- 1Department of Infectious Diseases, The Third People’s Hospital of Longgang Shenzhen, Shenzhen, China
- 2Department of Breast Surgical Oncology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Clinical Medicine School of Zhengzhou University, Zhengzhou, China
Objective: Breast cancer is one of the most common causes of death among women. Statins, typically used for cholesterol management, have been hypothesized to reduce recurrence and mortality rates in breast cancer. However, this association remains a subject of debate. This study evaluates the potential impact of statins on breast cancer recurrence and mortality.
Methods: A comprehensive search was conducted in the PubMed, EMBASE, and Cochrane databases for articles published up to June 2023. These articles examined the effect of statins on breast cancer recurrence and mortality both before and after diagnosis. The analysis was performed using random-effects models, calculating pooled hazard ratios (HR) and their 95% confidence intervals (CI).
Results: A total of 31 cohort studies, involving 261,834 female breast cancer patients, were included in this analysis. It was found that statin use prior to diagnosis was associated with a decrease in overall mortality (HR, 0.8; 95% CI, 0.69–0.93; I2 = 77.6%; P = 0.001) and breast cancer-specific mortality (HR, 0.76; 95% CI, 0.67–0.87; I2 = 72.7%; P = 0.005). Additionally, statin use after diagnosis was observed to reduce the recurrence of breast cancer (HR, 0.71; 95% CI, 0.61–0.82; I2 = 60%; P = 0.003), overall mortality (HR, 0.81; 95% CI, 0.70–0.92; I2 = 80.7%; P < 0.001), and breast cancer-specific mortality (HR, 0.76; 95% CI, 0.67–0.86; I2 = 74.5%; P < 0.001).
Conclusions: The findings of this study indicate that statin usage, both before and after breast cancer diagnosis, may be associated with reduced risks of overall and breast cancer-specific mortality, as well as lower recurrence rates.
Introduction
Breast cancer remains a major health issue for women worldwide. It is a leading cause of cancer-related deaths (1, 2), representing a quarter of all female cancer fatalities. In America, it ranks second only to lung cancer in terms of mortality rates (3, 4). While developed countries have seen a decrease in breast cancer incidence and improved outcomes, the situation is worsening in developing nations (5) due to limited treatment options. This highlights the urgent need for new and effective treatments.
Statins, primarily prescribed for treating dyslipidemia to reduce the risk of atherosclerosis and cardiovascular events (6, 7), are now increasingly being explored for their potential anti-cancer properties, extending beyond their well-known cardiovascular benefits (8, 9). Initial studies suggest that statins may be effective in a variety of cancers (10), potentially by inhibiting cell proliferation and inducing apoptosis (11, 12). The efficacy of statins appears to depend on their lipophilicity, dosage, and duration of treatment (10). However, due to patient diversity, there are discrepancies between laboratory studies and clinical data. Some studies have indicated a correlation between statin use and reduced breast cancer mortality (13–15), while others have found no such association (16–18). This study, therefore, aims to clarify the relationship between statin use and the risk of mortality or recurrence in women with breast cancer.
Methods
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Search strategy and inclusion criteria
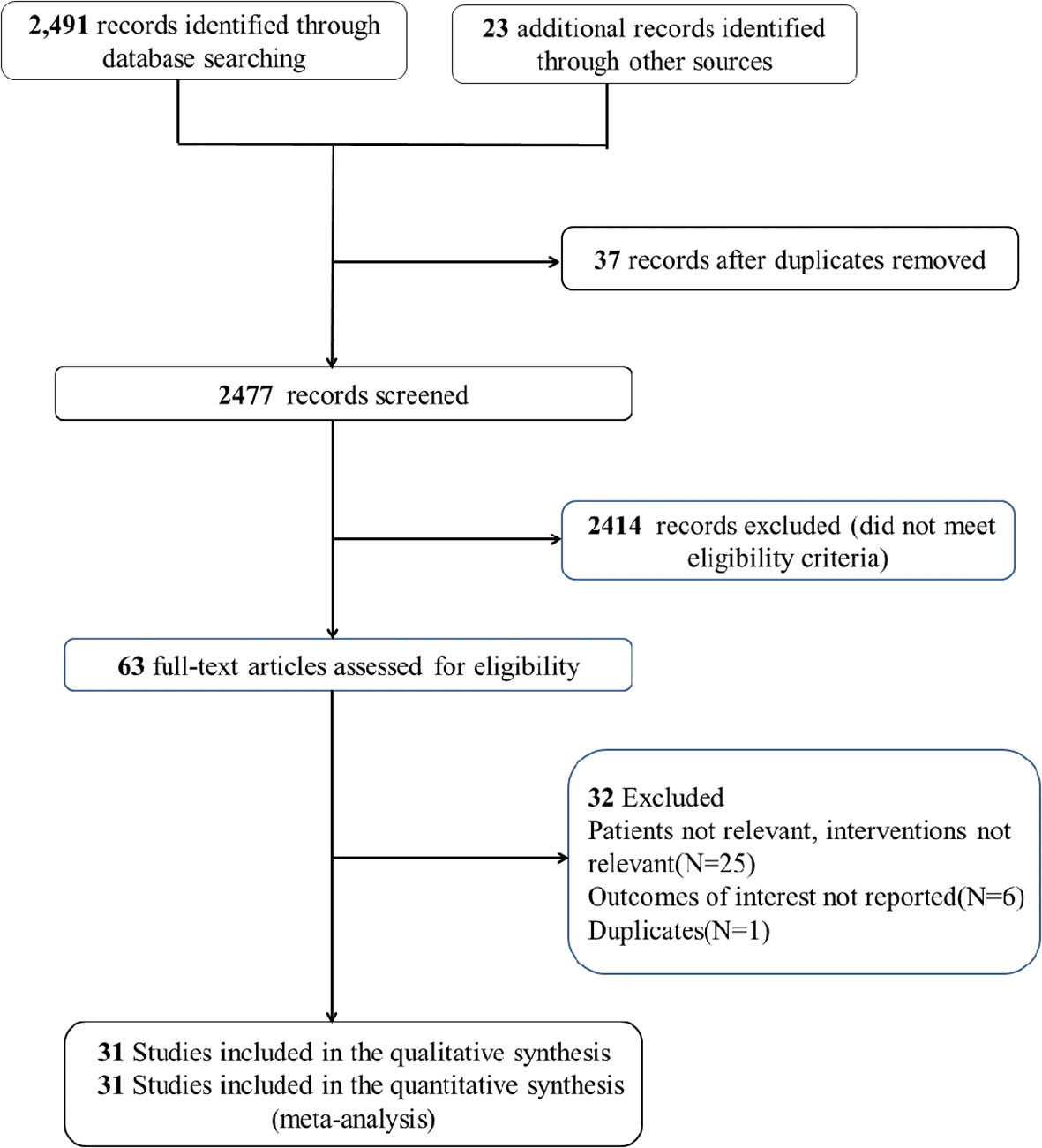
An extensive search was performed in the PubMed, EMBASE, and Cochrane CENTRAL databases for studies published up to 1 June, 2023. A combination of MeSH/Emtree terms and title/abstract keywords was used, focusing on “statins” and “breast cancer” (detailed in Stable 1). Included studies met these criteria: (1) involved adult patients diagnosed with breast cancer; (2) compared groups with and without statin use; (3) examined the relationship between statins and breast cancer mortality or recurrence; (4) provided detailed data on hazard ratios (HR) and 95% confidence intervals (CI) for outcomes such as recurrence, breast cancer-specific mortality, and all-cause mortality; and (5) were observational studies written in English. Exclusions were made for case reports, reviews, and preprints (search process illustrated in Figure 1).
