- 1Department of General Surgery, the General Hospital of Southern Theater Command, People’s Liberation Army (PLA), Guangzhou, China
- 2Department of Pathology, The General Hospital of Southern Theater Command, People’s Liberation Army (PLA), Guangzhou, China
- 3Medical Affairs Department, Acornmed Biotechnology Co., Ltd, Beijing, China
In this report, we present a case study of a 64-year-old female who was diagnosed with gastrointestinal stromal tumors (GISTs) and subsequently developed liver metastases despite undergoing radical resection. Next-generation sequencing (NGS) assays indicated that the tumor lacked KIT/PDGFRA/SDH/RAS-P (RAS pathways, RAS-P) mutations, thereby classifying this patient as quadruple WT GIST (qGIST). Treatment with imatinib was initiated, and after 2.5 months, recurrence of the tumor and multiple metastases around the surgical site were observed. Consequently, the patient was switched to sunitinib treatment and responded well. Although she responded well to sunitinib, the patient died of tumor dissemination within 4 months. This case study highlights the potential efficacy of imatinib and the VEGFR-TKI sunitinib in treating qGIST patients harboring a TP53 missense mutation.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common of all sarcomas (1). Most GISTs occur in the stomach (60–65%), and the second most common site is the small intestine (20–25%); the rectum, colon, esophagus, and other sites are rare (1, 2). GISTs are a heterogeneous group of tumors that includes a variety of molecular entities. Approximately 85-90% of GISTs harbor KIT or PDGFRA mutations, and the remaining GISTs that do not harbor any mutation of the KIT and PDGFRA genes are defined as wild-type (WT) GISTs. Among WT GISTs, 20-40% of tumors are SDH-deficient GISTs that show loss of function of the succinate dehydrogenase complex (SDH). Approximately 15% of KIT/PDGFRA WT cases harbor mutations in BRAF/RAS or NF1 and are referred to as RAS-pathway (RAS-P) mutant GISTs. In addition, a small subset of all GISTs (5%) that lack KIT/PDGFRA/SDH/RAS-P (RAS pathways, RAS-P) mutations can be referred to as quadruple WT GISTs or quadruple negative GISTs (3). To date, a variety of sporadic somatic molecular events in quadruple WT GISTs have been reported, including TP53, MEN1, MAX, CHD4, FGFR1, CTDNN2, CBL, ARID1A, BCOR, and APC (4).
TP53 is a critical tumor suppressor gene that encodes a 64 kDa protein (5, 6). Mutations in TP53 not only impair its antitumor activity but also confer mutant p53 protein oncogenic properties. TP53 gene mutations are found in a variety of tumors (6). However, information about mutations in TP53 that occur in quadruple WT GISTs is limited. Recently, the frequency of mutant TP53 in GISTs was reported to be 3.5%, and only one quadruple WT GIST case harbored a TP53 mutation (p.V172F) (5). In addition, approaches for p53-targeted therapy in quadruple WT GISTs are rare.
To our knowledge, imatinib, which targets KIT/PDGFRA, has been used as a standard first-line treatment for patients with localized and advanced GISTs (7). However, 50-60% of patients with GISTs may exhibit primary or secondary resistance to imatinib (8). In particular, WT GIST is known to be generally unresponsive to the tyrosine kinase inhibitor imatinib used for non-WT GIST (9, 10). In addition, there is no consensus on the treatment of qGIST owing to the variety of molecular subtypes. Thus, identifying gene mutations in different patients is necessary to guide therapy and improve prognosis by matching targeted drugs.
Here, we present a quadruple WT GIST case that harbored a TP53 missense mutation identified by next-generation sequencing. The patient progressed rapidly after imatinib treatment and subsequently achieved remission with the VEGFR-TKI sunitinib, which is a p53-targeted therapy. This case provides new insights into the rare treatment of qGIST.
