- 1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Rebecca and John Moores Cancer Center, University of California San Diego Health, San Diego, CA, United States
- 2Gynecologic Oncology, Stephenson Cancer Center, The University of Oklahoma College of Medicine, Oklahoma, OK, United States
- 3Gynecologic Oncology, HonorHealth Research Institute, University of Arizona College of Medicine, Creighton University School of Medicine, Phoenix, AZ, United States
- 4Obstetrics and Gynecology, University of Cincinnati Cancer Center, Cincinnati, OH, United States
- 5Center for Cancer Research, National Cancer Institute, Bethesda, MD, United States
- 6Division of Gynecologic Oncology, The Ohio State University and The James Comprehensive Cancer Center, Columbus, OH, United States
- 7Gynecologic Oncology, US Oncology Research, Texas Oncology, The Woodlands, TX, United States
The definition of “platinum-resistant ovarian cancer” has evolved; it now also reflects cancers for which platinum treatment is no longer an option. Standard of care for platinum-resistant ovarian cancer is single-agent, non-platinum chemotherapy with or without bevacizumab, which produces modest response rates, with the greatest benefits achieved using weekly paclitaxel. Several recent phase 3 trials of pretreated patients with prior bevacizumab exposure failed to meet their primary efficacy endpoints, highlighting the challenge in improving clinical outcomes among these patients. Combination treatment with antiangiogenics has improved outcomes, whereas combination strategies with immune checkpoint inhibitors have yielded modest results. Despite extensive translational research, there has been a lack of reliable and established biomarkers that predict treatment response in platinum-resistant ovarian cancer. Additionally, in the platinum-resistant setting, implications for the time between the penultimate dose of platinum therapy and platinum retreatment remain an area of debate. Addressing the unmet need for an effective treatment in the platinum-resistant setting requires thoughtful clinical trial design based on a growing understanding of the disease. Recent cancer drug approvals highlight the value of incorporating molecular phenotypes to better define patients who are more likely to respond to novel therapies. Clinical trials designed per the Gynecologic Cancer InterGroup recommendations—which advocate against relying solely upon the platinum-free interval—will help advance our understanding of recurrent ovarian cancer response where platinum rechallenge in the platinum-resistant setting may be considered. The inclusion of biomarkers in clinical trials will improve patient stratification and potentially demonstrate correlations with biomarker expression and duration of response. With the efficacy of antibody-drug conjugates shown for the treatment of some solid and hematologic cancers, current trials are evaluating the use of various novel conjugates in the setting of platinum-resistant ovarian cancer. Emerging novel treatments coupled with combination trials and biomarker explorations offer encouraging results for potential strategies to improve response rates and prolong progression-free survival in this population with high unmet need. This review outlines existing data from contemporary clinical trials of patients with platinum-resistant ovarian cancer and suggests historical synthetic benchmarks for non-randomized trials.
1 Introduction: Current landscape in platinum-resistant ovarian cancer
Although surgery and platinum-based chemotherapy are effective treatment strategies for advanced-stage epithelial ovarian cancer, most tumors will relapse within several years and develop resistance to platinum-based therapy (1). This state, termed platinum-resistant ovarian cancer (PROC), is defined as progression within 6 months of the last platinum-based regimen (2, 3). Despite advances in ovarian cancer treatment over the past decade, PROC remains a lethal disease with limited therapeutic options (2).
The current standard treatment for PROC is single-agent non-platinum chemotherapy—the most common of which are pegylated liposomal doxorubicin (PLD), paclitaxel, gemcitabine, and topotecan—with or without bevacizumab (2). As monotherapies, the clinical benefit of non-platinum single agents is modest in pretreated patients, with low objective response rates (ORR, 6%–13%), median progression-free survival (mPFS) of <6 months, and overall survival (OS) of <1 year (4–6). However, adding bevacizumab markedly increases clinical benefit (ORR, 31%; mPFS, 6.7 months; median OS [mOS], 16.6 months), particularly when combined with weekly paclitaxel (7, 8). Among tumors with BRCA mutations or homologous recombination deficiency (HRD), poly[adenosine diphosphate-ribose] polymerase inhibitors (PARPi) have changed the front-line treatment landscape. The role of PARPi in PROC is limited. For tumors that are PARPi naïve and BRCA mutated, the response rate in a single-arm study approached 30% (9). For tumors that are BRCA wild-type, PARPi are not considered a treatment option, as the response rate is <5% (2, 9). Most recently, mirvetuximab soravtansine, an antibody-drug conjugate (ADC) targeting folate receptor alpha (FRα), was approved by the US Food and Drug Administration (FDA) for patients with PROC and high FRα expression (10).
Bevacizumab, a monoclonal antibody that inhibits vascular endothelial growth factor (VEGF), received approval in the US and Europe in 2014 for use in combination with chemotherapy in PROC based on the results of the phase 3, randomized AURELIA trial (7, 11, 12). In AURELIA, treatment with bevacizumab combined with PLD, paclitaxel, or topotecan resulted in a longer mPFS compared to chemotherapy alone (6.7 vs 3.4 months [hazard ratio (HR), 0.48; 95% CI, 0.38–0.60; P<.001]) (7). Furthermore, the ORR of chemotherapy plus bevacizumab was also higher than that of chemotherapy alone (30.9% vs 12.6% [95% CI, 9.6–27.0; P<.001]) (7). In a correspondence published after the primary AURELIA manuscript, the paclitaxel plus bevacizumab cohort exhibited the greatest benefit, with an ORR of 53.3% (8). Interestingly, the paclitaxel monotherapy cohort performed notably well, with an ORR of 30.2% (8). Importantly, the AURELIA study population was limited to patients with ≤2 prior anticancer regimens, excluded tumors that progressed on platinum-based therapy, and included few patients with prior bevacizumab exposure (7). Single-agent bevacizumab first showed efficacy in the platinum-resistant, later-line treatment setting (13).
Bevacizumab is approved for first-line treatment of stage III/IV ovarian cancer in platinum-sensitive ovarian cancer (PSOC) and in PROC (<2 prior lines of therapy), impacting the relevance of the AURELIA data in contemporary patient cohorts (11). The AURELIA patient population may no longer accurately represent the PROC population, making response comparisons between historical and contemporary cohorts challenging.
When PROC progresses after treatment with bevacizumab-containing regimens, single-agent non-platinum chemotherapy is usually implemented (2). The efficacy of single-agent non-platinum chemotherapy with (n=52) or without (n=51) bevacizumab in Japanese patients with PROC that recurred after a bevacizumab-containing chemotherapy regimen was investigated in the open-label, randomized, phase 2 JGOG3023 trial (14). Although patients receiving chemotherapy plus bevacizumab had a numerically greater mOS than those receiving chemotherapy alone (15.3 vs 11.3 months [HR, 0.67; 95% CI, 0.38–1.17; P=.1556]) and a higher ORR (25.0% vs 13.7% [P=.0599]), the results were not significant, likely due to a small sample size (14).
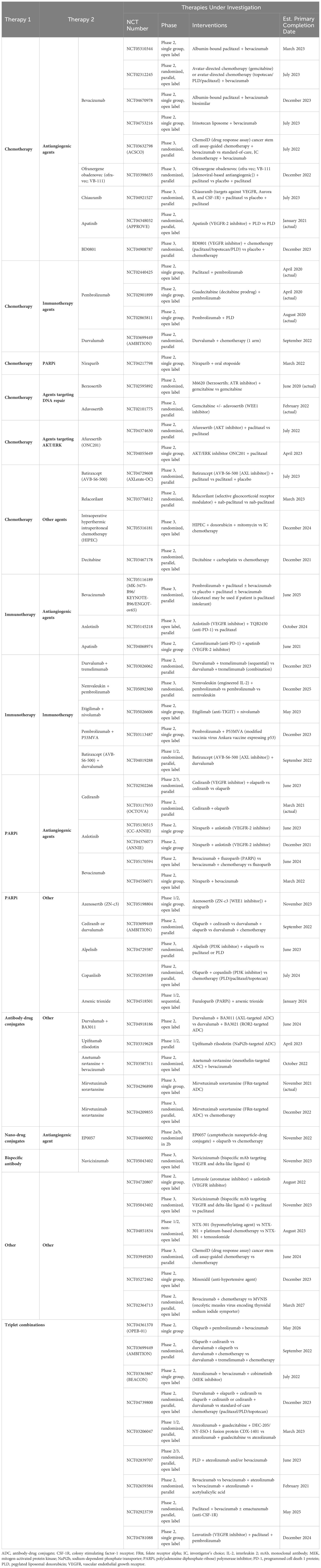
Recent phase 3 trials investigating novel regimens (CORAIL, JAVELIN Ovarian 200, NINJA, FORWARD I) failed to meet their primary efficacy endpoints, highlighting the challenges of treating PROC (Table 1) (6, 15–17). These trials enrolled pretreated patients (≥1 prior line of therapy) with prior bevacizumab exposure ranging from 26%–49% (6, 15–17). Response rates in control groups that received standard-of-care, single-agent chemotherapy ranged from 4%–13%, with a mPFS between 3.5–4.4 months, and a mOS between 11–13 months (Table 1) (6, 15–17).
