
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 31 October 2023
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1260116
 Pengcheng Zuo1
Pengcheng Zuo1 Yiying Mai1
Yiying Mai1 Zhuang Jiang1
Zhuang Jiang1 Bochao Zhang1
Bochao Zhang1 Yujin Wang1
Yujin Wang1 Mingxin Zhang1
Mingxin Zhang1 Zhen Wu1
Zhen Wu1 Junting Zhang1
Junting Zhang1 Liwei Zhang1,2*
Liwei Zhang1,2*Objective: Primary adult choroid plexus carcinomas (PACPCs) are extremely rare brain tumors. The existing literature primarily comprises case reports, which limits our understanding of this uncommon disease. This study aims to describe the clinical characteristics and prognosis of PACPCs, as well as to identify optimal treatment strategies.
Methods: We conducted a comprehensive analysis of clinical data from 7 patients with PACPCs who underwent surgical treatment at the Department of Neurosurgery, Beijing Tiantan Hospital, between March 2011 and March 2023. Additionally, a thorough search of the PubMed database was performed using the keywords “choroid plexus carcinoma” or “choroid plexus carcinomas” within the time frame of August 1975 to April 2023, which yielded a total of 28 identified cases. Subsequently, we evaluated risk factors for progression-free survival (PFS) and overall survival (OS) based on the pooled cases.
Results: The pooled cohort, consisting of 7 cases from our institution and 28 cases from the literature, included 20 males and 15 females with a mean age of 44.3 ± 14.7 years (range: 21-73 years). Gross-total resection (GTR) and non-GTR were achieved in 22 (62.9%) and 13 (37.1%) patients, respectively. Radiotherapy and chemotherapy were administered to 29 (90.6%) and 13 (40.6%) patients, respectively. After a mean follow-up of 21.0 ± 26.7 months (range: 2-132 months), 18 patients were alive, and 11 patients had died. The multivariate Cox regression model demonstrated that non-GTR (HR 5.262, 95% CI 1.350-20.516, p=0.017) was a negative prognostic factor for OS. However, we did not find any risk factors for PFS.
Conclusion: Complete surgical resection should be considered as the primary treatment approach for this rare disease. Chemotherapy and radiotherapy appear to have limited effectiveness in treating this condition. Further research with large cohorts is needed to validate our conclusions.
Choroid plexus tumors (CPTs) are extremely rare, accounting for only 0.3%-0.6% of brain tumors (1). Choroid plexus carcinomas (CPCs), classified as Grade III tumors by the World Health Organization, represent the most aggressive form of CPTs. They exhibit distinct malignant features, such as a high number of mitotic figures, dense cellularity, loss of clear papillary growth pattern, presence of necrosis, and infiltration into the surrounding brain tissue (2). It primarily occurs in children and rarely affects adults (3). An increasing number of studies have delved into the research of pediatric CPCs (4, 5). However, due to the rarity of adult CPCs, there is a scarcity of literature that systematically investigates the rare disease. In light of this, we have collected cases of primary adult choroid plexus carcinomas (PACPCs) from our institution and extensively gathered reported cases from the literature, aiming to describe the clinical characteristics and prognosis of this rare disease and provide the current optimal treatment approach.
