- 1Department of Cancer Center, Chengdu Seventh People's Hospital (Affiliated Cancer Hospital of Chengdu Medical College), Chengdu, China
- 2Center for Geriatric Medicine Assessment and Treatment, The Fourth People's Hospital of Chengdu, Chengdu, China
- 3Department of General Surgery, Chengdu Public Health Clinical Medical Center, Sichuan, Chengdu, China
Nasopharyngeal carcinoma (NPC) is a malignant tumor characterized by the malignant transformation of nasopharyngeal epithelial cells. It is highly sensitive to radiation therapy, making radiotherapy the primary treatment modality. However, 60-80% of patients are initially diagnosed with locally advanced NPC (LA-NPC), where radiotherapy alone often fails to achieve desirable outcomes. Therefore, combining radiotherapy with chemotherapy has emerged as an effective strategy to optimize treatment for LA-NPC patients. Among the various chemotherapy regimens, concurrent chemoradiotherapy (CCRT) using platinum-based drugs has been established as the most commonly utilized approach for LA-NPC patients. The extensive utilization of platinum drugs in clinical settings underscores their therapeutic potential and emphasizes ongoing efforts in the development of novel platinum-based complexes for anticancer therapy. The aim of this review is to elucidate the remarkable advances made in the field of platinum-based therapies for nasopharyngeal carcinoma, emphasizing their transformative impact on patient prognosis.
1 Introduction
Nasopharyngeal carcinoma (NPC) is a prevalent malignant epithelial tumor, with a high incidence in Southern China and Southeast Asia. It is closely linked to Epstein-Barr virus infection, further underscoring the need for effective therapeutic interventions (1). Due to the intricate anatomical location and complex structures involved, surgical access has been limited in the treatment of NPC. Consequently, radiation therapy has remained the primary treatment modality for this type of cancer, owing to its high sensitivity to radiation. In the era of conventional two-dimensional radiotherapy (2D-RT), early-stage NPC patients achieved favorable outcomes with radical radiotherapy alone, yielded 5-year overall survival rates ranging from 86% to 97% (2–4). However, 60-80% of patients were initially diagnosed with locally advanced NPC (LA-NPC), which is associated with a higher risk of local-regional recurrence and distant metastasis (5). For these patients with locally advanced disease, radical radiation therapy alone yielded 5-year overall survival rates of 58% to 77%, with a significant propensity for distant metastasis as the primary cause of treatment failure (6–9).
Over the past few decades, photon-based radiotherapy techniques have evolved from conventional radiotherapy to three-dimensional conformal radiotherapy (3D-CRT), and subsequently to intensity-modulated radiotherapy (IMRT). Technological advancements and equipment updates have enhanced the dosimetric characteristics of radiation, resulting in improved local control and survival rates, as well as reduced occurrence of adverse reactions in nasopharyngeal carcinoma. IMRT allows for more precise coverage of the tumor while better sparing critical organs, yielding significantly superior outcomes compared to conventional 2D-RT (10). The introduction of IMRT has led to an approximate 5-6% improvement in local control rates for locally advanced NPC (11). Currently, IMRT has become the most widely employed radiotherapy technique for the treatment of nasopharyngeal carcinoma. While radiation therapy alone has shown promise in treating early-stage NPC, locally advanced cases face significant challenges, including frequent local recurrence and distant metastasis, leading to suboptimal treatment outcomes. To overcome these hurdles, the integration of radiation therapy with chemotherapy has emerged as a powerful strategy.
Concurrent chemoradiotherapy (CCRT), utilizing platinum-based agents, has now established itself as the primary and standard therapeutic approach for locally advanced NPC. The anticancer mechanisms of platinum-based drugs primarily involve the inhibition of DNA synthesis through the activation of various signaling pathways, ultimately leading to apoptosis-mediated tumor regression (12). Capitalizing on this knowledge, extensive research efforts have been dedicated to the synthesis and evaluation of platinum-based complexes as potential antitumor agents. Clinical practice has witnessed the successful application of platinum drugs such as cisplatin, carboplatin, oxaliplatin, and nedaplatin in the management of NPC. Additionally, novel platinum drugs, including lobaplatin and nedaplatin, hold considerable promise in further optimizing treatment outcomes (13, 14).
This review aims to shed light on the remarkable progress achieved in the field of platinum-based therapy for NPC, underlining its transformative impact on patient outcomes. A comprehensive understanding of the clinical applications of platinum drugs will pave the way for future advancements, fostering the development of novel and more effective therapeutic strategies to combat this challenging disease.
2 The evolution of platinum-based synchronous chemotherapy in conjunction with radiation therapy
Cisplatin, a first-generation platinum-based drug, is widely recognized as one of the most extensively employed anti-tumor agents in clinical settings. Its versatility as a backbone chemotherapy drug across various malignancies has remarkably elevated patient survival rates and cure rates (15, 16). The groundbreaking Intergroup-0099 study (16) revolutionized the treatment landscape for LA-NPC patients by introducing concurrent cisplatin-based chemotherapy concomitant with radiotherapy. The study demonstrated the significant augmentation of radiotherapy efficacy through synchronous cisplatin-based chemotherapy, leading to improved patient survival outcomes. This seminal research has become a cornerstone in the establishment of the prevailing standard of care for LA-NPC. In the concurrent chemoradiotherapy group, patients received regular 100 mg/m2 doses of synchronized cisplatin chemotherapy at three-week intervals during radiotherapy. CCRT substantially enhanced local control rates among LA-NPC patients and markedly improved the 3-year overall survival (OS) rate compared to radiotherapy alone (76% vs. 46%, p < 0.001). Further analysis of updated reports revealed a strikingly significant difference in 5-year survival outcomes between the two study groups: the CCRT group exhibited a robust rate of 67%, while the radiotherapy alone group only achieved 37% (p = 0.001) (17). These results have been reaffirmed through subsequent large-scale phase III clinical trials (18–23), with long-term survival data coinciding with the 10-year follow-up (24), further highlighting the superior efficacy of CCRT over radiotherapy alone. Additionally, non-randomized controlled studies (25–28) have consistently reported the advantageous therapeutic effect of CCRT compared to radiotherapy alone. Altogether, the integration of synchronous cisplatin-based chemotherapy has significantly enhanced long-term survival outcomes for patients, bestowing valuable survival benefits upon those diagnosed with LA-NPC and solidifying its position as a fundamental cornerstone within the standard treatment paradigm for this condition.