Case presentation
A 64-year-old female presented at Southern Theater General Hospital for right abdominal pain that had persisted for over 3 months. Computed tomography (CT) imaging on July 8, 2022, showed a mass on the lower edge of the right upper abdominal liver lobe approximately 33 X 48 mm, and the boundary was unclear (Figure 1). The adjacent peritoneum, colon and liver were not clearly demarcated, the adjacent peritoneum was slightly thickened, its internal density was uneven, and it seemed to have punctate calcification foci and a slightly irregular low density. Thus, the CT result indicated the possibility of colon exophytic malignant stromal tumors. The tumor and part of the liver were surgically removed in July 2022 (Figure 1).
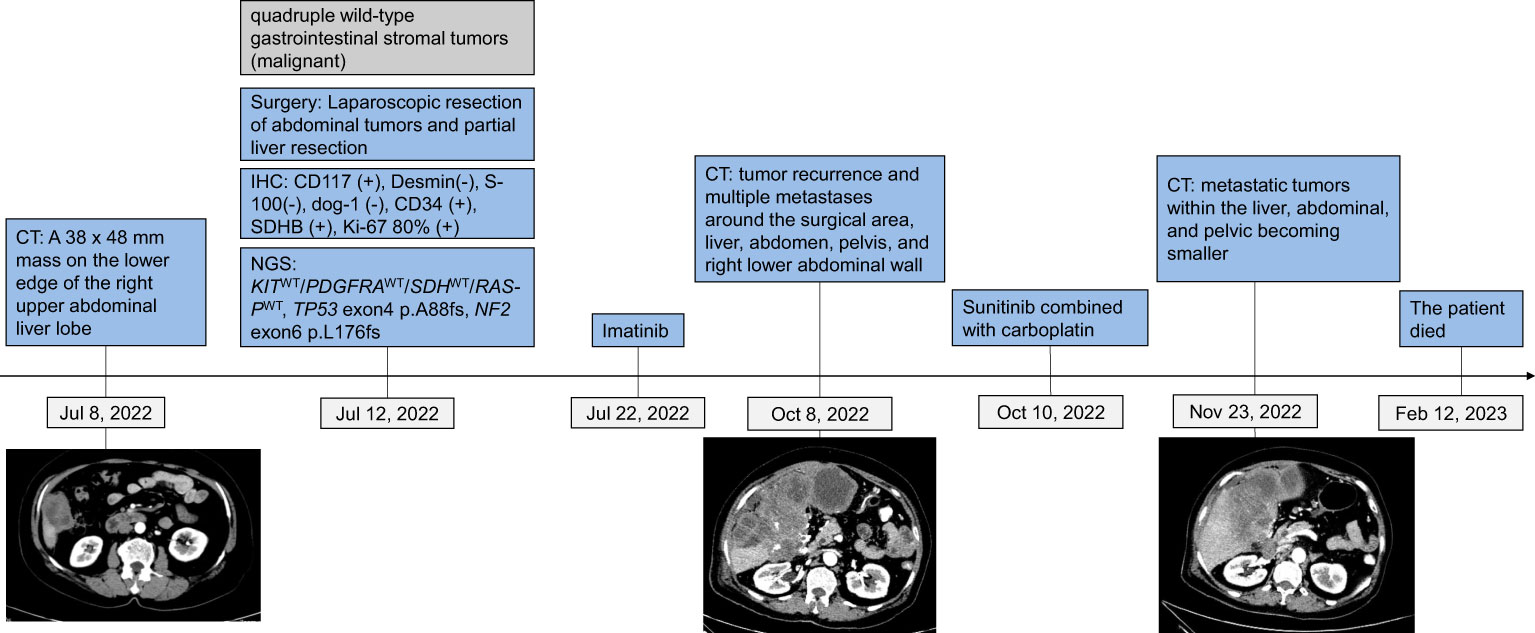
Figure 1 Tumor progression and treatment of a patient with quadruple wild-type gastrointestinal stromal tumors.
