aforementioned limitations, histopathology remains the gold standard for determining the presence of ENE, and in most cases, it can be determined and categorized as macroscopic (major) or microscopic (minor) (5).
2.2 ENE on imaging
ENE can also be visualized on pre-treatment morphologic imaging such as computed tomographic scans (CT) (18), magnetic resonance imaging (MRI) (19), ultrasound scans (US) (20) and positron emission tomography-computed tomographic scans (PET-CT) (21). Whilst the role of CT and MRI is well-established in the diagnosis of iENE, the value of PET-CT or US is questioned by many (22). There are several other issues that remain unresolved in defining or diagnosing iENE. Firstly, there is no clear consensus on the terminology, definitions or diagnostic criteria. iENE is often defined as an involved LN on imaging with an unequivocally ill-defined nodal border (Figure 2), i.e., clearly discernible loss of the sharp plane between LN capsule and surrounding fat (23–26). Faraji et al. (27) performed a retrospective analysis of preoperative CT images of patients with HPV-associated HNSCC and concluded that irregular nodal margins and absence of perinodal fat plane were the most specific and sensitive features for iENE. The terms “conglomerate”, “matted” and “coalescent” have all been used to describe radiographically poorly delineated aggregates of two or more LNs, where iENE occurs between abutting nodes with loss of the intervening nodal planes (Figure 3, pattern 2; Figure 4A) (5, 26, 30). However, there are limitations and some differences between these terms, even though many might use and interpret them interchangeably (31, 32). Another area of uncertainty in iENE reporting is the presence of central nodal necrosis. Some studies have recognized central nodal necrosis as a significant predictor of ENE, but others have dismissed it as an intranodal characteristic rather than a feature of ENE (33–35). It is possible that nodal necrosis is only an association with ENE since both might indicate an aggressive tumor phenotype).
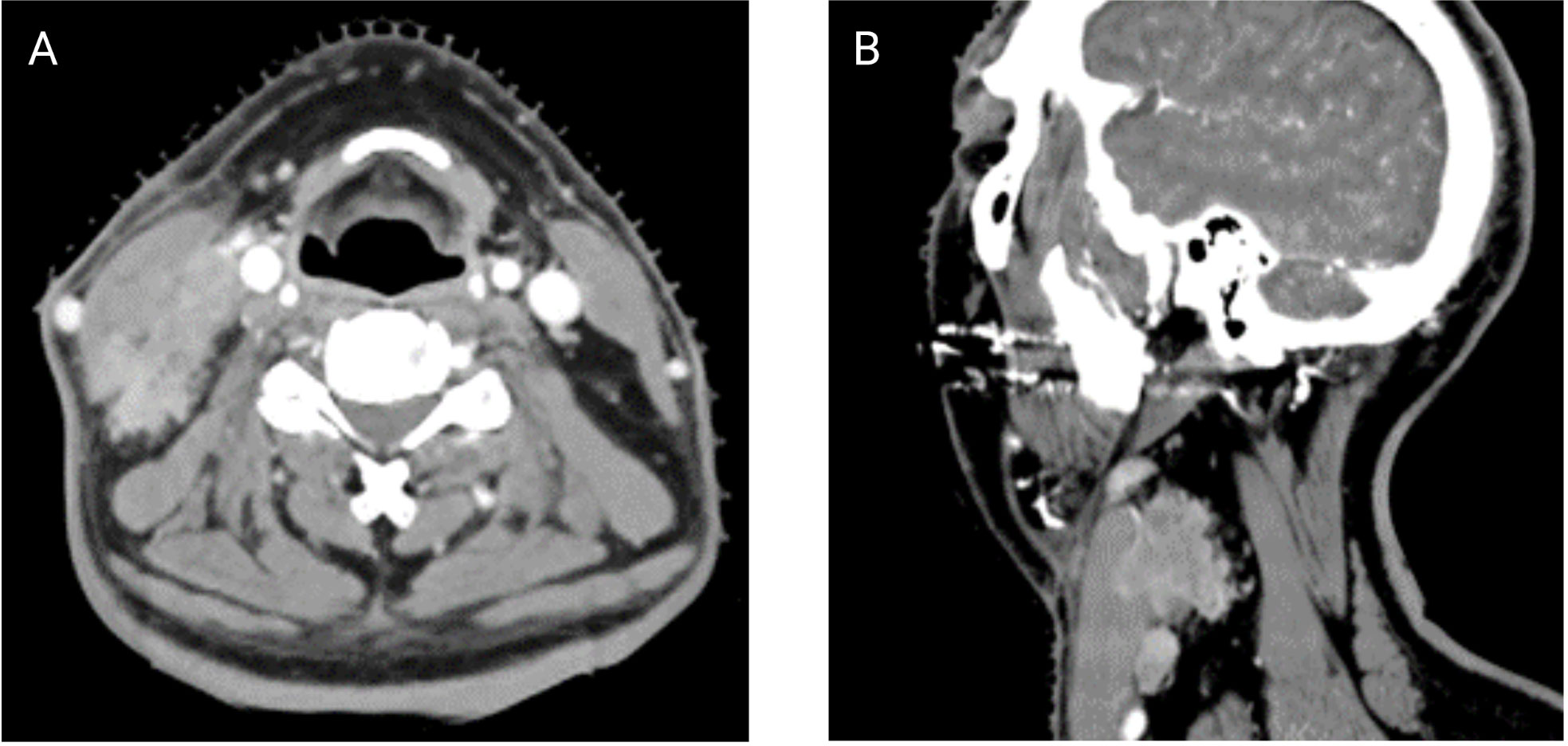
Figure 2 Contrasted axial (A) and sagittal (B) CT scans of a patient with clear ENE (Images kindly provided by Dr. Santiago Medrano).
Figure 3 Pattern/Grade of radiologically imaged ENE – depicting the extent of image-identified ENE (iENE) based on Hoebers et al. (28) and Chin et al. (29) (Images kindly provided by Dr. Eugene Yu).
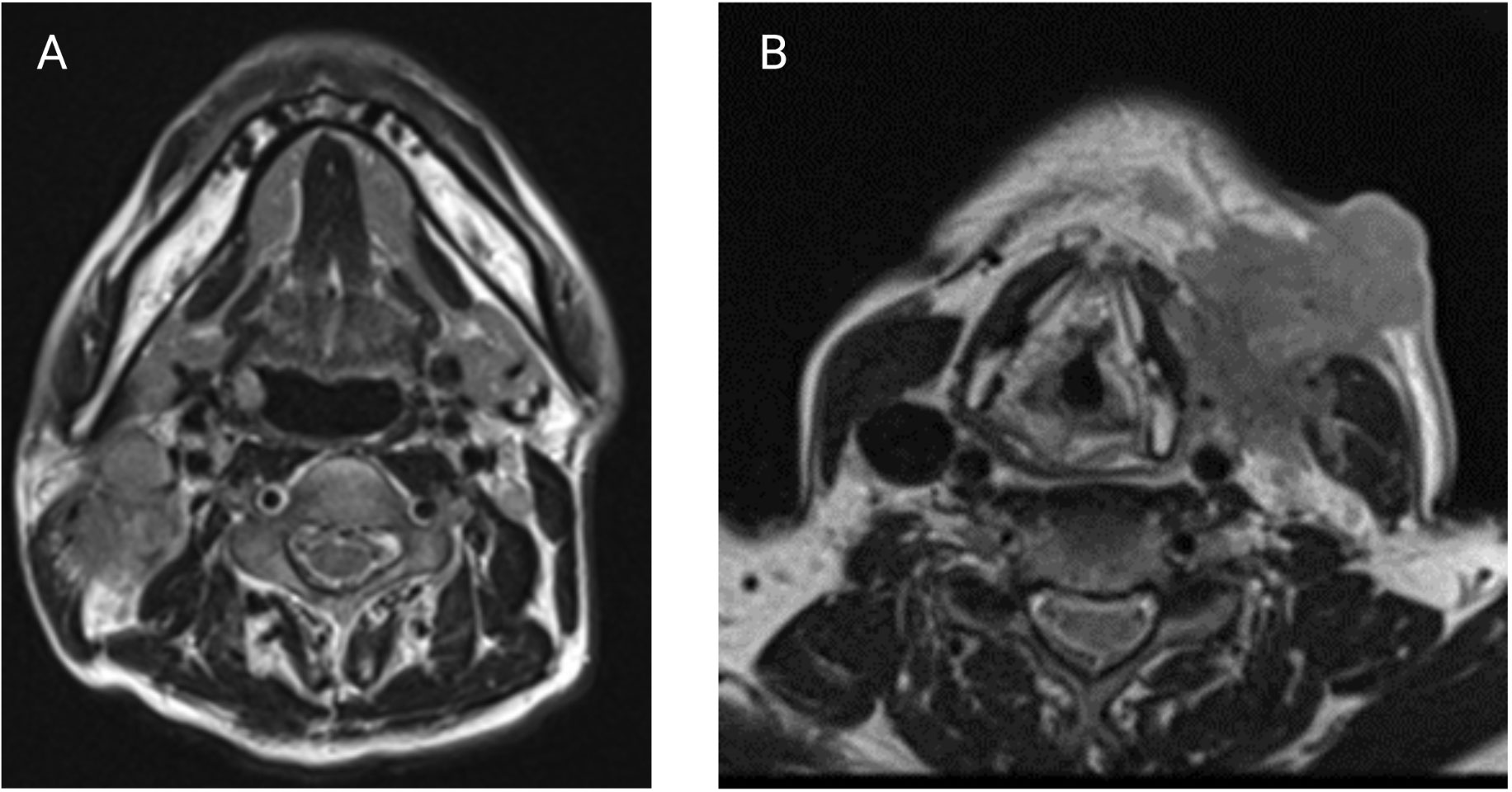
Figure 4 (A) Axial T2-weighted MRI showing a coalescent right level II nodal mass suspicious for iENE. (B) Axial T2-weighted MRI with a large L level III nodal mass with clear invasion of the sternocleidomastoid and extension into overlying subcutaneous fat and skin. (Images kindly provided by Dr. Eugene Yu).