3 Synchronous cisplatin chemotherapy
3.1 Choice of chemotherapy regimen and dosage for synchronous cisplatin monotherapy
In clinical practice, chemotherapy can cause both short-term and long-term toxicity, making it challenging for patients to tolerate high-intensity synchronous chemoradiotherapy. Studies have reported that approximately 29%-48% of patients are unable to complete the full three-cycle synchronous cisplatin chemotherapy (18, 20, 29). Determining the optimal regimen and dosage of cisplatin in combination with radiotherapy for synchronous chemotherapy remains a topic of debate, influenced by factors such as toxic reactions, patient preferences, and physician expertise. In the era of conventional radiotherapy (2D radiotherapy), the dosage of cisplatin administered during radiotherapy plays a crucial role in the prognosis of LA-NPC patients undergoing only CCRT. Retrospective studies (29–33) have previously suggested that administering a synchronous cisplatin dosage of 200mg/m2 during radiotherapy can provide survival benefits for patients. Recently, a prospective clinical study (34) demonstrated promising survival outcomes in low-risk LA-NPC patients (EBV-DNA < 4000 copies/ml) treated with 200mg/m2 cisplatin administration during radiotherapy, achieving an outstanding 3-year progression-free survival (PFS) rate of 88%. Thus, there seems to be a consensus on administering cisplatin at a dosage of ≥200mg/m2 during radiotherapy.
During concurrent radiotherapy and cisplatin administration, two common approaches are used: the weekly dosing regimen and the three-week dosing regimen. The weekly dosing regimen involves administering synchronous cisplatin at a dose of 30-40mg/m2 weekly during the course of radiotherapy, while the three-week dosing regimen entails administering synchronous cisplatin at a dose of 80-100mg/m2 every three weeks. A review of the literature indicates that the survival outcomes between the two regimens are similar (32, 35–39). The 5-year OS, disease-free survival (DFS), locoregional recurrence-free survival (LRRFS), and distant metastasis-free survival (DMFS) rates for the three-week regimen range from 85.2% to 91%, 63.8% to 92.6%, 92.0% to 96.7%, and 76.1% to 95.6%, respectively. For the weekly regimen, the respective rates range from 68.9% to 96.7%, 64.9% to 90.7%, 91.0% to 96.3%, and 80.1% to 96.7% (Table 1). Common grade 3-4 adverse events during treatment include anemia, thrombocytopenia, leukopenia, gastrointestinal reactions (such as nausea and vomiting), and mucositis. Among the five studies that reported the incidence of adverse events, four (35, 36, 38, 39) found no significant difference in grade 3-4 adverse events between the two groups. Only one study reported a lower incidence of grade 3-4 mucositis and nausea/vomiting in the weekly regimen group but a higher incidence of thrombocytopenia compared to the three-week regimen group. However, due to the limitations of retrospective studies, larger prospective phase III clinical trials are required to further validate the current research findings. Additionally, the three-week cisplatin schedule offers convenience in terms of administration frequency compared to weekly cisplatin during radiotherapy, potentially reducing hospitalization time. Consequently, the three-week regimen is often preferred in clinical practice.

Table 1 Clinical studies comparing three-week regimens with single-week regimens of simultaneous cisplatin chemotherapy in LA-NPC.
However, CCRT alone may not provide sufficient therapeutic intensity for LA-NPC patients with high-risk factors. Results from several large phase III clinical studies have confirmed the clinical importance of adding induction chemotherapy (IC) to cisplatin-based CCRT for early eradication of distant microscopic metastatic lesions, improvement of distant tumor control rates, and enhanced survival (40–44). A study found that in the era of 3D-CRT and IMRT, IC greatly reduced tumor volume, and clinical complete remission was observed in 11.3% of patients and clinical partial remission in 79.6% of patients (42). The NCCN guidelines also recommend that IC followed by CCRT as the standard treatment for LA-NPC. For patients with NPC undergoing IC followed by CCRT, several studies have suggested that a synchronous cisplatin dose exceeding 200mg/m2 can yield survival benefits (45–47). However, divergent conclusions have been reported in other studies. Peng et al.’s research (47) indicated that a dose of less than 200mg/m2 during CCRT following IC can enhance patients’ 4-year overall survival (OS) and distant metastasis-free survival (DMFS). Conversely, Lv et al.’s study (48) proposed that there is no significant difference in survival outcomes between patients receiving a cisplatin concurrent dose of ≥200 mg/m2 and those receiving <200 mg/m2. Further validation through large-scale Phase III clinical trials is still warranted.
3.2 Synchronous chemotherapy with cisplatin in combination with other drugs
During radiotherapy, synchronous chemotherapy regimens based on cisplatin, in combination with two or three drugs, have been continuously explored for LA-NPC. The most common regimen is fluorouracil combined with cisplatin (PF) (49), and other studies have also investigated the safety and efficacy of regimens including docetaxel, fluorouracil combined with cisplatin (TPF) (50), TP regimen (51–53), and gemcitabine combined with cisplatin (GP) (54, 55). Furthermore, studies have reported on the efficacy of raltitrexed plus cisplatin (56), cetuximab plus cisplatin (57, 58), and nimotuzumab plus cisplatin (14, 59, 60). Overall, the addition of chemotherapy or targeted drugs to cisplatin-based synchronous chemotherapy did not increase efficacy compared to cisplatin alone. In addition, some uncommon single-agent synchronous chemotherapy regimens have been reported for the treatment of nasopharyngeal carcinoma, including paclitaxel (61), S-1 (47, 48, 62, 63), cetuximab (64), and nimotuzumab (65), but they did not demonstrate superior efficacy or lower toxicity compared to single-agent cisplatin.
Firstly, the relatively small sample sizes of these studies may result in insufficient statistical power and potential selection bias, thereby compromising the robustness of the research findings. Secondly, some studies are retrospective in nature and predominantly conducted within a single center, necessitating multicenter, prospective, large-scale randomized clinical trials to further investigate the efficacy of these regimens. Currently, these approaches lack substantial research evidence to support their use. Overall, the synchronous chemotherapy combining cisplatin with other drugs does not appear to enhance efficacy; instead, it may lead to more severe hematological or non-hematological toxicities.