We conducted a retrospective analysis of 12 cases of PACPCs. All patients underwent surgery at Beijing Tiantan Hospital between March 2011 and March 2023. However, 4 patients were lost to follow-up, and 1 patient was diagnosed with secondary choroid plexus carcinoma associated with lung cancer. After excluding these 5 patients, a total of 7 cases were included in our study. Comprehensive systemic examinations ruled out potential additional tumors in these 7 patients. The collected clinical data consisted of age, sex, tumor location, extent of tumor resection, histopathological examinations, treatment protocol, and clinical outcomes. Pre- and postoperative MRI images were used to assess the extent of tumor excision, categorized as gross total resection (GTR) or non-GTR. Follow-up was conducted through telephone interviews. The pathological diagnosis of PACPCs was confirmed by the Department of Neuropathology at Beijing Neurosurgical Institute, following the 2021 World Health Organization Classification of Tumors of the Central Nervous System. To perform a pooled analysis of PACPCs, we conducted a search in the PubMed database from August 1975 to April 2023 using the keywords “choroid plexus carcinoma” or “choroid plexus carcinomas,” resulting in the inclusion of a total of 28 cases. Cox regression models were employed to evaluate variables and their association with progression-free survival (PFS) and overall survival (OS). The Kaplan-Meier method was used to determine differences in OS and PFS, with p-values calculated using the log-rank test. Statistical analyses were conducted using the SPSS Statistical Package software, with a significance level set at p < 0.05. It’s worth noting that the pooled cases with incomplete data were subsequently excluded from our analysis. Consequently, our final analysis included 29 cases with complete data for assessing risk factors related to OS and 25 cases for analyzing risk factors related to PFS.
The pooled cohort (including 7 cases from our institute and 28 cases from the literature) included 20 (57.1%) males and 15 (42.9%) females with a mean age of 44.3 ± 14.7 years (range: 21-73 years). The lesion locations included lateral ventricle (n=20), fourth ventricle (n=7), third ventricle (n=2), multiple lesions (n=2), cerebellopontine angle (n=1), occipital lobe (n=1), temporal lobe (n=1) and temporoparietal lobe (n=1). All patients’ lesions showed evident enhancement on imaging examinations (32 cases available). Gross-total resection (GTR) and non-GTR were achieved in 22 (62.9%) and 13 (37.1%) patients, respectively. Radiotherapy and chemotherapy were administered to 29 (90.6%) and 13 (40.6%) patients, respectively (Table 1). 7 (25%) cases presented with tumor dissemination or metastasis. The summary data of the cohort was presented in Table 2.
After a mean follow-up of 21.0 ± 26.7 months (range: 2-132 months), 18 patients were alive and 11 patients were dead. The univariate cox regression analysis revealed that non-GTR (HR 4.611, 95% CI 1.342-15.839, p=0.015) was a only risk factor to predict a poor OS (Table 3). Multivariate cox regression analysis confirmed that non-GTR (HR 5.262,95% CI 1.350-20.516, p=0.017) was the independent risk factor in OS (Table 3). Kaplan-Meier analysis also showed that non-GTR (p=0.0067) predicted a poor OS (Figures 1A), Median OS of non-GTR was 11 months, approximately 70% of patients who underwent complete surgical resection survived We did not observe any significant effect of radiotherapy or chemotherapy in prolonging the OS in this disease (Figures 1B, C). However, Median Survival Time of CT and no CT was 38 months and 48 months, respectively.

Figure 1 Kaplan-Meier survival curves illustrating patients who underwent gross total resection (GTR) exhibited a significantly improved OS compared to Non-GTR group (A). However, there was no statistically significant difference between patients who received chemotherapy (CT) and patients who did not receive CT (B), and patients who received radiotherapy (RT) and patients who did not receive RT (C).
After a median follow-up of 18.5 ± 20.8 months (range: 2-84 months), 10 (40%) patients suffered tumor recurrence. However, we did not identify any significant risk factors that influence the PFS of this disease (Table 4 and Figure 2). However, Median PFS of GTR and non-GTR was 48 VS 13 months, respectively. Median PFS of CT and no CT was 84 months VS 35 months, respectively.