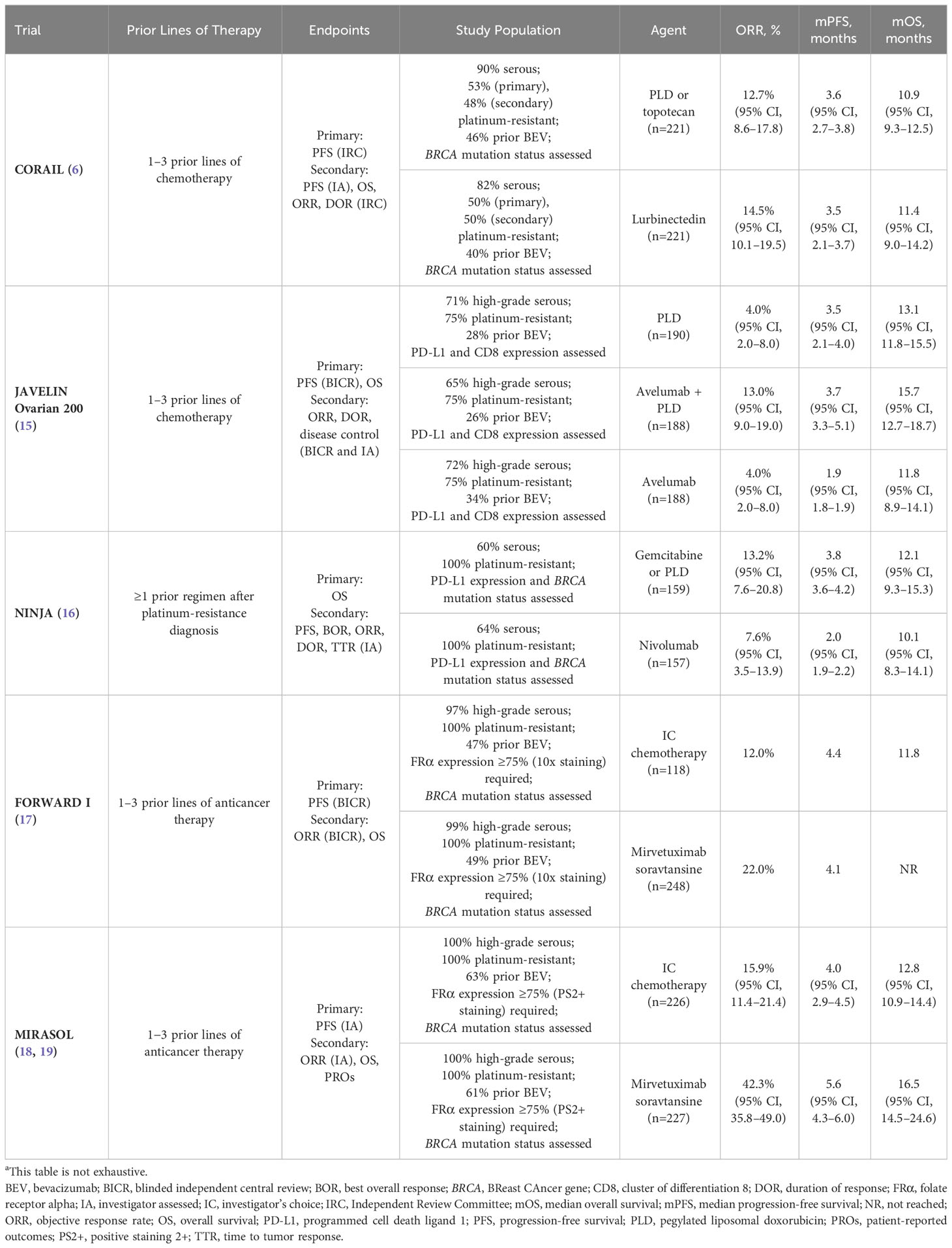
Table 1 Recent phase 3 trials in patients with platinum-resistant recurrent ovarian cancer comparing single-agent chemotherapy to an experimental agenta.
2 Drug development in platinum-resistant ovarian cancer
Since the breakthroughs of paclitaxel, topotecan, and PLD in the 1990s, ovarian cancer treatments stagnated until 2014. Bevacizumab and PARPi transformed the front-line treatment of high-grade epithelial ovarian cancer, resulting in multiple new FDA approvals granted between 2014–2020 (Figure 1) (12). This shifted the ovarian cancer treatment paradigm to include combination and maintenance regimens after initial diagnosis and surgical resection. Conversely, 1 PROC treatment has been approved since the approval of bevacizumab plus chemotherapy in 2014 (10, 12).
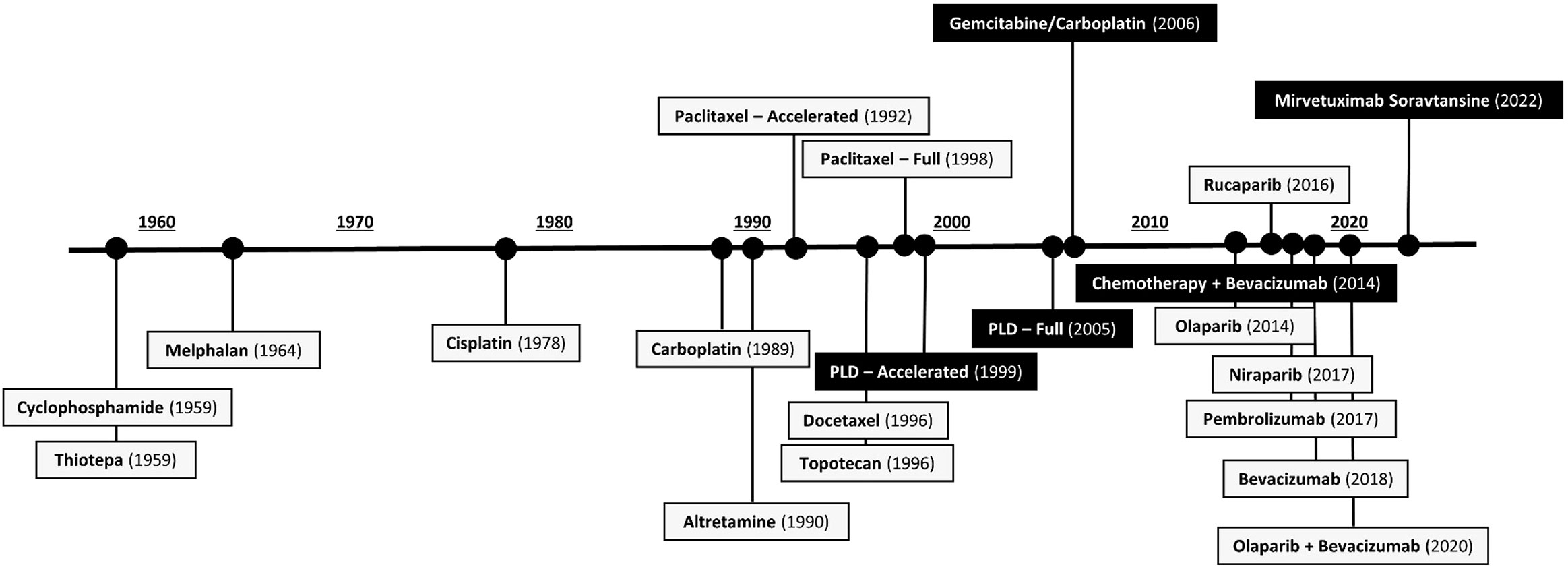
Figure 1 FDA-Approved Agents in Ovarian Cancer by Initial Approval Date (10, 20, 21). Figure 1 shows all drugs approved (and currently approved, as of November 2022) for the treatment of ovarian cancer. Agents in black boxes are indicated for the treatment of platinum-resistant ovarian cancer. FDA, US Food and Drug Administration; PLD, pegylated liposomal doxorubicin.