4 Synchronous chemotherapy with other platinum agents
Cisplatin-based synchronous chemotherapy regimens are associated with increased acute and late toxicities during radiotherapy, including severe gastrointestinal reactions (such as nausea and vomiting), renal toxicity (66), and ototoxicity (67, 68), posing limitations to the use of cisplatin. Furthermore, some patients develop resistance to cisplatin, which reduces its effectiveness, particularly when tumors recur (69). As a result, there is a growing demand for other platinum-based chemotherapy agents that can provide similar efficacy to NPC but with fewer side effects. Platinum derivatives such as nedaplatin, lobaplatin, and carboplatin have been explored as alternatives to cisplatin for the treatment of NPC.
4.1 Carboplatin
Carboplatin, a second-generation platinum agent, is also utilized for the treatment of various malignancies. A small retrospective study involving 75 LA-NPC patients (70) demonstrated poorer 3-year survival outcomes in the group receiving 2 cycles of synchronous carboplatin chemotherapy. A non-inferiority clinical trial (71) comparing the efficacy of cisplatin and carboplatin in synchronous chemoradiotherapy for LA-NPC revealed no significant differences in 3-year overall survival (OS; P=0.98) and disease-free survival (DFS; P=0.96) between the synchronous carboplatin and cisplatin groups. However, data from their latest multicenter study (72) indicated that adding adjuvant chemotherapy to carboplatin-based synchronous chemoradiotherapy did not significantly improve short-term efficacy but increased toxicity. Another phase II clinical trial (73) demonstrated favorable outcomes with carboplatin-based synchronous chemoradiotherapy for LA-NPC, with a 3-year OS rate of 83.6% and a PFS rate of 65.3%. Additionally, patients exhibited good compliance. Nevertheless, there is still controversy regarding the evidence supporting the equivalence of second-generation platinum agent carboplatin to cisplatin.
4.2 Nedaplatin
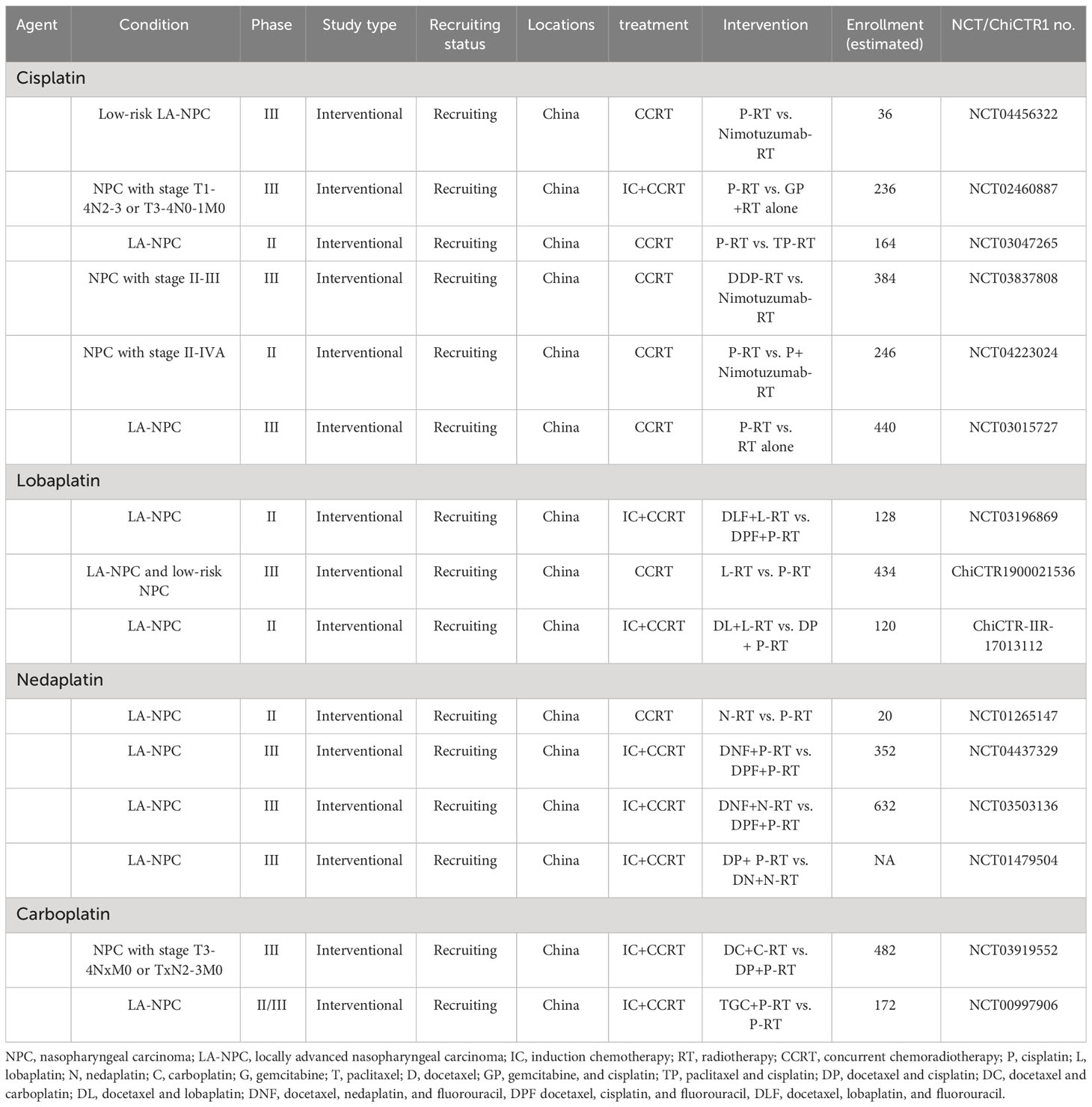
Nedaplatin is a cisplatin analog with similar antitumor mechanisms and therapeutic effects, but it does not require hydration to protect the kidneys. Several studies (74–76) have compared the efficacy of nedaplatin and cisplatin in synchronous chemoradiotherapy, suggesting that nedaplatin may be a promising alternative to cisplatin, as it is effective and safe for treating NPC. The results of a randomized Phase III controlled trial (77) indicated that for stage II-IVB NPC patients, nedaplatin-based CCRT is not inferior to cisplatin-based CCRT in terms of the 2-year progression-free survival (PFS). Moreover, the cisplatin group had a higher incidence of Grade 3-4 adverse events. The recently updated 5-year follow-up results (78) support the initial findings. Additionally, from a cost-effectiveness analysis perspective, nedaplatin-based synchronous chemoradiotherapy holds an advantage (79). Overall, nedaplatin appears to be one of the potential alternatives to cisplatin in synchronous chemoradiotherapy for LA-NPC. Ongoing studies such as NCT04472403, NCT01479504, NCT01265147, NCT04437329, and NCT03503136 are further evaluating the efficacy of nedaplatin in NPC, and there are also ongoing explorations of combination regimens involving nedaplatin (80, 81).
