
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 07 November 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1256012
This article is part of the Research Topic Transarterial Chemoembolization for Hepatocellular Carcinoma Patients View all 13 articles
Purpose: Liver abscess is a rare and serious complication after transarterial chemoembolization (TACE) for liver cancer; however, its impact on the prognosis is unclear. This retrospective study examined the outcomes of patients with liver abscess formation following TACE for malignant liver tumors to elucidate the impact of liver abscess formation on the prognosis of these patients.
Methods: From January 2017 to January 2022, 1,387 patients with malignant tumors underwent 3,341 sessions of TACE at our hospital. Clinical characteristics of patients at baseline and follow-up were examined, including treatment and outcome of liver abscess, tumor response to the TACE leading to liver abscess, and overall survival time.
Results: Of 1,387 patients, 15 (1.1%) patients with liver abscess complications after TACE resulted in a total of 16 (0.5%) cases of liver abscess after 3,341 TACE sessions (including one patient with two events). After antibiotic or percutaneous catheter drainage (PCD) treatment, all the infections associated with liver abscesses were controlled. In the PCD group, eight patients died before drainage tube removal, one retained the drainage tube until the end of follow-up, and five underwent drainage tube removal; the mean drainage tube removal time was 149.17 ± 134.19 days. The efficacy of TACE leading to liver abscess was evaluated as partial response (18.75%), stable disease (37.5%), and progressive disease (43.75%). Eleven patients died during the follow-up period owing to causes unrelated to infections caused by liver abscesses. The survival rates at 3 months, 6 months, 1 year, and 5 years were 86.7%, 50.9%, 25.5%, and 17%, respectively.
Conclusion: Patients with liver abscess formation following TACE for malignant liver tumors experienced prolonged drainage tube removal time after PCD; while this condition did not directly cause death, it indirectly contributed to a poor prognosis in these patients.
Transarterial chemoembolization (TACE) is an effective method for the treatment of unresectable malignant liver tumors (1, 2). With the development of embolization concepts and advances in embolization materials, TACE has become the preferred non-surgical treatment for unresectable primary liver cancer and liver metastases (3). It can embolize multiple lesions in a single course of treatment and can be applied repeatedly to the same patient (4).
However, TACE has a series of complications, including post-embolization syndrome (PES), hepatic impairment, and leukopenia, which are common and mild (5). In contrast, a liver abscess is a rare and serious complication. Its incidence is approximately 0.22%–4.46% (5, 6), and it may prolong hospital stay, delay tumor treatment, and even lead to death from severe infection (7–9). The risk factors for liver abscess after TACE include diabetes, biliary abnormalities, large tumor size, and portal vein occlusion (10–13).
Antibiotics combined with percutaneous catheter drainage (PCD) is the mainstream method for the treatment of liver abscess, but there are only a few studies on the therapeutic effect of liver abscess after TACE (8, 9, 12, 13). In addition, the relationship between TACE-induced liver abscess formation and tumor prognosis remains poorly understood. Abscess formation may promote tumor necrosis, but its inflammatory microenvironment may trigger tumor proliferation, migration, and other malignant processes.
Thus, this study aimed to retrospectively examine the outcomes of patients who developed liver abscesses following TACE to elucidate the impact of liver abscess formation on the prognosis of these patients.
This study was approved by the ethics committee of our hospital and complies with the principles of the Declaration of Helsinki. Owing to the retrospective nature of this study, the informed consent requirement was waived. The data were anonymized to protect patient privacy. Data were extracted from electronic medical records of patients with malignant liver tumors treated with TACE between January 2017 and January 2022. The characteristics of patients with complicated liver abscesses were reviewed.
The variables of interest included sex, age, comorbidities (e.g., diabetes), tumor status (primary/secondary), tumor diameter, liver function (Child–Pugh class), major surgical history, TACE status (session count, embolic materials used, and session duration), and liver abscess status (clinical symptoms, diagnosis time, and treatment method).
Follow-up assessments included the following variables: liver abscess healed, tumor response, drainage tube removal time, and survival status.
The diagnostic criteria for liver abscess included contrast-enhanced computed tomography (CT) images showing one or more focal hypodense lesions in the liver that may contain gas and the observation of persistent fever or chills. In addition, one of the following two conditions had to be met: a) blood culture was positive for bacteria; b) aspirated fluid contained a typical purulent substance or was positive for pus culture.