Data extraction
Key information was extracted from selected studies, including the first author, publication year, study methodology, duration of follow-up, sample size, average age of participants, details of the statin intervention, and study outcomes.
Statistical analysis
The data for this meta-analysis were processed using STATA 12.0 software. To assess the impact of statins on breast cancer prognosis, HR and 95% CI from selected studies were extracted. Study variations were evaluated using the Q test and I2 index. A P-value less than 0.05 and I2 greater than 50% indicated significant heterogeneity, prompting the use of random-effects models for combined outcomes. Additionally, sensitivity analysis, Egger’s test, and funnel plots were employed to assess the robustness and potential publication bias of the findings.
Results
Study selection and characteristics
A total of 31 studies (13–43) were included in this meta-analysis, encompassing data from 261,834 female breast cancer patients. Among these, five studies (16, 23, 27, 28, 32) provided data on the impact of statin use prior to diagnosis on breast cancer-specific mortality, while another five (16, 23, 28, 32, 37) discussed its effects on all-cause mortality. Additionally, 13 studies (13, 18, 21, 25, 30, 34–36, 38, 40–43) reported on the influence of statin use after diagnosis on breast cancer recurrence. A further 12 articles (14, 16–18, 21, 22, 26, 28, 29, 31, 33, 39) and 19 studies (13–22, 24, 26–31, 36, 39) provided results regarding the effects of post-diagnosis statin use on overall mortality and breast cancer-induced mortality, respectively. The primary details from these studies are summarized in Table 1.
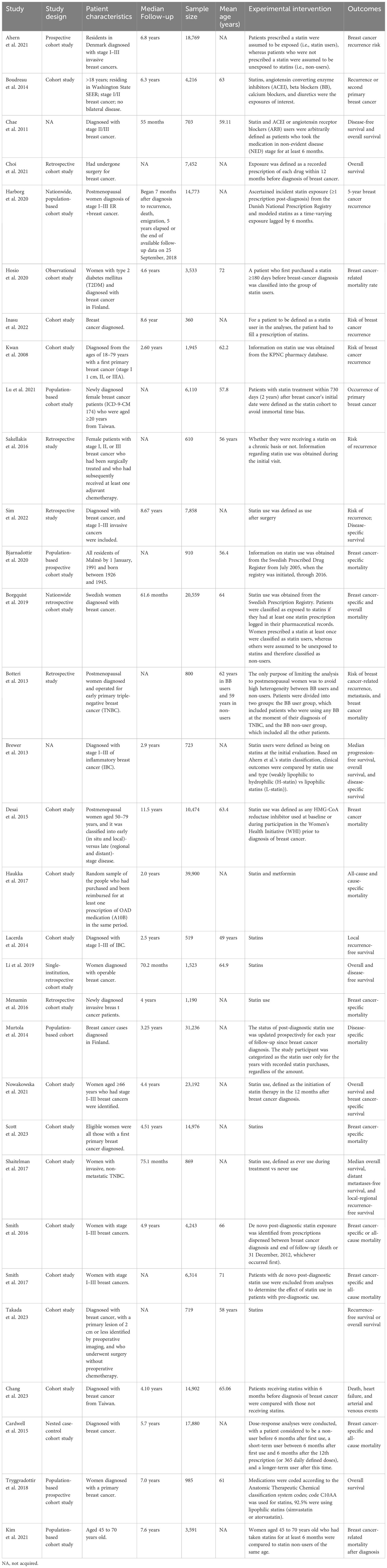
Table 1 Characteristics of the included studies in the meta-analysis of statin vs non-statin use in adult breast cancer patients.
Outcomes
The meta-analysis revealed that statin use, compared to non-use, was associated with a lower risk of overall mortality (HR, 0.8; 95% CI, 0.69–0.93; I2 = 77.6%; P = 0.001; Figure 2) and breast cancer-specific mortality prior to diagnosis (HR, 0.76; 95% CI, 0.67–0.87; I2 = 72.7%; P = 0.005; Figure 3). Post-diagnosis statin use also appeared to decrease breast cancer recurrence (HR, 0.71; 95% CI, 0.61–0.82; I2 = 60%; P < 0.003; Figure 4), overall mortality (HR, 0.81; 95% CI, 0.70–0.92; I2 = 80.7%; P < 0.001; Figure 5), and breast cancer-specific mortality (HR, 0.76; 95% CI, 0.67–0.86; I2 = 74.5%; P < 0.001; Figure 6). Sensitivity analysis indicated consistent results across studies (refer to Supplementary Figures 2, 5, 8, 11, 14). Egger’s tests and funnel plots suggested no significant publication bias (Egger’s tests: Supplementary Figure 3, P = 0.821; Supplementary Figure 6, P = 0.229; Supplementary Figure 9, P < 0.01; Supplementary Figure 12, P = 0.698; Supplementary Figure 15, P = 0.148; and funnel plots: Supplementary Figures 1, 4, 7, 10, 13).