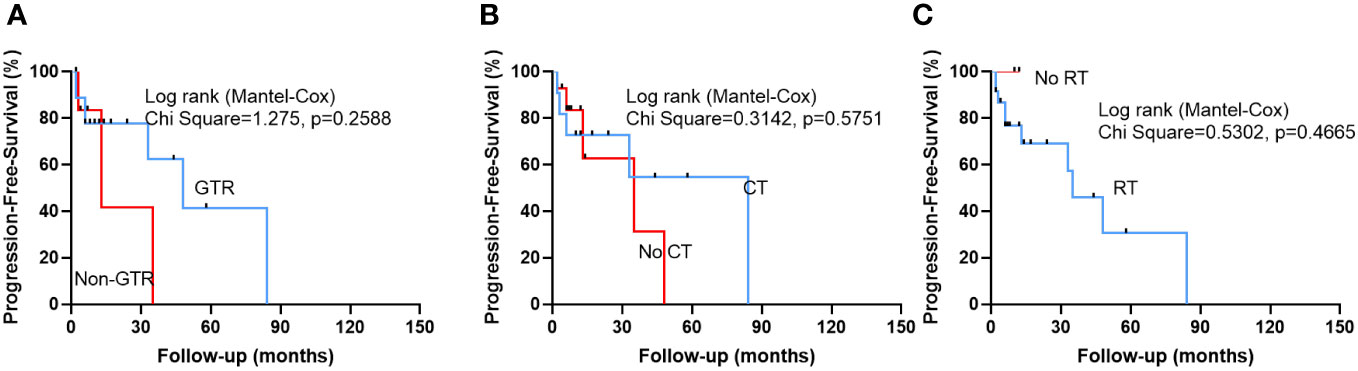
Figure 2 Kaplan-Meier survival curves show that there was no statistically significant difference between patients who achieved GTR and patients who did not achieve GTR, and patients who received CT and patients who did not receive CT (B), and patients who received radiotherapy (RT) and patients who did not receive RT.
A 60-year-old female patient presented with a complaint of headaches persisting for the past 5 months and was admitted to the Department of Neurosurgery at Beijing Tiantan Hospital. A preoperative cranial MRI revealed a cystic-solid mass located within the third ventricle. The cystic wall and the solid portion of the mass showed isointense on both T1- and T2-weighted images. Following the administration of a contrast agent, significant enhancement was observed (Figures 3A–C). Neurological examination indicated a decline in bilateral visual acuity. The transfrontal-transventricular approach was employed, and the tumor was completely excised (Figures 3D–F). Postoperative pathology revealed a significant presence of pleomorphic tumor cells with a blurring of papillary architecture (Figure 