In our clinical practice, broad-spectrum antibiotics were applied to patients with suspected liver abscesses after TACE (14). If the infection was not effectively controlled after simple antibiotic treatment, PCD was performed. Infection control was defined as the disappearance of associated clinical symptoms and infection-related indicators returned to normal. The criteria for post-PCD tube removal included the absence of clinical symptoms and a drainage volume of <10 mL/day for three consecutive days and the follow-up CT scan findings showing no evidence of the abscess or <2 cm in size.
All patients with liver abscess formation after TACE were followed up. All patients underwent contrast-enhanced CT/magnetic resonance imaging (MRI) scanning 10–60 days after liver abscess formation to determine liver abscess healed and tumor response. Subsequently, patients were followed up every 2–3 months. Patients who could not visit the hospital were followed up via telephone. Survival was the outcome of interest. If patients developed a second liver abscess, it was recorded as a follow-up endpoint for the first liver abscess. The deadline for the follow-up was December 31, 2022.
Liver abscess healed was defined as infection under control, which no longer needed PCD or intravenous antibiotics. Tumor response represented the therapeutic efficacy of TACE leading to liver abscess formation, which was determined based on contrast-enhanced CT/MRI findings within 1–3 months after TACE using the modified Response Evaluation Criteria in Solid Tumors (15).
Data were expressed as means ± standard deviations or percentages. Statistical analysis was performed using SPSS 26.0 (IBM SPSS, Armonk, NY, USA).
A total of 1,387 patients with malignant liver tumors underwent 3,341 TACE sessions at our hospital between January 2017 and January 2022. Among them, 15 (1.1%) cases were complicated with liver abscess, resulting in a total of 16 (0.5%) liver abscess cases after 3,341 TACE sessions (including one patient with two events) (Figures 1A–C). The patients’ baseline characteristics are presented in Table 1.
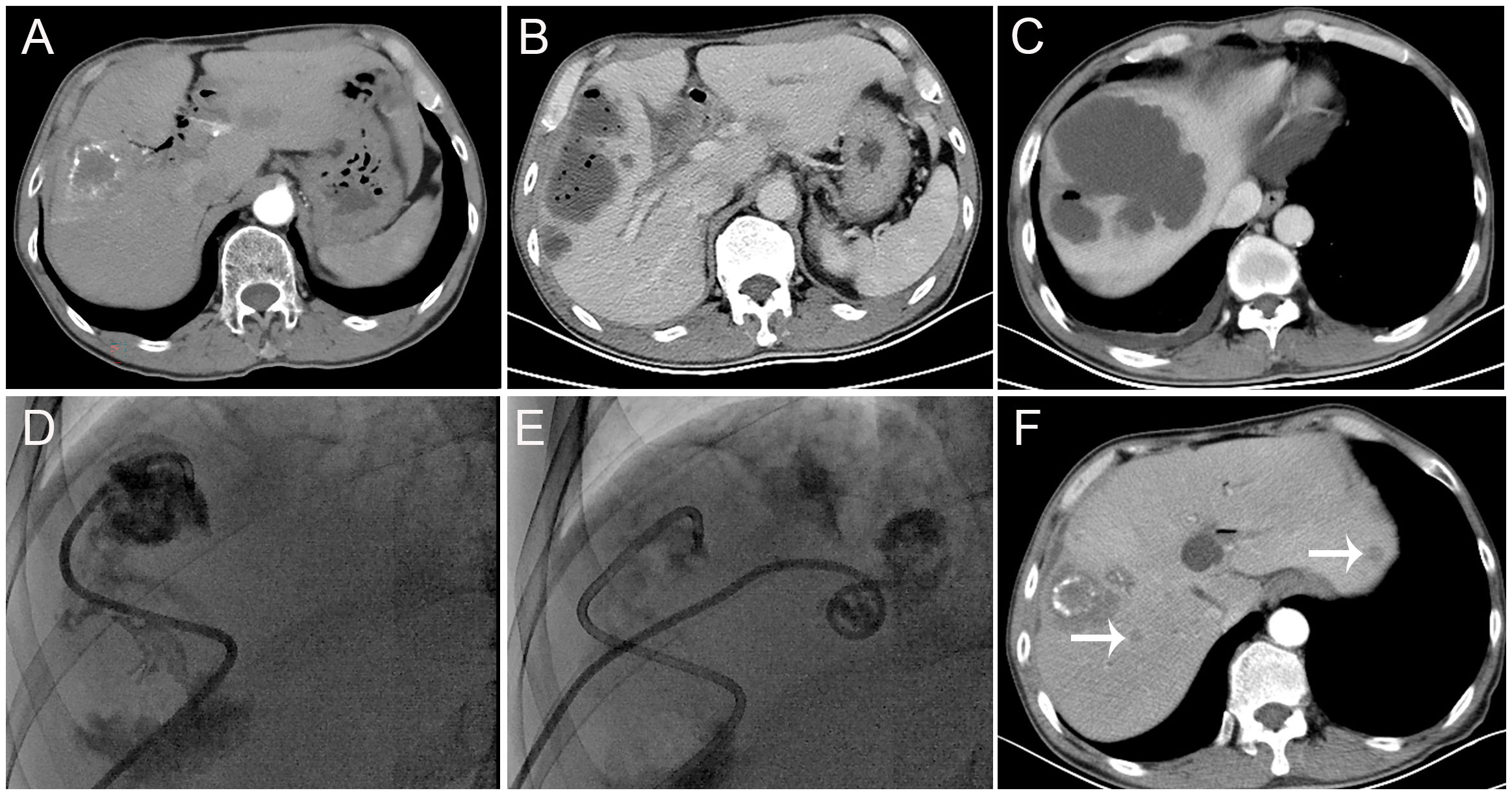
Figure 1 Findings from a 62-year-old patient with liver metastasis of cholangiocarcinoma and a history of biliary-enteric anastomosis and one previous transarterial chemoembolization (TACE) session. (A) Before the TACE session, contrast-enhanced computed tomography scans revealed hypodense lesions in segment V of the liver, with no significant enhancement. A small amount of iodized-oil emulsion deposit was observed, and the intrahepatic bile duct showed multiple pneumobilia and dilatations. (B, C) Thirteen days after this TACE session, contrast-enhanced computed tomography scans revealed the formation of multiple gas-containing liver abscesses. (D, E) Subsequently, the patient underwent percutaneous drainage (PCD). (F) One month after PCD, contrast-enhanced computed tomography scans showed an increase in tumor size and multiple new metastases in the liver (arrow).