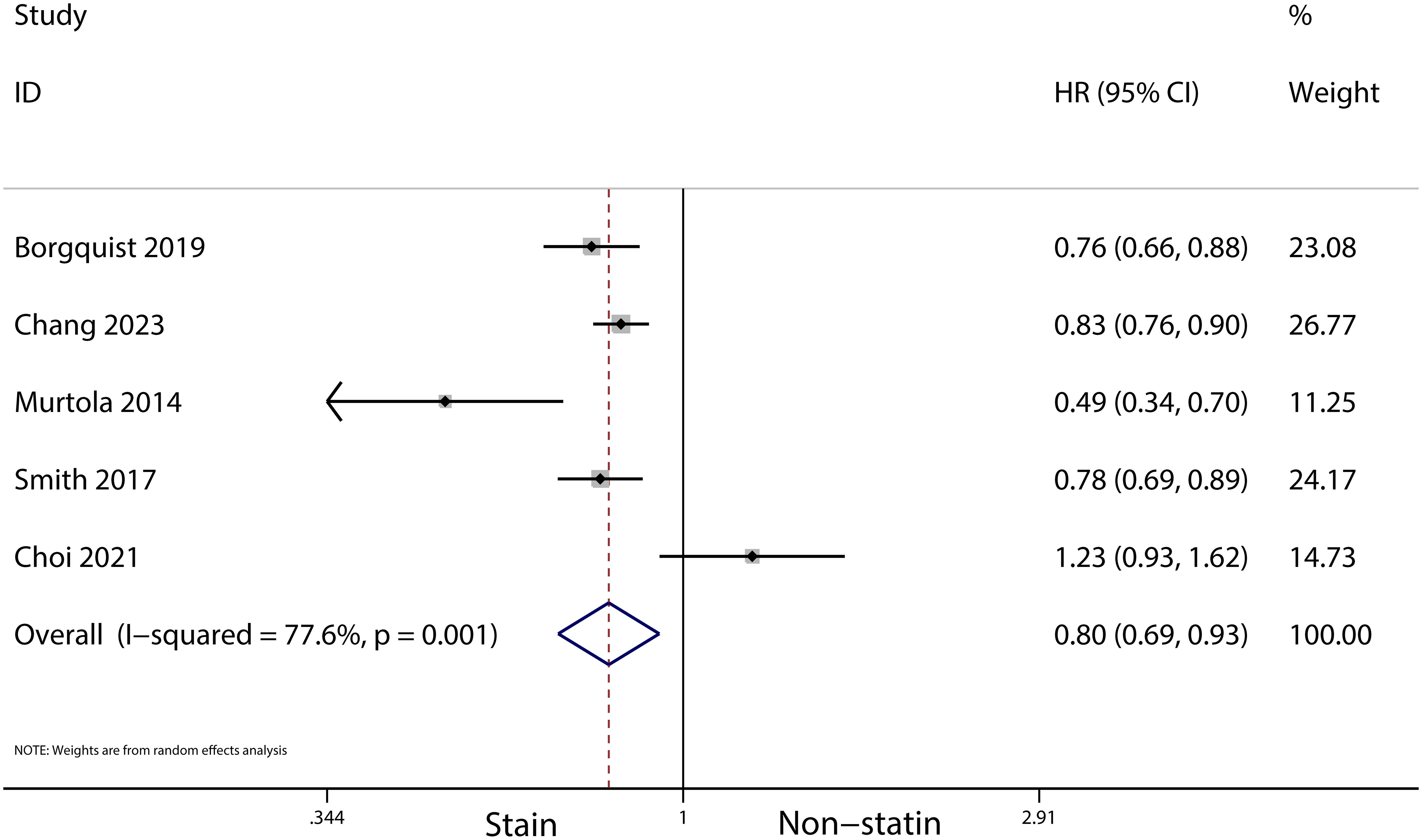
Figure 2 Forest plot of the HR of this meta-analysis for the overall mortality between statin and non-statin use in patients with prior to breast cancer.

Figure 3 Forest plot of the HR of this meta-analysis for breast cancer-specific mortality in patients with prior to diagnosis breast cancer.
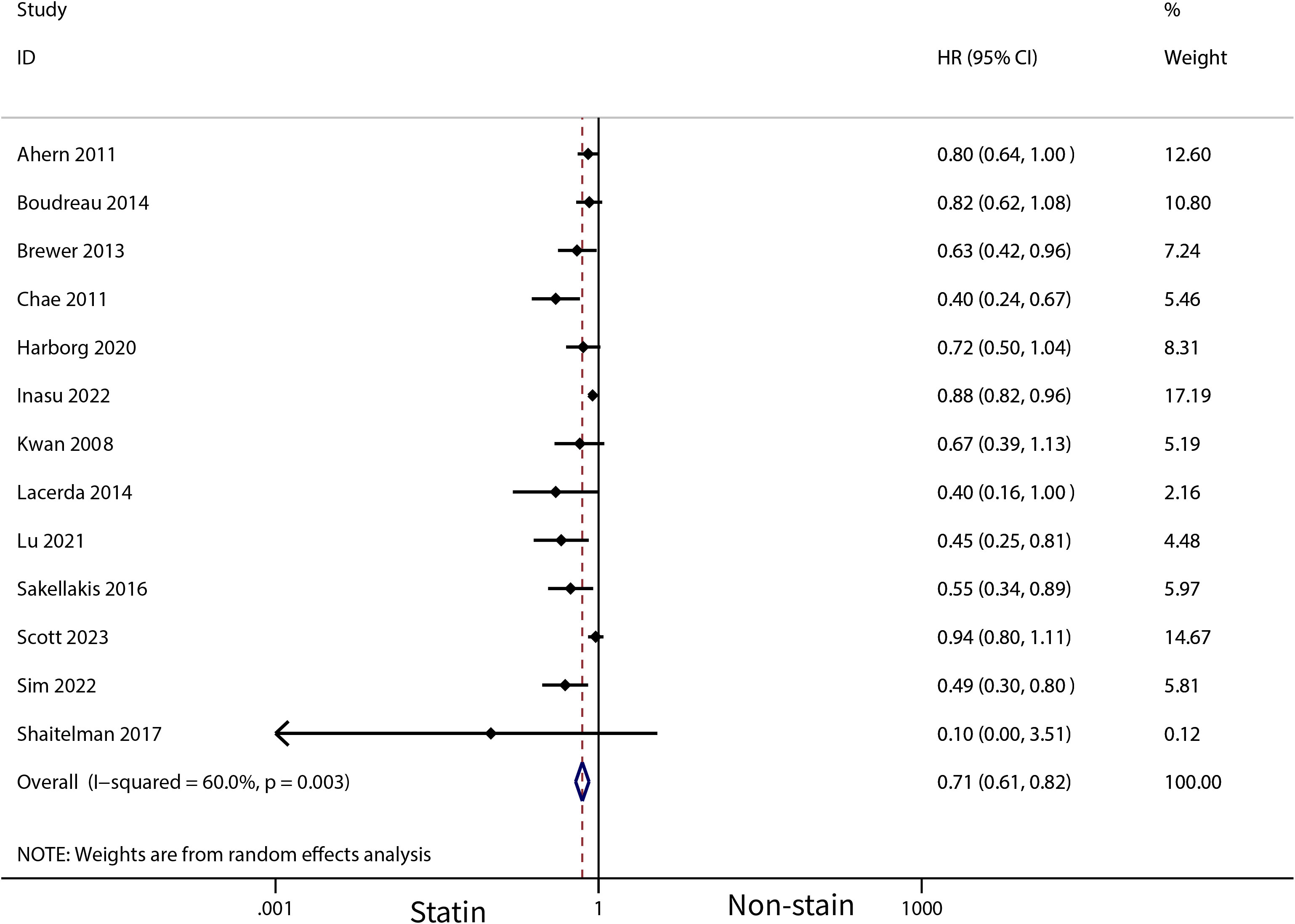
Figure 4 Forest plot of the HR of this meta-analysis for breast cancer recurrence in patients with post-diagnosis breast cancer.