Limited efficacy signals in PROC clinical trials underscore the challenge of drug development for these patients, and the implications of prior bevacizumab or PARPi exposure on response are unclear. Trials evaluating combination treatment with immunotherapy and chemotherapy in PROC also yielded disappointing results. Lurbinectedin, a cytotoxic agent that inhibits oncogenic transcription, was evaluated vs chemotherapy (PLD or topotecan) in the open-label, phase 3 CORAIL trial in patients with PROC, including those with prior bevacizumab exposure (lurbinectedin arm, 40%; PLD/topotecan arm, 46%) (6). CORAIL failed to meet the primary endpoint of significant improvement in PFS between lurbinectedin and PLD/topotecan (mPFS, 3.5 vs 3.6 months [P=.6059]) (6). JAVELIN Ovarian 200, an open-label, phase 3 trial evaluating avelumab (anti–programmed cell death-ligand 1 [PD-L1]) monoclonal antibody) combined with PLD vs PLD alone, was not superior in mPFS (3.7 vs 3.5 months [HR, 0.78; repeated 93.1% CI, 0.59–1.24; one-sided P=.030]) or mOS (15.7 vs 13.1 months [HR, 0.89; repeated 88.85% CI, 0.74–1.24; one-sided P=.21]) (15). Similarly, trials evaluating single-agent immune checkpoint inhibitors have reported either modest or statistically insignificant benefits, with response rates below 13% (15, 16, 22). NINJA, an open-label, phase 3 trial of the anti–programmed cell death 1 protein (PD-1) monoclonal antibody nivolumab vs chemotherapy, failed to demonstrate any statistical difference in OS (16). Indeed, mOS in the nivolumab arm was shorter compared with chemotherapy (10.1 vs 12.1 months [HR, 1.0; 95% CI, 0.8–1.3; P=.808]) (16). Trials evaluating monotherapies (eg, PARPi, immunotherapies) in PROC have also yielded disappointing results (Table 2) (9, 22, 23, 25, 27, 28). In the open-label, phase 2 KEYNOTE-100 trial in patients with advanced recurrent ovarian cancer, the anti-PD-L1 monoclonal antibody pembrolizumab showed a modest ORR of 7.4% in cohort A (1–3 prior regimens; platinum-free interval [PFI] or treatment-free interval [TFI] 3–12 months) and 9.9% in cohort B (4–6 prior regimens; PFI or TFI ≥3 months) (22). However, a prespecified analysis of KEYNOTE-100 found that higher PD-L1 expression (combined positive score ≥10) correlated with higher ORR, regardless of clinical features such as number of prior treatment lines and degree of platinum sensitivity (22).
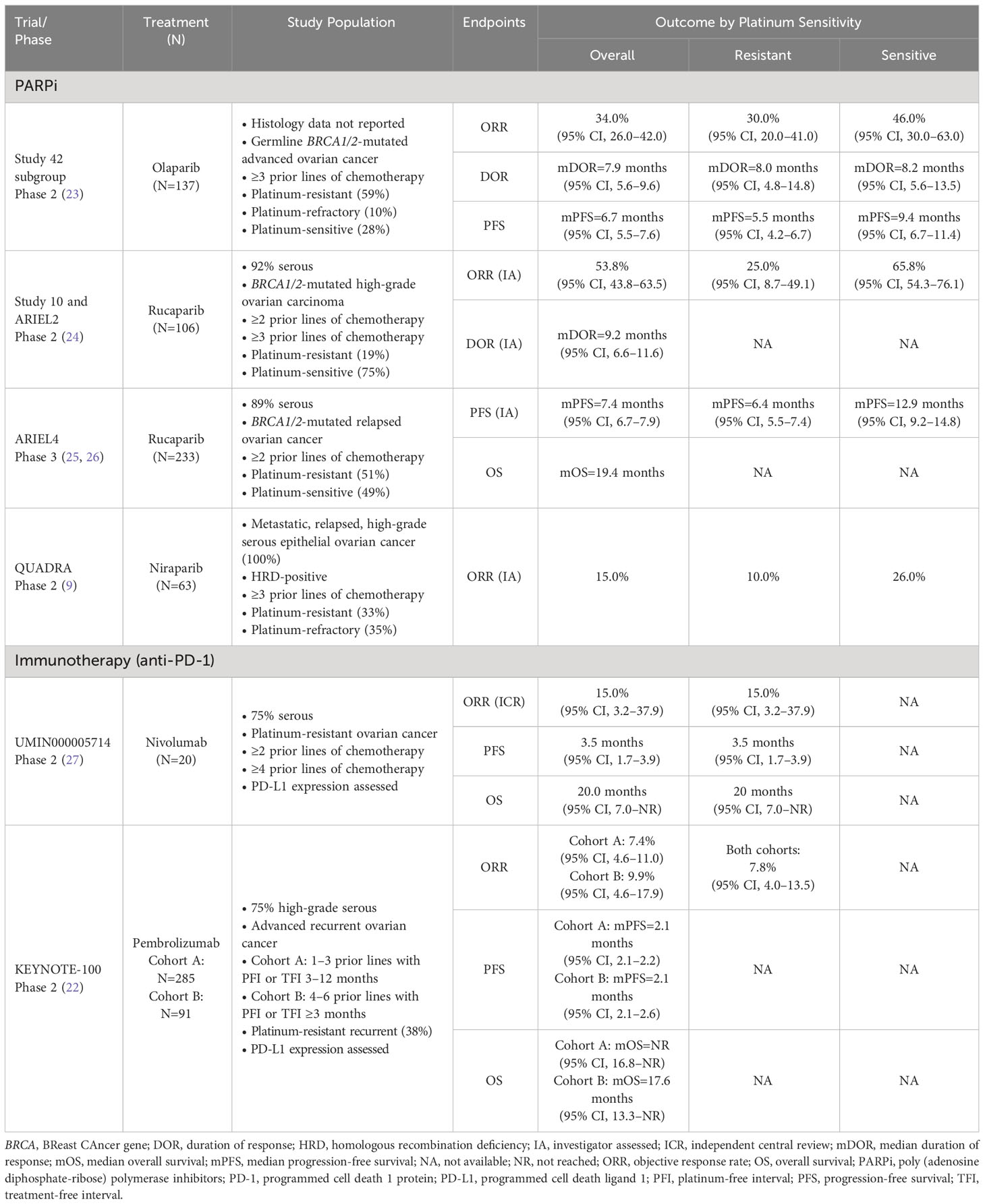
Table 2 Select monotherapy clinical trials with results in heavily pretreated patients with platinum-resistant ovarian cancer.
3 Opportunities and challenges
Numerous unsuccessful clinical trials in PROC underscore the need to identify effective treatments. Novel combinations using existing therapies may improve outcomes. Recent phase 2 and 3 clinical trials of combination therapies are summarized in Table 3.
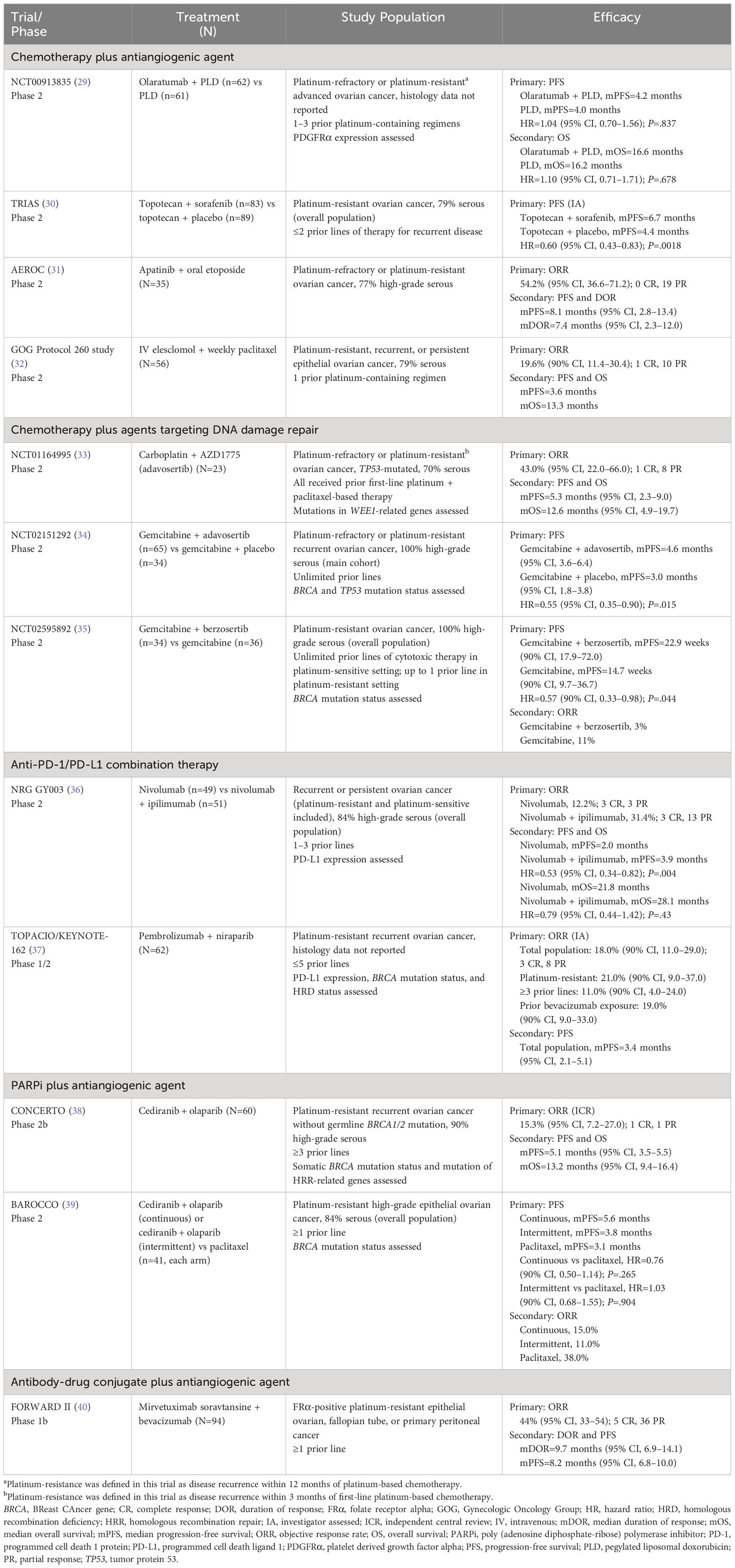
Table 3 Recent clinical trials using combination therapy approaches in pretreated patients with platinum-resistant ovarian cancer.