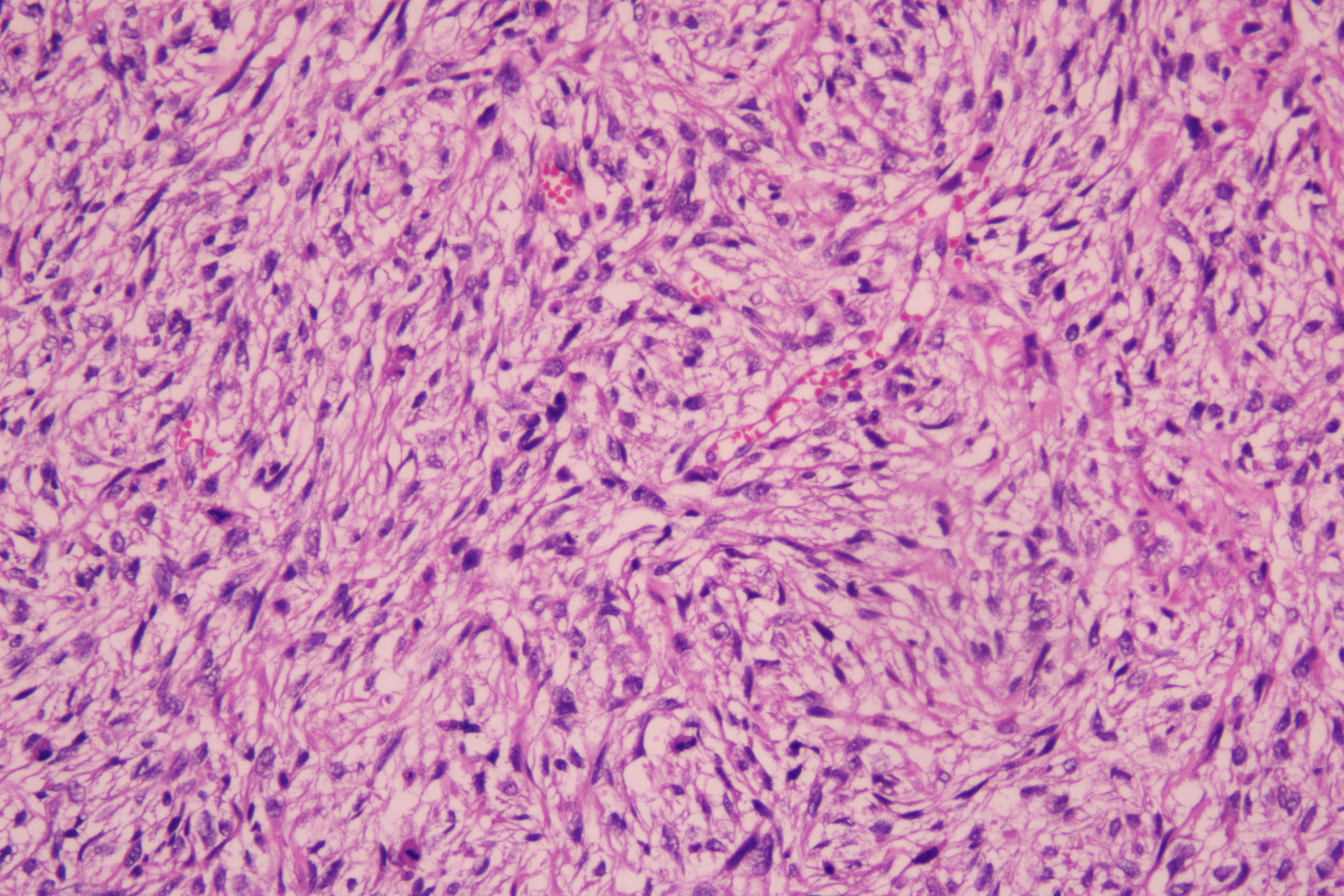
Histopathological examination of the excised mass showed spindle cells and epithelioid cells were mixed. The spindle cells were arranged in bundles, in a braided and palisade manner, with varying cell density, no significant pleomorphism, and visible nuclear vacuoles. Epithelioid cells were distributed in sheets with clear cell boundaries. Multinucleated giant cells were observed in some areas and mitotic images could be seen. Fibrous demarcation was observed between the spindle cells and epithelioid cells. Focal stroma exhibited myxoid alteration (Figure 2).