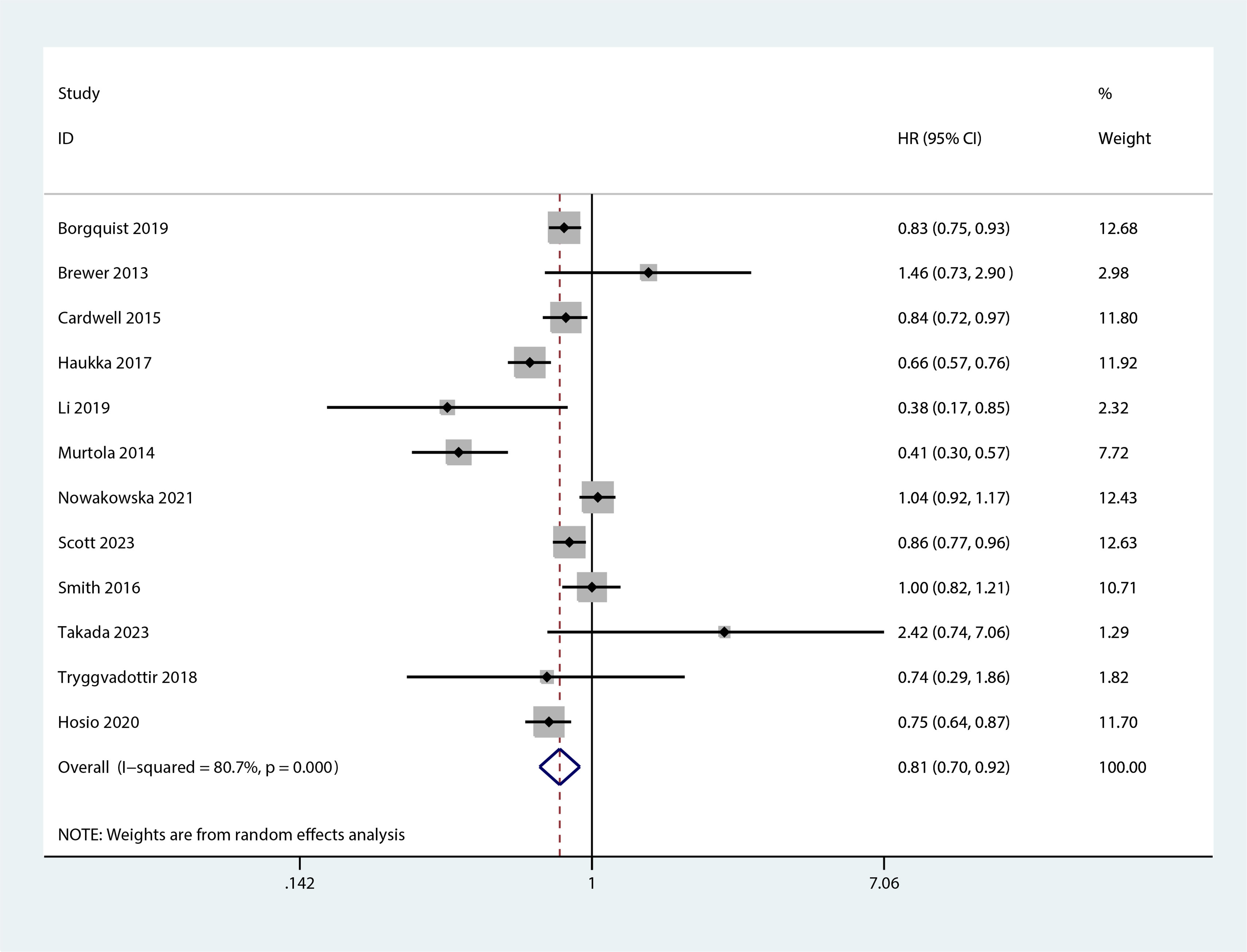
Figure 5 Forest plot of the HR of this meta-analysis for the overall mortality between statin and non-statin use in patients with post-diagnosis breast cancer.
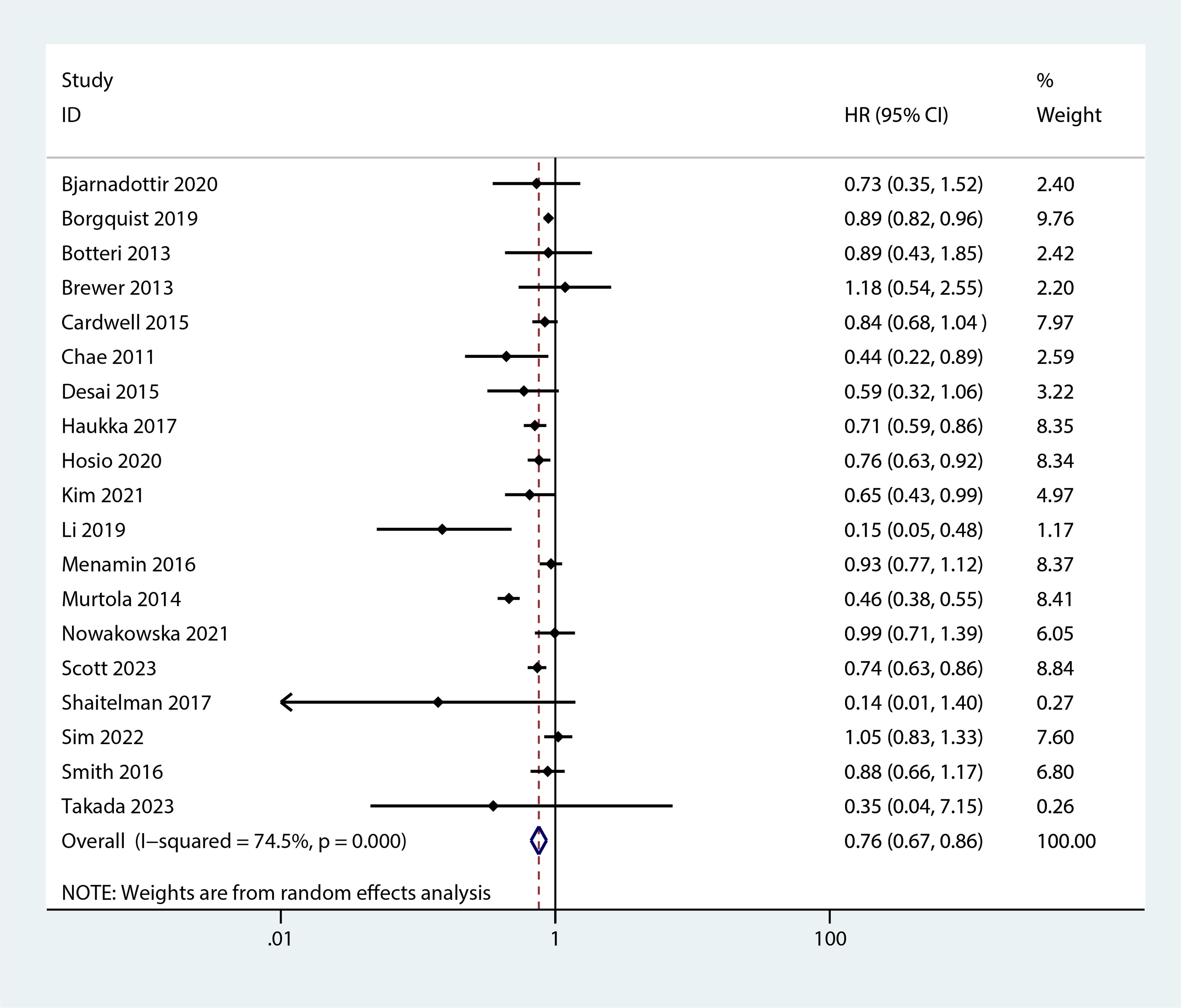
Figure 6 Forest plot of the HR of this meta-analysis for breast cancer-specific mortality prior to diagnosis in patients with post-diagnosis breast cancer.