Antiangiogenic strategies have been pivotal in treating ovarian cancer, and combination therapies show promising results (3). TRIAS, a phase 2 clinical trial that evaluated a sequential combination of sorafenib—a non-selective oral multi-kinase inhibitor of VEGF—with topotecan, was the first to significantly improve OS in patients with PROC or platinum-refractory ovarian cancer (ie, cancer that progressed during platinum therapy) (30). PFS also significantly improved (30). In the open-label, phase 2b CONCERTO trial, the combination of olaparib and cediranib—an antiangiogenic targeting VEGF—demonstrated modest efficacy (ORR, 15.3%; mPFS, 5.1 months; mOS, 13.2 months) in heavily treated patients (all ≥3 prior lines of therapy) with non-germline-BRCA1/2-mutated PROC (38). However, this combination failed to demonstrate superior PFS vs standard-of-care paclitaxel in the open-label, phase 2 BAROCCO trial in a less heavily treated (60%, ≥3 prior lines of therapy) PROC population, of whom 12% had a BRCA mutation (39).
Immunotherapeutic approaches may hold promise in ovarian cancer; however, combination strategies using immune checkpoint inhibitors have yielded modest results to date (3). In the open-label, phase 2 NRG GY003 trial, patients with recurrent or persistent ovarian cancer (PFI <12 months and PROC) who received a 4-dose induction of combination nivolumab/ipilimumab followed by nivolumab exhibited a significantly higher objective response than those receiving nivolumab alone (31.4% vs 12.2% [odds ratio, 3.28; 85% CI, 1.54–infinity; P=.034]); however, mPFS remained low with and without nivolumab/ipilimumab induction (3.9 vs 2.0 months [HR, 0.53; 95% CI, 0.34–0.82; P=.0041]) (36). Despite the short induction, combination nivolumab/ipilimumab treatment yielded more grade ≥3 treatment-related adverse events (TRAEs), including increased pancreatic/liver enzymes, anemia, and colitis or diarrhea (36). The open-label, phase 1/2 TOPACIO/KEYNOTE-162 trial enrolled patients with recurrent PROC (n=30; 48%) or those deemed ineligible for platinum-based therapy (37). The immune checkpoint inhibitor/PARPi combination of pembrolizumab/niraparib demonstrated efficacy across all study populations (ORR overall, 18%; ORR PROC, 21%), irrespective of BRCA or HRD status, prior bevacizumab, or tumor mutational burden (37). Several chemotherapy/immunotherapy combinations are currently under investigation, in addition to trials combining anti-PD-1/PD-L1, antiangiogenic therapy, and DNA-damaging agents (3, 41). The phase 2 OPAL trial investigating dostarlimab/bevacizumab/niraparib combination has shown clinical activity in patients with PROC, most of whom had BRCA wild-type or HRD test negative tumors (42). The phase 2 MOONSTONE/GOG-3032 trial of niraparib plus dostarlimab did not reach the threshold for second-stage accrual at interim analysis due to a low ORR (29.3%) (43). The phase 2 CAPRI trial showed that olaparib plus ceralasertib yielded no objective responses (ie, complete or partial), but showed improved survival outcomes in a subgroup of patients with BRCA mutations (44).
Lastly, the phase 1b FORWARD II trial investigated the combination of the ADC mirvetuximab soravtansine with bevacizumab and found encouraging efficacy (ORR, 44% [95% CI, 33–54]), comparable to standard of care (40).
Of note, baseline clinical characteristics such as number of prior lines of chemotherapy, prior treatment regimens (eg, bevacizumab), histologic subtype, and mutational status are important to consider when interpreting efficacy outcomes in PROC. Therefore, much of this information has been described in Tables 1-3, which report outcomes in relevant clinical trials in PROC.
4 New approaches to drug development
Addressing the unmet need in PROC requires thoughtful clinical trial designs and novel effective agents. Recent cancer drug approvals emphasize incorporating molecular phenotypes into clinical trial designs to better define patient and tumor characteristics that may benefit from the study drug. The era of precision medicine has witnessed tumor-agnostic approvals, based on expression of a common biomarker rather than simply the tumor type (defined by primary site of origin) (45). In 2017, the FDA approved pembrolizumab for unresectable/metastatic solid tumors progressing after prior treatment in adult and pediatric patients based on microsatellite instability-high status or mismatch repair deficiency (dMMR), irrespective of tumor type (46). In 2020, pembrolizumab received approval to treat adult and pediatric patients with unresectable/metastatic tumor mutational burden-high solid tumors (excluding central nervous system cancers) and progression after prior therapy (46). Similarly, larotrectinib and entrectinib, 2 selective inhibitors of tropomyosin receptor kinases, received approval for treating unresectable/metastatic solid tumors with a neurotrophic tyrosine receptor kinase gene fusion in adult and pediatric patients, regardless of tumor origin (47, 48). Additional recent tumor-agnostic approvals include dabrafenib plus trametinib (based on BRAF V600E or V600K mutations) and dostarlimab (based on dMMR) (49–51).
Unfortunately, high-grade serous ovarian cancers are not commonly associated with specific driver mutations, but rather by chromosomal instability and copy number alterations (52). Considering histology in this cancer type, while recognizing that some patients (eg, those with high FRα expression) may benefit from targeted treatment, is necessary (45). Tumor-agnostic indications may be most appropriate for drugs with high response rates and for rare tumors (45). As our understanding of ovarian cancer evolves, we must include biomarkers, companion diagnostics, and molecular profiling in clinical trial designs.
Further, umbrella and basket protocol designs can compare different novel regimens or incorporate different patient populations within 1 trial. Notably, in the investigator-initiated, phase 2 AMBITION trial in PROC, patients with HRD test positive tumors were randomized to combination olaparib/cediranib or olaparib/durvalumab, while those with HRD test negative tumors were randomized by PD-L1 expression to durvalumab/chemotherapy, durvalumab/tremelimumab at 75 mg/chemotherapy, or durvalumab/tremelimumab at 300 mg/chemotherapy (53). Additionally, the open-label, phase 2 BOUQUET trial is an ongoing biomarker-driven trial evaluating multiple biomarker-based therapies in patients with persistent or recurrent rare epithelial ovarian tumors, including but not limited to low-grade serous ovarian carcinoma, clear cell carcinoma, mucinous carcinoma, and carcinosarcoma (54). Data from this trial may inform biomarker-driven patient stratification and treatment selection.
Finally, clinical trials in the PROC setting may have limited generalizability to real-world patient populations due to eligibility criteria that restrict enrollment to moderately pretreated individuals with potentially fewer comorbidities. Additionally, some trials lack an appropriately diverse patient population, limiting our ability to understand clinical benefit across certain racial and ethnic groups. There are active initiatives to address these shortcomings by both ensuring diversity in enrollment while simultaneously mandating more practical inclusion and exclusion criteria in PROC trials.
