
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 29 September 2023
Sec. Cancer Molecular Targets and Therapeutics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1258287
This article is part of the Research TopicTherapeutic Advances in Immune Checkpoint Inhibitors for Cancer TreatmentView all 9 articles
Background: Immune agents targeting Programmed cell death-1 (PD-1) are a new type of cancer treatment drugs. By inhibiting the interaction between PD-1 and PD-L1, the ability of the immune system to attack tumor cells is enhanced. These immune preparations have shown significant efficacy in the treatment of various malignant tumors. However, like other drugs, immune preparations targeting PD-1 may also cause side effects, including arthralgia. Therefore, we conduct a meta-analysis to assess whether immune-checkpoint inhibitors targeting programmed cell death-1 in lung cancer patients will lead to arthralgia adverse events.
Methods: We conducted a comprehensive search across multiple databases, including PubMed, Medline (Ovid), Web of Science, Cochrane, Embase, Scopus, CKNI, Wang fang, VIP database, Sino Med, and Clinical Trails, to identify relevant studies. The search encompassed articles published up until June 20th, 2023. The primary outcome is adverse events about arthralgia and secondary outcomes are any other related with arthralgia. Data extraction was carried out by two independent individuals, and the Cochrane Risk of Bias tool version 2.0 was employed to assess the included studies. The systematic review and meta-analysis were conducted using RevMan 5.3 software.
Results: 12 studies are included in the meta-analysis. All included studies were determined to have a low risk of random sequence generation bias. The meta-analysis result showed that arthralgia RR = 1.11, 95% CI [0.88, 1.40], I2 = 56%, back pain RR = 1.86, 95% CI [1.07, 3.26], I2 = 84%, myalgia RR = 0.49, 95% CI [0.27, 0.88], I2 = 86% and muscular pain RR = 1.97, 95% CI [1.40, 2.77], I2 = 23%.
Conclusion: The use of targeted inhibitors may lead to an increased incidence of back pain, while potentially reducing the occurrence of myalgia. On the other hand, immune-checkpoint inhibitors targeting programmed cell death-1 in lung cancer patients may not cause arthralgia and muscular pain.
Lung cancer is a prevalent malignant tumor, and its treatment options include surgery, radiotherapy, chemotherapy, and immunotherapy (1). Immunotherapy has emerged as a new treatment option for lung cancer by activating the patient’s immune system and enhancing their ability to attack tumors. One of the commonly used immunotherapeutic drugs is the immunocheckpoint inhibitor targeting Programmed cell death -1 (PD-1) (2). However, this drug can also cause adverse events, including arthralgia (3).
PD-1 inhibitors are immune checkpoint inhibitors that activate the immune system and enhance its ability to attack tumors by inhibiting the binding of PD-1 and its ligand PD-L1 (4). However, PD-1 inhibitors can also cause adverse events, including arthralgia. Arthralgia is a common adverse event of PD-1 inhibitor treatment, characterized by joint swelling, pain, stiffness, and other symptoms. These symptoms can affect the patient’s quality of life and even hinder the progress of treatment (5).
The mechanism of arthralgia is not entirely clear, but it may be related to the immune system activation caused by PD-1 inhibitors. PD-1 inhibitors can activate the immune system, causing immune cells to attack normal tissues and trigger inflammatory reactions. This inflammatory reaction may affect the normal function of joints, leading to arthralgia and other symptoms (6, 7).
When patients experience adverse events such as arthralgia, doctors usually take appropriate treatment measures based on the severity of symptoms and individual patient conditions (8). Nonsteroidal anti-inflammatory drugs, steroids, and other drugs can be given to alleviate symptoms. During the treatment process, doctors also need to closely monitor the changes in the patient’s condition and adjust the treatment plan in a timely manner. Nonsteroidal anti-inflammatory drugs are commonly used to relieve arthralgia (9). The drug can alleviate arthralgia and other symptoms by inhibiting inflammatory reactions. However, Nonsteroidal anti-inflammatory drugs can also cause adverse events, such as gastrointestinal bleeding, renal function damage, etc. Therefore, when using Nonsteroidal anti-inflammatory drugs, attention should be paid to the dosage and medication time to avoid unnecessary adverse events. In addition to drug treatment, patients can also take some self-management measures to alleviate arthralgia and other symptoms. For example, appropriate exercise can be performed to maintain joint flexibility and mobility; Pay attention to diet and avoid excessive intake of fat and sugar; Maintain a good mindset and avoid excessive anxiety and tension (10).