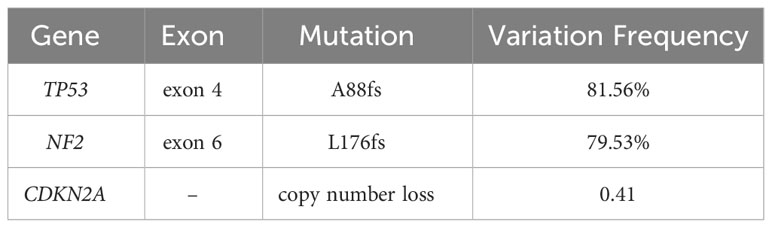
On immunohistochemistry, the tumor cells were positive for CD117, negative for Desmin and S-100 (Figure 3), negative for dog-1, positive for CD34, and positive for SDHB. The Ki-67 labeling index was 80%. The mutation status of the tissue sample was detected by using the Next-generation sequencing (NGS)-based Acorn808 gene panel (Acornmed, Beijing, China), which covers all the coding exons of 808 tumor-related genes that are frequently mutated in solid tumors. The NGS results revealed no mutations in the exons corresponding to the c-KIT and PDGFRA genes, including exons 9, 11, 13 and 17 of KIT and exons 12, 14 and 18 of PDGFRA. This patient lacked mutations in RAS-P (NF-1, BRAF, RAS). Therefore, this GIST patient had quadruple WT GIST (KITWT/PDGFRAWT/SDHWT/RAS-PWT). Interestingly, NGS revealed the presence of a TP53 exon 4 p. A88fs (81.56% abundance in tissue) and NF2 exon 5 p. L176fs (79.53% abundance in tissue) somatic mutation (Table 1).
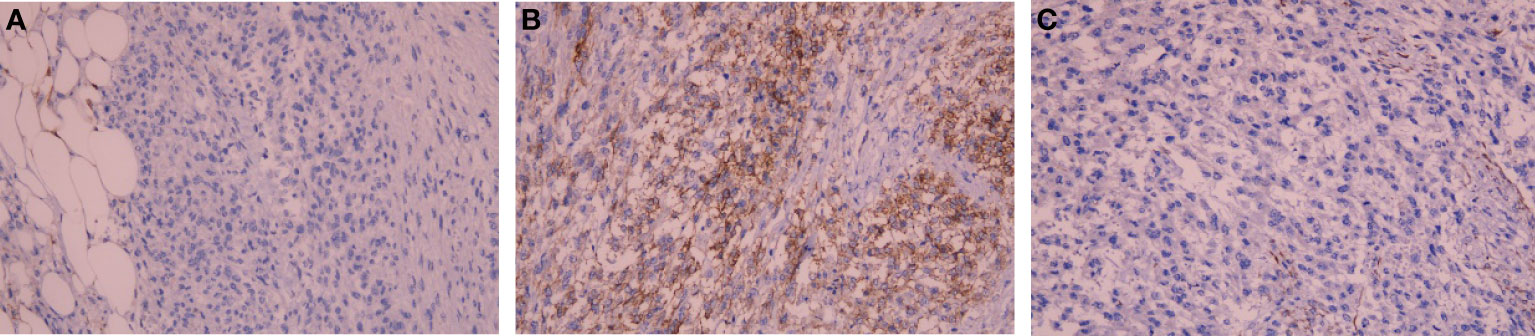
Figure 3 Photomicrograph showing negative immunohistochemical stains of (A) Desmin and (B) S-100, positive immunohistochemical stains of (C) CD117 (IHC x200).