4.3 Lobaplatin
Lobaplatin is a third-generation platinum agent. Previous studies have reported that lobaplatin can overcome certain forms of resistance caused by other platinum agents (82). The results of a Phase II trial (83) validated the efficacy and safety of lobaplatin-based induction chemotherapy followed by CCRT in the treatment of LA-NPC. Subsequently, the results of a large Phase III randomized non-inferiority controlled trial (84) demonstrated that lobaplatin-based induction chemotherapy plus CCRT for LA-NPC had similar survival outcomes and side effects compared to cisplatin-based treatment. A subsequent commentary (85) indicated that lobaplatin is not inferior to cisplatin and has lower toxicity, making it a promising alternative to cisplatin. Additionally, ongoing clinical studies such as NCT04472403, NCT03196869, ChiCTR1900021536, and ChiCTR-IIR-17013112 aim to further evaluate the benefits and risks of lobaplatin in nasopharyngeal carcinoma and validate the value of these treatment strategies (Table 2).
4.4 Oxaliplatin
A phase III clinical study (86) explored the efficacy of oxaliplatin monotherapy combined with synchronous chemotherapy compared to radiation therapy alone. The findings indicated that the oxaliplatin group demonstrated a more favorable short-term survival profile; however, extensive randomized trials are warranted to thoroughly evaluate its comparative effectiveness against cisplatin.
5 Platinum and sequential treatment approach
The results of several large phase III clinical studies confirm that the addition of cisplatin-based induction chemotherapy (IC) to cisplatin-based CCRT is important for the early eradication of distant microscopic metastatic lesions, the improvement of distant tumor control and the enhancement of survival (40, 41, 43, 44, 47). The administration of 2-4 cycles of IC followed by CCRT has been shown to increase treatment-related toxicity and hinder patients’ ability to withstand subsequent high-intensity CRRT. Studies have reported that following IC, approximately 8% to 13% of patients do not complete the intended two cycles of synchronous cisplatin chemotherapy, while 22% to 39% do not complete three cycles (100mg/m2 cisplatin every three weeks) of CCRT (40, 44, 87). In addition, NPC patients receiving IC plus CRRT treatment exhibit higher rates of grade 3 or 4 adverse events compared to those receiving CRRT alone, with 20% to 40% of patients unable to complete the originally planned course of synchronous radiotherapy due to severe toxicity (44, 87, 88). However, interruptions and extensions of radiotherapy have been shown to have detrimental effects on patient survival (89). The dosage of cisplatin administered during concurrent chemoradiotherapy (CCRT-DDP) is a significant prognostic factor for LA-NPC patients, and a dose of 200mg/m2 of synchronous cisplatin may already be deemed adequate (29, 30, 34, 46, 47). While induction chemotherapy is also cisplatin-based, there is currently a lack of research focused on examining the association between the dosage of cisplatin administered during the entire treatment course and the survival outcomes of LA-NPC patients receiving IC followed by CCRT. While there is currently a lack of investigation on the therapeutic efficacy of other platinum-based drug dosages in LA-NPC patients, regarding lobaplatin and nedaplatin, significant research is warranted. In terms of toxicity reactions, it appears that lobaplatin and nedaplatin may offer a potential reduction in severe acute adverse effects compared to cisplatin. However, further exploration is required through robust head-to-head large-scale studies. Currently, there is a dearth of research exploring the impact of platinum-based drug dosages on the therapeutic efficacy of lobaplatin and nedaplatin in LA-NPC patients. However, concerning toxicity reactions, it appears that both lobaplatin and nedaplatin might offer a potential reduction in severe acute adverse effects compared to cisplatin (90). Nevertheless, further investigation is required through extensive head-to-head studies with large sample sizes to validate the finding.
6 Conclusions and prospects
In regard to the treatment of NPC, platinum-based chemotherapy agents play a pivotal role. At present, platinum-based concurrent chemoradiotherapy, particularly cisplatin-based regimens, stands as the standard treatment for NPC. Nevertheless, investigations have revealed that combining cisplatin with other drugs in synchronous chemotherapy fails to enhance overall survival rates and may, in fact, increase the incidence of adverse reactions. Consequently, further research is imperative to elucidate the optimal dosage and regimen for cisplatin monotherapy in synchronous chemotherapy protocols for NPC. Furthermore, while there may be no substantial disparities in efficacy and toxicity between weekly and three-week regimens, the latter offers improved convenience and reduced hospitalization duration.
Distinct species of platinum-based chemotherapeutic agents possess individual merits. In an ideal scenario, alternative platinum agents should be able to replace cisplatin while boasting comparable activity, efficacy, and decreased toxicity. However, debates regarding the equivalence of second-generation platinum salts, such as carboplatin, to cisplatin in NPC patients remain unresolved. Nedaplatin, with its advantageous low cost and capacity to serve as a substitute for cisplatin-resistant patients or those intolerant to cisplatin’s side effects, represents a prospective alternative. Notably, lobaplatin, a novel generation platinum derivative, has exhibited remarkable efficacy in the management of NPC, matching cisplatin in therapeutic outcomes while demonstrating a lower incidence of toxic reactions. Nonetheless, due to its higher price compared to cisplatin, the inclusion of lobaplatin in medical insurance coverage could potentially mitigate the economic burden on patients. Lobaplatin and nedaplatin may serve as compelling avenues for advancing research in upgrading synchronous chemoradiotherapy strategies for locally advanced NPC. However, when substituting platinum salts, further study is crucial to explore alternative individualized treatment strategies, including administration dosage and regimens, aimed at alleviating long-term toxic reactions and economic burdens faced by LA-NPC patients.
Furthermore, there is ongoing exploration of platinum-based chemotherapy combined with immune checkpoint inhibitors as a first-line treatment strategy for recurrent/metastatic NPC, demonstrating notable advantages (91–93). In the case of locally advanced NPC, several ongoing clinical trials, including NCT03700476, NCT04557020, NCT04447612, NCT04447326, NCT04782765, and NCT03734809, are continuously investigating the potential advantages of integrating immunotherapy into platinum-based IC. This emerging combination holds significant potential as a prospective therapeutic option for the future management of LA-NPC.