5 Accelerated approvals, benefits, and pitfalls
The FDA’s accelerated approval program (and analogous non-US programs) can expedite approvals of drugs that address unmet needs for serious or life-threatening conditions (55). Accelerated approval can be based on a surrogate or intermediate clinical endpoint “reasonably likely to predict clinical benefit,” providing earlier evaluation of clinical benefit based on well-controlled phase 2 studies (55). Although OS is the most objective benchmark for demonstrating clinical benefit in oncology clinical trials, it requires a larger sample size and longer follow-up compared with more rapidly assessed endpoints (eg, time to tumor progression and PFS) (56). Since ORR can be assessed using a single-arm trial, it is the most common surrogate endpoint for accelerated approvals (57). Duration of response (DOR) is also occasionally used to support ORR (57). The gold standard for endpoints in PROC randomized trials remains OS, but when OS is confounded by long post-progression survival (>18 months) and crossover (common in trial participants), PFS is the preferred endpoint. Open-label studies where PFS is a key endpoint should utilize placebo controls and blinded independent central review to objectify clinical activity.
Several treatments for ovarian cancer have benefitted from accelerated approval (58). PLD’s accelerated approval occurred in 1999 based on 3 phase 2 studies, with confirmation from a randomized phase 3 trial (58, 59). However, some accelerated approvals in oncology do not demonstrate clinical benefit in confirmatory studies (57). Notably, bevacizumab received accelerated approval for metastatic breast cancer in 2008 based on improved PFS in an open-label, phase 3 clinical trial (60, 61). Subsequent placebo-controlled, double-blind confirmatory trials failed to confirm that the magnitude of the effect on PFS constituted a clinical benefit and showed more TRAEs compared to chemotherapy, which led to a revoked approval for this indication (62).
In ovarian cancer, the voluntary withdrawals of rucaparib, olaparib, and niraparib for recurrent, late-line treatment demonstrate the vulnerabilities of accelerated approval (63–65). These withdrawals stemmed from non-hypothesis-tested subset analyses, which suggested a detrimental effect on OS with PARPi exposure when compared to chemotherapy.
Although post-approval studies typically take years to complete (median [range], 3.4 years [0.5–12.6]), as of November 2022, only 21 indications (for 16 different drugs) with accelerated approvals have been withdrawn, while 88 were verified in confirmatory studies (57, 66, 67). While accelerated approvals usually endure, choosing appropriate surrogate endpoints and designing proper single-arm trials that can predict meaningful treatment effects remain challenging.
6 Moving away from “platinum-resistant ovarian cancer”
Over the past decade, the definition of “platinum resistant” has shifted from utilizing only the historical definition of disease recurrence <6 months after the completion of last platinum-based chemotherapy (2, 68). In clinical trials and practice, PFI-based classification has been accepted for predicting chemotherapy response, disease prognosis, and patient selection and stratification (68). However, platinum response is neither binary, nor accurately represented by an arbitrary cutoff based solely on the time of diagnosis of recurrence (68, 69). Further, evidence suggests that PFI may not be optimal for predicting clinical response in all cases. Previous trials such as AURELIA have shown clinical benefit independent of PFI (7). Thus, a PFI of <6 months may not equate with resistance to platinum agents. A recent meta-analysis reported a 36% response rate to platinum-based regimens vs a 16% response rate to non-platinum-based regimens in a PROC population (70). Therefore, platinum rechallenge remains a viable option for some historically defined patients with PROC. Although the term “PROC” has evolved in clinical practice, it is still useful for regulatory approval due to previously discussed uncertainties, where platinum retreatment may not be the best option.
Several factors can affect time to relapse and platinum sensitivity. Variable timing and application of tools to detect recurrence (eg, cancer antigen-125, computed tomography scan, positron emission tomography scan) can skew the time to relapse and impact historical “platinum sensitive” vs “platinum resistant” designations (68, 69). Further, the tumor’s molecular profile can influence response. Patients with BRCA1/2-mutated recurrent ovarian cancer may respond to platinum-based and other chemotherapy agents that induce direct DNA damage (69, 71, 72). In ovarian cancer, the type, number, and outcome of prior therapies are important considerations when assessing potential response to further treatments (68). However, it is unclear what impact maintenance therapy with targeted and biological agents may have on subsequent treatment response (68). Other influential factors include histological subtype, prior surgical interventions, symptoms at recurrence, and the molecular profile beyond BRCA (eg, HRD status) (68, 69).
These factors have been incorporated in a new proposed classification system for patients with recurrent ovarian cancer and in the Gynecologic Cancer InterGroup (GCIG) consensus recommendations for recurrent ovarian cancer clinical trials (68, 69). The GCIG recommends TFI replaces PFI, with specific reporting of the TFI from last platinum dose (TFIp) and the specific method used to diagnose recurrence (68). Additionally, the GCIG recommends recording the TFI from last non-platinum therapy (TFInp) and last biological agent (TFIb), where applicable (68). The GCIG also notes that biomarkers will likely play a more predictive role than TFIp regarding treatment response (68).
Historically, developing new PROC regimens without reliable and established biomarkers for treatment response has been challenging. The recent validation of FRα as a predictive biomarker for the ADC mirvetuximab soravtansine heralds a new strategy for drug development where tumor surface antigens may be identified, quantified, and targeted. For trials targeting immune mechanisms, biomarkers such as tumor-infiltrating lymphocytes and tumor mutational burden (a tumor’s total number of somatic coding mutations) may guide which patients may respond to immunotherapy (3); however, these biomarkers have not been validated.
Including biomarkers in future clinical trials will help establish their relevance in ovarian cancer and their relationship to efficacy endpoints, including DOR. This will help identify measures to inform patient response beyond the 6-month PFI cutoff.
7 Future perspectives
Developing effective therapies for patients with PROC is complex and necessitates consideration of tumor and patient heterogeneity. Ongoing and recently completed clinical trials feature novel agents, which utilize combination treatments and targeted, biomarker-based therapies (Table 4).
Since ADCs have succeeded in treating other cancers, the use of this novel approach of targeting cytotoxic payloads to tumor cells is also being evaluated in PROC (73).
To date, the only ADC that has been evaluated for PROC in a pivotal trial is mirvetuximab soravtansine, an ADC that targets FRα, which is minimally expressed on normal tissues but overexpressed in >80% of epithelial ovarian tumors (73–75). In the open-label, phase 3 FORWARD I trial, mirvetuximab soravtansine did not meet its primary endpoint of PFS, but both ORR and OS favored mirvetuximab soravtansine over chemotherapy (ORR, 24% vs 10% [P=.014]; OS, 17.3 vs 12.0 months [HR, 0.71; 95% CI, 0.49–1.02; [P=.063]) in a subgroup of patients with high FRα expression, although these endpoints were not statistically significant (17). Importantly, mirvetuximab soravtansine displayed improved tolerability compared to chemotherapy; grade ≥3 TRAEs (25.1% vs 44.0%), dose reductions (19.8% vs 30.3%), and discontinuations (4.5% vs 8.3%) occurred more frequently in the chemotherapy group (17). In the pivotal, single-arm SORAYA trial of patients with FRα-high PROC, mirvetuximab soravtansine demonstrated an ORR of 32.4% (median DOR [mDOR], 6.9 months), with clinical benefit maintained across prespecified subgroups, including patients with 3 prior therapy lines (ORR, 30.2%; mDOR, 7.4 months) and with prior PARPi exposure (ORR, 38.0%; mDOR, 5.7 months) (76). The confirmatory, randomized, phase 3 MIRASOL trial evaluated the efficacy and safety profile of single-agent mirvetuximab soravtansine vs single-agent chemotherapy (77). Compared with chemotherapy, mirvetuximab soravtansine showed statistically significant improvements in investigator-assessed PFS (mPFS, 5.6 vs 4.0 months [HR, 0.65; 95% CI, 0.52–0.81; [P<.0001]), investigator-assessed ORR (42.3% vs 15.9% [odds ratio, 3.81; 95% CI, 2.44–5.94; [P<.0001]), and OS (mOS, 16.5 vs 12.8 months [HR, 0.67; 95% CI, 0.50–0.89; [P=.0046]) (18, 19). No new safety signals occurred with mirvetuximab soravtansine and improved tolerability with mirvetuximab soravtansine vs chemotherapy was demonstrated by comparatively fewer grade ≥3 treatment-emergent adverse events ([TEAEs] 42% vs 54%), serious adverse events (24% vs 33%), and TEAEs leading to discontinuation (9% vs 16%) (18, 19).
Additional ADCs targeting other proteins are being evaluated in earlier-stage clinical trials in PROC, including mesothelin (anetumab ravtansine), sodium-dependent phosphate transporter (NaPi2b; upifitamab rilsodotin), dipeptidase 3/DPEP3 (tamrintamab pamozirine), mucin 16/MUC16 (sofituzumab vedotin), tissue factor (tisotumab vedotin, recently approved for recurrent/metastatic cervical cancer), and Trop-2 (SKB264, datopotamab deruxtecan, sacituzumab govitecan) (73, 78–80).