3G) and the immunohistochemistry results showed a Ki-67 index of 50%. After discharge, the patient received both radiotherapy and chemotherapy. However, during subsequent follow-up, the patient, unfortunately, died 12 months after the procedure.
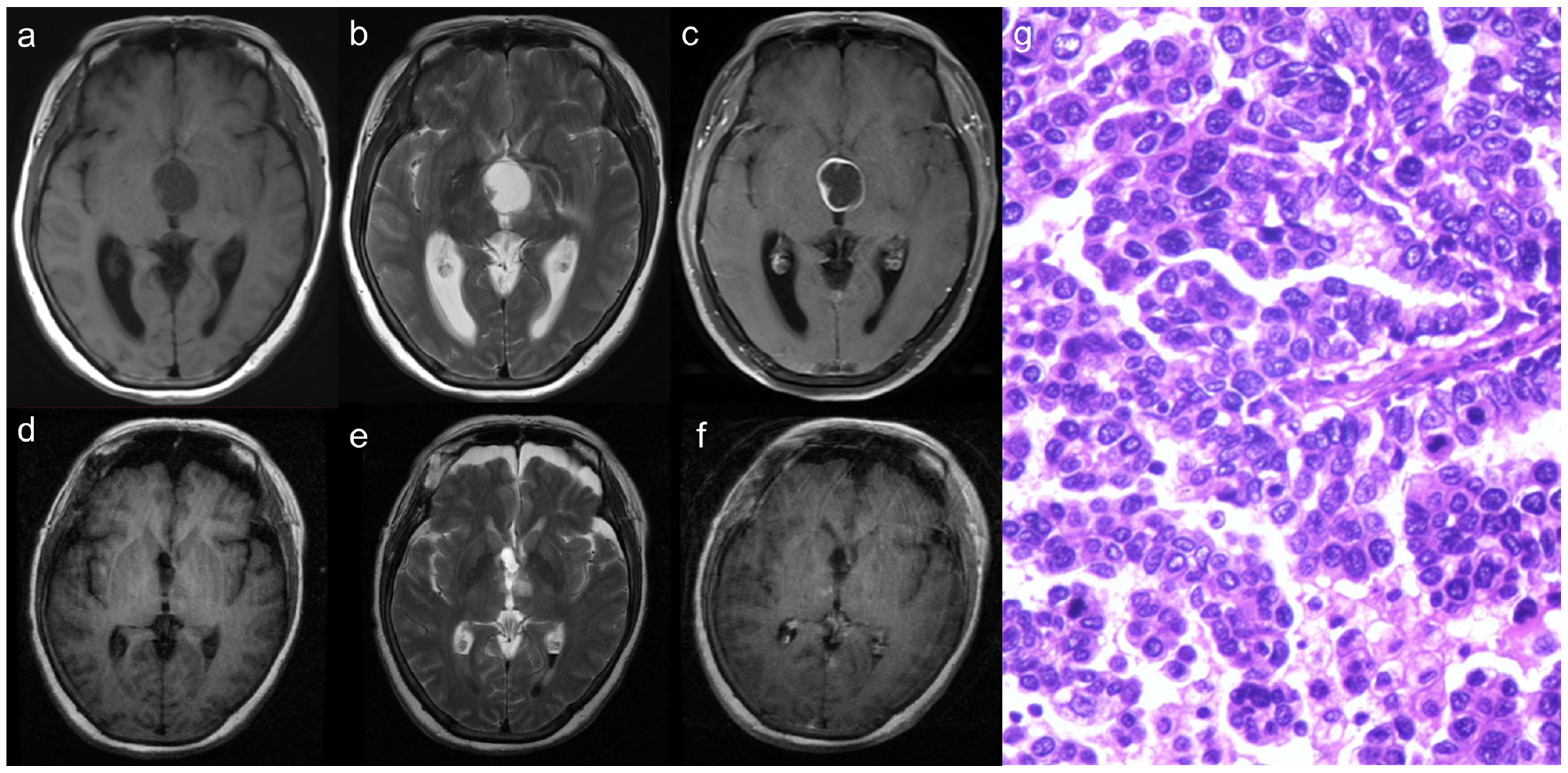
Figure 3 The solid part of the tumor demonstrates isointense on both T1 and T2-weighted images (A, B), with significant enhancement observed following contrast agent injection (C). After surgery, the tumor was resected totally (D–F). Histopathology demonstrated that a significant presence of pleomorphic tumor cells with a blurring of papillary architecture (G).
Choroid plexus carcinoma (CPC) is extremely rare and is predominantly found in children, occurring rarely in adults (32). Due to its rarity, there is currently a lack of data on the incidence of this condition. From 2011 to 2023, our center treated a total of approximately 500 patients with Choroid plexus tumors (CPTs), of which 68 cases were diagnosed as CPCs, accounting for approximately 13.6% of all CPTs. Among the 68 cases of CPCs, 12 cases were adults, representing approximately 2.4% of all 500 cases.