Unfortunately, the use of imaging for ENE diagnosis prior to treatment is complicated by reports of low sensitivity, and poor negative predictive value (Figure 5). Studies show that iENE demonstrates a sensitivity of 60-80% and a specificity of 72-96% to predict pENE (17, 36, 37). Maxwell et al. (38) assessed CT scans from 65 surgically treated HNSCC patients, with two radiologists scoring the likelihood of iENE using a 5-point scale. That method demonstrated high inter-rater variability and poor performance, with an area under the curve (AUC) of the receiver operating characteristic ranged from 0.65–0.69 (38). This has stimulated research into methods to improve iENE reporting, and as a result, various imaging features have been combined into grading or classification systems, Table 1. Chin et al. (29, 39), Hoebers et al. (28) and Lu et al. (31) have all classified iENE into three patterns (Figure 3; Table 1), while Ai et al. (40) categorized the presence of iENE into just two grades. All four systems identified grade 1 as iENE limited to perinodal fat only. Moreover, both Hoebers’ and Lu’s systems aligned well for grade 2 (coalescent or matted nodes) and grade 3 (ENE into adjacent structures like muscles, nerves, skin, etc.). Ai et al. omitted coalescent or matted nodes as feature from their classification (40).
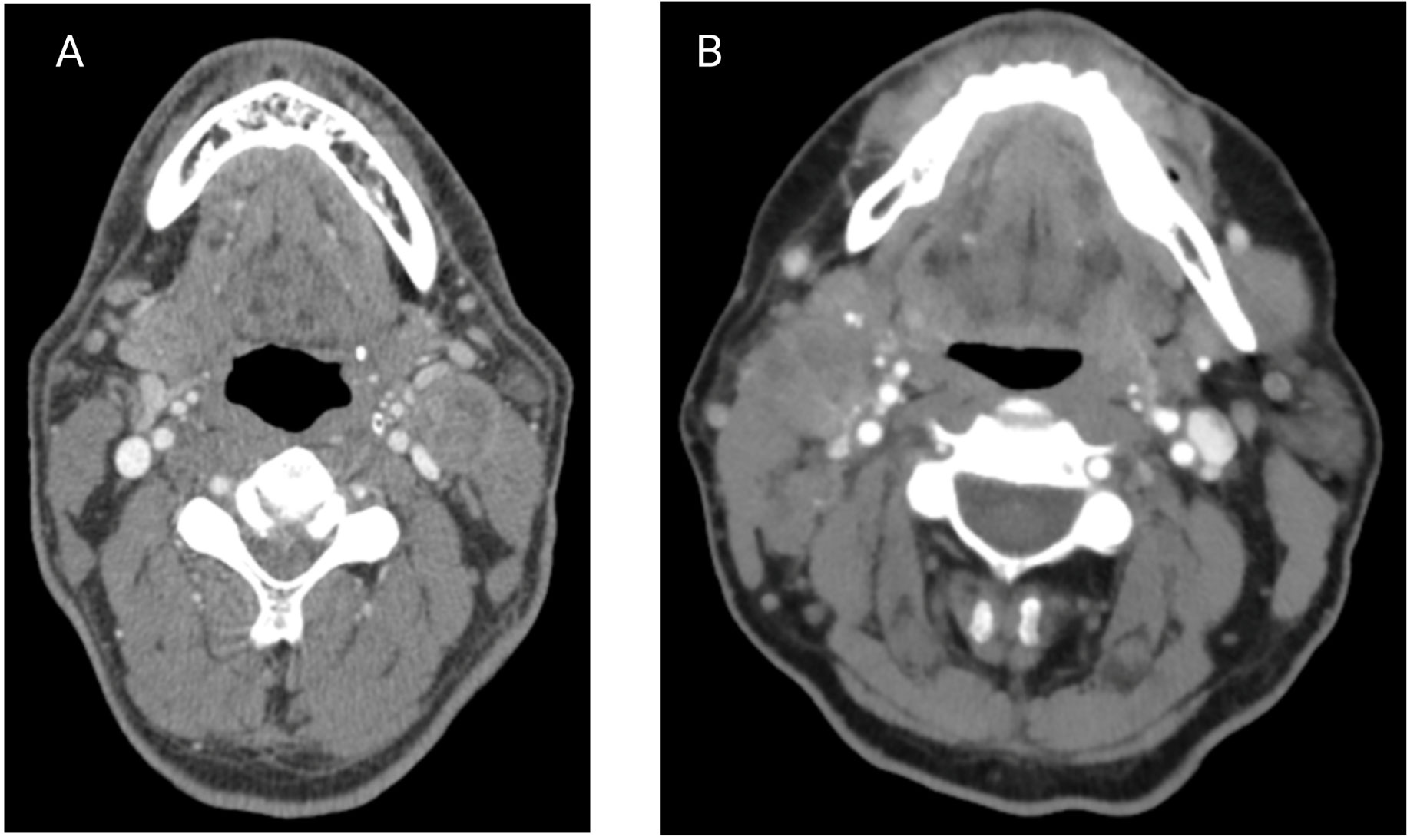
Figure 5 (A) Contrasted axial CT scan of a patient with a L level II node suspicious for iENE, which was later confirmed pathologically. (B) Contrasted axial CT scan of a patient with a R level II node suspicious for iENE, but ENE was not found on pathology. (Images kindly provided by Dr. James Bates).
2.3 Clinical ENE
Clinical ENE (cENE) was defined by the AJCC 8th edition of the TNM staging manual as: 1) unambiguous evidence of gross ENE on clinical examination, i.e. invasion of skin, infiltration of musculature or dense tethering to adjacent structures or cranial nerve, brachial plexus, sympathetic trunk or phrenic nerve invasion with dysfunction, and 2) strong radiographic evidence of ENE to support the clinical examination (9) (Figure 4B). Clinical ENE will typically correlate with grade 3 in the Hoebers and Lu classification systems (28, 31, 41). As such, this designation is reserved for only the most obvious, and relatively uncommon, cases of ENE.
3 Prognostic impact of ENE
3.1 Prognostic impact of pENE
pENE typically indicates a poorer prognosis (42, 43). While some of the older studies concluded that there was no relationship between pENE and survival (44, 45), these studies exhibited significant weakness due to insufficient statistical power, heterogeneity in adjuvant treatment strategies, and variable pathological interpretation of pENE (46, 47). More recent studies examining the prognostic importance of pENE in HNSCC have reported up to 50% lower relative overall and disease-specific survival (DSS) for patients with pENE (43, 48–55). Several publications (43, 46, 52, 56) have demonstrated that pENE is a poor prognostic indicator for distant metastasis (DM) (pooled OR 2.18, 95% CI 1.23–3.87), and loco-regional recurrence (LRR) (pooled OR 1.33, 95% CI 0.86–2.07). Other studies have concluded that pENE is a better predictor of OS than either resection margins (50, 57, 58) or TNM staging (43, 57, 58).