Author contributions
FZ: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. YW: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. HW: Conceptualization, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet (2019) 394:64–80. doi: 10.1016/S0140-6736(19)30956-0
2. Yi JL, Gao L, Huang XD, Li SY, Luo JW, Cai WM, et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: Ten-year experience of a single institution. Int J Radiat Oncol Biol Phys (2006) 65:161–8. doi: 10.1016/j.ijrobp.2005.12.003
3. Xiao WW, Han F, Lu TX, Chen CY, Huang Y, Zhao C. Treatment outcomes after radiotherapy alone for patients with early-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys (2009) 74:1070–6. doi: 10.1016/j.ijrobp.2008.09.008
4. Song CH, Wu HG, Heo DS, Kim KH, Sung MW, Park CI. Treatment outcomes for radiotherapy alone are comparable with neoadjuvant chemotherapy followed by radiotherapy in early-stage nasopharyngeal carcinoma. Laryngoscope (2008) 118:663–70. doi: 10.1097/MLG.0b013e3181626cfe
5. Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou ZX, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer. (2014) 33:381–7. doi: 10.5732/cjc.014.10086
6. Mao YP, Xie FY, Liu LZ, Sun Y, Li L, Tang LL, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys (2009) 73:1326–34. doi: 10.1016/j.ijrobp.2008.07.062
7. Takiar V, Ma D, Garden AS, Li J, Rosenthal DI, Beadle BM, et al. Disease control and toxicity outcomes for T4 carcinoma of the nasopharynx treated with intensity-modulated radiotherapy. Head Neck. (2016) 38 Suppl 1:E925–33. doi: 10.1002/hed.24128
8. Chua DT, Ma J, Sham JS, Mai HQ, Choy DT, Hong MH, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J Clin Oncol (2005) 23:1118–24. doi: 10.1200/JCO.2005.12.081
9. Liu MZ, Tang LL, Zong JF, Huang Y, Sun Y, Mao YP, et al. Evaluation of sixth edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement. Int J Radiat Oncol Biol Phys (2008) 70:1115–23. doi: 10.1016/j.ijrobp.2007.07.2353
10. Moon SH, Cho KH, Lee CG, Keum KC, Kim YS, Wu HG, et al. IMRT vs. 2D-radiotherapy or 3D-conformal radiotherapy of nasopharyngeal carcinoma: Survival outcome in a Korean multi-institutional retrospective study (KROG 11-06). Strahlenther Onkol. (2016) 192:377–85. doi: 10.1007/s00066-016-0959-y
11. Zhang B, Mo Z, Du W, Wang Y, Liu L, Wei Y. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: A systematic review and meta-analysis. Oral Oncol (2015) 51:1041–6. doi: 10.1016/j.oraloncology.2015.08.005
12. Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. (Review). Oncol Rep (2003) 10:1663–82. doi: 10.3892/or.10.6.1663
13. Wang X, Wang X, Guo Z. Functionalization of platinum complexes for biomedical applications. Acc Chem Res (2015) 48:2622–31. doi: 10.1021/acs.accounts.5b00203
14. Wang L, Zhuang H, Xu X, Zhou J, Jiao Y. Efficacy and survival analysis of nimotuzumab combined with concurrent chemoradiotherapy in the treatment of locally advanced nasopharyngeal carcinoma. Front Oncol (2023) 13:1129649. doi: 10.3389/fonc.2023.1129649
15. Rancoule C, Guy JB, Vallard A, Ben Mrad M, Rehailia A, Magné N. [50th anniversary of cisplatin]. Bull Cancer. (2017) 104:167–76. doi: 10.1016/j.bulcan.2016.11.011
16. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol (1998) 16:1310–7. doi: 10.1200/JCO.1998.16.4.1310
17. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Superiority of 5-year survival with chemo-radiotherapy (CT-RT) vs radiotherapy in patients (Pts) with locally advanced nasopharyngeal cancer (NPC). Intergroup (0099) (SWOG 8892, RTOG 8817, ECOG 2388) phase III study: final report (abstract no. 905). Proc Am Soc Clin Oncol 20(2001):227a.
18. Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol (2005) 23:6730–8. doi: 10.1200/JCO.2005.16.790
19. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Final overall survival analysis of gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma: A multicenter, randomized phase III trial. J Clin Oncol (2022) 40:2420–5. doi: 10.1200/JCO.22.00327
20. Lee AW, Lau WH, Tung SY, Chua DT, Chappell R, Xu L, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol (2005) 23:6966–75. doi: 10.1200/JCO.2004.00.7542
21. Chan AT, Leung SF, Ngan RK, Teo PM, Lau WH, Kwan WH, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst (2005) 97:536–9. doi: 10.1093/jnci/dji084
22. Chan AT, Teo PM, Ngan RK, Leung TW, Lau WH, Zee B, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol (2002) 20:2038–44. doi: 10.1200/JCO.2002.08.149
23. Chen Y, Liu MZ, Liang SB, Zong JF, Mao YP, Tang LL, et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of China. Int J Radiat Oncol Biol Phys (2008) 71:1356–64. doi: 10.1016/j.ijrobp.2007.12.028
24. Lee AWM, Tung SY, Ng WT, Lee V, Ngan RKC, Choi HCW, et al. A multicenter, phase 3, randomized trial of concurrent chemoradiotherapy plus adjuvant chemotherapy versus radiotherapy alone in patients with regionally advanced nasopharyngeal carcinoma: 10-year outcomes for efficacy and toxicity. Cancer (2017) 123:4147–57. doi: 10.1002/cncr.30850
25. Cheng SH, Liu TW, Jian JJ, Tsai SY, Hao SP, Huang CH, et al. Concomitant chemotherapy and radiotherapy for locally advanced nasopharyngeal carcinoma. Cancer J Sci Am (1997) 3:100–6.