The histopathological diagnosis of CPC is featured increased cell density, increased mitotic figures (usually≥5 per 10 high- power fields) and necrosis (33). While choroid plexus papillomas have distinct papillary structures, the papillary features in CPC are often not evident or even lost (34). CPC primarily needs to be differentiated from three other types of intraventricular brain tumors. First are the choroid plexus papilloma. Both of these tumors exhibit distinct papillary structures, have a lower proliferation index (Ki-67, range:0.98%-2.22%) (35), while CPC lacks clear papillary features and often has a higher proliferation index (Ki-67≥50%) (36). The second type of tumor requiring differentiation is ependymoma, particularly the papillary variant. It can be distinguished by its characteristic cytological and morphological features on histological examination. These features include stippled chromatin, ependymal rosettes (37). The third type is papillary meningioma, a rare high-grade (WHO grade III) variant of meningioma that can occur in the choroid plexus. This tumor is histologically described as having a pseudopapillary or perivascular pattern (38), which distinguishes it from CPC. The results of immunohistochemical studies of CPC exhibit considerable variability. Gottschalk (39) et al. reported varying degrees of positive immunoreactions in CPP or CPC for several markers, including cytokeratin, S-100 protein, vimentin, epithelial membrane antigen, and others. Notably, Inamura (40) et al. and Osada et al (18) both found that the levels of CA19-9 decreased rapidly after the removal of the tumor. CA19-9 shows promise in detecting the recurrence of CPC. We found that 25% of the cases exhibited tumor dissemination in the cerebrospinal fluid or even distant metastases, indicating that adult CPC demonstrates a more malignant biological behavior.
Surgery is the primary approach for treating pediatric CPCs. Sun et al. conducted an analysis of clinical data from 102 pediatric patients (age ≤ 18 years) with CPCs and found that complete surgical resection significantly improves the OS of these patients (4). In our study, complete surgical resection was also found to be advantageous in improving OS of PACPCs. Furthermore, complete surgical resection improved the median PFS of these patients. However, it’s important to note that GTR did not exhibit a significant statistical difference in improving PFS. Based on these findings, if feasible, efforts should be made to achieve complete surgical resection in this rare disease.
Chemotherapy plays an important role in pediatric CPCs. A previous study conducted a statistical analysis of 135 cases of pediatric CPCs to explore the role of radiotherapy and chemotherapy. The authors found that chemotherapy alone or in combination with radiotherapy contributed to improving OS. However, radiotherapy alone did not improve OS (5). Another study showed that chemotherapy play a significant role in achieving long-term survival, but unfortunately, it cannot completely prevent the occurrence of recurrences (41). In our study, there was no significant statistical difference observed in the OS and PFS between patients who received chemotherapy and those who did not. Despite the lack of statistical difference, we found that the median PFS of patients who received chemotherapy was higher than that of patients who did not receive chemotherapy. This finding may suggest a potential role of chemotherapy in CPC. Regarding radiotherapy, due to the fact that the majority of patients (90.6%) in our cases received radiotherapy, we cannot accurately determine the role played by radiotherapy in this disease.
Overall, adult CPC has a poor prognosis, and complete surgical resection is currently the most effective approach to improve patient OS. The roles of radiotherapy and chemotherapy require further validation through more cases.