Based on its prognostic importance, pENE is considered an indication for intensification in treatment protocols for HNSCC patients. A pooled subset analysis of two landmark trials [EORTC 22931 (7) and RTOG 9501 (8)] cemented the paradigm of treatment intensification for patients with pENE (6). The addition of concomitant high-dose cisplatin to RT in patients with pENE and/or positive margins reduced the risk of LRR and death by 42% and 30% respectively compared to adjuvant RT alone (6). Although these trials did not analyze pENE and positive margins separately, they still support the role of intensified treatment in pENE cases as over 50% of enrolled patients had pENE (5, 6). Importantly, this adjuvant therapy for cases with pENE attenuates the reported negative impacts of pENE on prognosis (5, 59). It should be noted however that these studies consisted mainly of non-oropharyngeal cancer and so recruited cases were highly likely to be mainly HPV-negative.
3.2 Prognostic impact of the extent of ENE
The significance of the extent of pENE remains unclear. This controversy potentially stems from the lack of a universally accepted pENE definition and is further confounded by variations in sample processing and interpretation (5, 47, 49). While macroscopic pENE consistently indicates poor prognosis in HNSCC (60–62), the prognostic significance of microscopic pENE has not been widely proven (49, 50, 60, 63). Carter et al. (63) and Brasilino de Carvalho (60) found that macroscopic pENE increased the risk of recurrence (RR 3.5, 95% CI 1.7–7.0), and worsened recurrence-free survival (RFS), but they found that microscopic pENE had no impact (RR 1.3, 95% CI 0.6–3.0). Similarly, Clark et al. (62) studied a mixed cohort of HNSCC with advanced nodal stage and reported that while microscopic pENE had a similar risk of regional recurrence as no pENE, those with macroscopic pENE fared significantly worse (RR 4.3; 95% CI 1.87–9.89). However, the authors noted that patients with microscopic pENE had intermediate DSS outcomes between those with no pENE and those with macroscopic pENE, but the authors did not report actual values (62). Moreover, Jose et al. (49) analysed survival outcomes in a mixed cohort of HNSCC patients (71% had laryngeal and hypopharyngeal cancers), and demonstrated no statistical difference in RFS between microscopic and macroscopic pENE. It is apparent that there is differential impact of pENE by its extent. However, optimal cutoff of pENE extent remains debatable. The 8th edition AJCC/UICC (TNM8) staging manual did not include a cut-off for the extent of pENE in its definition, but they recommended documenting pENE grade as minor (≤2mm) or major (>2mm) ENE (64). Wreesmann et al. (65) and Mamic et al. (13) used ROC curve analysis to define discriminatory thresholds of 1.7 and 1.9 mm respectively in patients with oral cavity cancers, supporting the AJCC recommendation of 2 mm cutoff threshold. de Almeida et al. also showed prognostic difference in minor vs major pENE using a 2 mm cutoff (66). Similarly, Kwon et al. (11) and Arun et al. (16) found that empirically defined pENE extension thresholds of 2 mm (in a mixed cohort of HNSCC) and 5 mm (in oral cavity cancers) respectively, were able to produce significant survival differences (HR 3.8, 95% CI 2.0-7.2, and HR 95% CI). However, other studies did not support the arbitrary 2 mm extension threshold (15, 16). At the least, future efforts to better define and standardize pENE criteria would be well-served by agreeing upon a consistent terminology, i.e., major/minor or macroscopic/microscopic. Since ENE is most often diagnosed by a pathologist using a microscope and very rarely by the naked eye, even when greater than 2mm, we suggest the use of the terms major/minor as opposed to microscopic/macroscopic.
3.3 Prognostic impact of pENE in HPV-associated oropharyngeal squamous cell carcinomas
The impact of pENE on prognosis of HPV-associated OPSCC is widely questioned (67–69). Multiple retrospective single-centre studies (67, 69–79), two multi-centric studies (80, 81), one small national cancer database (NCDB) study (82), and two systematic reviews (46, 59) failed to demonstrate a negative prognostic impact for pENE in HPV-associated OPSCC treated with surgery and post-operative adjuvant therapy and suggest that the addition of chemotherapy to adjuvant radiation may not be necessary in such cases. Consequently the AJCC/UICC staging system excluded pENE for those patients (5, 64, 83) However, it must be noted that most of these studies were small and arguably inadequately powered to detect statistical significance for pENE in HPV-associated OPSCC, especially in this group of patients with significantly better survival and fewer events (5, 83, 84). Furthermore, the use of adjuvant chemoradiotherapy may have mitigated the negative prognostic impact of the pENE.
More recently, increasing evidence has suggested that pENE (especially >1 mm) in HPV-associated OPSCC does indeed negatively impact survival and regional control (83–90). Multivariate analysis of 92 p16-positive patients found pENE was an independent risk factor for overall survival (OS) and disease progression (87). Several other retrospective analyses of patients with HPV-associated OPSCC show that pENE is associated with worse survival, albeit with moderate effect size (5-11%) (84, 88–90). Importantly, a recent systematic review by Benchitrit et al. (83) pooling 1349 patients from 6 studies, concluded that pENE is associated with a relative reduction in OS of 89% (HR 1.89, 95% CI 1.15-3.13) in HPV-associated OPSCC. Moreover, the phase II ECOG 3311 trial (91) demonstrated that arm D, in which 88% of low risk HPV-associated OPSCC patients had pENE >1 mm, showed significantly poorer 2-year progression-free survival (PFS) of 90.7% (90% CI, 86.2 to 95.4) compared to arm A patients (2 year PFS= 96.9% (90% CI, 91.9 to 100%), who had no pENE, and arm B and C patients who had <1 mm pENE (2year PFS 94.9% (90% CI, 91.3 to 98.6]) and 96.0% (90% CI, 92.8 to 99.3) respectively. These differences in OS are further underscored by the fact that arm D patients received significantly more intensive tri-modal therapy (surgery, 66Gy of RT and cisplatin), compared to arm A patients (surgery only), and arm B and C (surgery and RT only at 50 or 60 Gy) (91).
3.4 Prognostic impact of iENE
Since the evidence on pENE only applies to surgically treated patients, there is great interest in the prognostic value of iENE, which could be applied to a wider cohort of HNSCC patients (5, 59, 83). In a systematic review, Benchetrit et al. (83) pooled iENE data from 1468 patients with HPV-associated OPSCC and demonstrated that iENE led to worse OS (HR 2.64, 95% CI 1.46-4.78), with a greater contribution by increased risk of distant failure (HR 3.83, 95% CI 1.88-7.80) than locoregional failure (HR, 2.03, 95% CI 0.86-4.79). A similar trend is also seen in the nasopharyngeal (30, 31, 39, 92) and HPV-negative HNSCC (41) literature: one large systematic review (93) that pooled data from 7532 patients with nasopharyngeal cancer found that iENE was associated with worse OS (HR 1.85-2.62) and distant metastasis-free survival (HR 2.07 -3.14). iENE has also been shown to be an independent factor associated with worse survival and distant control in patients with HPV-negative tumours in the oropharynx (41), oral cavity (23) and in mixed cohorts of HNSCC (94–96). Together, this literature points to a higher prognostic impact for iENE than pENE, possibly indicating that unequivocal iENE correlates with more advanced pENE (23, 83).
4 Management of ENE in head and neck cancer
To date, identification of pENE in HNSCC is critical in determining optimal treatment. Moreover, the identification of pENE also dictates the radiation dose. Peters et al. (97) conducted a prospective randomized trial to evaluate the optimal dose of radiotherapy in patients with locally advanced HNSCC. In this study, lower risk patients were randomized to 57.6 Gy vs 63 Gy, and those who were higher risk (typically pENE or positive margins) were randomized to 63 Gy vs 68.4 Gy. The study demonstrated that patients who had pENE had better regional control with doses ≥63 Gy (97). These results were further validated in a prospective clinical trial in 288 patients with locally advanced HNSCC (98). High risk patients with pENE or ≥2 risk factors received a higher dose, 63 Gy over either 5 or 7 weeks and showed that altered (accelerated) fractionation trended towards improved loco-regional control and OS.