For patients using statins for more than a year after diagnosis, a further reduced risk of recurrence was noted (HR, 0.7; 95% CI, 0.55–0.90; I2 = 37.3%, P = 0.203; Figure 7). Additionally, statin use for over six months post-diagnosis was linked with a lower risk of cancer-related death (HR, 0.71; 95% CI, 0.54–0.93, I2 = 87.7%, P < 0.001; Figure 8). Murtola et al. (28) observed that increased intensity of statin use post-diagnosis was associated with greater reductions in cancer-related deaths, especially in patients with metastatic tumors. The lowest intensity of statin use (14–183 defined daily doses/year) correlated with the smallest reduction in cancer deaths in metastatic patients (HR, 0.66; 95% CI, 0.4–1.09), while medium (184–300 defined daily doses/year) and high intensity (301 or more defined daily doses/year) were associated with greater reductions in mortality (HR, 0.43; 95% CI, 0.2–0.9 and HR, 0.42; 95% CI, 0.19–0.94, respectively).
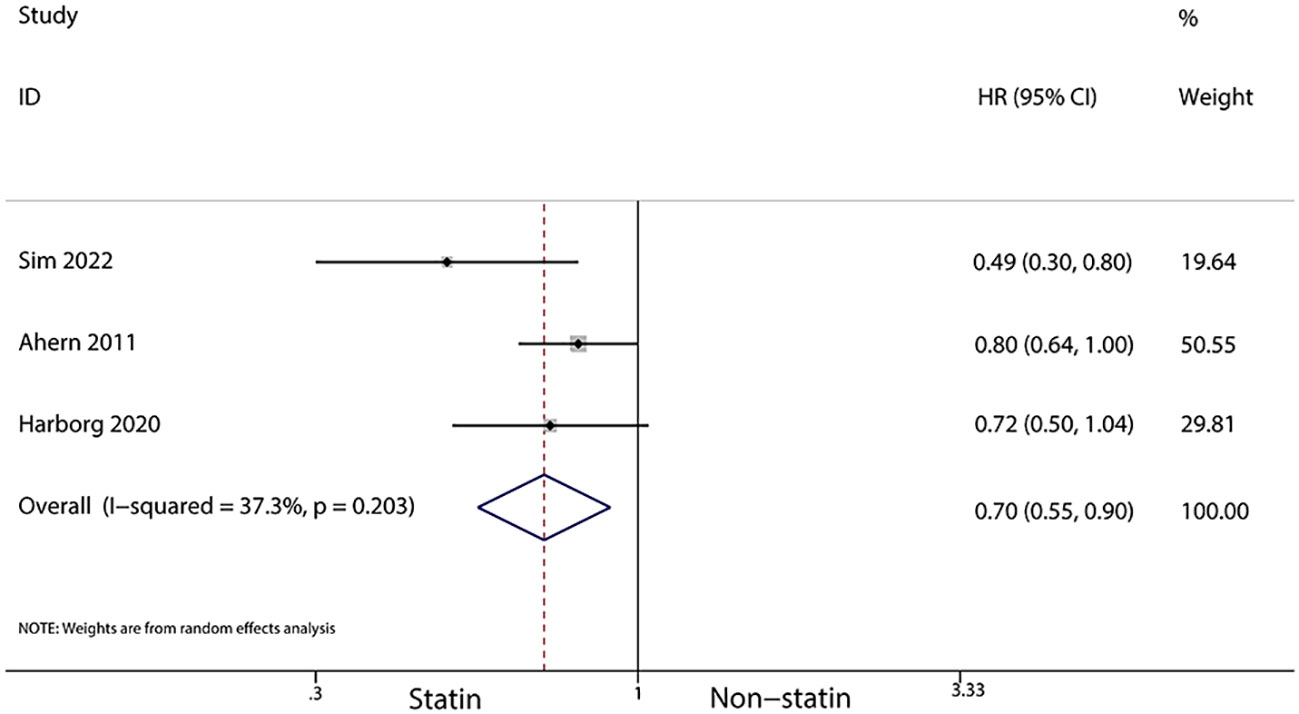
Figure 7 Forest plot of the HR of this meta-analysis of statins for more than a year after diagnosis for breast cancer recurrence in patients with breast cancer.
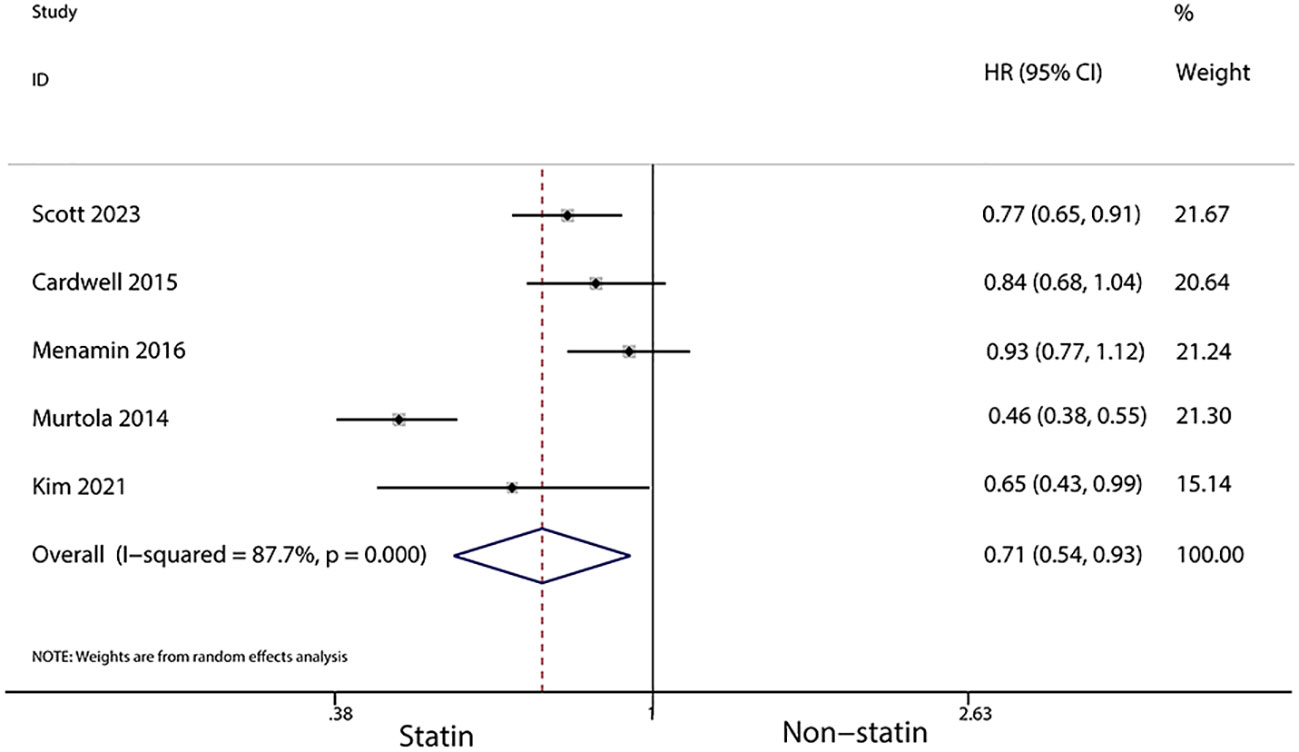
Figure 8 Forest plot of the HR of this meta-analysis of statin use for over six months post-diagnosis for cancer-related death in patients with breast cancer.