When lung cancer patients receive PD-1 inhibitor treatment, they may experience adverse events such as arthralgia, which require timely diagnosis and treatment. Doctors need to develop personalized treatment plans based on the individual situation of patients to improve treatment effectiveness and reduce the occurrence of adverse events (11). Patients also need to actively cooperate with the doctor’s treatment, take self-management measures, alleviate arthralgia and other symptoms, and improve the quality of life (12). Therefore, we conduct a systematic review and meta-analysis to assess the arthralgia adverse events due to immune-checkpoint inhibitors targeting programmed cell death-1 in lung cancer patients.
A thorough search of multiple databases, including PubMed, Medline (Ovid), Web of Science, Cochrane, Embase, Scopus, CKNI, Wang fang, VIP database, Sino Med, and Clinical Trails, was conducted to identify relevant studies. The search was conducted until June 20th, 2023. The search strategy employed for Medline (Ovid) is outlined in Table 1.
a) Randomized controlled trials (RCTs);
b) patients have adverse events with arthralgia or related symptoms;
c) Patients using immune-checkpoint inhibitors targeting programmed cell death-1 in lung cancer patients as the main treatment.
a) Not report related outcomes.
b) Studies with an observational design were included in the analysis.
c) Insufficient information regarding the baseline characteristics was noted.
Adverse events about arthralgia
a) Any other related with arthralgia
b) Other symptoms related to bone pain including back pain, myalgia, muscular pain and etc.
Two independent reviewers (Defang Zou and Xiaoping Wang) conducted data extraction using a standardized form that included study demographics, baseline characteristics, study design, intervention methods, outcome measures, and results. In case of any discrepancies, the reviewers resolved them through discussion, and a third review author was consulted if necessary.
The risk of bias in the included studies was assessed by two authors using the Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019) risk of bias assessment tool. Any discrepancies were resolved through consensus. The assessment tool evaluated seven items, including random sequence generation, assignment concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The items were categorized as green, yellow, and red colors and “+”, “-”, “?”, indicating “low,” “high,” and “unclear” risk of bias.
To assess publication bias related to the primary outcome measures, funnel plots were generated using RevMan 5.3 software.
The Review Manager software (RevMan version 5.3, Cochrane Collaboration, Oxford, UK) was utilized to conduct statistical analyses. The effect size for merging the continuous variables in the study was determined using WMD and a 95% CI. For merging the binary variables, RR and a 95% CI were used as the effect size. Initially, a heterogeneity test was conducted on the included studies. Sensitivity analysis was performed to investigate any significant clinical or methodological heterogeneity. Statistical heterogeneity was assessed using I2 and P values.
The presence of heterogeneity among trial results was evaluated using the P value and I² statistic. When more than two articles were included, heterogeneity was assessed. If the I² value exceeded 50%, the random effect model was used based on clinical heterogeneity. To identify the source of heterogeneity, subgroup analysis, sensitivity analysis, and funnel plots were employed. The statistical calculations were performed using RevMan 5.3 software.
We conducted a comprehensive search on 8 databases, namely PubMed, Medline (Ovid), Web of Science, Cochrane, Embase, Scopus, CKNI, Wang fang, VIP database, Sino Med, and Clinical Trials, until June 16th, 2023. A total of 3120 records were identified through the database searching process, while an additional 8 records were found through other sources. After removing duplicates, we collected 625 unique records after duplicates removed. Out of these, 51 articles were assessed for eligibility, and ultimately, 12 studies were finally included in the meta-analysis (Figure 1).
12 studies characteristics information are collected in Table 2. The difference is discussed by the third author or the whole group. The characteristics of include studies can be found in Table 2.
All inclueded are low risk of random sequence generation bias. Two studies are unclear about allocation concealment. Most studies are unclear of performance bias and detection bias except one studies mentioned the use of blinding methods. All studies are low risk of attritions bias and reporting bias. Some studies are unclear of other bias such are lost of follow-up (Figure 2).