26. Cheng SH, Jian JJ, Tsai SY, Yen KL, Chu NM, Chan KY, et al. Long-term survival of nasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy. Int J Radiat Oncol Biol Phys (2000) 48:1323–30. doi: 10.1016/S0360-3016(00)00779-3
27. Lin JC, Chen KY, Jan JS, Hsu CY. Partially hyperfractionated accelerated radiotherapy and concurrent chemotherapy for advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys (1996) 36:1127–36. doi: 10.1016/S0360-3016(96)00384-7
28. Lin JC, Jan JS, Hsu CY. Pilot study of concurrent chemotherapy and radiotherapy for stage IV nasopharyngeal cancer. Am J Clin Oncol (1997) 20:6–10. doi: 10.1097/00000421-199702000-00002
29. Lee AW, Tung SY, Ngan RK, Chappell R, Chua DT, Lu TX, et al. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer. (2011) 47:656–66. doi: 10.1016/j.ejca.2010.10.026
30. Loong HH, Ma BB, Leung SF, Mo F, Hui EP, Kam MK, et al. Prognostic significance of the total dose of cisplatin administered during concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Radiother Oncol (2012) 104:300–4. doi: 10.1016/j.radonc.2011.12.022
31. Wei W, Huang Z, Li S, Chen H, Zhang G, Li S, et al. Pretreatment Epstein-Barr virus DNA load and cumulative cisplatin dose intensity affect long-term outcome of nasopharyngeal carcinoma treated with concurrent chemotherapy: experience of an institute in an endemic area. Oncol Res Treat (2014) 37:88–95. doi: 10.1159/000360178
32. Lee JY, Sun JM, Oh DR, Lim SH, Goo J, Lee SH, et al. Comparison of weekly versus triweekly cisplatin delivered concurrently with radiation therapy in patients withlocally advanced nasopharyngeal cancer: A multicenter randomized phase II trial (KCSG-HN10-02). Radiother Oncol (2016) 118:244–50. doi: 10.1016/j.radonc.2015.11.030
33. Peng H, Chen L, Zhang Y, Li WF, Mao YP, Zhang F, et al. Prognostic value of the cumulative cisplatin dose during concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: A secondary analysis of a prospective phase III clinical trial. Oncologist (2016) 21:1369–76. doi: 10.1634/theoncologist.2016-0105
34. Li XY, Luo DH, Guo L, Mo HY, Sun R, Guo SS, et al. Deintensified chemoradiotherapy for pretreatment epstein-barr virus DNA-selected low-risk locoregionally advanced nasopharyngeal carcinoma: A phase II randomized noninferiority trial. J Clin Oncol (2022) 40:1163–73. doi: 10.1200/JCO.21.01467
35. Tao CJ, Lin L, Zhou GQ, Tang LL, Chen L, Mao YP, et al. Comparison of long-term survival and toxicity of cisplatin delivered weekly versus every three weeks concurrently with intensity-modulated radiotherapy in nasopharyngeal carcinoma. PloS One (2014) 9:e110765. doi: 10.1371/journal.pone.0110765
36. Meng DF, Sun R, Peng LX, Huang YS, Yang Q, Luo DH, et al. A comparison of weekly versus 3-weekly cisplatin during concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma using intensity modulated radiation therapy: a matched study. J Cancer. (2018) 9:92–9. doi: 10.7150/jca.21357
37. Zhu Q, Hu H, Tang LQ, You R, Zhao JJ, Weng DS, et al. Weekly versus triweekly cisplatin plus intensity-modulated radiotherapy in locally advanced nasopharyngeal carcinoma: A propensity score analysis with a large cohort. J Cancer. (2018) 9:3447–55. doi: 10.7150/jca.26110
38. Wang K, Dong J, He S, Wang X, Jiang C, Hu P, et al. Comparison of weekly and triweekly cisplatin regimens during concurrent chemoradiotherapy for nasopharyngeal carcinoma. BMC Cancer. (2019) 19:482. doi: 10.1186/s12885-019-5688-z
39. Gundog M, Basaran H, Bozkurt O, Eroglu C. A comparison of cisplatin cumulative dose and cisplatin schedule in patients treated with concurrent chemo-radiotherapy in nasopharyngeal carcinoma. Braz J Otorhinolaryngol (2020) 86:676–86. doi: 10.1016/j.bjorl.2019.04.008
40. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol (2016) 17:1509–20. doi: 10.1016/S1470-2045(16)30410-7
41. Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicentre randomised controlled trial. Eur J Cancer. (2017) 75:14–23. doi: 10.1016/j.ejca.2016.12.039
42. Peng H, Chen L, Li WF, Guo R, Mao YP, Zhang Y, et al. Tumor response to neoadjuvant chemotherapy predicts long-term survival outcomes in patients with locoregionally advanced nasopharyngeal carcinoma: A secondary analysis of a randomized phase 3 clinical trial. Cancer (2017) 123:1643–52. doi: 10.1002/cncr.30520
43. Frikha M, Auperin A, Tao Y, Elloumi F, Toumi N, Blanchard P, et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02). Ann Oncol (2018) 29:731–6. doi: 10.1093/annonc/mdx770
44. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med (2019) 381:1124–35. doi: 10.1056/NEJMoa1905287
45. Liu SL, Sun XS, Yan JJ, Chen QY, Lin HX, Wen YF, et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients based on induction chemotherapy response. Radiother Oncol (2019) 137:83–94. doi: 10.1016/j.radonc.2019.04.020
46. Jiang YT, Chen KH, Yang J, Liang ZG, Li L, Qu S, et al. Efficiency of high cumulative cisplatin dose in high- and low-risk patients with locoregionally advanced nasopharyngeal carcinoma. Cancer Med (2022) 11:715–27. doi: 10.1002/cam4.4477
47. Wen DW, Li ZX, Chen FP, Lin L, Peng BY, Kou J, et al. Individualized cumulative cisplatin dose for locoregionally-advanced nasopharyngeal carcinoma patients receiving induction chemotherapy and concurrent chemoradiotherapy. Oral Oncol (2020) 107:104675. doi: 10.1016/j.oraloncology.2020.104675
48. Lv JW, Qi ZY, Zhou GQ, He XJ, Chen YP, Mao YP, et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients receiving additional induction chemotherapy. Cancer Sci (2018) 109:751–63. doi: 10.1111/cas.13474
49. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol (2003) 21:631–7. doi: 10.1200/JCO.2003.06.158
50. Komatsu M, Arai Y, Yabuki K, Sano D, Shiono O, Sakuma Y, et al. Concurrent chemoradiotherapy with docetaxel, cisplatin and 5-fluorouracil (TPF) in patients with nasopharyngeal carcinoma. Anticancer Res (2015) 35:6861–7.