Complete surgical resection should be considered as the primary treatment approach for this rare disease. Chemotherapy appears to have limited effectiveness in treating this condition. Due to the fact that the majority of patients underwent radiation therapy, we cannot assess the specific impact of radiotherapy on the disease. Further research with large cohorts is needed to validate our conclusions.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by human research ethics committee of Beijing Tiantan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
PZ: Writing – original draft. YM: Formal Analysis, Writing – original draft. ZJ: Formal Analysis, Writing – original draft. BZ: Data curation, Writing – original draft. YW: Investigation, Writing – original draft. MZ: Investigation, Writing – original draft. ZW: Resources, Writing – review & editing. JZ: Resources, Writing – review & editing. LZ: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Multicenter clinical big data study and multi-path tumorigenesis mechanisms and precision treatment research on brainstem glioma (JINGYIYAN2018-7).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sun MZ, Oh MC, Ivan ME, Kaur G, Safaee M, Kim JM, et al. Current management of choroid plexus carcinomas. Neurosurgical review. (2014) 37(2):179–92. doi: 10.1007/s10143-013-0499-1
2. Jeibmann A, Wrede B, Peters O, Wolff JE, Paulus W, Hasselblatt M. Malignant progression in choroid plexus papillomas. J Neurosurg (2007) 107(3 Suppl):199–202. doi: 10.3171/ped-07/09/199
3. Witten AJ, Mendenhall SK, DeWitt LS, Vortmeyer A, Cohen-Gadol A. Cerebellopontine angle primary choroid plexus carcinoma present in an adult: case report and literature review. Cureus. (2021) 13(2):e13268. doi: 10.7759/cureus.13268
4. Sun MZ, Ivan ME, Clark AJ, Oh MC, Delance AR, Oh T, et al. Gross total resection improves overall survival in children with choroid plexus carcinoma. J Neurooncol. (2014) 116(1):179–85. doi: 10.1007/s11060-013-1281-5
5. Sun MZ, Ivan ME, Oh MC, Delance AR, Clark AJ, Safaee M, et al. Effects of adjuvant chemotherapy and radiation on overall survival in children with choroid plexus carcinoma. J Neurooncol. (2014) 120(2):353–60. doi: 10.1007/s11060-014-1559-2
6. Jo IY, Yeo SG, Oh HJ, Oh JS. Choroid plexus carcinoma with leptomeningeal spread in an adult: a case report and review of the literature. J Med Case Rep (2021) 15(1):286. doi: 10.1186/s13256-021-02887-2
7. Crea A, Bianco A, Cossandi C, Forgnone S, Fornaro R, Crobeddu E, et al. Choroid plexus carcinoma in adults: literature review and first report of a location into the third ventricle. World Neurosurg (2020) 133:302–7. doi: 10.1016/j.wneu.2019.10.051
8. Kim T, Park MR, Hong EK, Gwak HS. Choroid plexus carcinoma in adults: two case reports. Brain tumor Res Treat (2019) 7(1):48–52. doi: 10.14791/btrt.2019.7.e23
9. Pellerino A, Cassoni P, Boldorini R, Pinessi L, Rudà R. Response to combined radiotherapy and chemotherapy of a leptomeningeal spread from choroid plexus carcinoma: case report. Neurological Sci Off J Ital Neurological Soc Ital Soc Clin Neurophysiology. (2015) 36(4):639–41. doi: 10.1007/s10072-014-1983-2
10. Ozdogan S, Gergin YE, Gergin S, Senol O, Tiryaki M, Tatarli N, et al. Choroid plexus carcinoma in adults: an extremely rare case. Pan Afr Med J (2015) 20:302. doi: 10.11604/pamj.2015.20.302.5854
11. Guo P, Tang W, Li S, Li H, Cheng L, Feng Y, et al. Choroid plexus carcinoma in the external ventricle of an adult. J craniofacial surgery. (2015) 26(7):e664–6. doi: 10.1097/scs.0000000000002103
12. Bohara M, Hirabaru M, Fujio S, Higashi M, Yonezawa H, Karki P, et al. Choroid plexus tumors: experience of 10 cases with special references to adult cases. Neurologia medico-chirurgica. (2015) 55(12):891–900. doi: 10.2176/nmc.oa.2015-0126