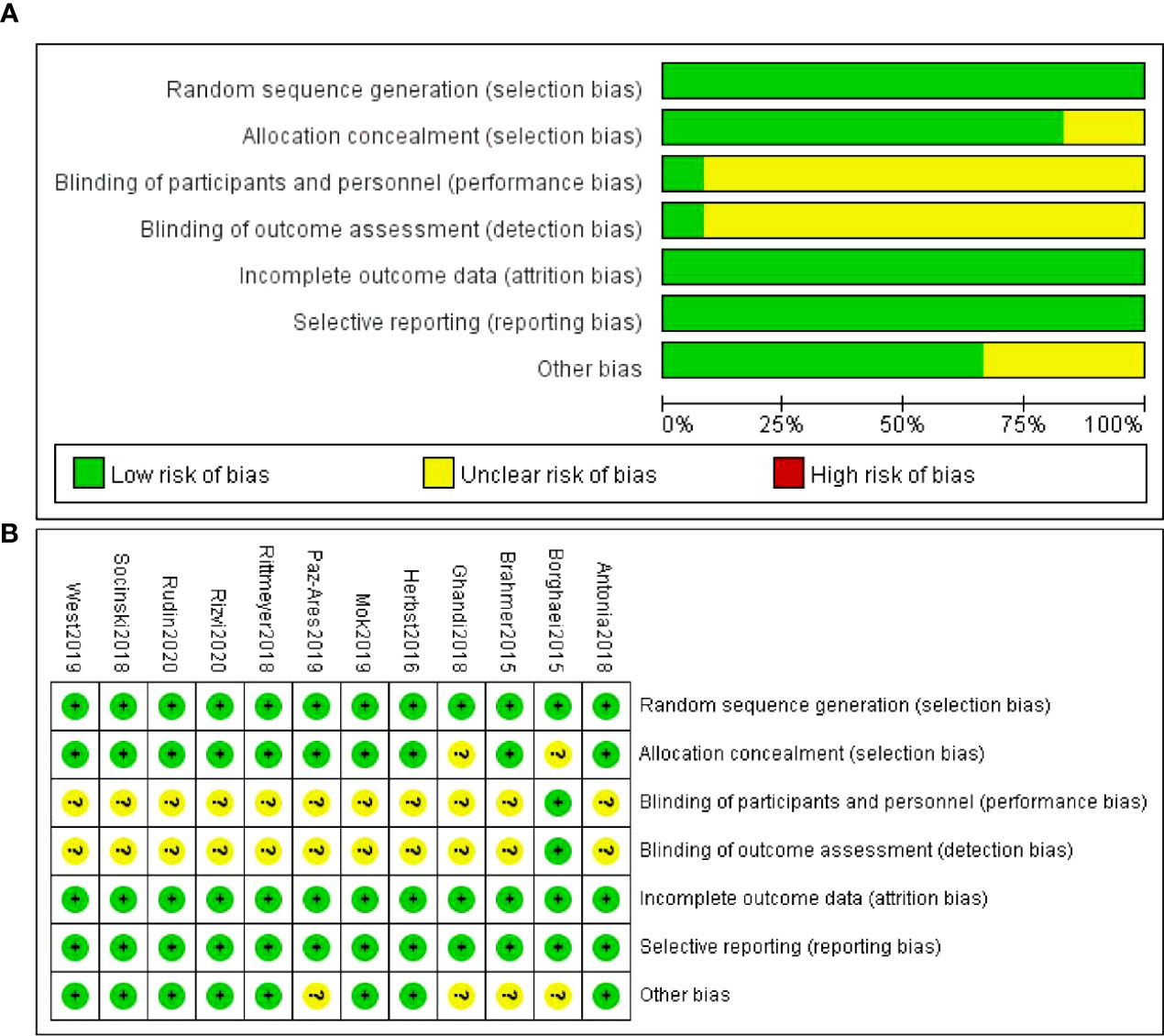
Figure 2 Quality assessment of the included studies. (A) Risk of bias summary (B) Risk of bias graph.
There were 10 studies which discussed arthralgia. The forest plot risk ratio RR = 1.11, 95% CI [0.88, 1.40], I2 = 56%. The asymmetrical shape of the funnel plot suggested the possibility of publication bias. To address this concern, a sensitivity analysis was performed and revealed that all values included in the literature fell within a reasonable range (Figure 3).
There were 5 studies which discussed back pain. The forest plot risk ratio RR = 1.86, 95% CI [1.07, 3.26], I2 = 84%. The asymmetrical shape of the funnel plot suggested the possibility of publication bias. To address this concern, a sensitivity analysis was performed and revealed that all values included in the literature fell within a reasonable range (Figure 4).
There were 7 studies which discussed myalgia. The forest plot risk ratio RR = 0.49, 95% CI [0.27, 0.88], I2 = 86%. The asymmetrical shape of the funnel plot suggested the possibility of publication bias. To address this concern, a sensitivity analysis was performed and revealed that all values included in the literature fell within a reasonable range (Figure 5).
There were 3 studies which discussed muscular pain. The forest plot risk ratio RR = 1.97, 95% CI [1.40, 2.77], I2 = 23%. The asymmetrical shape of the funnel plot suggested the possibility of publication bias. To address this concern, a sensitivity analysis was performed and revealed that all values included in the literature fell within a reasonable range (Figure 6).