According to the NCCN guidelines Version 2. 2022 Gastrointestinal stromal tumors (GISTs), TKIs could offer a treatment option with relatively good responses for wild-type GISTs. Thus, postoperative adjuvant imatinib therapy was recommended for this patient. The patient received imatinib (400 mg/day) treatment from July 22, 2022, to October 7, 2022. The CT result on October 8, 2022, indicated tumor recurrence and multiple metastases around the surgical area, liver, abdomen, pelvis, and right lower abdominal wall, with a lesion size of 14*11*8 cm in the liver area.
Considering that sunitib is the standard second-line therapy for GISTs, and TP53 exon 4 p. A88fs indicates that patients with solid tumors might be sensitive to VEGFR-TKIs (11), we treated the patient with sunitinib (2 weeks of treatment at a dose of 37.5 mg/day and 1 week of rest) in combination with carboplatin (AUC 6, IV, d1, q21d). The patient responded well to sunitinib at first, with metastatic tumors in the liver, abdominal, and pelvic becoming smaller and a lesion in the liver region measuring 10*8*7 cm; however, she died of tumor dissemination on February 12, 2023.
Discussion
Here, we show a rare quadruple WT GIST with TP53 mutation. This quadruple WT GIST patient’s disease progressed rapidly after initial imatinib treatment; however, after switching to sunitinib, the disease responded well. GISTs are uncommon neoplasms that originate from the interstitial cells of Cajal located within the gastrointestinal tract. GISTs are commonly present in the stomach (60-65% of cases), followed by the small intestine (20-25%), while occurrence in other locations, such as the rectum, colon, and esophagus, is infrequent (1). In this case, the tumor originated from the abdominal region, with its location potentially impacting the patient’s prognosis, which has rarely been reported. KIT and PDGFRA mutations are the most common mutations found in GISTs, accounting for approximately 85-90% of cases. There is a rare subtype of GISTs that do not harbor any mutations in the KIT, PDGFRA, SDH, and RAS-P genes, and these are defined as quadruple WT GISTs (1, 3).
TP53 gene mutations have been found in a small percentage of GISTs, particularly in the quadruple WT subgroup. The TP53 gene, also known as tumor protein 53, is a well-known tumor suppressor gene that is frequently mutated in many different types of cancer (4). At present, the exact role of TP53 mutations in the development and progression of GISTs remains unclear. However, it is believed that TP53 mutations may play a role in the development of GISTs by promoting genomic instability and thereby facilitating the acquisition of additional genetic alterations that contribute to tumor development and progression (11). Studies have suggested that TP53 mutations may be associated with poorer prognosis and decreased survival in patients with GISTs (6). Additionally, preclinical studies have suggested that TP53 mutations may confer resistance to certain therapies, such as imatinib, which is a standard treatment for GISTs (5). While TP53 mutations are not common in GISTs, their association with qWT GISTs and possible involvement in tumorigenesis and treatment resistance merits further investigation.
Although imatinib treatment for GIST has been recognized as the model of precision oncology, there is still substantial confusion on how to manage quadruple WT GISTs (2). The discovery of the TP53 mutation and CDKN2A copy number loss may be the reason for the patient’s rapid disease progression following imatinib therapy. The poor prognostic effect of TP53 gene overexpression and its association with higher malignant risk in GIST have already been described (11). As is common knowledge, the TP53 gene plays a role in the activation of DNA repair and apoptosis initiation. For several decades, the role of TP53 dysregulation in carcinogenesis has been explored in human tumors and linked to both tumor-suppressing and oncogenic function loss (12).Tumors with p53 mutations often develop more rapidly, respond poorly to anticancer therapy and have a poor prognosis (5, 6). Another possibly relevant mechanism of GIST progression toward more aggressive biological behavior is a partial deletion of chromosome 9, which plays an important role in the transition from low- to high-risk GIST (5). The loss of the cell cycle inhibitor p16 (CDKN2A), which is a result of this deletion, is one potential outcome. Inactivating mutations in this gene or its deletion appear to be required to increase cell cycle activity and, as a result, progress from low- to high-risk GIST (5).