Discussion
This meta-analysis synthesized data from 31 studies involving 2,611,014 women diagnosed with breast cancer. It focused on assessing the impact of statins on the risk of overall mortality, breast cancer-specific mortality, and the recurrence of breast cancer. Our findings suggest a beneficial effect of statins, both prior to and after diagnosis, in reducing overall mortality, breast cancer-specific mortality, and the recurrence of the disease. These results are in line with a previous study (44) that reviewed 23 articles involving 178,712 breast cancer patients, which also suggested that statin use could decrease overall mortality, disease-induced mortality, and recurrence in women with breast cancer. However, the earlier meta-analysis (44) was limited to post-diagnosis statin use and did not include data on pre-diagnosis use. Other research (45) has indicated that statins may reduce disease-induced mortality and recurrence in breast cancer patients, but did not differentiate between pre- and post-diagnosis use of statins, and found no significant impact on the risk of developing breast cancer. Additionally, a study (46) involving seven studies and 197,048 women with breast cancer indicated that statins might reduce both disease-induced and overall mortality. In addition, a randomized controlled study (47) showed that statin use after diagnosis was associated with a reduction in all-cause mortality (HR, 1.16; 95% CI, 0.7–1.91). Furthermore, there is evidence (48) suggesting an enhanced effect on breast cancer reduction when pitavastatin is used alongside neoadjuvant chemotherapy protocols.
Ongoing research is examining the mechanisms through which statins may decrease mortality in breast cancer patients. The mevalonate pathway, a common feature in cancerous lesions, is upregulated and can be inhibited by statins, exerting an anti-cancer effect. Statins also have an association with ferroptosis (49) and can impair autophagy flux, leading to breast cancer cell death via the ERK1/2 and Akt pathways (50, 51). Preclinical studies suggest that statins can reduce the activity of breast cancer cells, limit their proliferation, and induce apoptosis (52). Atorvastatin may enhance antitumor immunity by inhibiting extracellular vesicles programmed death-ligand 1 (PD-L1) (53). Furthermore, altering cholesterol abundance in cancer cells could affect cellular membrane fluidity and signal transduction, influencing cancer progression (54). The role of statins in three estrogen receptor-positive cancers (breast, endometrial, and ovarian) is currently under investigation, with studies reporting a beneficial role in reducing incidence and mortality (55). In patients with estrogen-dependent breast cancer, post-diagnostic statin use has been linked to reduced cancer-specific mortality (56), potentially by disrupting hormone receptor-related pathways (57). The combination of metformin and simvastatin is reported to enhance the expression of prolyl hydroxylase 2, decreasing hypoxia-inducible factor 1α expression induced in endothelin 1, leading to more effective antiangiogenic and anti-cancer effects (58).
This meta-analysis has some limitations. Firstly, the specific types of statins used were not clearly detailed in the original studies, and different types of statins might yield different results. Secondly, while we included studies focusing on the effect of statins on the prognosis of breast cancer patients, the dosage of statins varied across these studies. Lastly, the studies included were observational, which may introduce more confounding bias and loss of follow-up.
Conclusions
In conclusion, the results of this meta-analysis indicate that the use of statins may beneficially impact the recurrence of breast cancer, as well as reduce all-cause mortality and breast cancer-specific mortality, irrespective of whether statins are used before or after a breast cancer diagnosis. However, these findings underscore the necessity for additional high-quality research to more definitively ascertain the effects of statins on the prognosis of women with breast cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
XJ: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. YL: Formal Analysis, Methodology, Software, Writing – original draft. ZX: Data curation, Investigation, Methodology, Writing – review & editing. QM: Formal Analysis, Software, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1256747/full#supplementary-material