51. He XY, Hu CS, Ying HM, Wu YR, Zhu GP, Liu TF. Paclitaxel with cisplatin in concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol (2010) 267:773–8. doi: 10.1007/s00405-009-1112-7
52. Baykara M, Buyukberber S, Ozturk B, Coskun U, Kaplan MA, Unsal DK, et al. Efficacy and safety of concurrent chemoradiotherapy with cisplatin and docetaxel in patients with locally advanced nasopharyngeal cancers. Tumori (2013) 99:469–73. doi: 10.1177/030089161309900405
53. Chen X, Hong Y, Feng J, Ye J, Zheng P, Guan X, et al. Concurrent chemoradiotherapy comparison of taxanes and platinum versus 5-fluorouracil and platinum in nasopharyngeal carcinoma treatment. Chin Med J (Engl) (2014) 127:142–9. doi: 10.3901/JME.2014.09.142
54. He X, Ou D, Ying H, Zhu G, Hu C, Liu T. Experience with combination of cisplatin plus gemcitabine chemotherapy and intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol (2012) 269:1027–33. doi: 10.1007/s00405-011-1720-x
55. Gu MF, Liu LZ, He LJ, Yuan WX, Zhang R, Luo GY, et al. Sequential chemoradiotherapy with gemcitabine and cisplatin for locoregionally advanced nasopharyngeal carcinoma. Int J Cancer. (2013) 132:215–23. doi: 10.1002/ijc.27638
56. Wu Y, Wei X, Yuan Z, Xu H, Li Y, Li Y, et al. Phase II study of induction chemotherapy followed by concurrent chemoradiotherapy with raltitrexed and cisplatin in locally advanced nasopharyngeal carcinoma. Chin J Cancer Res (2020) 32:665–72. doi: 10.21147/j.issn.1000-9604.2020.05.11
57. Ma BBY, Kam MKM, Leung SF, Hui EP, King AD, Chan SL, et al. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol (2012) 23:1287–92. doi: 10.1093/annonc/mdr401
58. Li Y, Chen QY, Tang LQ, Liu LT, Guo SS, Guo L, et al. Concurrent chemoradiotherapy with or without cetuximab for stage II to IVb nasopharyngeal carcinoma: a case-control study. BMC Cancer. (2017) 17:567. doi: 10.1186/s12885-017-3552-6
59. Cai Z, Chen D, Qiu W, Liang C, Huang Y, Zhou J, et al. Concurrent chemoradiotherapy combined with nimotuzumab in stage III-IVa nasopharyngeal carcinoma: a retrospective analysis. J Cancer Res Clin Oncol (2022) 149(6):2327–44. doi: 10.21203/rs.3.rs-1316434/v1
60. Zong JF, Liang QD, Lu QJ, Liu YH, Xu HC, Chen BJ, et al. Comparison of radiotherapy combined with nimotuzumab vs. chemoradiotherapy for locally recurrent nasopharyngeal carcinoma. BMC Cancer. (2021) 21:1274. doi: 10.1186/s12885-021-08995-y
61. Hu W, Ding W, Yang H, Shao M, Wang B, Wang J, et al. Weekly paclitaxel with concurrent radiotherapy followed by adjuvant chemotherapy in locally advanced nasopharyngeal carcinoma. Radiother Oncol (2009) 93:488–91. doi: 10.1016/j.radonc.2009.06.030
62. Fengtong J, Jiangtao F, Yating W, Lili W, Jianbo C, Xiaofei W. Effects of S-1 combined with radiotherapy in the treatment of nasopharyngeal cancer: A meta-analysis based on randomized controlled trials. Open Med (Wars). (2017) 12:107–14. doi: 10.1515/med-2017-0017
63. Wen L, You C, Lu X, Zhang L. Phase II trial of concurrent chemoradiotherapy with S-1 versus weekly cisplatin for locoregionally advanced nasopharyngeal carcinoma. Mol Clin Oncol (2015) 3:687–91. doi: 10.3892/mco.2015.529
64. Xu T, Liu Y, Dou S, Li F, Guan X, Zhu G. Weekly cetuximab concurrent with IMRT aggravated radiation-induced oral mucositis in locally advanced nasopharyngeal carcinoma: Results of a randomized phase II study. Oral Oncol (2015) 51:875–9. doi: 10.1016/j.oraloncology.2015.06.008
65. Li HM, Li P, Qian YJ, Wu X, Xie L, Wang F, et al. A retrospective paired study: efficacy and toxicity of nimotuzumab versus cisplatin concurrent with radiotherapy in nasopharyngeal carcinoma. BMC Cancer. (2016) 16:946. doi: 10.1186/s12885-016-2974-x
66. Driessen CM, Uijen MJ, van der Graaf WT, van Opstal CC, Kaanders JH, Nijenhuis T, et al. Degree of nephrotoxicity after intermediate- or high-dose cisplatin-based chemoradiotherapy in patients with locally advanced head and neck cancer. Head Neck. (2016) 38 Suppl 1:E1575–81. doi: 10.1002/hed.24281
67. Yip PL, Mok KCJ, Ho HS, Lee WYV, Wong ACL, Lau CT, et al. Sensorineural hearing loss in nasopharyngeal carcinoma survivors in the modern treatment era - the early and late effects of radiation and cisplatin. Clin Oncol (R Coll Radiol). (2022) 34:e160–e7. doi: 10.1016/j.clon.2021.10.013
68. Low WK, Toh ST, Wee J, Fook-Chong SM, Wang DY. Sensorineural hearing loss after radiotherapy and chemoradiotherapy: a single, blinded, randomized study. J Clin Oncol (2006) 24:1904–9. doi: 10.1200/JCO.2005.05.0096
69. Amable L. Cisplatin resistance and opportunities for precision medicine. Pharmacol Res (2016) 106:27–36. doi: 10.1016/j.phrs.2016.01.001
70. Yau TK, Lee AW, Wong DH, Pang ES, Ng WT, Yeung RM, et al. Treatment of Stage IV(A-B) nasopharyngeal carcinoma by induction-concurrent chemoradiotherapy and accelerated fractionation: impact of chemotherapy schemes. Int J Radiat Oncol Biol Phys (2006) 66:1004–10. doi: 10.1016/j.ijrobp.2006.06.016
71. Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, Sumitsawan Y, Tharavichitkul E, Sukthomya V, et al. Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non-inferiority, open trial. Eur J Cancer. (2007) 43:1399–406. doi: 10.1016/j.ejca.2007.03.022
72. Chitapanarux I, Kittichest R, Tungkasamit T, Asakit T, Chomprasert K, Chakrabandhu S, et al. Two-year outcome of concurrent chemoradiation with carboplatin with or without adjuvant carboplatin/fluorouracil in nasopharyngeal cancer: A multicenter randomized trial. Curr Probl Cancer. (2021) 45:100620. doi: 10.1016/j.currproblcancer.2020.100620
73. Songthong A, Chakkabat C, Kannarunimit D, Lertbutsayanukul C. Efficacy of intensity-modulated radiotherapy with concurrent carboplatin in nasopharyngeal carcinoma. Radiol Oncol (2015) 49:155–62. doi: 10.2478/raon-2014-0044
74. Tang C, Wu F, Wang R, Lu H, Li G, Liu M, et al. Comparison between nedaplatin and cisplatin plus docetaxel combined with intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma: a multicenter randomized phase II clinical trial. Am J Cancer Res (2016) 6:2064–75.