The identification of ENE is also important in HPV-associated OPSCC, a disease setting typically associated with a favorable prognosis (99, 100). The pre-treatment detection of ENE in those patients plays a crucial role in identifying patients who may benefit from treatment de-escalation or intensified treatment approaches. For example, in ECOG 3311, even though patients with matted nodes on radiology were excluded from participating, nearly 30% of recruited patients had pENE mandating an escalation of adjuvant treatment, mainly for pENE >1mm (91). In the ORATOR trial, which compared primary RT versus surgery for patients with early OPSCC (mostly p16-positive) and no signs of iENE, nearly 24% of the surgery group had high risk features (positive margins or/and pENE) and ended up receiving tri-modal therapy (101). A recent analysis of a large national cancer database demonstrated pENE in up to 28% of patients who underwent transoral robotic surgery for HPV-associated OPSCC (102). These patients may not have required tri-modal therapy, if ENE could have been reliably identified before surgery, and definitive chemoradiotherapy recommended instead.
Based on conflicting evidence regarding the prognostic significance of pENE in HPV-associated OPSCC, there have been some studies exploring the de-escalation of treatment in surgically treated patients. The AVOID study attempted to omit chemotherapy for surgically treated HPV-associated OPSCC patients with no pENE and showed an excellent 2-year PFS rate (92.1%, 95% CI 80.2%-97.0%) (103). However, if one extrapolates the results of the ECOG 3311 one can surmise that in patients with pENE <1mm, dose reduction may be feasible, albeit that did not result in major improvements in patient reported functional outcomes (91). However, patients with pENE >1mm appear to have poorer outcomes than those without, despite receiving significantly more intensive treatment with cisplatin and higher doses of RT. In summary, although there may be a group of patients with HPV-associated OPSCC with low burden of ENE (possibly those with less than 2mm of ENE) who may not actually benefit from chemotherapy, that subgroup has not been adequately identified and therefore the treatment of such patients with adjuvant RT alone is not appropriate outside of a clinical trial.
5 Gaps in knowledge and future considerations
5.1 pENE definitions and terminology
The recent changes to the AJCC/UICC TNM system (64) were well received by the head and neck oncologic community worldwide (104), but the criteria for “unambiguous”, clinically-overt ENE (105) remain vague. Moreover, there is significant uncertainty regarding the diagnostic criteria of pENE, leading to heterogeneity when making a diagnosis. This may be contributing to the conflicting evidence commonly encountered in pENE research. There is still no consensus among pathologists on a preferred terminology for pENE, and a constellation of terms like extranodal extension, extracapsular spread, or extranodal spread are used interchangeably. Furthermore, while most pathologists will agree that extension of tumor cells in the perinodal fat and soft tissue is diagnostic for pENE, there is still significant uncertainty around determining pENE in challenging cases with matted nodes, nodal hilar involvement or in cases with direct extension of primary tumor into a node. Moreover, there is still no agreement between pathologists whether HPV status should be taken into consideration when interpreting pENE features. These challenging issues are best addressed first by consensus, to standardize the definitions, terminologies and synoptic reporting used for pENE, especially for microscopic versus macroscopic, in both HPV-associated and HPV-negative HNSCC. The pathology community should also come to agreement on a standardized lymph node processing and sampling methodology for pENE (5, 10). This may help facilitate much needed research on the prognostic power of pENE in both HPV-associated and -negative HNSCC.
5.2 iENE definitions and terminology
The importance of a common language for defining iENE at the time of diagnosis has been poorly addressed so far, and the lack of standardized definitions and nomenclature has contributed to conflicting evidence, both in clinical trials and real-world data (5). However, a rational roadmap to develop a standardized nomenclature for iENE faces some challenges. Widespread agreement in the radiology community regarding the diagnostic criteria for ENE on imaging is still lacking. Features like nodal size, central nodal necrosis and capsular thickening are still being debated as criteria for iENE. Moreover, there is still no consensus regarding interpreting findings like matted/coalescent nodes, or the impact of HPV status on iENE features. Furthermore, there is still no conclusive evidence regarding the best imaging modality for iENE identification. There are several published classification systems for iENE in head and neck cancer as shown in Table 1, but none of these systems have been widely adopted in routine clinical practice. Research into assessing, improving and validating these systems is needed, to enable wide adoption into clinical practice and there is a need for better appreciation of the impact of ENE, at least in its worst form, on the outcome of patients rather than disregarding it.
Thus, there is a pressing need for standardized diagnostic criteria for iENE, which could improve reproducibility and facilitate research and widespread clinical implementation. In our view, an international consensus process aiming to standardize the iENE criteria and address these gaps in the literature on iENE is needed.
5.3 Prognostic impact of iENE and pENE in HPV-associated and HPV-negative HNSCC
Robust and large-scale studies are needed to quantify the prognostic impact of pENE and iENE in HPV- associated and HPV-negative HNSCC. Such studies need to be adequately powered to definitively address the prognostic significance of the different grades of pENE, and to validate the commonly used 2 mm threshold, especially in HPV-associated tumors. This could then be integrated into the TNM system, de-escalation trials, and everyday practice.
5.4 Artificial intelligence for iENE
Recent advances in artificial intelligence (AI) may hold promise for use in the diagnosis of iENE and outcome prediction. Kann et al. (106) trained a 3-dimensional convolutional neural network using 2875 CT-segmented LN samples, correlated with pathology samples to act as a ground truth. They demonstrated an improvement in the AUC to 0.91 with a sensitivity of 88% (false negative rate: 12%), and specificity of 85% (false positive rate: 15%) (106). They later validated this approach using two external cohorts, consisting of a total of 200 LNs (107). The algorithm achieved an AUC of 0.84 (83.1% accuracy) and 0.90 (88.6% accuracy) in the two cohorts, outperforming two independent radiologists’ AUCs of 0.70 and 0.71 in the first cohort, and 0.60 and 0.82 in the second cohort respectively. The diagnostic accuracy and inter-rater variability of both radiologists improved when they were supported with deep learning assistance. Ariji et al. (108) also developed a deep learning algorithm and compared performance to radiologists. Once again, the deep learning system achieved high accuracy (84.0%) for diagnosing iENE, using a set of AI-determined features. In comparison, the radiologists’ accuracies based on a set of radiological criteria - minor axis ≥ 11 mm, central necrosis, and irregular borders- were 55.7%, 51.1% and 62.6% respectively (108). These efforts are still in early stages of development and will need to undergo wider external validation before routine implementation in clinical practice.
5.5 Biomarker discoveries for iENE
In the last decade, advances in biomarker technologies have led to multiple discoveries in HNSCC diagnosis and prognosis. There are currently several promising molecular biomarkers that could potentially be used for predicting pENE before commencement of treatment. However, these are still in early stages of development, with a high rate of false discoveries (46, 109). External validation in larger cohorts, and in some cases, better biomarkers are needed to confirm these associations and their clinical impact before being incorporated into clinical treatment paradigms.
6 Conclusion
Extranodal extension is associated with aggressive cancer behavior and poor prognosis. There are challenges in accurately identifying and classifying ENE, both on histopathologic examination and on pre-treatment imaging. Although earlier single institutional studies (72, 76) suggested lack of impact of pENE on HPV-associated OPSCC, likely due to selection bias and small sample size, more recent large studies indicate pENE is prognostic in this disease (83, 88, 89). iENE also has a negative prognostic impact, particularly on distant control. One of the major challenges is how to reduce the risk of distant metastasis in ENE+ patients (89, 110). International consensus is needed on definitions, terminology, and diagnostic criteria for both pENE and iENE in HNSCC. Moreover, large-scale studies are necessary to determine their prognostic impact in HPV-associated and HPV-negative cases.
Author contributions
CH: Conceptualization, Writing – original draft, Writing – review & editing. AA-F: Conceptualization, Writing – original draft, Writing – review and editing. DM: Writing – original draft, Writing – review and editing. LM: Conceptualization, Writing – original draft, Writing – review and editing. SB: Conceptualization, Writing – original draft, Writing – review and editing. JB: Conceptualization, Writing – original draft, Writing – review and editing. AL: Conceptualization, Writing – original draft, Writing – review and editing. PB: Conceptualization, Writing – original draft, Writing – review and editing. PS: Conceptualization, Writing – original draft, Writing – review and editing. PN: Conceptualization, Writing – original draft, Writing – review and editing. SH: Writing – review and editing. WL: Writing – review and editing. BO: Writing – review and editing. HM: Conceptualization, Writing – original draft, Writing – review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. Preparation of this manuscript was supported in part by the NCI Cancer Center Support Grant (P30CA225520) awarded to the University of Oklahoma Stephenson Cancer Center (SCC), and a grant from the Oklahoma Tobacco Settlement Endowment Trust (R23-02).