Imatinib is the standard treatment for GIST, however, it is already known that the absence of KIT or PDGFRA mutations confers greater resistance to imatinib (10, 13). In addition, preclinical studies have described that TP53 mutations in GIST may not be sensitive to imatinib treatment (5). It appears that most KIT/PDGFRA WT GISTs have an indolent clinical behavior that is not predicted by conventional risk stratification (13). And the patient underwent rapid progression after standard treatment with imatinib, suggesting that imatinib may not be suitable as a first-line treatment for qGIST. Considering sunitinib as a standard second line treatment and NGS detection of TP53 mutation, the patient received treatment with sunitinib, with a significant remission. Currently, it has been documented that patients with TP53 mutations potentially respond significantly to VEGFR TKI therapy (11). However, up to 50-60% of GISTs show primary or secondary resistance to imatinib (8). Advanced resistant GIST responds to second-line sunitinib, third-line regorafenib, and the novel broad-spectrum TKI ripretinib (1, 8). Combined with the gene test results, the patient was given sunitinib after first-line imatinib resistance. Encouragingly, the patient initially responded well with smaller metastatic tumors in the liver, abdomen and pelvis than before, and the size of the lesion in the liver region was reduced to 10*8*7 cm after sunitinib treatment (the size of the tumor in the liver metastasis after imatinib treatment was 14*11*8 cm). It may be worthwhile to use sunitinib as a first-line treatment option for patients with qGIST. Unfortunately, the patient succumbed to tumor dissemination, which was possibly related to sunitinib resistance after remission, highlighting the need for more careful monitoring and treatment. Recent studies have shown that the p53 pathway may be involved in the resistance of renal cell carcinoma cells to sunitinib, and that p53-positive cases tend to be associated with poor progression-free survival (PFS) after first-line sunitinib treatment (14). A clinical trial study (IMmotion151) also showed TP53 mutation was significantly associated with poor PFS in metastatic RCC (mRCC) treated with first-line sunitinib (14). Further research is evidently required to delve into qGIST, especially in combination with TP53 mutation.
Conclusions
In conclusion, we reported a case of a 64-year-old female with GIST who developed liver metastases despite surgery. Genetic testing revealed that the tumor lacked specific mutations, classifying it as quadruple qGIST. While treated with imatinib, the tumor recurred, but the patient responded well to sunitinib. However, she passed away due to tumor dissemination within four months. This case suggests that qGIST patients with TP53 mutations may benefit from treatment with the VEGFR-TKI sunitinib, but further research is needed to optimize treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LC: Funding acquisition, Supervision, Writing – review and editing, Conceptualization, Resources. YC: Writing – original draft, Investigation. JC: Writing – review and editing, Data curation. LL: Writing – review and editing, Methodology. XM: Formal Analysis, Project administration, Software, Writing – review and editing. YS: Formal Analysis, Software, Writing – review and editing. YZ: Formal Analysis, Software, Writing – review and editing. HC: Writing – review & editing, LH: Data curation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