Given the heterogeneity of PROC, combination therapies may improve clinical outcomes. Chemotherapy is being explored in combination with antiangiogenics, immune checkpoint inhibitors, PARPi, agents targeting DNA damage repair, agents targeting AXL and AKT/ERK, and a glucocorticoid receptor modulator (Table 4). Non-chemotherapeutic immunotherapy regimens being investigated include existing and novel immune checkpoint inhibitors combined with antiangiogenics, an engineered IL-2, an anti-TIGIT monoclonal antibody, and a p53 vaccine. PARPi are being studied in non-chemotherapeutic combinations with antiangiogenics and inhibitors of PI3K and WEE1. Triplet combinations utilizing various agents, including PARPi, anti-PD-1/PD-L1, and antiangiogenics, are underway. Other ongoing and recently completed phase 2 and 3 trials are summarized in Table 4.
These novel PROC treatments may improve response rates and prolong PFS for patients with few options. However, improving the treatment paradigm will require overcoming several obstacles. Disease heterogeneity is a major challenge that may have influenced prior trial failure (41). Identifying a well-defined homogeneous population will be critical to ensure impactful trial outcomes. Increased testing for biomarkers such as tumor mutational burden and dMMR, particularly in mucinous, clear cell, and endometrioid histologies, is encouraged, as is participation in appropriate clinical trials (81). Another challenge in PROC is the frailty of patients—due to age, comorbidities, and/or toxicity from multiple rounds of prior therapy—who are frequently recruited for trials and often have rapidly progressive disease (68). In 1 study, 30%–50% of patients discontinued due to progressive disease before receiving sufficient doses of therapy, and therefore had no chance of clinical benefit (15). Focusing on more specific populations may improve outcomes by excluding patients unlikely to be positively impacted.
8 Conclusion
Addressing the unmet need for effective therapies in heavily pretreated PROC is an ongoing challenge. As research elucidates the molecular mechanisms of ovarian cancer progression, new insights will provide guidance on developing novel targeted therapies.
Author contributions
RE: Writing – review and editing, conceptualization. KM: Writing – review and editing, conceptualization. BM: Writing – review and editing, conceptualization. TH: Writing – review and editing, conceptualization. CA: Writing – review and editing, conceptualization. DO: Writing – review and editing, conceptualization. RC: Writing – review and editing, conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This narrative review article was funded by ImmunoGen, Inc., who also contributed to the conception of the work.
Acknowledgments
Medical writing and editorial support were provided by PRECISIONscientia, of Yardley, PA, USA and funded by ImmunoGen, Inc.
Conflict of interest
RE reports receiving institution research support from AstraZeneca, Clovis Oncology, Eisai, Merck, and Novocure; and consulting and advisory fees from AstraZeneca; Cardiff Oncology; Clovis Oncology; Eisai; Elevar; Genentech/Roche; GSK/Tesaro; ImmunoGen, Inc; Merck; Mersana; and Myriad. KM reports advisory board participation for Addi; Alkermes; Aravive, Inc; AstraZeneca; Blueprint Pharma; Caris; Celsion Corporation; Clovis; Eisai Co, Ltd; Eli Lilly and Company; EMD Serono; Genentech, Inc/Roche; GlaxoSmithKline plc/Tesaro; I-MAB Biopharma Co, Ltd; ImmunoGen, Inc; InxMed Co, Ltd; Jiangsu Hengrui Pharmaceuticals Company Ltd; Merck & Co, Inc; Mereo Biopharma Group plc; Mersana Therapeutics, Inc; Myriad Genetics, Inc; Novartis Pharmaceuticals Corporation; Novocure GmbH; Onconova Therapeutics, Inc; OncXerna Therapeutics, Inc; Tarveda Therapeutics; VBL Therapeutics; and Verastem, Inc; research funding from Eli Lilly and Company; GlaxoSmithKline plc/Tesaro; Merck & Co, Inc; PTC Therapeutics; and Verastem, Inc; and employment by GOG Partners, NRG Ovarian Cancer Committee Chair, and employment by The GOG Foundation Inc uncompensated. BM reports receiving speaker and/or consultant fees from Acrivon; Adaptimmune; Amgen; Aravive; AstraZeneca; Bayer; Clovis; Eisai; Elevar; Genmab/Seagen; the GOG Foundation; Gradalis; Heng Rui; ImmunoGen, Inc; Laekna; MacroGenics; Merck; Mersana; Myriad; Novartis; Novocure; OncoC4; Panavance; Pieris; Pfizer; Roche/Genentech; Sorrento; TESARO/GSK; VBL; Verastem; and Zentalis; and consultant and/or investigator fees from US Oncology Research. TH reports advisory board participation for AstraZeneca; Caris; Clovis; Eisai Co, Ltd; Epsilogen Ltd; Genentech, Inc; GlaxoSmithKline plc; Johnson & Johnson; Merck & Co, Inc; Mersana Therapeutics, Inc; and Seagen Inc. CA reports nothing to disclose. DO reports receiving institutional funds from AbbVie; Advaxis; Agenus, Inc; Alkermes; Aravive, Inc; Arcus Biosciences, Inc; AstraZeneca; BeiGene USA, Inc; Boston Biomedical; Bristol Myers Squibb; Clovis Oncology; Deciphera Pharma; Eisai; EMD Serono, Inc; Exelixis; Genentech, Inc; Genmab; GlaxoSmithKline; GOG Foundation; Hoffmann-La Roche, Inc; ImmunoGen, Inc; Incyte Corporation; IOVANCE Biotherapeutics; Karyopharm; Leap Therapeutics, Inc; Ludwig Institute for Ca; Merck & Co, Inc; Merck Sharp & Dohme Corp; Mersana Therapeutics, Inc; NCI; Novartis; Novocure; NRG Oncology; OncoC4, Inc; OncoQuest, Inc; Pfizer, Inc; Precision Therapeutics, Inc; Prelude Therapeutics; Regeneron Pharmaceuticals, Inc; RTOG; Rubius Therapeutics; Seattle Genetics Seagen; Sutro Biopharma; SWOG; TESARO; and Verastem, Inc; and consultant and/or advisory board personal fees from AbbVie; Adaptimmune; Agenus, Inc; Arquer Diagnostics; Arcus Biosciences, Inc; AstraZeneca; Atossa Therapeutics; Boston Biomedical; Cardiff Oncology; Celcuity; Clovis Oncology; Corcept Therapeutics; Duality Bio; Eisai; Elevar; Exelixis; Genentech, Inc; Genelux; GlaxoSmithKline; GOG Foundation; Hoffmann-La Roche, Inc; ImmunoGen, Inc; Imvax; InterVenn; INXMED; IOVANCE Biotherapeutics; Janssen; Jazz Pharmaceuticals; Laekna; Leap Therapeutics, Inc; Luzsana Biotechnology; Merck & Co, Inc; Merck Sharp & Dohme Corp; Mersana Therapeutics, Inc; Myriad; Novartis; Novocure; OncoC4, Inc; Onconova; Regeneron Pharmaceuticals, Inc; Replimmune; R-PHARM US, LLC; Roche Diagnostics; Seattle Genetics Seagen; Sorrento; Sutro Biopharma; Tarveda Therapeutics; Toray; Trillium; Umoja; Verastem, Inc; VBL Therapeutics; Vincerx Pharma; Xencor; and Zentalis. RC reports research funding from AbbVie Inc; AstraZeneca; Clovis; Genmab A/S; ImmunoGen, Inc; Merck & Co, Inc; Mersana Therapeutics, Inc; Roche/Genentech, Inc; and Toray; and scientific steering committee participation with AbbVie Inc; Agenus Inc; Aravive, Inc; AstraZeneca; Clovis; Deciphera Pharmaceuticals, Inc; Epsilogen Ltd; Genentech, Inc; Genmab A/S/Seagen Inc; GlaxoSmithKline plc; ImmunoGen, Inc; Karyopharm Therapeutics Inc; Merck & Co, Inc; Mirati Therapeutics, Inc; Novocure GmbH; OncXerna Therapeutics, Inc; and Toray.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chien J, Kuang R, Landen C, Shridhar V. Platinum-sensitive recurrence in ovarian cancer: the role of tumor microenvironment. Front Oncol (2013) 3:251. doi: 10.3389/fonc.2013.00251
2. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. Version 2. Plymouth Meeting, PA: National Comprehensive Cancer Network (2022), July 13, 2022.