Acknowledgments
The authors thank Dr. Eugene Yu, Department of Medical Imaging, University of Toronto, Toronto, Canada and Dr. Santiago Medrano-Martorell, Department of Radiology, Hospital Clínic de Barcelona, Barcelona, Spain for providing images used in this publication. Figures were created in Biorender.com.
Conflict of interest
HM reports grants from UK National Institute of Health research, Cancer Research UK, the UK Medical Research Council, and AstraZeneca; advisory board fees from AstraZeneca, MSD, Merck, Nanobiotix, and Seagen; and is Director of Warwickshire head neck clinic and Docpsert Health.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Willis RA. Epidermoid carcinoma of the head and neck, with special reference to metastasis. J Pathol Bacteriol (1930) 33(3):501–26. doi: 10.1002/path.1700330302
2. Bennett SH, Futrell JW, Roth JA, Hoye RC, Ketcham AS. Prognostic significance of histologic host response in cancer of the larynx or hypopharynx. Cancer (1971) 28(5):1255–65. doi: 10.1002/1097-0142(1971)28:5<1255::aid-cncr2820280524>3.0.co;2-a
3. Routman DM, Funk RK, Tangsriwong K, Lin A, Keeney MG, Garcia JJ, et al. Relapse rates with surgery alone in human papillomavirus-related intermediate- and high-risk group oropharynx squamous cell cancer: A multi-institutional review. Int J Radiat Oncol Biol Phys (2017) 99(4):938–46. doi: 10.1016/j.ijrobp.2017.06.2453
4. Bhattacharya P, Mukherjee R. Lymph node extracapsular extension as a marker of aggressive phenotype: Classification, prognosis and associated molecular biomarkers. Eur J Surg Oncol (2021) 47(4):721–31. doi: 10.1016/j.ejso.2020.09.005
5. Huang SH, Chernock R, O’Sullivan B, Fakhry C. Assessment criteria and clinical implications of extranodal extension in head and neck cancer. Am Soc Clin Oncol Educ Book (2021) 41:265–78. doi: 10.1200/EDBK_320939
6. Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck (2005) 27(10):843–50. doi: 10.1002/hed.20279
7. Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med (2004) 350(19):1945–52. doi: 10.1056/NEJMoa032641
8. Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med (2004) 350(19):1937–44. doi: 10.1056/NEJMoa032646
9. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer (2017).
10. Bullock MJ, Beitler JJ, Carlson DL, Fonseca I, Hunt JL, Katabi N, et al. Data set for the reporting of nodal excisions and neck dissection specimens for head and neck tumors: explanations and recommendations of the guidelines from the international collaboration on cancer reporting. Arch Pathol Lab Med (2019) 143(4):452–62. doi: 10.5858/arpa.2018-0421-SA
11. Kwon M, Roh JL, Lee J, Cho KJ, Choi SH, Nam SY, et al. Extranodal extension and thickness of metastatic lymph node as a significant prognostic marker of recurrence and survival in head and neck squamous cell carcinoma. J Craniomaxillofac Surg (2015) 43(6):769–78. doi: 10.1016/j.jcms.2015.04.021
12. Wreesmann VB, Katabi N, Palmer FL, Montero PH, Migliacci JC, Gonen M, et al. Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head Neck (2016) 38 Suppl 1:E1192–9. doi: 10.1002/hed.24190
13. Mamic M, Lucijanic M, Manojlovic L, Muller D, Suton P, Luksic I. Prognostic significance of extranodal extension in oral cavity squamous cell carcinoma with occult neck metastases. Int J Oral Maxillofac Surg (2021) 50(3):309–15. doi: 10.1016/j.ijom.2020.07.006
14. Tirelli G, Tofanelli M, Sacchet E, Bussani R, Shafiei V, Gatto A, et al. Extranodal extension in head and neck squamous cell cancer: is there a role for further stratification? Br J Oral Maxillofac Surg (2021) 59(5):567–72. doi: 10.1016/j.bjoms.2020.09.015
15. Greenberg JS, Fowler R, Gomez J, Mo V, Roberts D, El Naggar AK, et al. Extent of extracapsular spread: a critical prognosticator in oral tongue cancer. Cancer (2003) 97(6):1464–70. doi: 10.1002/cncr.11202
16. Arun I, Maity N, Hameed S, Jain PV, Manikantan K, Sharan R, et al. Lymph node characteristics and their prognostic significance in oral squamous cell carcinoma. Head Neck (2021) 43(2):520–33. doi: 10.1002/hed.26499
17. Abdel-Halim CN, Rosenberg T, Dyrvig AK, Hoilund-Carlsen PF, Sorensen JA, Rohde M, et al. Diagnostic accuracy of imaging modalities in detection of histopathological extranodal extension: A systematic review and meta-analysis. Oral Oncol (2021) 114:105169. doi: 10.1016/j.oraloncology.2020.105169
18. Carvalho P, Baldwin D, Carter R, Parsons C. Accuracy of CT in detecting squamous carcinoma metastases in cervical lymph nodes. Clin Radiol (1991) 44(2):79–81. doi: 10.1016/s0009-9260(05)80500-8
19. Kimura Y, Sumi M, Sakihama N, Tanaka F, Takahashi H, Nakamura T. MR imaging criteria for the prediction of extranodal spread of metastatic cancer in the neck. AJNR Am J Neuroradiol (2008) 29(7):1355–9. doi: 10.3174/ajnr.A1088
20. Steinkamp HJ, Beck A, Werk M, Rademaker J, Felix R. [Extracapsular spread of cervical lymph node metastases: diagnostic relevance of ultrasound examinations]. Ultraschall Med (2003) 24(5):323–30. doi: 10.1055/s-2003-42914
21. Chun BJ, Yoo Ie R, Joo YH, Nam IC, Cho JH, Kim CS, et al. Efficacy of 18F-fluorodeoxyglucose positron emission tomography/CT imaging for extracapsular spread of laryngeal squamous cell carcinoma. Head Neck (2016) 38(2):290–3. doi: 10.1002/hed.23889
22. Su Z, Duan Z, Pan W, Wu C, Jia Y, Han B, et al. Predicting extracapsular spread of head and neck cancers using different imaging techniques: a systematic review and meta-analysis. Int J Oral Maxillofac Surg (2016) 45(4):413–21. doi: 10.1016/j.ijom.2015.11.021
23. Almulla A, Noel CW, Lu L, Xu W, O’Sullivan B, Goldstein DP, et al. Radiologic-pathologic correlation of extranodal extension in patients with squamous cell carcinoma of the oral cavity: implications for future editions of the TNM classification. Int J Radiat Oncol Biol Phys (2018) 102(4):698–708. doi: 10.1016/j.ijrobp.2018.05.020
24. Chai RL, Rath TJ, Johnson JT, Ferris RL, Kubicek GJ, Duvvuri U, et al. Accuracy of computed tomography in the prediction of extracapsular spread of lymph node metastases in squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg (2013) 139(11):1187–94. doi: 10.1001/jamaoto.2013.4491
25. Prabhu RS, Magliocca KR, Hanasoge S, Aiken AH, Hudgins PA, Hall WA, et al. Accuracy of computed tomography for predicting pathologic nodal extracapsular extension in patients with head-and-neck cancer undergoing initial surgical resection. Int J Radiat Oncol Biol Phys (2014) 88(1):122–9. doi: 10.1016/j.ijrobp.2013.10.002
26. Spector ME, Gallagher KK, Light E, Ibrahim M, Chanowski EJ, Moyer JS, et al. Matted nodes: poor prognostic marker in oropharyngeal squamous cell carcinoma independent of HPV and EGFR status. Head Neck (2012) 34(12):1727–33. doi: 10.1002/hed.21997
27. Faraji F, Aygun N, Coquia SF, Gourin CG, Tan M, Rooper LM, et al. Computed tomography performance in predicting extranodal extension in HPV-positive oropharynx cancer. Laryngoscope (2020) 130(6):1479–86. doi: 10.1002/lary.28237
28. Hoebers F, Yu E, O’Sullivan B, Postma AA, Palm WM, Bartlett E, et al. Augmenting inter-rater concordance of radiologic extranodal extension in HPV-positive oropharyngeal carcinoma: A multicenter study. Head Neck (2022) 44(11):2361–9. doi: 10.1002/hed.27130