13. Yip C-M, Tseng H-H, Hsu SSSS.Choroid plexus carcinoma: a rare tumor in adult. Surg Sci (2014) 5:146–9.
14. Kishore S, Negi G, Meena H, Anuradha K, Pathak PV, Bansal K. Choroid plexus carcinoma in an adult. J Neurosci Rural Pract (2012) 3(1):71–3. doi: 10.4103/0976-3147.91952
15. Misaki K, Nakada M, Mohri M, Hayashi Y, Hamada J. MGMT promoter methylation and temozolomide response in choroid plexus carcinoma. Brain tumor pathology. (2011) 28(3):259–63. doi: 10.1007/s10014-011-0033-5
16. Lozier AP, Arbaje YM, Scheithauer BW. Supratentorial, extraventricular choroid plexus carcinoma in an adult: case report. Neurosurgery. (2009) 65(4):E816–7. doi: 10.1227/01.Neu.0000348291.48810.C2
17. Fernández Calvo O, Gallegos Sancho MI, Quindós Varela M, Paredes Velázquez L, Dopico Vázquez D, Antón Aparicio LM. Choroid plexus carcinoma: a rare condition and its therapeutic management. Clin Trans Oncol Off Publ Fed Spanish Oncol Societies Natl Cancer Institute Mexico. (2007) 9(6):401–3. doi: 10.1007/s12094-007-0073-9
18. Osada H, Mori K, Yamamoto T, Nakao Y, Wada R, Maeda M. Choroid plexus carcinoma secreting carbohydrate antigen 19-9 in an adult. Case Rep Neurologia medico-chirurgica. (2006) 46(5):251–3. doi: 10.2176/nmc.46.251
19. Han SR, Yee GT, Joo M, Whang CJ. Choroid plexus carcinoma in an adult. J Korean Neurosurg Soc (2006) 40(2):122.
20. Guan J, Liu G, Guo G, Geng Y, Cheng Y, Li Y, et al. Choroid plexus papillary carcinoma associated with hemorrhage. A Case Rep neuroradiology J (2006) 19(5):616–20. doi: 10.1177/197140090601900510
21. Fabi A, Salesi N, Di Cocco B, Vidiri A, Visca P, Pace A, et al. Choroid plexus carcinoma in the adult: is there a role for chemotherapy? J Exp Clin Cancer Res CR. (2005) 24(3):493–6.
22. Yusuf İ, GÜRKANLAR D, ÖNGÜRÜ Ö, TİMURKAYNAK EJTN. Choroid plexus carcinoma in an adult patient: case report. Turk Neurosurg (2005) 15(2):105–8.
23. Wyatt SS, Price RA, Holthouse D, Elsaleh H. Choroid plexus carcinoma in an adult. Australas radiology. (2001) 45(3):369–71. doi: 10.1046/j.1440-1673.2001.00941.x
24. Kohmura E, Maruno M, Sawada K, Arita N, Yoshimine T. Usefulness of synaptophysin immunohistochemistry in an adult case of choroid plexus carcinoma. Neurological Res (2000) 22(5):478–80. doi: 10.1080/01616412.2000.11740704
25. Hashizume A, Kodama Y, Hotta T, Yuki K, Taniguchi E, Eguchi K, et al. Choroid plexus carcinoma in the lateral ventricle–case report. Neurologia medico-chirurgica. (1995) 35(10):742–4. doi: 10.2176/nmc.35.742
26. Başkaya MK, Erekul S, Egemen N, Gökalp HZ, Sertçelik A. Choroid plexus carcinoma of the lateral ventricle. Rep Case an adult. Clin Neurol neurosurgery. (1994) 96(1):47–51. doi: 10.1016/0303-8467(94)90029-9
27. Matsuda M, Uzura S, Nakasu S, Handa J. Primary carcinoma of the choroid plexus in the lateral ventricle. Surg neurology. (1991) 36(4):294–9. doi: 10.1016/0090-3019(91)90091-m
28. Itoh Y, Kowada M, Mineura K. Choroid plexus carcinoma. Report of a case with positron emission tomographic study. Neuroradiology. (1986) 28(4):374. doi: 10.1007/bf00333452
29. Carpenter DB, Michelsen WJ, Hays AP. Carcinoma of the choroid plexus. Case Rep J Neurosurg (1982) 56(5):722–7. doi: 10.3171/jns.1982.56.5.0722