75. Liu T, Sun Q, Chen J, Li B, Qin W, Wang F, et al. Neoadjuvant chemotherapy with fluorouracil plus nedaplatin or cisplatin for locally advanced nasopharyngeal carcinoma: a retrospective study. J Cancer. (2018) 9:3676–82. doi: 10.7150/jca.27198
76. Zhan ZJ, Tao HY, Qiu WZ, Liu ZY, Zhang RX, Liao K, et al. Clinical value of nedaplatin-based chemotherapy combined with radiotherapy for locoregional advanced nasopharyngeal carcinoma: a retrospective, propensity score-matched analysis. J Cancer. (2020) 11:6782–9. doi: 10.7150/jca.47090
77. Tang LQ, Chen DP, Guo L, Mo HY, Huang Y, Guo SS, et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol (2018) 19:461–73. doi: 10.1016/S1470-2045(18)30104-9
78. Tang QN, Liu LT, Qi B, Guo SS, Luo DH, Sun R, et al. Effect of concurrent chemoradiotherapy with nedaplatin vs cisplatin on the long-term outcomes of survival and toxic effects among patients with stage II to IVB nasopharyngeal carcinoma: A 5-year follow-up secondary analysis of a randomized clinical trial. JAMA Netw Open (2021) 4:e2138470. doi: 10.1001/jamanetworkopen.2021.38470
79. Liao W, Huang J, Wu Q, Zhu G, Wang X, Wen F, et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma: A cost-effectiveness analysis. Oral Oncol (2019) 93:15–20. doi: 10.1016/j.oraloncology.2019.04.003
80. Xu J, He X, Cheng K, Guo W, Bian X, Jiang X, et al. Concurrent chemoradiotherapy with nedaplatin plus paclitaxel or fluorouracil for locoregionally advanced nasopharyngeal carcinoma: Survival and toxicity. Head Neck. (2014) 36:1474–80. doi: 10.1002/hed.23487
81. Hu Y, Fu JT, Shi D, Feng B, Shi Z. Clinical efficacy and safety of gemcitabine plus nedaplatin in the treatment of advanced nasopharyngeal carcinoma. J Cancer Res Ther (2016) 12:C252–c5. doi: 10.4103/0973-1482.200750
82. McKeage MJ. Lobaplatin: a new antitumour platinum drug. Expert Opin Investig Drugs (2001) 10:119–28. doi: 10.1517/13543784.10.1.119
83. Ke LR, Xia WX, Qiu WZ, Huang XJ, Yang J, Yu YH, et al. Safety and efficacy of lobaplatin combined with 5-fluorouracil as first-line induction chemotherapy followed by lobaplatin-radiotherapy in locally advanced nasopharyngeal carcinoma: preliminary results of a prospective phase II trial. BMC Cancer. (2017) 17:134. doi: 10.1186/s12885-017-3080-4
84. Lv X, Cao X, Xia WX, Liu KY, Qiang MY, Guo L, et al. Induction chemotherapy with lobaplatin and fluorouracil versus cisplatin and fluorouracil followed by chemoradiotherapy in patients with stage III-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised, controlled, phase 3 trial. Lancet Oncol (2021) 22:716–26. doi: 10.1016/S1470-2045(21)00075-9
85. Cavalieri S, Licitra L. Next generation platinum salt in nasopharygeal carcinoma. Lancet Oncol (2021) 22:577–8. doi: 10.1016/S1470-2045(21)00182-0
86. Zhang L, Zhao C, Peng PJ, Lu LX, Huang PY, Han F, et al. Phase III study comparing standard radiotherapy with or without weekly oxaliplatin in treatment of locoregionally advanced nasopharyngeal carcinoma: preliminary results. J Clin Oncol (2005) 23:8461–8. doi: 10.1200/JCO.2004.00.3863
87. Yang Q, Cao SM, Guo L, Hua YJ, Huang PY, Zhang XL, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer. (2019) 119:87–96. doi: 10.1016/j.ejca.2019.07.007
88. Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol (2009) 27:3684–90. doi: 10.1200/JCO.2008.19.9109
89. Kwong DL, Sham JS, Chua DT, Choy DT, Au GK, Wu PM. The effect of interruptions and prolonged treatment time in radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys (1997) 39:703–10. doi: 10.1016/S0360-3016(97)00339-8
90. Liang X, Liu Q, Yao W, Yang S. Efficacy and toxicity of three concurrent chemoradiotherapy regimens in treating nasopharyngeal carcinoma: Comparison among cisplatin, nedaplatin, and lobaplatin. Med (Baltimore). (2022) 101:e31187. doi: 10.1097/MD.0000000000031187
91. Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med (2021) 27:1536–43. doi: 10.1038/s41591-021-01444-0
92. Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol (2021) 22:1162–74. doi: 10.1016/S1470-2045(21)00302-8
Keywords: nasopharyngeal carcinoma, concurrent chemoradiotherapy, chemotherapy, Platinum, Cisplatin
Citation: Zhu F, Wu Y and Wang H (2023) Advance in integrating platinum-based chemotherapy with radiotherapy for locally advanced nasopharyngeal carcinoma. Front. Oncol. 13:1259331. doi: 10.3389/fonc.2023.1259331
Received: 15 July 2023; Accepted: 12 September 2023;
Published: 28 September 2023.
Edited by:
Paolo Bossi, Humanitas Research Hospital, ItalyReviewed by:
Maria Cossu Rocca, European Institute of Oncology (IEO), ItalyCopyright © 2023 Zhu, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Wang, NDIwMTQzNDQ5QHFxLmNvbQ==
 Fubin Zhu
Fubin Zhu Yidan Wu2
Yidan Wu2