29. Chin O, Alshafai L, O’Sullivan B, Su J, Hope A, Bartlett E, et al. Inter-rater concordance and operating definitions of radiologic nodal feature assessment in human papillomavirus-positive oropharyngeal carcinoma. Oral Oncol (2022) 125:105716. doi: 10.1016/j.oraloncology.2022.105716
30. Hu Y, Lu T, Huang SH, Lin S, Chen Y, Fang Y, et al. High-grade radiologic extra-nodal extension predicts distant metastasis in stage II nasopharyngeal carcinoma. Head Neck (2019) 41(9):3317–27. doi: 10.1002/hed.25842
31. Lu T, Hu Y, Xiao Y, Guo Q, Huang SH, O’Sullivan B, et al. Prognostic value of radiologic extranodal extension and its potential role in future N classification for nasopharyngeal carcinoma. Oral Oncol (2019) 99:104438. doi: 10.1016/j.oraloncology.2019.09.030
32. Sananmuang T, Yu E, Su J, O’Sullivan B, Rathod S, Chan B, et al. Pre- and post-radiotherapy radiologic nodal features and oropharyngeal cancer outcomes. Laryngoscope (2021) 131(4):E1162–E71. doi: 10.1002/lary.29045
33. Mahajan A, Chand A, Agarwal U, Patil V, Vaish R, Noronha V, et al. Prognostic value of radiological extranodal extension detected by computed tomography for predicting outcomes in patients with locally advanced head and neck squamous cell cancer treated with radical concurrent chemoradiotherapy. Front Oncol (2022) 12:814895. doi: 10.3389/fonc.2022.814895
34. Aiken AH, Poliashenko S, Beitler JJ, Chen AY, Baugnon KL, Corey AS, et al. Accuracy of preoperative imaging in detecting nodal extracapsular spread in oral cavity squamous cell carcinoma. AJNR Am J Neuroradiol (2015) 36(9):1776–81. doi: 10.3174/ajnr.A4372
35. Randall DR, Lysack JT, Hudon ME, Guggisberg K, Nakoneshny SC, Wayne Matthews T, et al. Diagnostic utility of central node necrosis in predicting extracapsular spread among oral cavity squamous cell carcinoma. Head Neck (2015) 37(1):92–6. doi: 10.1002/hed.23562
36. Park SI, Guenette JP, Suh CH, Hanna GJ, Chung SR, Baek JH, et al. The diagnostic performance of CT and MRI for detecting extranodal extension in patients with head and neck squamous cell carcinoma: a systematic review and diagnostic meta-analysis. Eur Radiol (2021) 31(4):2048–61. doi: 10.1007/s00330-020-07281-y
37. Yan F, Byun YJ, Nguyen SA, Stalcup ST, Day TA. Predictive value of computed tomography in identifying extranodal extension in human papillomavirus-positive versus human papillomavirus-negative head and neck cancer. Head Neck (2020) 42(9):2687–95. doi: 10.1002/hed.26281
38. Maxwell JH, Rath TJ, Byrd JK, Albergotti WG, Wang H, Duvvuri U, et al. Accuracy of computed tomography to predict extracapsular spread in p16-positive squamous cell carcinoma. Laryngoscope (2015) 125(7):1613–8. doi: 10.1002/lary.25140
39. Chin O, Yu E, O’Sullivan B, Su J, Tellier A, Siu L, et al. Prognostic importance of radiologic extranodal extension in nasopharyngeal carcinoma treated in a Canadian cohort. Radiother Oncol (2021) 165:94–102. doi: 10.1016/j.radonc.2021.10.018
40. Ai QY, King AD, Poon DMC, Mo FKF, Hui EP, Tong M, et al. Extranodal extension is a criterion for poor outcome in patients with metastatic nodes from cancer of the nasopharynx. Oral Oncol (2019) 88:124–30. doi: 10.1016/j.oraloncology.2018.11.007
41. Pilar A, Yu E, Su J, O’Sullivan B, Bartlett E, Waldron JN, et al. Prognostic value of clinical and radiologic extranodal extension and their role in the 8th edition TNM cN classification for HPV-negative oropharyngeal carcinoma. Oral Oncol (2021) 114:105167. doi: 10.1016/j.oraloncology.2020.105167
42. Shah JP, Cendon RA, Farr HW, Strong EW. Carcinoma of the oral cavity. factors affecting treatment failure at the primary site and neck. Am J Surg (1976) 132(4):504–7. doi: 10.1016/0002-9610(76)90328-7
43. Johnson JT, Barnes EL, Myers EN, Schramm VL Jr., Borochovitz D, Sigler BA. The extracapsular spread of tumors in cervical node metastasis. Arch Otolaryngol (1981) 107(12):725–9. doi: 10.1001/archotol.1981.00790480001001
44. Mamelle G, Pampurik J, Luboinski B, Lancar R, Lusinchi A, Bosq J. Lymph node prognostic factors in head and neck squamous cell carcinomas. Am J Surg (1994) 168(5):494–8. doi: 10.1016/s0002-9610(05)80109-6
45. Pinsolle J, Pinsolle V, Majoufre C, Duroux S, Demeaux H, Siberchicot F. Prognostic value of histologic findings in neck dissections for squamous cell carcinoma. Arch Otolaryngol Head Neck Surg (1997) 123(2):145–8. doi: 10.1001/archotol.1997.01900020023003
46. Mermod M, Tolstonog G, Simon C, Monnier Y. Extracapsular spread in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol (2016) 62:60–71. doi: 10.1016/j.oraloncology.2016.10.003
47. Ferlito A, Rinaldo A, Devaney KO, MacLennan K, Myers JN, Petruzzelli GJ, et al. Prognostic significance of microscopic and macroscopic extracapsular spread from metastatic tumor in the cervical lymph nodes. Oral Oncol (2002) 38(8):747–51. doi: 10.1016/s1368-8375(02)00052-0
48. Shaw RJ, Lowe D, Woolgar JA, Brown JS, Vaughan ED, Evans C, et al. Extracapsular spread in oral squamous cell carcinoma. Head Neck (2010) 32(6):714–22. doi: 10.1002/hed.21244
49. Jose J, Coatesworth AP, Johnston C, MacLennan K. Cervical node metastases in squamous cell carcinoma of the upper aerodigestive tract: the significance of extracapsular spread and soft tissue deposits. Head Neck (2003) 25(6):451–6. doi: 10.1002/hed.10214
50. Woolgar JA, Rogers SN, Lowe D, Brown JS, Vaughan ED. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncol (2003) 39(2):130–7. doi: 10.1016/s1368-8375(02)00030-1
51. Andersen PE, Warren F, Spiro J, Burningham A, Wong R, Wax MK, et al. Results of selective neck dissection in management of the node-positive neck. Arch Otolaryngol Head Neck Surg (2002) 128(10):1180–4. doi: 10.1001/archotol.128.10.1180
52. Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer (2001) 92(12):3030–6. doi: 10.1002/1097-0142(20011215)92:12<3030::aid-cncr10148>3.0.co;2-p
53. Snow GB, Annyas AA, van Slooten EA, Bartelink H, Hart AA. Prognostic factors of neck node metastasis. Clin Otolaryngol Allied Sci (1982) 7(3):185–92. doi: 10.1111/j.1365-2273.1982.tb01581.x
54. Shimizu K, Inoue H, Saitoh M, Ohtsuki N, Ishida H, Makino K, et al. Distribution and impact of lymph node metastases in oropharyngeal cancer. Acta Otolaryngol (2006) 126(8):872–7. doi: 10.1080/00016480500504259
55. Dunne AA, Muller HH, Eisele DW, Kessel K, Moll R, Werner JA. Meta-analysis of the prognostic significance of perinodal spread in head and neck squamous cell carcinomas (HNSCC) patients. Eur J Cancer (2006) 42(12):1863–8. doi: 10.1016/j.ejca.2006.01.062
56. Vaidya AM, Petruzzelli GJ, Clark J, Emami B. Patterns of spread in recurrent head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg (2001) 125(4):393–6. doi: 10.1067/mhn.2001.117715
57. Liao CT, Wang HM, Chang JT, Ng SH, Hsueh C, Lee LY, et al. Analysis of risk factors for distant metastases in squamous cell carcinoma of the oral cavity. Cancer (2007) 110(7):1501–8. doi: 10.1002/cncr.22959
58. Jan JC, Hsu WH, Liu SA, Wong YK, Poon CK, Jiang RS, et al. Prognostic factors in patients with buccal squamous cell carcinoma: 10-year experience. J Oral Maxillofac Surg (2011) 69(2):396–404. doi: 10.1016/j.joms.2010.05.017