Eight (50%) TACE sessions were “first” sessions; the mean number of sessions received was 2.31 ± 2.2 (range, 1–9). The mean maximum tumor diameter was 7.09 ± 2.86 (range, 2.8–12.4) cm. Five, one, and 10 TACE sessions were performed using iodized-oil emulsion (IOE), IOE and gelatin sponge particles, and drug-eluting beads (100–300 μm), respectively. The mean TACE duration was 109.06 ± 37.87 (range, 60–180) min. The mean interval from TACE to the diagnosis of liver abscess formation was 14.19 ± 9.21 (range, 3–40) days. The most common symptom was fever (93.8%), followed by chills (43.8%) and abdominal pain (31.3%). One patient received antibiotics only, while 14 patients received PCD combined with antibiotic treatment (Figures 1D, E). The infection caused by liver abscesses was effectively controlled in all patients after treatment. Enterococcus faecalis (31.25%) was the most common bacterial species detected in blood/pus cultures (Table 2).
Follow-up assessments after PCD revealed six (40%) abscess cases were healed, nine (60%) abscess cases did not heal completely, eight patients died before drainage tube removal, one patient retained the drainage tube until the end of the follow-up, and five patients underwent drainage tube removal. The mean drainage tube removal time was 149.17 ± 134.19 (range, 73–420) days. A total of zero, three (18.75%), six (37.5%), and seven (43.75%) cases showed complete response (CR), partial response, stable disease, and progressive disease (PD), respectively (Figure 1F). Eleven patients died during the follow-up period owing to causes unrelated to infections caused by liver abscesses (Table 3). The survival rates at 3 months, 6 months, 1 year, and 5 years were 86.7%, 50.9%, 25.5%, and 17%, respectively.
PCD is an effective treatment for liver abscess formation after TACE to control infection (13, 16); however, our study showed that its cure rate was low, mainly due to the inability to remove the drainage tube. In addition, the drainage tube removal time in this study was longer than that in patients with community-acquired liver abscesses (17, 18). Delayed or failed extubation may be the result of the connection between abscesses and bile ducts or even biloma formation (19).