Author XM, YS, HC, and YZ were employed by the company Acornmed Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Blay J-Y, Kang Y-K, Nishida T, von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Primers (2021) 7:22. doi: 10.1038/s41572-021-00254-5
2. Luo Y, Wu Y, Chang X, Huang B, Luo D, Zhang J, et al. Identification of a novel FGFR2-KIAA1217 fusion in esophageal gastrointestinal stromal tumours: A case report. Front Oncol (2022) 12:884814. doi: 10.3389/fonc.2022.884814
3. Pantaleo MA, Nannini M, Corless CL, Heinrich MC. Quadruple wild-type (WT) GIST: defining the subset of GIST that lacks abnormalities of KIT, PDGFRA, SDH, or RAS signaling pathways. Cancer Med (2015) 4:101–3. doi: 10.1002/cam4.325
4. Astolfi A, Pantaleo MA, Indio V, Urbini M, Nannini M. The emerging role of the FGF/FGFR pathway in gastrointestinal stromal tumor. IJMS (2020) 21:3313. doi: 10.3390/ijms21093313
5. Ihle MA, Huss S, Jeske W, Hartmann W, Merkelbach-Bruse S, Schildhaus H-U, et al. Expression of cell cycle regulators and frequency of TP53 mutations in high risk gastrointestinal stromal tumors prior to adjuvant imatinib treatment. PloS One (2018) 13:e0193048. doi: 10.1371/journal.pone.0193048
6. Hu J, Cao J, Topatana W, Juengpanich S, Li S, Zhang B, et al. Targeting mutant p53 for cancer therapy: direct and indirect strategies. J Hematol Oncol (2021) 14:157. doi: 10.1186/s13045-021-01169-0
7. Huang W, Yuan W, Ren L, Xu C, Luo R, Zhou Y, et al. A novel fusion between CDC42BPB and ALK in a patient with quadruple wild-type gastrointestinal stromal tumor. Molec Gen Gen Med (2022) 10:e1881. doi: 10.1002/mgg3.1881
8. Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. JCO (2008) 26:5352–9. doi: 10.1200/JCO.2007.15.7461
9. Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, George S, et al. Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors. JAMA Oncol (2016) 2:922–8. doi: 10.1001/jamaoncol.2016.0256
10. Wong NACS, Giger OT, ten Hoopen R, Casey RT, Russell K, Faulkner C. Next-generation sequencing demonstrates the rarity of short kinase variants specific to quadruple wild-type gastrointestinal stromal tumours. J Clin Pathol (2021) 74:194–7. doi: 10.1136/jclinpath-2020-206613
11. Li AM, Boichard A, Kurzrock R. Mutated TP53 is a marker of increased VEGF expression: analysis of 7,525 pan-cancer tissues. Cancer Biol Ther (2019) 21:95–100. doi: 10.1080/15384047.2019.1665956
12. Pantaleo MA, Urbini M, Indio V, Ravegnini G, Nannini M, De M, et al. Genome-wide analyses identifies MEN1 and MAX mutations and a neuroendocrine-like molecular heterogeneity in quadruple WT GIST. Mol Cancer Res (2017) 15:553–62. doi: 10.1158/1541-7786.MCR-16-0376
13. Nannini M, Biasco G, Astolfi A, Pantaleo MA. An overview on molecular biology of KIT/PDGFRA wild type (WT) gastrointestinal stromal tumours (GIST). J Med Genet (2013) 50:653–61. doi: 10.1136/jmedgenet-2013-101695
Keywords: case report, quadruple WT GIST, next-generation sequencing, TP53 mutation, p53-targeted therapy
Citation: Chen Y, Chen J, Long L, Han L, Mi X, Song Y, Cheng H, Zhang Y and Cheng L (2023) Case Report: A novel TP53 mutation in a patient with quadruple wild-type gastrointestinal stromal tumor. Front. Oncol. 13:1260706. doi: 10.3389/fonc.2023.1260706
Received: 18 July 2023; Accepted: 23 October 2023;
Published: 09 November 2023.
Edited by:
Laura Gatti, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Luigi Tornillo, University of Basel, SwitzerlandLucia Guadalupe Taja Chayeb, National Institute of Cancerology (INCAN), Mexico
Copyright © 2023 Chen, Chen, Long, Han, Mi, Song, Cheng, Zhang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyang Cheng, Y2hlbmdsaXlhbmcyMDIzQHNpbmEuY29t
 Yuhong Chen1
Yuhong Chen1 Liansheng Long
Liansheng Long Liyang Cheng
Liyang Cheng