59. Tassone P, Crawley M, Bovenzi C, Zhan T, Keane W, Cognetti D, et al. Pathologic markers in surgically treated HPV-associated oropharyngeal cancer: retrospective study, systematic review, and meta-analysis. Ann Otol Rhinol Laryngol (2017) 126(5):365–74. doi: 10.1177/0003489417693014
60. Brasilino de Carvalho M. Quantitative analysis of the extent of extracapsular invasion and its prognostic significance: a prospective study of 170 cases of carcinoma of the larynx and hypopharynx. Head Neck (1998) 20(1):16–21. doi: 10.1002/(sici)1097-0347(199801)20:1<16::aid-hed3>3.0.co;2-6
61. Carter RL, Barr LC, O’Brien CJ, Soo KC, Shaw HJ. Transcapsular spread of metastatic squamous cell carcinoma from cervical lymph nodes. Am J Surg (1985) 150(4):495–9. doi: 10.1016/0002-9610(85)90162-x
62. Clark J, Li W, Smith G, Shannon K, Clifford A, McNeil E, et al. Outcome of treatment for advanced cervical metastatic squamous cell carcinoma. Head Neck (2005) 27(2):87–94. doi: 10.1002/hed.20129
63. Carter RL, Bliss JM, Soo KC, O’Brien CJ. Radical neck dissections for squamous carcinomas: pathological findings and their clinical implications with particular reference to transcapsular spread. Int J Radiat Oncol Biol Phys (1987) 13(6):825–32. doi: 10.1016/0360-3016(87)90094-0
64. Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin (2017) 67(2):122–37. doi: 10.3322/caac.21389
65. Wreesmann VB, Katabi N, Palmer FL, Montero PH, Migliacci JC, Gonen M, et al. Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head Neck (2016) 38 Suppl 1(Suppl 1):E1192–9. doi: 10.1002/hed.24190
66. de Almeida JR, Truong T, Khan NM, Su JS, Irish J, Gilbert R, et al. Treatment implications of postoperative chemoradiotherapy for squamous cell carcinoma of the oral cavity with minor and major extranodal extension. Oral Oncol (2020) 110:104845. doi: 10.1016/j.oraloncology.2020.104845
67. Iyer NG, Dogan S, Palmer F, Rahmati R, Nixon IJ, Lee N, et al. Detailed analysis of clinicopathologic factors demonstrate distinct difference in outcome and prognostic factors between surgically treated HPV-positive and negative oropharyngeal cancer. Ann Surg Oncol (2015) 22(13):4411–21. doi: 10.1245/s10434-015-4525-0
68. Sinha P, Lewis JS Jr., Piccirillo JF, Kallogjeri D, Haughey BH. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer (2012) 118(14):3519–30. doi: 10.1002/cncr.26671
69. Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope (2012) 122 Suppl 2:S13–33. doi: 10.1002/lary.23493
70. Rich JT, Milov S, Lewis JS Jr., Thorstad WL, Adkins DR, Haughey BH. Transoral laser microsurgery (TLM) +/- adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope (2009) 119(9):1709–19. doi: 10.1002/lary.20552
71. Wilkie MD, Upile NS, Lau AS, Williams SP, Sheard J, Helliwell TR, et al. Transoral laser microsurgery for oropharyngeal squamous cell carcinoma: A paradigm shift in therapeutic approach. Head Neck (2016) 38(8):1263–70. doi: 10.1002/hed.24432
72. Sinha P, Kallogjeri D, Gay H, Thorstad WL, Lewis JS Jr., Chernock R, et al. High metastatic node number, not extracapsular spread or N-classification is a node-related prognosticator in transorally-resected, neck-dissected p16-positive oropharynx cancer. Oral Oncol (2015) 51(5):514–20. doi: 10.1016/j.oraloncology.2015.02.098
73. Kumar B, Cipolla MJ, Old MO, Brown NV, Kang SY, Dziegielewski PT, et al. Surgical management of oropharyngeal squamous cell carcinoma: Survival and functional outcomes. Head Neck (2016) 38 Suppl 1:E1794–802. doi: 10.1002/hed.24319
74. Klozar J, Koslabova E, Kratochvil V, Salakova M, Tachezy R. Nodal status is not a prognostic factor in patients with HPV-positive oral/oropharyngeal tumors. J Surg Oncol (2013) 107(6):625–33. doi: 10.1002/jso.23292
75. Kaczmar JM, Tan KS, Heitjan DF, Lin A, Ahn PH, Newman JG, et al. HPV-related oropharyngeal cancer: Risk factors for treatment failure in patients managed with primary transoral robotic surgery. Head Neck (2016) 38(1):59–65. doi: 10.1002/hed.23850
76. Maxwell JH, Ferris RL, Gooding W, Cunningham D, Mehta V, Kim S, et al. Extracapsular spread in head and neck carcinoma: impact of site and human papillomavirus status. Cancer (2013) 119(18):3302–8. doi: 10.1002/cncr.28169
77. Hobelmann KC, Topf MC, Bar-Ad V, Luginbuhl AJ, Keane WM, Curry JM, et al. AJCC-8ed nodal staging does not predict outcomes in surgically managed HPV-associated oropharyngeal cancer. Oral Oncol (2018) 82:138–43. doi: 10.1016/j.oraloncology.2018.05.016
78. Park YM, Kang MS, Koh YW, Choi EC, Kim SH. Does p16+ Predict a favorable prognosis for oropharyngeal cancer? Risk factors for treatment failure for patients who underwent surgery-based therapy. Ann Surg Oncol (2019) 26(2):547–54. doi: 10.1245/s10434-018-6806-x
79. Kharytaniuk N, Molony P, Boyle S, O’Leary G, Werner R, Heffron C, et al. Association of extracapsular spread with survival according to human papillomavirus status in oropharynx squamous cell carcinoma and carcinoma of unknown primary site. JAMA Otolaryngol Head Neck Surg (2016) 142(7):683–90. doi: 10.1001/jamaoto.2016.0882
80. Haughey BH, Hinni ML, Salassa JR, Hayden RE, Grant DG, Rich JT, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head Neck (2011) 33(12):1683–94. doi: 10.1002/hed.21669
81. Haughey BH, Sinha P, Kallogjeri D, Goldberg RL, Lewis JS Jr., Piccirillo JF, et al. Pathology-based staging for HPV-positive squamous carcinoma of the oropharynx. Oral Oncol (2016) 62:11–9. doi: 10.1016/j.oraloncology.2016.09.004
82. Amini A, Jasem J, Jones BL, Robin TP, McDermott JD, Bhatia S, et al. Predictors of overall survival in human papillomavirus-associated oropharyngeal cancer using the National Cancer Data Base. Oral Oncol (2016) 56:1–7. doi: 10.1016/j.oraloncology.2016.02.011
83. Benchetrit L, Torabi SJ, Givi B, Haughey B, Judson BL. Prognostic significance of extranodal extension in HPV-mediated oropharyngeal carcinoma: A systematic review and meta-analysis. Otolaryngol Head Neck Surg (2021) 164(4):720–32. doi: 10.1177/0194599820951176
84. Bauer E, Mazul A, Chernock R, Rich J, Jackson RS, Paniello R, et al. Extranodal extension is a strong prognosticator in HPV-positive oropharyngeal squamous cell carcinoma. Laryngoscope (2020) 130(4):939–45. doi: 10.1002/lary.28059
85. Beltz A, Gosswein D, Zimmer S, Limburg I, Wunsch D, Gribko A, et al. Staging of oropharyngeal squamous cell carcinoma of the head and neck: Prognostic features and power of the 8th edition of the UICC staging manual. Eur J Surg Oncol (2019) 45(6):1046–53. doi: 10.1016/j.ejso.2019.02.032
86. Kompelli AR, Morgan P, Li H, Harris W, Day TA, Neskey DM. Prognostic impact of high-risk pathologic features in HPV-related oropharyngeal squamous cell carcinoma and tobacco use. Otolaryngol Head Neck Surg (2019) 160(5):855–61. doi: 10.1177/0194599818818446
87. Freitag J, Wald T, Kuhnt T, Gradistanac T, Kolb M, Dietz A, et al. Extracapsular extension of neck nodes and absence of human papillomavirus 16-DNA are predictors of impaired survival in p16-positive oropharyngeal squamous cell carcinoma. Cancer (2020) 126(9):1856–72. doi: 10.1002/cncr.32667