One study has argued that complete liquefactive necrosis of tumor lesions may be indicative of a good prognosis and that the discharge of necrotic tumor tissue fluid via the drainage tube may have positive implications for tumor treatment (13). However, the findings of our study were not consistent with previous findings. In this study, no cases were evaluated as CR, while cases with PD accounted for 43.75%. Furthermore, 11 patients died during the follow-up period. The formation of a liver abscess delayed subsequent treatment and was bound to affect the prognosis, although the infection caused by the abscess could be controlled. Liver abscess was not directly related to death, but it led indirectly to poor prognosis in these patients.
History of biliary-enteric anastomosis is an important risk factor for liver abscess formation (6, 9, 10). In this study, 53.3% of the patients had a history of biliary surgery. Some studies have found that Oddi sphincter dysfunction or incision permitted retrograde intestinal bacteria entry into and colonization of the bile duct (8, 20). Meanwhile, the local toxicity of chemoembolic agents and the embolization of bile duct feeding vessels resulting from TACE may lead to bile duct injury (21). In this study, patients had received an average of 2.31 ± 2.24 TACE sessions at the time of liver abscess formation; among them, one patient developed liver abscesses after the second and ninth TACE sessions. Bile duct injury following TACE may enable opportunistic pathogens to colonize the bile duct and enter the liver parenchyma, where they undergo rapid proliferation within the local ischemic and hypoxic microenvironment after TACE, thus leading to liver abscess formation (6, 22, 23).
Diabetes is also thought to be a predisposing factor for liver abscess formation after TACE.
Diabetes is also thought to be a predisposing factor for liver abscess formation after TACE (9, 10). On the one hand, the chronic inflammatory state that arises in diabetes leads to continuous reactive oxygen species (ROS) production, and ROS stimulate the expression of pro-inflammatory cytokines by activating transcription factors such as nuclear factor-kappa B (24). In addition, in diabetic patients, excess adipose tissue secretes pro-inflammatory cytokines, further amplifying oxidative stress (25). On the other hand, hyperglycemia in diabetes is thought to cause dysfunction of the immune response, which fails to control the spread of invading pathogens in diabetic subjects, through the following mechanisms: impairment of cytokine production, leukocyte recruitment inhibition, defects in pathogen recognition, neutrophil dysfunction, macrophage dysfunction, natural killer cell dysfunction, and inhibition of antibodies and complement effector (26).
In this study, 87.5% of the tumors had a maximum diameter of >5 cm. Large tumor size and extensive use of embolic materials may have simultaneously increased the extent of intrahepatic necrosis and the risk of abscess formation (9). In addition, portal vein tumor thrombosis and gelatin sponge use have been associated with liver abscess formation (8). Owing to the small sample size, we could not examine the association between these risk factors and liver abscess formation.
It has been suggested that patients with liver metastases are more likely to develop liver abscesses after TACE compared with hepatocellular carcinoma (HCC) (27). In HCC, high concentrations of chemoembolic agents in the tumor tissue after infusion can be achieved because the arteries that feed the tumor and intratumoral blood space are mostly dilated. On the contrary, the feeding artery and the intratumoral blood space of metastatic liver tumors are not usually dilated, thus decreasing the intratumoral concentration of chemoembolic agents. This may result in higher concentrations of the chemoembolic agents in the surrounding liver parenchyma and initiation of biliary epithelial damage. Additionally, HCC is mostly noted in cirrhotic livers that are known to have dilation of the perivascular plexus, which can serve as a porto-arterial shunt and compensate for the decreased arterial flow (27, 28). Therefore, they may be the reasons for the higher incidence of liver abscesses in patients with liver metastases.
Prevention of the occurrence of liver abscesses after TACE has been the focus of clinical attention. Incidentally, it is unreasonable and unnecessary to use antibiotics prophylactically for all patients with TACE. It has been shown that prophylactic antibiotics reduce the incidence of liver abscess formation in patients with bile duct injury (29, 30). Moreover, previous studies have shown that the incidence of liver abscess formation does not increase among patients without bile duct injury who do not receive prophylactic antibiotics (30, 31). Therefore, stratification according to risk factors is more reasonable for the administration of prophylactic antibiotics before TACE.