Immune agents targeting Programmed cell death -1 (PD-1) are a new class of cancer drugs, which have shown excellent efficacy in the treatment of a variety of malignant tumors (25). Although these drugs have achieved great success in anti-tumor immunotherapy, their activation of the immune system may also lead to a series of autoimmune reactions, including arthralgia. However, they may also cause some side effects including arthralgia (26).
Arthralgia is a common adverse reaction. Clinical studies have shown that about 5% to 10% of cancer patients receiving targeted PD-1 immunotherapy will have arthralgia symptoms (27). This arthralgia is usually mild to moderate and can affect multiple joints, including hands, knees, shoulders, etc. It may lead to limited mobility, discomfort, and pain. The exact mechanism of arthralgia caused by targeted PD-1 immune agents is still unclear, but several hypotheses can explain this phenomenon.
Firstly, the activation of the immune system may lead to autoimmune reactions, triggering inflammatory reactions in joint tissue (28). Secondly, drugs may interfere with the Immune tolerance mechanism, causing the immune system to attack normal joint tissues. Finally, arthralgia may also be due to the influence of drugs on the neural network, resulting in sensory abnormalities and changes in pain signal transmission (26). These indicates the use of immune checkpoint inhibitors may bring a series of adverse events. Therefore, our research highlights the necessity to assess the adverse of immune-checkpoint inhibitors for lung cancer patients.
In our study, we conducted a comprehensive search across 8 databases, including PubMed, Medline (Ovid), Web of Science, Cochrane, Embase, Scopus, CKNI, Wang fang, VIP database, Sino Med, and Clinical Trials, until June 16th, 2023. A total of 3120 records were identified through the database searching process, with an additional 8 records found through other sources. After removing duplicates, we obtained 625 unique records. Among these, 51 articles were assessed for eligibility, resulting in the inclusion of 12 studies in the meta-analysis. All included studies were determined to have a low risk of random sequence generation bias. The meta-analysis result showed that arthralgia RR = 1.11, 95% CI [0.88, 1.40], I2 = 56%, back pain RR = 1.86, 95% CI [1.07, 3.26], I2 = 84%, myalgia RR = 0.49, 95% CI [0.27, 0.88], I2 = 86% and muscular pain RR = 1.97, 95% CI [1.40, 2.77], I2 = 23%. The findings indicate that the use of targeted inhibitors may lead to an increased incidence of back pain, while potentially reducing the occurrence of myalgia. However, immune-checkpoint inhibitors targeting programmed cell death-1 in lung cancer patients may not cause arthralgia and muscular pain.
This study holds significant implications for future clinical rehabilitation research. In order to enhance our comprehension of the arthralgia adverse events due to immune-checkpoint inhibitors targeting programmed cell death-1 in lung cancer patients. Our research findings can help lung cancer patients who are using immune-checkpoint inhibitors to prevent joint pain in advance. In addition, It is suggested that forthcoming studies incorporate extended follow-up periods and include a greater number of randomized controlled trials and mechanism research. Moreover, it was observed that numerous studies examined in our analysis either insufficiently reported or ambiguously reported crucial methodological particulars, such as randomization/allocation concealment and blinding methods. To enhance the reporting quality in future trials, we recommend adherence to the Consolidated Standards of Reporting Trials (CONSORT) statement (29).