3. Indini A, Nigro O, Lengyel CG, Ghidini M, Petrillo A, Lopez S, et al. Immune-checkpoint inhibitors in platinum-resistant ovarian cancer. Cancers (Basel) (2021) 13(7):1663. doi: 10.3390/cancers13071663
4. Mutch DG, Orlando M, Goss T, Teneriello MG, Gordon AN, McMeekin SD, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol (2007) 25(19):2811–8. doi: 10.1200/JCO.2006.09.6735
5. ten Bokkel Huinink W, Gore M, Carmichael J, Gordon A, Malfetano J, Hudson I, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol (1997) 15(6):2183–93. doi: 10.1200/JCO.1997.15.6.2183
6. Gaillard S, Oaknin A, Ray-Coquard I, Vergote I, Scambia G, Colombo N, et al. Lurbinectedin versus pegylated liposomal doxorubicin or topotecan in patients with platinum-resistant ovarian cancer: A multicenter, randomized, controlled, open-label phase 3 study (CORAIL). Gynecol Oncol (2021) 163(2):237–45. doi: 10.1016/j.ygyno.2021.08.032
7. Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol (2014) 32(13):1302–8. doi: 10.1200/JCO.2013.51.4489
8. Poveda AM, Selle F, Hilpert F, Reuss A, Savarese A, Vergote I, et al. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: analysis by chemotherapy cohort of the randomized phase III AURELIA trial. J Clin Oncol (2015) 33(32):3836–8. doi: 10.1200/JCO.2015.63.1408
9. Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol (2019) 20(5):636–48. doi: 10.1016/S1470-2045(19)30029-4
12. Nero C, Ciccarone F, Pietragalla A, Duranti S, Daniele G, Salutari V, et al. Ovarian cancer treatments strategy: focus on PARP inhibitors and immune check point inhibitors. Cancers (Basel) (2021) 13(6):1298. doi: 10.3390/cancers13061298
13. Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol (2007) 25(33):5165–71. doi: 10.1200/JCO.2007.11.5345
14. Shoji T, Enomoto T, Abe M, Okamoto A, Nagasawa T, Oishi T, et al. Efficacy and safety of standard of care with/without bevacizumab for platinum-resistant ovarian/fallopian tube/peritoneal cancer previously treated with bevacizumab: The Japanese Gynecologic Oncology Group study JGOG3023. Cancer Sci (2022) 113(1):240–50. doi: 10.1111/cas.15185
15. Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol (2021) 22(7):1034–46. doi: 10.1016/S1470-2045(21)00216-3
16. Hamanishi J, Takeshima N, Katsumata N, Ushijima K, Kimura T, Takeuchi S, et al. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: open-label, randomized trial in Japan (NINJA). J Clin Oncol (2021) 39(33):3671–81. doi: 10.1200/JCO.21.00334
17. Moore KN, Oza AM, Colombo N, Oaknin A, Scambia G, Lorusso D, et al. randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann Oncol (2021) 32(6):757–65. doi: 10.1016/j.annonc.2021.02.017
18. Moore KN, Angelergues A, Konecny GE, Banerjee SN, Pignata S, Colombo N, et al. Phase III MIRASOL (GOG 3045/ENGOT-ov55) study: Initial report of mirvetuximab soravtansine vs. investigator's choice of chemotherapy in platinum-resistant, advanced high-grade epithelial ovarian, primary peritoneal, or fallopian tube cancers with high folate receptor-alpha expression. J Clin Oncol (2023) 41(17_suppl):LBA5507–LBA. doi: 10.1200/JCO.2023.41.17_suppl.LBA5507
19. Moore KN, Angelergues A, Konecny G, Banerjee S, Pignata S, Colombo N, et al., in: Presented at: 2023 American Society of Clinical Oncology Annual Meeting, Chicago, IL. LBA 5507, June 2-6, 2023.
21. Monk BJ. Current Landscape in Ovarian Cancer: PARP Inhibitors and Targeted Therapy. CCO. Updated December 3, 2020. Available at: https://www.clinicaloptions.com/oncology/programs/parp-inhibitors-in-ovarian-cancer/module/text-module/page-1 (Accessed January 12, 2023).
22. Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol (2019) 30(7):1080–7. doi: 10.1093/annonc/mdz135
23. Domchek SM, Aghajanian C, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol (2016) 140(2):199–203. doi: 10.1016/j.ygyno.2015.12.020
24. Oza AM, Tinker AV, Oaknin A, Shapira-Frommer R, McNeish IA, Swisher EM, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol (2017) 147(2):267–75. doi: 10.1016/j.ygyno.2017.08.022
25. Kristeleit R, Lisyanskaya A, Fedenko A, Dvorkin M, de Melo AC, Shparyk Y, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial. Lancet Oncol (2022) 23(4):465–78. doi: 10.1016/S1470-2045(22)00122-X
26. Clovis Oncology Inc. Rubraca Direct Healthcare Provider Letter. Rubraca® (Rucaparib) for treatment of BRCA-mutated ovarian cancer after 2 or more chemotherapies is voluntarily withdrawn in the US. Boulder, CO: Clovis Oncology, Inc (2022).
27. Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol (2015) 33(34):4015–22. doi: 10.1200/JCO.2015.62.3397
28. Oza AM, Selle F, Davidenko I, Korach J, Mendiola C, Pautier P, et al. Efficacy and safety of bevacizumab-containing therapy in newly diagnosed ovarian cancer: ROSiA single-arm phase 3B study. Int J Gynecol Cancer (2017) 27(1):50–8. doi: 10.1097/IGC.0000000000000836
29. McGuire WP, Penson RT, Gore M, Herraez AC, Peterson P, Shahir A, et al. Randomized phase II study of the PDGFRα antibody olaratumab plus liposomal doxorubicin versus liposomal doxorubicin alone in patients with platinum-refractory or platinum-resistant advanced ovarian cancer. BMC Cancer (2018) 18(1):1292. doi: 10.1186/s12885-018-5198-4
30. Chekerov R, Hilpert F, Mahner S, El-Balat A, Harter P, De Gregorio N, et al. Sorafenib plus topotecan versus placebo plus topotecan for platinum-resistant ovarian cancer (TRIAS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol (2018) 19(9):1247–58. doi: 10.1016/S1470-2045(18)30372-3
31. Lan CY, Wang Y, Xiong Y, Li JD, Shen JX, Li YF, et al. Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study. Lancet Oncol (2018) 19(9):1239–46. doi: 10.1016/S1470-2045(18)30349-8
32. Monk BJ, Kauderer JT, Moxley KM, Bonebrake AJ, Dewdney SB, Secord AA, et al. A phase II evaluation of elesclomol sodium and weekly paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube or primary peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol (2018) 151(3):422–7. doi: 10.1016/j.ygyno.2018.10.001
33. Leijen S, van Geel RM, Sonke GS, de Jong D, Rosenberg EH, Marchetti S, et al. Phase II study of WEE1 inhibitor AZD1775 plus carboplatin in patients with TP53-mutated ovarian cancer refractory or resistant to first-line therapy within 3 months. J Clin Oncol (2016) 34(36):4354–61. doi: 10.1200/JCO.2016.67.5942
34. Lheureux S, Cristea MC, Bruce JP, Garg S, Cabanero M, Mantia-Smaldone G, et al. Adavosertib plus gemcitabine for platinum-resistant or platinum-refractory recurrent ovarian cancer: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet (2021) 397(10271):281–92. doi: 10.1016/S0140-6736(20)32554-X
35. Konstantinopoulos PA, Cheng SC, Wahner Hendrickson AE, Penson RT, Schumer ST, Doyle LA, et al. Berzosertib plus gemcitabine versus gemcitabine alone in platinum-resistant high-grade serous ovarian cancer: a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol (2020) 21(7):957–68. doi: 10.1016/S1470-2045(20)30180-7
36. Zamarin D, Burger RA, Sill MW, Powell DJ Jr., Lankes HA, Feldman MD, et al. Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: an NRG oncology study. J Clin Oncol (2020) 38(16):1814–23. doi: 10.1200/JCO.19.02059
37. Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol (2019) 5(8):1141–9. doi: 10.1001/jamaoncol.2019.1048
38. Lee JM, Moore RG, Ghamande S, Park MS, Diaz JP, Chapman J, et al. CedIranib in combination with olaparib in patients without a germline BRCA1/2 mutation and with recurrent platinum-resistant ovarian cancer: Phase IIb CONCERTO trial. Clin Cancer Res (2022) 28(19):4186–93. doi: 10.1158/1078-0432.CCR-21-1733
39. Colombo N, Tomao F, Benedetti Panici P, Nicoletto MO, Tognon G, Bologna A, et al. Randomized phase II trial of weekly paclitaxel vs. cedIranib-olaparib (continuous or intermittent schedule) in platinum-resistant high-grade epithelial ovarian cancer. Gynecol Oncol (2022) 164(3):505–13. doi: 10.1016/j.ygyno.2022.01.015
40. Gilbert L, Oaknin A, Matulonis UA, Mantia-Smaldone GM, Lim PC, Castro CM, et al. Safety and efficacy of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol Oncol (2023) 170:241–7. doi: 10.1016/j.ygyno.2023.01.020
41. Le Saux O, Ray-Coquard I, Labidi-Galy SI. Challenges for immunotherapy for the treatment of platinum resistant ovarian cancer. Semin Cancer Biol (2021) 77:127–43. doi: 10.1016/j.semcancer.2020.08.017
42. Liu J, Gaillard S, Wahner Hendrickson A, Moroney J, Yeku O, Diver E, et al. , in: Presented at: 2021 SGO Annual Meeting on Women's Cancer, March 19-25, 2022.