In this study, 15 of 1,387 (1.1%) patients undergoing TACE subsequently developed liver abscesses; this rate was similar to that previously reported (5–13, 16, 19, 27, 32–36) (Table 4). Although the incidence of liver abscess formation after TACE is low, it is necessary to grasp its clinical symptoms. In our study, the most common symptom reported by patients was fever (93.8%), followed by chills (43.8%) and abdominal pain (31.3%). However, PES characterized by fever, right upper quadrant pain, nausea, and vomiting is the most common adverse event, affecting approximately 60%–80% of patients. PES generally occurs immediately after TACE, with fever peaking within 2 days after TACE, and it is often self-limiting and does not require antibiotic treatment (37–40). In contrast, in this study, the mean interval from TACE to the discovery of liver abscess was 14.19 ± 9.21 (range, 3–40) days, suggesting that the onset of liver abscess formation occurred later than PES at the time point after TACE. Furthermore, chills are frequently observed among patients with liver abscesses but not in those with PES, which may help differentiate between these outcomes.
This study has some limitations. First, as this was a single-center study, the results cannot be generalized. Second, as this was a retrospective study, our data integrity may not be as high as that in prospective studies or clinical trials. Third, as some patients after TACE were subsequently lost to follow-up, this study may have underestimated the incidence of liver abscess formation following TACE. Fourth, the low incidence of post-TACE liver abscess and small sample size precluded comparative analyses of prognosis in patients without liver abscess formation. Moreover, we could not use univariate and multivariate Cox regression statistical methods to reach more robust conclusions.
In conclusion, patients with liver abscess formation following TACE for malignant liver tumors had a long drainage tube removal time after PCD and even required continuous canalization. Although liver abscess formation was not directly related to death, it indirectly led to poor prognosis in these patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
This study protocol was reviewed and approved by the Ethics Committee of Shengjing Hospital of China Medical University (protocol number 2022PS1175K). The studies were conducted in accordance with the local legislation and institutional requirements. Owing to the retrospective nature of this study, the informed consent requirement was waived.
YW: Writing – original draft. ZC: Data curation, Funding acquisition, Writing – review & editing. JHZ: Formal Analysis, Writing – review & editing. ZL: Data curation, Writing – original draft. JZ: Writing – review & editing.
The study was supported by the National Natural Science Foundation of China (Grant No. 81901856) and the 345 Talent Project of the Shengjing Hospital of China Medical University.
We are grateful to all the authors for their contributions to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatol (Baltimore Md) (2003) 37(2):429–42. doi: 10.1053/jhep.2003.50047
2. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatol (Baltimore Md) (2010) 52(2):762–73. doi: 10.1002/hep.23725
3. Zhong BY, Jin ZC, Chen JJ, Zhu HD, Zhu XL. Role of transarterial chemoembolization in the treatment of hepatocellular carcinoma. J Clin Trans hepatol (2023) 11(2):480–9. doi: 10.14218/jcth.2022.00293
4. Xia J, Ren Z, Ye S, Sharma D, Lin Z, Gan Y, et al. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol (2006) 59:40712. doi: 10.016/j.ejrad.2006.03.002
5. Xia J, Ren Z, Ye S, Sharma D, Lin Z, Gan Y, et al. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J radiol (2006) 59(3):407–12. doi: 10.1016/j.ejrad.2006.03.002
6. Kim W, Clark TW, Baum RA, Soulen MC. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc interventional Radiol JVIR (2001) 12(8):965–8. doi: 10.1016/s1051-0443(07)61577-2
7. Reed RA, Teitelbaum GP, Daniels JR, Pentecost MJ, Katz MD. Prevalence of infection following hepatic chemoembolization with cross-linked collagen with administration of prophylactic antibiotics. J Vasc interventional Radiol JVIR (1994) 5(2):367–71. doi: 10.1016/s1051-0443(94)71504-9