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
DZ: Writing – original draft. XPW: Data curation, Writing – original draft. XiW: Investigation, Writing – original draft. CL: Data curation, Writing – original draft. AW: Writing – review & editing. XiaW: Writing – review & editing. YY: Writing – review & editing. YS: Data curation, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol (2017) 28(10):2377–85. doi: 10.1093/annonc/mdx286
2. Xu C, Chen Y-P, Du X-J, Liu J-Q, Huang C-L, Chen L, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ (2018) 363:k4226. doi: 10.1136/bmj.k4226
3. Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol (2019) 5(9):1310–7. doi: 10.1001/jamaoncol.2019.1022
4. Zhong H, Zhou J, Xu D, Zeng X. Rheumatic immune-related adverse events induced by immune checkpoint inhibitors. Asia Pac J Clin Oncol (2021) 17(3):178–85. doi: 10.1111/ajco.13346
5. Cappelli LC, Gutierrez AK, Bingham CO 3rd, Shah AA. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: A systematic review of the literature. Arthritis Care Res (Hoboken) (2017) 69(11):1751–63. doi: 10.1002/acr.23177
6. Msaouel P, Oromendia C, Siefker-Radtke AO, Tannir NM, Subudhi SK, Gao J, et al. Evaluation of technology-enabled monitoring of patient-reported outcomes to detect and treat toxic effects linked to immune checkpoint inhibitors. JAMA Netw Open (2021) 4(8):e2122998. doi: 10.1001/jamanetworkopen.2021.22998
7. Lai-Kwon J, Khoo C, Lo S, Milne D, Mohamed M, Raleigh J, et al. The survivorship experience for patients with metastatic melanoma on immune checkpoint and BRAF-MEK inhibitors. J Cancer Surviv (2019) 13(4):503–11. doi: 10.1007/s11764-019-00770-0
8. Abdel-Rahman O, Eltobgy M, Oweira H, Giryes A, Tekbas A, Decker M. Immune-related musculoskeletal toxicities among cancer patients treated with immune checkpoint inhibitors: a systematic review. Immunotherapy (2017) 9(14):1175–83. doi: 10.2217/imt-2017-0108
9. Dey A, Manolios N, Long GV, Carlino MS, Kefford R, Schrieber L. Musculoskeletal immune-related adverse events with the use of checkpoint inhibitors in Malignancy. Intern Med J (2022) 52(5):818–27. doi: 10.1111/imj.15123
10. Schulz TU, Zierold S, Sachse MM, Pesch G, Tomsitz D, Schilbach K, et al. Persistent immune-related adverse events after cessation of checkpoint inhibitor therapy: Prevalence and impact on patients' health-related quality of life. Eur J Cancer (2022) 176:88–99. doi: 10.1016/j.ejca.2022.08.029
11. Steven NM, Fisher BA. Management of rheumatic complications of immune checkpoint inhibitor therapy - an oncological perspective. Rheumatol (Oxford) (2019) 58(Suppl 7):vii29–39. doi: 10.1093/rheumatology/kez536
12. Kostine M, Truchetet ME, Schaeverbeke T. Clinical characteristics of rheumatic syndromes associated with checkpoint inhibitors therapy. Rheumatol (Oxford) (2019) 58(Suppl 7):vii68–74. doi: 10.1093/rheumatology/kez295
13. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
14. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
15. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
16. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
17. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med (2018) 378(24):2288–301. doi: 10.1056/NEJMoa1716948
18. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
19. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
20. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
21. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(7):924–37. doi: 10.1016/S1470-2045(19)30167-6
22. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet (2019) 394(10212):1929–39. doi: 10.1016/S0140-6736(19)32222-6
23. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer. JAMA Oncol (2020) 6(5):661–74. doi: 10.1001/jamaoncol.2020.0237
24. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol (2020) 38(21):2369–79. doi: 10.1200/JCO.20.00793
25. Crout TM, Lennep DS, Kishore S, Majithia V. Systemic vasculitis associated with immune check point inhibition: analysis and review. Curr Rheumatol Rep (2019) 21(6):28. doi: 10.1007/s11926-019-0828-7
26. Ghosh N, Couette N, van Binsbergen WH, Weinmann SC, Jivanelli B, Shea B, et al. Identification of outcome domains in immune checkpoint inhibitor-induced inflammatory arthritis and polymyalgia rheumatica: A scoping review by the OMERACT irAE working group. Semin Arthritis Rheumatol (2023) 58:152110. doi: 10.1016/j.semarthrit.2022.152110
27. Leipe J, Christ LA, Arnoldi AP, Mille E, Berger F, Heppt M, et al. Characteristics and treatment of new-onset arthritis after checkpoint inhibitor therapy. RMD Open (2018) 4(2):e000714. doi: 10.1136/rmdopen-2018-000714
28. Almutairi AR, McBride A, Slack M, Erstad BL, Abraham I. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: A systematic review and meta-analysis. Front Oncol (2020) 10:91. doi: 10.3389/fonc.2020.00091
Keywords: immune-checkpoint inhibitors, programmed cell death-1, lung cancer patients, systematic review, meta-analysis
Citation: Zou D, Wang X, Sun Y, Wang X, Lu C, Wang A, Wang X and Yang Y (2023) Arthralgia adverse events due to immune-checkpoint inhibitors for lung cancer patients: a systematic review and meta-analysis. Front. Oncol. 13:1258287. doi: 10.3389/fonc.2023.1258287
Received: 13 July 2023; Accepted: 10 August 2023;
Published: 29 September 2023.
Edited by:
Guanghua Rong, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Pengpeng Zhang, Nanjing Medical University, ChinaCopyright © 2023 Zou, Wang, Sun, Wang, Lu, Wang, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Yang, NjMyMzg3MDExQHFxLmNvbQ==; Xia Wang, d2FuZ3hpYTc5MDRAMTYzLmNvbQ==; Aiyun Wang, d2FuZ2FpeXVuQG5qdWNtLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.