References
1. Fahad Ullah M. Breast cancer: current perspectives on the disease status. Adv Exp Med Biol (2019) 1152:51–64.
2. Kolak A, Kamińska M, Sygit K, Budny A, Surdyka D, Kukiełka-Budny B, et al. Primary and secondary prevention of breast cancer. Ann Agric Environ Med (2017) 24(4):549–53. doi: 10.26444/aaem/75943
3. Ghoncheh M MZ, Salehiniya H. Epidemiology, incidence and mortality of breast cancer in Asia. Asian Pac J Cancer Prev (2016) 17(S3):47–52. doi: 10.7314/APJCP.2016.17.S3.47
4. Coughlin SS. Breast cancer epidemiology, prevention, and screening. Adv Exp Med Biol (2019) 1152:9–29. doi: 10.1007/978-3-030-20301-6_2
5. Winters S, Martin C, Murphy D, Shokar NK. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci (2017) 151:1–32. doi: 10.1016/bs.pmbts.2017.07.002
6. Faubion SS, Kapoor E, Moyer AM, Hodis HN, Miller VM. Statin therapy: does sex matter? Menopause (2019) 26(12):1425–35. doi: 10.1097/GME.0000000000001412
7. Rocha K, Pereira BMV, Rodrigues AC. An update on efflux and uptake transporters as determinants of statin response. Expert Opin Drug Metab Toxicol (2018) 14(6):613–24. doi: 10.1080/17425255.2018.1482276
8. Ahmadi M, Amiri S, Pecic S, Machaj F, Rosik J, Łos MJ, et al. Pleiotropic effects of statins: A focus on cancer. Biochim Biophys Acta (BBA) - Mol Basis Dis (2020) 1866(12):165968. doi: 10.1016/j.bbadis.2020.165968
9. Fatehi Hassanabad A. Current perspectives on statins as potential anti-cancer therapeutics: clinical outcomes and underlying molecular mechanisms. Trans Lung Cancer Res (2019) 8(5):692–9. doi: 10.21037/tlcr.2019.09.08
10. Barbalata CI TL, Achim M, Tomuta I, Porfire AS. Statins in risk-reduction and treatment of cancer. World J Clin Oncol (2020) 11(8):573–88. doi: 10.5306/wjco.v11.i8.573
11. Hu M-B, Zhang JW, Gao JB, Qi YW, Gao Y, Xu L, et al. Atorvastatin induces autophagy in MDA-MB-231 breast cancer cells. Ultrastructural Pathol (2018) 42(5):409–15. doi: 10.1080/01913123.2018.1522406
12. Huang SW, Chyuan IT, Shiue C, Yu MC, Hsu YF, Hsu MJ. Lovastatin-mediated MCF-7 cancer cell death involves LKB1-AMPK-p38MAPK-p53-survivin signalling cascade. J Cell Mol Med (2019) 24(2):1822–36. doi: 10.1111/jcmm.14879
13. Sim Y, Lim C, Phyu N, Tan KTB, Chew LST, Wong CY, et al. The impact of statin use and breast cancer recurrence - A retrospective study in Singapore. Front Oncol (2022) 12. doi: 10.3389/fonc.2022.835320
14. Takada K, Kashiwagi S, Iimori N, Kouhashi R, Yabumoto A, Goto W, et al. Impact of oral statin therapy on clinical outcomes in patients with cT1 breast cancer. BMC Cancer (2023) 23(1):224. doi: 10.1186/s12885-023-10631-w
15. Bjarnadottir O, Feldt M, Inasu M, Bendahl PO, Elebro K, Kimbung S, et al. Statin use, HMGCR expression, and breast cancer survival - The Malmö Diet and Cancer Study. Sci Rep (2020) 10(1):558. doi: 10.1038/s41598-019-57323-9
16. Borgquist S, Broberg P, Tojjar J, Olsson H. Statin use and breast cancer survival – a Swedish nationwide study. BMC Cancer (2019) 19(1):54. doi: 10.1186/s12885-018-5263-z
17. Haukka J, Niskanen L, Auvinen A. Risk of cause-specific death in individuals with cancer-modifying role diabetes, statins and metformin. Int J Cancer (2017) 141(12):2437–49. doi: 10.1002/ijc.31016
18. Scott OW, TinTin S, Harborg S, Kuper-Hommel MJJ, Lawrenson R, Elwood JM. Post-diagnostic statin use and breast cancer-specific mortality: a population-based cohort study. Breast Cancer Res Treat (2023) 199(1):195–206. doi: 10.1007/s10549-022-06815-w
19. Kim DS, Ahn HS, Kim HJ. Statin use and incidence and mortality of breast and gynecology cancer: A cohort study using the National Health Insurance claims database. Int J Cancer (2021) 150(7):1156–65. doi: 10.1002/ijc.33869
20. Botteri E, Munzone E, Rotmensz N, Cipolla C, De Giorgi V, Santillo B, et al. Therapeutic effect of β-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat (2013) 140(3):567–75. doi: 10.1007/s10549-013-2654-3
21. Brewer TM, Masuda H, Liu DD, Shen Y, Liu P, Iwamoto T, et al. Statin use in primary inflammatory breast cancer: a cohort study. Br J Cancer (2013) 109(2):318–24. doi: 10.1038/bjc.2013.342
22. Cardwell CR HB, Hughes C, Murray LJ. Statin use after diagnosis of breast cancer and survival: a population-based cohort study. Epidemiology (2015) 26(1):68–78. doi: 10.1097/EDE.0000000000000189
23. Chang W-T, Lin HW, Lin SH, Li YH. Association of statin use with cancer- and noncancer-associated survival among patients with breast cancer in Asia. JAMA Network Open (2023) 6(4):e239515. doi: 10.1001/jamanetworkopen.2023.9515
24. Desai P, Lehman A, Chlebowski RT, Kwan ML, Arun M, Manson JE, et al. Statins and breast cancer stage and mortality in the Women’s Health Initiative. Cancer Causes Control (2015) 26(4):529–39. doi: 10.1007/s10552-015-0530-7
25. Lacerda L, Reddy JP, Liu D, Larson R, Li L, Masuda H, et al. Simvastatin radiosensitizes differentiated and stem-like breast cancer cell lines and is associated with improved local control in inflammatory breast cancer patients treated with postmastectomy radiation. Stem Cells Trans Med (2014) 3(7):849–56. doi: 10.5966/sctm.2013-0204
26. Li YR, Ro V, Steel L, Carrigan E, Nguyen J, Williams A, et al. Impact of long-term lipid-lowering therapy on clinical outcomes in breast cancer. Breast Cancer Res Treat (2019) 176(3):669–77. doi: 10.1007/s10549-019-05267-z
27. Mc Menamin ÚC, Murray LJ, Hughes CM, Cardwell CR. Statin use and breast cancer survival: a nationwide cohort study in Scotland. BMC Cancer (2016) 16(1). doi: 10.1186/s12885-016-2651-0
28. Wright JM, Visvanathan K, Artama M, Vainio H, Pukkala E. Statin use and breast cancer survival: A nationwide cohort study from Finland. PloS One (2014) 9(10):e110231. doi: 10.1371/journal.pone.0110231
29. Nowakowska MK, Lei X, Thompson MT, Shaitelman SF, Wehner MR, Woodward WA, et al. Association of statin use with clinical outcomes in patients with triple-negative breast cancer. Cancer (2021) 127(22):4142–50. doi: 10.1002/cncr.33797
30. Shaitelman SF, Stauder MC, Allen P, Reddy S, Lakoski S, Atkinson B, et al. Impact of statin use on outcomes in triple negative breast cancer. J Cancer (2017) 8(11):2026–32. doi: 10.7150/jca.18743
31. Smith A, Murphy L, Sharp L, O'Connor D, Gallagher WM, Bennett K, et al. De novo post-diagnosis statin use, breast cancer-specific and overall mortality in women with stage I–III breast cancer. Br J Cancer (2016) 115(5):592–8. doi: 10.1038/bjc.2016.232
32. Smith A, Murphy L, Zgaga L, Barron TI, Bennett K. Pre-diagnostic statin use, lymph node status and mortality in women with stages I–III breast cancer. Br J Cancer (2017)117(4):588–96. doi: 10.1038/bjc.2017.227