8. Song SY, Chung JW, Han JK, Lim HG, Koh YH, Park JH, et al. Liver abscess after transcatheter oily chemoembolization for hepatic tumors: incidence, predisposing factors, and clinical outcome. J Vasc interventional Radiol JVIR (2001) 12(3):313–20. doi: 10.1016/s1051-0443(07)61910-1
9. Sun W, Xu F, Li X, Li CR. A case series of liver abscess formation after transcatheter arterial chemoembolization for hepatic tumors. Chin Med J (Engl) (2017) 130(11):1314–9. doi: 10.4103/0366-6999.206345
10. Shin JU, Kim KM, Shin SW, Min SY, Park SU, Sinn DH, et al. A prediction model for liver abscess developing after transarterial chemoembolization in patients with hepatocellular carcinoma. Digestive liver Dis (2014) 46(9):813–7. doi: 10.1016/j.dld.2014.05.003
11. de Baère T, Roche A, Amenabar JM, Lagrange C, Ducreux M, Rougier P, et al. Liver abscess formation after local treatment of liver tumors. Hepatol (Baltimore Md) (1996) 23(6):1436–40. doi: 10.1002/hep.510230620
12. Arslan M, Degirmencioglu S. Liver abscesses after transcatheter arterial embolization. J Int Med Res (2019) 47(3):1124–30. doi: 10.1177/0300060518816875
13. Jia Z, Tu J, Cao C, Wang W, Zhou W, Ji J, et al. Liver abscess following transarterial chemoembolization for the treatment of hepatocellular carcinoma: A retrospective analysis of 23 cases. J Cancer Res Ther (2018) 14(Supplement):S628–s633. doi: 10.4103/0973-1482.199385
14. Luo M, Yang XX, Tan B, Zhou XP, Xia HM, Xue J, et al. Distribution of common pathogens in patients with pyogenic liver abscess in China: a meta-analysis. Eur J Clin Microbiol Infect Dis (2016) 35(10):1557–65. doi: 10.1007/s10096-016-2712-y
15. Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J hepatol (2020) 72(2):288–306. doi: 10.1016/j.jhep.2019.09.026
16. Lv WF, Lu D, He YS, Xiao JK, Zhou CZ, Cheng DL. Liver abscess formation following transarterial chemoembolization: clinical features, risk factors, bacteria spectrum, and percutaneous catheter drainage. Medicine (2016) 95(17):e3503. doi: 10.1097/md.0000000000003503
17. Jindal A, Pandey A, Sharma MK, Mukund A, Vijayaraghavan R, Arora V, et al. Management practices and predictors of outcome of liver abscess in adults: A series of 1630 patients from a liver unit. J Clin Exp hepatol (2021) 11(3):312–20. doi: 10.1016/j.jceh.2020.10.002
18. Haider SJ, Tarulli M, McNulty NJ, Hoffer EK. Liver abscesses: factors that influence outcome of percutaneous drainage. AJR Am J roentgenol (2017) 209(1):205–13. doi: 10.2214/ajr.16.17713
19. Zhu M, Li G, Chen Y, Gong G, Guo W. Clinical features and treatment of hepatic abscesses with biloma formation after transcatheter arterial chemoembolization. Arab J Gastroenterol (2022) 23(1):32–8. doi: 10.1016/j.ajg.2021.12.002
20. Halpenny DF, Torreggiani WC. The infectious complications of interventional radiology based procedures in gastroenterology and hepatology. J gastrointestinal liver Dis JGLD. (2011) 20(1):71–5.
21. Xu H, Yu X, Hu J. The risk assessment and clinical research of bile duct injury after transcatheter arterial chemoembolization for hepatocellular carcinoma. Cancer Manage Res (2021) 13:5039–52. doi: 10.2147/cmar.S303172
22. Moon E, Tam MD, Kikano RN, Karuppasamy K. Prophylactic antibiotic guidelines in modern interventional radiology practice. Semin interventional radiol (2010) 27(4):327–37. doi: 10.1055/s-0030-1267853
23. Geschwind JF, Kaushik S, Ramsey DE, Choti MA, Fishman EK, Kobeiter H. Influence of a new prophylactic antibiotic therapy on the incidence of liver abscesses after chemoembolization treatment of liver tumors. J Vasc interventional Radiol JVIR (2002) 13(11):1163–6. doi: 10.1016/s1051-0443(07)61959-9
24. Caturano A, D'Angelo M, Mormone A, Russo V, Mollica MP, Salvatore T, et al. Oxidative stress in type 2 diabetes: impacts from pathogenesis to lifestyle modifications. Curr Issues Mol Biol (2023) 45(8):6651–66. doi: 10.3390/cimb45080420
25. Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J physiology pathophysiol Pharmacol (2019) 11(3):45–63.
26. Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev (2020) 16(5):442–9. doi: 10.2174/1573399815666191024085838
27. Sakamoto I, Aso N, Nagaoki K, Matsuoka Y, Uetani M, Ashizawa K, et al. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics Rev Publ Radiological Soc North America Inc. (1998) 18(3):605–19. doi: 10.1148/radiographics.18.3.9599386
28. Lee HN, Hyun D. Complications related to transarterial treatment of hepatocellular carcinoma: A comprehensive review. Korean J radiol (2023) 24(3):204–23. doi: 10.3348/kjr.2022.0395
29. Wang Q, Hodavance M, Ronald J, Suhocki PV, Kim CY. Minimal risk of biliary tract complications, including hepatic abscess, after transarterial embolization for hepatocellular carcinoma using concentrated antibiotics mixed with particles. Cardiovasc interventional radiol (2018) 41(9):1391–8. doi: 10.1007/s00270-018-1989-x
30. Watchmaker JM, Lipnik AJ, Fritsche MR, Baker JC, Mouli SK, Geevarghese S, et al. Are prophylactic antibiotics necessary prior to transarterial chemoembolization for hepatocellular carcinoma in patients with native biliary anatomy? J Surg Oncol (2018) 117(6):1312–7. doi: 10.1002/jso.24993
31. Chen J, Wu G. Retrospective Analysis of Chinese Patients with Hepatocellular Carcinoma (HCC) undergoing Transcatheter Arterial Chemoembolization (TACE) with or without Prophylactic Antibiotic Therapy. J Coll Physicians Surgeons–Pakistan JCPSP (2018) 28(12):914–8. doi: 10.29271/jcpsp.2018.12.914
32. Huang SF, Ko CW, Chang CS, Chen GH. Liver abscess formation after transarterial chemoembolization for Malignant hepatic tumor. Hepato-gastroenterology (2003) 50(52):1115–8.
33. Tarazov PG, Polysalov VN, Prozorovskij KV, Grishchenkova IV, Rozengauz EV. Ischemic complications of transcatheter arterial chemoembolization in liver Malignancies. Acta radiologica (Stockholm Sweden 1987) (2000) 41(2):156–60. doi: 10.1080/028418500127344966
34. Gates J, Hartnell GG, Stuart KE, Clouse ME. Chemoembolization of hepatic neoplasms: safety, complications, and when to worry. Radiographics Rev Publ Radiological Soc North America Inc. (1999) 19(2):399–414. doi: 10.1148/radiographics.19.2.g99mr08399
35. Chen C, Chen PJ, Yang PM, Huang GT, Lai MY, Tsang YM, et al. Clinical and microbiological features of liver abscess after transarterial embolization for hepatocellular carcinoma. Am J gastroenterol (1997) 92(12):2257–9.
36. Farinati F, De Maria N, Marafin C, Herszènyi L, Del Prato S, Rinaldi M, et al. Unresectable hepatocellular carcinoma in cirrhosis: survival, prognostic factors, and unexpected side effects after transcatheter arterial chemoembolization. Digestive Dis Sci (1996) 41(12):2332–9. doi: 10.1007/bf02100123
37. Jun CH, Ki HS, Lee HK, Park KJ, Park SY, Cho SB, et al. Clinical significance and risk factors of postembolization fever in patients with hepatocellular carcinoma. World J gastroenterol (2013) 19(2):284–9. doi: 10.3748/wjg.v19.i2.284
38. Shim JH, Park JW, Choi JI, Kim HB, Lee WJ, Kim CM. Does postembolization fever after chemoembolization have prognostic significance for survival in patients with unresectable hepatocellular carcinoma? J Vasc interventional Radiol JVIR (2009) 20(2):209–16. doi: 10.1016/j.jvir.2008.10.021
39. Prajapati HJ, Rafi S, El-Rayes BF, Kauh JS, Kooby DA, Kim HS. Safety and feasibility of same-day discharge of patients with unresectable hepatocellular carcinoma treated with doxorubicin drug-eluting bead transcatheter chemoembolization. J Vasc interventional Radiol JVIR (2012) 23(10):1286–93.e1. doi: 10.1016/j.jvir.2012.07.003
Keywords: transarterial chemoembolization, percutaneous drainage, liver abscess, malignant liver tumors, prognosis
Citation: Wang Y, Chang Z, Zheng J, Liu Z and Zhang J (2023) The impact of liver abscess formation on prognosis of patients with malignant liver tumors after transarterial chemoembolization. Front. Oncol. 13:1256012. doi: 10.3389/fonc.2023.1256012
Received: 10 July 2023; Accepted: 23 October 2023;
Published: 07 November 2023.
Edited by:
John Gibbs, Hackensack Meridian Health, United StatesReviewed by:
Yahua Li, First Affiliated Hospital of Zhengzhou University, ChinaCopyright © 2023 Wang, Chang, Zheng, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhang, emhhbmdqdW5zanl5QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.