30. Vaquero J, Cabezudo J, Leunda G, Carrillo R, García Uría J. Primary carcinoma of the choroid plexus with metastatic dissemination within the central nervous system. Acta Neurochir (Wien) (1979) 51(1-2):105–11. doi: 10.1007/bf01401801 Cited in: Pubmed
31. Dohrmann GJ, Collias JC. Choroid plexus carcinoma. J Neurosurg (1975) 43(2):225–32. doi: 10.3171/jns.1975.43.2.0225
32. Wrede B, Liu P, Wolff JE. Chemotherapy improves the survival of patients with choroid plexus carcinoma: a meta-analysis of individual cases with choroid plexus tumors. J Neurooncol. (2007) 85(3):345–51. doi: 10.1007/s11060-007-9428-x
33. Gopal P, Parker JR, Debski R, Parker JC Jr.Choroid plexus carcinoma. Arch Pathol Lab Med (2008) 132(8):1350–4. doi: 10.5858/2008-132-1350-cpc
34. Chow E, Reardon DA, Shah AB, Jenkins JJ, Langston J, Heideman RL, et al. Pediatric choroid plexus neoplasms. Int J Radiat oncology biology physics. (1999) 44(2):249–54. doi: 10.1016/s0360-3016(98)00560-4
35. Sunada I, Tsuyuguchi N, Hara M, Ochi H. 18F-FDG and 11C-methionine PET in choroid plexus papilloma–report of three cases. Radiat Med (2002) 20(2):97–100.
36. Turkoglu E, Kertmen H, Sanli AM, Onder E, Gunaydin A, Gurses L, et al. Clinical outcome of adult choroid plexus tumors: retrospective analysis of a single institute. Acta neurochirurgica. (2014) 156(8):1461–8. doi: 10.1007/s00701-014-2138-1
37. Adesina AM. Intraoperative consultation in the diagnosis of pediatric brain tumors. Arch Pathol Lab Med (2005) 129(12):1653–60. doi: 10.5858/2005-129-1653-icitdo
38. Al-Sarraj S, King A, Martin AJ, Jarosz J, Lantos PL. Ultrastructural examination is essential for diagnosis of papillary meningioma. Histopathology. (2001) 38(4):318–24. doi: 10.1046/j.1365-2559.2001.01128.x
39. Gottschalk J, Jautzke G, Paulus W, Goebel S, Cervos-Navarro J. The use of immunomorphology to differentiate choroid plexus tumors from metastatic carcinomas. Cancer. (1993) 72(4):1343–9. doi: 10.1002/1097-0142(19930815)72:4<1343::aid-cncr2820720432>3.0.co;2-g
40. Inamura T, Nishio S, Miyagi Y, Kamikaseda K, Ueda K, Fukui M, et al. Primary choroid plexus carcinoma producing carbohydrate antigen 19-9. Clin neuropathology. (2000) 19(6):268–72.
41. Samuel TA, Parikh J, Sharma S, Giller CA, Sterling K, Kapoor S, et al. Recurrent adult choroid plexus carcinoma treated with high-dose chemotherapy and syngeneic stem cell (bone marrow) transplant. J neurological Surg Part A Cent Eur neurosurgery. (2013) 74 Suppl 1:e149–54. doi: 10.1055/s-0032-1333419
Keywords: primary, adult, choroid plexus carcinoma, gross-total resection, radiotherapy
Citation: Zuo P, Mai Y, Jiang Z, Zhang B, Wang Y, Zhang M, Wu Z, Zhang J and Zhang L (2023) Primary adult choroid plexus carcinomas: a single-center experience with a systematic review. Front. Oncol. 13:1260116. doi: 10.3389/fonc.2023.1260116
Received: 17 July 2023; Accepted: 20 October 2023;
Published: 31 October 2023.
Edited by:
Pankaj Pathak, National Institute of Neurological Disorders and Stroke (NIH), United StatesReviewed by:
Iolanda Boffa, Telethon Institute of Genetics and Medicine (TIGEM), ItalyCopyright © 2023 Zuo, Mai, Jiang, Zhang, Wang, Zhang, Wu, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liwei Zhang, emhhbmdsaXdlaXR0eXlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.