33. Tryggvadottir H, Huzell L, Gustbée E, Simonsson M, Markkula A, Jirström K, et al. Interactions between ABCB1 genotype and preoperative statin use impact clinical outcomes among breast cancer patients. Front Oncol (2018) 8. doi: 10.3389/fonc.2018.00428
34. Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, et al. Statin prescriptions and breast cancer recurrence risk: A Danish nationwide prospective cohort study. JNCI J Natl Cancer Institute (2011) 103(19):1461–8. doi: 10.1093/jnci/djr291
35. Boudreau DM, Yu O, Chubak J, Wirtz HS, Bowles EJ, Fujii M, et al. Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res Treat (2014) 144(2):405–16. doi: 10.1007/s10549-014-2870-5
36. Chae YK, Valsecchi ME, Kim J, Bianchi AL, Khemasuwan D, Desai A, et al. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Invest (2011) 29(9):585–93. doi: 10.3109/07357907.2011.616252
37. Choi M, Han J, Yang BR, Jang MJ, Kim M, Lee DW, et al. Association of insulin, metformin, and statin with mortality in breast cancer patients. Cancer Res Treat (2021) 53(1):65–76. doi: 10.4143/crt.2020.430
38. Harborg S, Heide-Jørgensen U, Ahern TP, Ewertz M, Cronin-Fenton D, Borgquist S. Statin use and breast cancer recurrence in postmenopausal women treated with adjuvant aromatase inhibitors: a Danish population-based cohort study. Breast Cancer Res Treat (2020) 183(1):153–60. doi: 10.1007/s10549-020-05749-5
39. Hosio M, Urpilainen E, Hautakoski A, Marttila M, Arffman M, Sund R, et al. Survival after breast cancer in women with type 2 diabetes using antidiabetic medication and statins: a retrospective cohort study. Acta Oncol (2020) 59(9):1110–7. doi: 10.1080/0284186X.2020.1769858
40. Inasu M, Feldt M, Jernström H, Borgquist S, Harborg S. Statin use and patterns of breast cancer recurrence in the Malmö Diet and Cancer Study. Breast (2022) 61:123–8. doi: 10.1016/j.breast.2022.01.003
41. Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat (2007) 109(3):573–9. doi: 10.1007/s10549-007-9683-8
42. Lu Y-C, Huang DW, Chen PT, Tsai CF, Lin MC, Lin CC, et al. Association between statin use and second cancer risk in breast cancer patients: a nationwide population-based cohort study. Breast Cancer Res Treat (2020) 185(3):773–83. doi: 10.1007/s10549-020-05969-9
43. Sakellakis M, Akinosoglou K, Kostaki A, Spyropoulou D, Koutras A. Statins and risk of breast cancer recurrence. Breast Cancer: Targets Ther (2016) 8:199–205. doi: 10.2147/BCTT.S116694
44. Zhao G, Ji Y, Ye Q, Ye X, Wo G, Chen X, et al. Effect of statins use on risk and prognosis of breast cancer: a meta-analysis. Anti-Cancer Drugs (2021) 33(1):e507–18. doi: 10.1097/CAD.0000000000001151
45. Lv H, Shi D, Fei M, Chen Y, Xie F, Wang Z, et al. Association between statin use and prognosis of breast cancer: A meta-analysis of cohort studies. Front Oncol (2020) 10. doi: 10.3389/fonc.2020.556243
46. Liu B, Yi Z, Guan X, Zeng YX, Ma F. The relationship between statins and breast cancer prognosis varies by statin type and exposure time: a meta-analysis. Breast Cancer Res Treat (2017) 164(1):1–11. doi: 10.1007/s10549-017-4246-0
47. Alarfi H, Youssef LA, Salamoon M. A prospective, randomized, placebo-controlled study of a combination of simvastatin and chemotherapy in metastatic breast cancer. J Oncol 2020 (2020) p:4174395. doi: 10.1155/2020/4174395
48. Dewidar SA, Hamdy O, Eltantawy A, El-Mesery M, El Gayar AM, Soliman MM. Effect of concomitant use of pitavastatin with neoadjuvant chemotherapy protocols in breast cancer patients: A randomized controlled clinical trial. Saudi Pharm J (2022) 30(10):1486–96. doi: 10.1016/j.jsps.2022.07.011
49. Jiang W, Hu JW, He XR, Jin WL, He XY. Statins: a repurposed drug to fight cancer. J Exp Clin Cancer Res (2021) 40(1):241. doi: 10.1186/s13046-021-02041-2
50. Yin L, He Z, Yi B, Xue L, Sun J. Simvastatin suppresses human breast cancer cell invasion by decreasing the expression of pituitary tumor-transforming gene 1. Front Pharmacol (2020) 11. doi: 10.3389/fphar.2020.574068
51. Castellanos-Esparza Y, Wu S, Huang L, Buquet C, Shen R, Sanchez Gonzalez B, et al. Synergistic promoting effects of pentoxifylline and simvastatin on the apoptosis of triple-negative MDA-MB-231 breast cancer cells. Int J Oncol (2018) 52(4):1246–54. doi: 10.3892/ijo.2018.4272
52. Bytautaite M, Petrikaite V. Comparative study of lipophilic statin activity in 2D and 3D in vitro models of human breast cancer cell lines MDA-MB-231 and MCF-7. OncoTargets Ther (2020) 13:13201–9. doi: 10.2147/OTT.S283033
53. Choe E-J, Lee CH, Bae JH, Park JM, Park SS, Baek MC. Atorvastatin enhances the efficacy of immune checkpoint therapy and suppresses the cellular and extracellular vesicle PD-L1. Pharmaceutics (2022) 14(8):1660. doi: 10.3390/pharmaceutics14081660
54. Centonze G, Natalini D, Piccolantonio A, Salemme V, Morellato A, Arina P, et al. Cholesterol and its derivatives: multifaceted players in breast cancer progression. Front Oncol (2022) 12. doi: 10.3389/fonc.2022.906670
55. Markowska A, Antoszczak M, Markowska J, Huczyński A. Statins: HMG-coA reductase inhibitors as potential anticancer agents against Malignant neoplasms in women. Pharmaceuticals (2020) 13(12):422. doi: 10.3390/ph13120422
56. Xu W-H, Zhou Y-H. The relationship between post-diagnostic statin usage and breast cancer prognosis varies by hormone receptor phenotype: a systemic review and meta-analysis. Arch Gynecol Obstetrics (2021) 304(5):1315–21. doi: 10.1007/s00404-021-06065-z
57. Hyder T, Marti JLG, Nasrazadani A, Brufsky AM. Statins and endocrine resistance in breast cancer. Cancer Drug Resistance (2021) 4(2):356–64. doi: 10.20517/cdr.2020.112
Keywords: statins, breast cancer, overall mortality, disease-induced mortality, recurrence
Citation: Jia X, Lu Y, Xu Z and Mu Q (2023) Impact of statin use on breast cancer recurrence and mortality before and after diagnosis: a systematic review and meta-analysis. Front. Oncol. 13:1256747. doi: 10.3389/fonc.2023.1256747
Received: 11 July 2023; Accepted: 23 November 2023;
Published: 18 December 2023.
Edited by:
Connie Irene Diakos, Royal North Shore Hospital, AustraliaReviewed by:
Carmine De Angelis, University of Naples Federico II, ItalyAdam Brufsky, University of Pittsburgh Medical Center, United States
Copyright © 2023 Jia, Lu, Xu and Mu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolin Jia, MTI5MDA0NTA2OUBxcS5jb20=
†These authors have contributed equally to this work
 Xiaolin Jia
Xiaolin Jia Ye Lu
Ye Lu Zili Xu3
Zili Xu3