43. Randall LM, O'Malley DM, Monk BJ, Coleman RL, Gaillard S, Adams SF, et al. MOONSTONE/GOG-3032: Interim analysis of a phase 2 study of niraparib + dostarlimab in patients (pts) with platinum-resistant ovarian cancer (PROC). J Clin Oncol (2022) 40(16_suppl:):5573–3. doi: 10.1200/JCO.2022.40.16_suppl.5573
44. Shah PD, Wethington SL, Pagan C, Latif N, Tanyi J, Martin LP, et al. Combination ATR and PARP Inhibitor (CAPRI): A phase 2 study of ceralasertib plus olaparib in patients with recurrent, platinum-resistant epithelial ovarian cancer. Gynecol Oncol (2021) 163(2):246–53. doi: 10.1016/j.ygyno.2021.08.024
45. Yan L, Zhang W. Precision medicine becomes reality-tumor type-agnostic therapy. Cancer Commun (Lond) (2018) 38(1):6. doi: 10.1186/s40880-018-0274-3
52. Kuo KT, Guan B, Feng Y, Mao TL, Chen X, Jinawath N, et al. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Res (2009) 69(9):4036–42. doi: 10.1158/0008-5472.CAN-08-3913
53. Lee JY, Kim BG, Kim JW, Lee JB, Park E, Joung JG, et al. Biomarker-guided targeted therapy in platinum-resistant ovarian cancer (AMBITION; KGOG 3045): a multicentre, open-label, five-arm, uncontrolled, umbrella trial. J Gynecol Oncol (2022) 33(4):e45. doi: 10.1016/S0090-8258(22)00345-6
54. Clinical Trials Identifier: NCT04931342. A study evaluating the efficacy and safety of biomarker-driven therapies in patients with persistent or recurrent rare epithelial ovarian tumors (BOUQUET) (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT04931342 (Accessed September 19, 2022).
55. US Department of Health and Human Services. Food and Drug Administration. Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics. Washington, DC: US Dept of Health and Human Services (2014).
56. Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist (2008) 13 Suppl 2:19–21. doi: 10.1634/theoncologist.13-S2-19
57. Beaver JA, Howie LJ, Pelosof L, Kim T, Liu J, Goldberg KB, et al. A 25-year experience of US food and drug administration accelerated approval of Malignant hematology and oncology drugs and biologics: A review. JAMA Oncol (2018) 4(6):849–56. doi: 10.1001/jamaoncol.2017.5618
58. Herzog TJ, Monk BJ. Bringing new medicines to women with epithelial ovarian cancer: what is the unmet medical need? Gynecol Oncol Res Pract (2017) 4:13. doi: 10.1186/s40661-017-0050-0
60. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med (2007) 357(26):2666–76. doi: 10.1056/NEJMoa072113
61. Vitry A, Nguyen T, Entwistle V, Roughead E. Regulatory withdrawal of medicines marketed with uncertain benefits: the bevacizumab case study. J Pharm Policy Pract (2015) 8:25. doi: 10.1186/s40545-015-0046-2
62. US Department of Health and Human Services. , in: Food and Drug Administration. Proposal to Withdraw Approval for the Breast Cancer Indication for AVASTIN (Bevacizumab). Decision of the Commissioners. Docket No. FDA-2010-N-0621, November 18, 2011.
66. US Food and Drug Administration. Verified Clinical Benefit. Cancer Accelerated Approvals. Updated October 11, 2022. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/verified-clinical-benefit-cancer-accelerated-approvals (Accessed October 11, 2022).
67. US Food and Drug Administration. Withdrawn. Cancer Accelerated Approvals. Updated October 11, 2022. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/withdrawn-cancer-accelerated-approvals (Accessed October 11, 2022).
68. Wilson MK, Pujade-Lauraine E, Aoki D, Mirza MR, Lorusso D, Oza AM, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recurrent disease. Ann Oncol (2017) 28(4):727–32. doi: 10.1093/annonc/mdw663
69. Alvarez RD, Matulonis UA, Herzog TJ, Coleman RL, Monk BJ, Markman M. Moving beyond the platinum sensitive/resistant paradigm for patients with recurrent ovarian cancer. Gynecol Oncol (2016) 141(3):405–9. doi: 10.1016/j.ygyno.2016.03.005
70. Rumyantsev A, Tyulyandina A, Fedyanin M, Pokataev I, Glazkova E, Israelyan E, et al. Platinum vs non-platinum chemotherapy for platinum-resistant ovarian cancer: A meta-analysis. J Clin Oncol (2022) 40(16 suppl):e17551. doi: 10.1200/JCO.2022.40.16_suppl.e17551
71. Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol (2012) 30(21):2654–63. doi: 10.1200/JCO.2011.39.8545
72. Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med (2018) 379(26):2495–505. doi: 10.1056/NEJMoa1810858
73. El Bairi K, Al Jarroudi O, Afqir S. Revisiting antibody-drug conjugates and their predictive biomarkers in platinum-resistant ovarian cancer. Semin Cancer Biol (2021) 77:42–55. doi: 10.1016/j.semcancer.2021.03.031
74. Markert S, Lassmann S, Gabriel B, Klar M, Werner M, Gitsch G, et al. Alpha-folate receptor expression in epithelial ovarian carcinoma and non-neoplastic ovarian tissue. Anticancer Res (2008) 28(6A):3567–72.
75. Moore KN, Martin LP, O'Malley DM, Matulonis UA, Konner JA, Perez RP, et al. Safety and activity of mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: A phase I expansion study. J Clin Oncol (2017) 35(10):1112–8. doi: 10.1200/JCO.2016.69.9538
76. Matulonis U, Lorusso D, Oaknin A, Pignata S, Dean A, Denys H, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol (2023) 41(13):2436–45. doi: 10.1200/JCO.22.01900
77. Clinicaltrials.gov Identifier: NCT04209855. A Study of Mirvetuximab Soravtansine vs. Investigator's Choice of Chemotherapy in Platinum-Resistant, Advanced High-Grade Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancers With High Folate Receptor-Alpha Expression (MIRASOL). Updated June 16, 2022. Available at: https://clinicaltrials.gov/ct2/show/NCT04209855 (Accessed August 12, 2022).
78. ClinicalTrials.gov Identifier: NCT04152499. Phase I-II, FIH, TROP2 ADC, Advanced Unresectable/Metastatic Solid Tumors, Refractory to Standard Therapies (A264). Updated July 12, 2022. Available at: https://clinicaltrials.gov/ct2/show/NCT04152499 (Accessed November 9, 2022).
79. ClinicalTrials.gov Identifier: NCT05489211. Study of Dato-Dxd as Monotherapy and in Combination with Anti-cancer Agents in Patients With Advanced Solid Tumours. Updated September 19, 2022. Available at: https://clinicaltrials.gov/ct2/show/NCT05489211 (Accessed November 9, 2022).
80. ClinicalTrials.gov Identifier: NCT01631552. Study of Sacituzumab Govitecan-hziy (IMMU-132) in Adults with Epithelial Cancer. Updated August 12, 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT01631552 (Accessed November 9, 2022).
Keywords: bevacizumab, biomarker, folate receptor alpha, mirvetuximab soravtansine, platinum-resistant ovarian cancer, and targeted therapy
Citation: Eskander RN, Moore KN, Monk BJ, Herzog TJ, Annunziata CM, O’Malley DM and Coleman RL (2023) Overcoming the challenges of drug development in platinum-resistant ovarian cancer. Front. Oncol. 13:1258228. doi: 10.3389/fonc.2023.1258228
Received: 13 July 2023; Accepted: 11 August 2023;
Published: 17 October 2023.
Edited by:
Fabio Martinelli, National Cancer Institute Foundation (IRCCS), ItalyReviewed by:
Sarah Taylor, University of Pittsburgh, United StatesMaria Lee, Seoul National University Hospital, Republic of Korea
Copyright © 2023 Eskander, Moore, Monk, Herzog, Annunziata, O’Malley and Coleman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramez N. Eskander, cmVza2FuZGVyQGhlYWx0aC51Y3NkLmVkdQ==
 Ramez N. Eskander
Ramez N. Eskander Kathleen N. Moore
Kathleen N. Moore Bradley J. Monk3
Bradley J. Monk3 Thomas J. Herzog
Thomas J. Herzog Christina M. Annunziata
Christina M. Annunziata David M. O’Malley
David M. O’Malley