88. Zhan KY, Eskander A, Kang SY, Old MO, Ozer E, Agrawal AA, et al. Appraisal of the AJCC 8th edition pathologic staging modifications for HPV-positive oropharyngeal cancer, a study of the National Cancer Data Base. Oral Oncol (2017) 73:152–9. doi: 10.1016/j.oraloncology.2017.08.020
89. An Y, Park HS, Kelly JR, Stahl JM, Yarbrough WG, Burtness BA, et al. The prognostic value of extranodal extension in human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer (2017) 123(14):2762–72. doi: 10.1002/cncr.30598
90. Miccio JA, Verma V, Kelly J, Kann BH, An Y, Park HS, et al. Impact of contralateral lymph nodal involvement and extranodal extension on survival of surgically managed HPV-positive oropharyngeal cancer staged with the AJCC eighth edition. Oral Oncol (2019) 99:104447. doi: 10.1016/j.oraloncology.2019.104447
91. Ferris RL, Flamand Y, Weinstein GS, Li S, Quon H, Mehra R, et al. Phase II randomized trial of transoral surgery and low-dose intensity modulated radiation therapy in resectable p16+ Locally advanced oropharynx cancer: an ECOG-ACRIN cancer research group trial (E3311). J Clin Oncol (2022) 40(2):138–49. doi: 10.1200/JCO.21.01752
92. Mao Y, Wang S, Lydiatt W, Shah JP, Colevas AD, Lee AWM, et al. Unambiguous advanced radiologic extranodal extension determined by MRI predicts worse outcomes in nasopharyngeal carcinoma: Potential improvement for future editions of N category systems. Radiother Oncol (2021) 157:114–21. doi: 10.1016/j.radonc.2021.01.015
93. Tsai TY, Chou YC, Lu YA, Kang CJ, Huang SF, Liao CT, et al. The prognostic value of radiologic extranodal extension in nasopharyngeal carcinoma: Systematic review and meta-analysis. Oral Oncol (2021) 122:105518. doi: 10.1016/j.oraloncology.2021.105518
94. Liu JT, Kann BH, De B, Buckstein M, Bakst RL, Genden EM, et al. Prognostic value of radiographic extracapsular extension in locally advanced head and neck squamous cell cancers. Oral Oncol (2016) 52:52–7. doi: 10.1016/j.oraloncology.2015.11.008
95. de Bree R, Ljumanovic R, Hazewinkel MJ, Witte BI, Castelijns JA. Radiologic extranodal spread and matted nodes: Important predictive factors for development of distant metastases in patients with high-risk head and neck cancer. Head Neck (2016) 38 Suppl 1:E1452–8. doi: 10.1002/hed.24257
96. Moon H, Choi YJ, Lee YS, Lee SW, Kim SB, Roh JL, et al. Value of extranodal extension detected by computed tomography for predicting clinical response after chemoradiotherapy in head and neck squamous cell cancer. Acta Otolaryngol (2018) 138(4):392–9. doi: 10.1080/00016489.2017.1395517
97. Peters LJ, Goepfert H, Ang KK, Byers RM, Maor MH, Guillamondegui O, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys (1993) 26(1):3–11. doi: 10.1016/0360-3016(93)90167-t
98. Ang KK, Trotti A, Brown BW, Garden AS, Foote RL, Morrison WH, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys (2001) 51(3):571–8. doi: 10.1016/s0360-3016(01)01690-x
99. Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med (2010) 363(1):24–35. doi: 10.1056/NEJMoa0912217
100. Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol (2015) 33(29):3235–42. doi: 10.1200/JCO.2015.61.6995
101. Nichols AC, Theurer J, Prisman E, Read N, Berthelet E, Tran E, et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol (2019) 20(10):1349–59. doi: 10.1016/S1470-2045(19)30410-3
102. Baliga S, Klamer B, Jhawar S, Gamez M, Mitchell D, Blakaj A, et al. Identification of clinical and socioeconomic predictors of adjuvant therapy after trans-oral robotic surgery in patients with oropharyngeal squamous cell carcinoma. Cancers (Basel) (2020) 12(9):2474. doi: 10.3390/cancers12092474
103. Swisher-McClure S, Lukens JN, Aggarwal C, Ahn P, Basu D, Bauml JM, et al. A phase 2 trial of alternative volumes of oropharyngeal irradiation for de-intensification (AVOID): omission of the resected primary tumor bed after transoral robotic surgery for human papilloma virus-related squamous cell carcinoma of the oropharynx. Int J Radiat Oncol Biol Phys (2020) 106(4):725–32. doi: 10.1016/j.ijrobp.2019.11.021
104. Zanoni DK, Patel SG, Shah JP. Changes in the 8th edition of the American Joint Committee on Cancer (AJCC) staging of head and neck cancer: rationale and implications. Curr Oncol Rep (2019) 21(6):52. doi: 10.1007/s11912-019-0799-x
105. Glastonbury CM, Mukherji SK, O’Sullivan B, Lydiatt WM. Setting the stage for 2018: how the changes in the american joint committee on cancer/union for international cancer control cancer staging manual eighth edition impact radiologists. AJNR Am J Neuroradiol (2017) 38(12):2231–7. doi: 10.3174/ajnr.A5409
106. Kann BH, Aneja S, Loganadane GV, Kelly JR, Smith SM, Decker RH, et al. Pretreatment identification of head and neck cancer nodal metastasis and extranodal extension using deep learning neural networks. Sci Rep (2018) 8(1):14036. doi: 10.1038/s41598-018-32441-y
107. Kann BH, Hicks DF, Payabvash S, Mahajan A, Du J, Gupta V, et al. Multi-institutional validation of deep learning for pretreatment identification of extranodal extension in head and neck squamous cell carcinoma. J Clin Oncol (2020) 38(12):1304–11. doi: 10.1200/JCO.19.02031
108. Ariji Y, Sugita Y, Nagao T, Nakayama A, Fukuda M, Kise Y, et al. CT evaluation of extranodal extension of cervical lymph node metastases in patients with oral squamous cell carcinoma using deep learning classification. Oral Radiol (2020) 36(2):148–55. doi: 10.1007/s11282-019-00391-4
109. Dhanda J, Triantafyllou A, Liloglou T, Kalirai H, Lloyd B, Hanlon R, et al. SERPINE1 and SMA expression at the invasive front predict extracapsular spread and survival in oral squamous cell carcinoma. Br J Cancer (2014) 111(11):2114–21. doi: 10.1038/bjc.2014.500
Keywords: extranodal extension, head and neck cancer, locally advanced head and neck cancer, head and neck pathology, head and neck squamous cell carcinoma
Citation: Henson CE, Abou-Foul AK, Morton DJ, McDowell L, Baliga S, Bates J, Lee A, Bonomo P, Szturz P, Nankivell P, Huang SH, Lydiatt WM, O’Sullivan B and Mehanna H (2023) Diagnostic challenges and prognostic implications of extranodal extension in head and neck cancer: a state of the art review and gap analysis. Front. Oncol. 13:1263347. doi: 10.3389/fonc.2023.1263347
Received: 19 July 2023; Accepted: 04 September 2023;
Published: 20 September 2023.
Edited by:
Gyorgy B. Halmos, University Medical Center Groningen, NetherlandsReviewed by:
Sandro J. Stoeckli, Cantonal Hospital St.Gallen, SwitzerlandMusaddiq Javvad Awan, Medical College of Wisconsin, United States
Copyright © 2023 Henson, Abou-Foul, Morton, McDowell, Baliga, Bates, Lee, Bonomo, Szturz, Nankivell, Huang, Lydiatt, O’Sullivan and Mehanna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christina E. Henson, Q2hyaXN0aW5hLWhlbnNvbkBvdWhzYy5lZHU=
†These authors share first authorship
 Christina E. Henson
Christina E. Henson Ahmad K. Abou-Foul
Ahmad K. Abou-Foul Daniel J. Morton3
Daniel J. Morton3 Lachlan McDowell
Lachlan McDowell Sujith Baliga
Sujith Baliga James Bates
James Bates Anna Lee
Anna Lee Pierluigi Bonomo
Pierluigi Bonomo Petr Szturz
Petr Szturz Paul Nankivell
Paul Nankivell Hisham Mehanna
Hisham Mehanna